Abstract
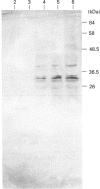
Cells from primary rat astrocyte cultures express a 36.5 kDa protein that cross-reacts with polyclonal antibodies to the catalytic subunit of rat hepatic glucose-6-phosphatase on Western blotting. Glucose-6-phosphate-hydrolysing activity of the order of 10 nmol/min per mg of total cellular protein can be demonstrated in cell homogenates. This activity shows latency, and is localized to the microsomal fraction. Kinetic analysis shows a Km of 15 mM and a Vmax. of 30 nmol/min per mg of microsomal protein in disrupted microsomes. Approx. 40% of the total phosphohydrolase activity is specific glucose-6-phosphatase, as judged by sensitivity to exposure to pH 5 at 37 degrees C. Previous reports that the brain microsomal glucose-6-phosphatase system does not distinguish glucose 6-phosphate and mannose 6-phosphate are confirmed in astrocyte microsomes. However, we demonstrate significant phosphomannose isomerase activity in brain microsomes, allowing for ready interconversion between mannose 6-phosphate and glucose 6-phosphate (Vmax. 15 nmol/min per mg of microsomal protein; apparent Km < 1 mM; pH optimum 5-6 for the two-step conversion). This finding invalidates the past inference from the failure of brain microsomes to distinguish mannose 6-phosphate and glucose 6-phosphate that the cerebral glucose-6-phosphatase system lacks a 'glucose 6-phosphate translocase' [Fishman and Karnovsky (1986) J. Neurochem. 46, 371-378]. Furthermore, light-scattering experiments confirm that a proportion of whole brain microsomes is readily permeable to glucose 6-phosphate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ali S. Y., Robinson N. Ultrastructural demonstration of glucose 6-phosphatase in cerebral cortex. Histochemistry. 1981;72(1):107–111. doi: 10.1007/BF00496785. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M., von Dippe P., Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988 Jun 15;263(17):8338–8343. [PubMed] [Google Scholar]
- Anchors J. M., Karnovsky M. L. Purification of cerebral glucose-6-phosphatase. An enzyme involved in sleep. J Biol Chem. 1975 Aug 25;250(16):6408–6416. [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981 Aug;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash V., Riskin A., Shafrir E., Waddell I. D., Burchell A. Kinetic and immunologic evidence for the absence of glucose-6-phosphatase in early human chorionic villi and term placenta. Biochim Biophys Acta. 1991 Jan 23;1073(1):161–167. doi: 10.1016/0304-4165(91)90197-o. [DOI] [PubMed] [Google Scholar]
- Bass L., Bodsch W., Robinson P. J., Young M. O. Metabolites of 2-deoxyglucose in rat brain at 12-24 h: bounds on kinetic constants. Am J Physiol. 1987 Oct;253(4 Pt 1):E453–E460. doi: 10.1152/ajpendo.1987.253.4.E453. [DOI] [PubMed] [Google Scholar]
- Berteloot A., Vidal H., van de Werve G. Rapid kinetics of liver microsomal glucose-6-phosphatase. Evidence for tight-coupling between glucose-6-phosphate transport and phosphohydrolase activity. J Biol Chem. 1991 Mar 25;266(9):5497–5507. [PubMed] [Google Scholar]
- Bignami A., Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. doi: 10.1177/25.6.69656. [DOI] [PubMed] [Google Scholar]
- Blair J. N., Burchell A. The mechanism of histone activation of the hepatic microsomal glucose-6-phosphatase system: a novel method to assay glucose-6-phosphatase activity. Biochim Biophys Acta. 1988 Feb 17;964(2):161–167. doi: 10.1016/0304-4165(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Bock E., Moller M., Nissen C., Sensenbrenner M. Glial fibrillary acidic protein in primary astroglial cell cultures derived from newborn rat brain. FEBS Lett. 1977 Nov 15;83(2):207–211. doi: 10.1016/0014-5793(77)81006-5. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Schultzberg M., Bartfai T., Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992 Feb;58(2):454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Burchell A., Burchell B. Stabilization of partially-purified glucose 6-phosphatase by fluoride. Is enzyme inactivation caused by dephosphorylation? FEBS Lett. 1980 Sep 8;118(2):180–184. doi: 10.1016/0014-5793(80)80214-6. [DOI] [PubMed] [Google Scholar]
- Burchell A. Molecular pathology of glucose-6-phosphatase. FASEB J. 1990 Sep;4(12):2978–2988. doi: 10.1096/fasebj.4.12.2168325. [DOI] [PubMed] [Google Scholar]
- Burchell A., Waddell I. D. The molecular basis of the hepatic microsomal glucose-6-phosphatase system. Biochim Biophys Acta. 1991 Apr 17;1092(2):129–137. doi: 10.1016/0167-4889(91)90146-o. [DOI] [PubMed] [Google Scholar]
- Butler M., Morell P. Sidedness of phospholipid synthesis on brain membranes. J Neurochem. 1982 Jul;39(1):155–164. doi: 10.1111/j.1471-4159.1982.tb04714.x. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin M., Pearce B., Morrow C., Murphy S. Effects of extracellular potassium on glycogen stores of astrocytes in vitro. J Neurochem. 1988 Dec;51(6):1846–1851. doi: 10.1111/j.1471-4159.1988.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin M., Pearce B., Morrow C., Murphy S. Effects of neurotransmitters on astrocyte glycogen stores in vitro. J Neurochem. 1988 Dec;51(6):1852–1857. doi: 10.1111/j.1471-4159.1988.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Countaway J. L., Waddell I. D., Burchell A., Arion W. J. The phosphohydrolase component of the hepatic microsomal glucose-6-phosphatase system is a 36.5-kilodalton polypeptide. J Biol Chem. 1988 Feb 25;263(6):2673–2678. [PubMed] [Google Scholar]
- Dienel G. A., Nelson T., Cruz N. F., Jay T., Crane A. M., Sokoloff L. Over-estimation of glucose-6-phosphatase activity in brain in vivo. Apparent difference in rates of [2-3H]glucose and [U-14C]glucose utilization is due to contamination of precursor pool with 14C-labeled products and incomplete recovery of 14C-labeled metabolites. J Biol Chem. 1988 Dec 25;263(36):19697–19708. [PubMed] [Google Scholar]
- Domin B. A., Serabjit-Singh C. J., Philpot R. M. Quantitation of rabbit cytochrome P-450, form 2, in microsomal preparations bound directly to nitrocellulose paper using a modified peroxidase-immunostaining procedure. Anal Biochem. 1984 Feb;136(2):390–396. doi: 10.1016/0003-2697(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Fellows L. K., Boutelle M. G., Fillenz M. Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J Neurochem. 1992 Dec;59(6):2141–2147. doi: 10.1111/j.1471-4159.1992.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fishman R. S., Karnovsky M. L. Apparent absence of a translocase in the cerebral glucose-6-phosphatase system. J Neurochem. 1986 Feb;46(2):371–378. doi: 10.1111/j.1471-4159.1986.tb12978.x. [DOI] [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Gamberucci A., Scott H. M., Burchell A., Benedetti A. Permeability of rat liver microsomal membrane to glucose 6-phosphate. Biochem J. 1992 Sep 15;286(Pt 3):813–817. doi: 10.1042/bj2860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart D. Z., Broderius M. A., Borson N. D., Drewes L. R. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):733–737. doi: 10.1073/pnas.89.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Mukherji B., Sloviter H. A. Metabolism of isolated rat brain perfused with glucose or mannose as substrate. J Neurochem. 1972 May;19(5):1279–1285. doi: 10.1111/j.1471-4159.1972.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Hardebo J. E., Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann Neurol. 1980 Jul;8(1):1–31. doi: 10.1002/ana.410080102. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Miller A. L. Deoxyglucose-6-phosphate stability in vivo and the deoxyglucose method. J Neurochem. 1987 Dec;49(6):1941–1960. doi: 10.1111/j.1471-4159.1987.tb02457.x. [DOI] [PubMed] [Google Scholar]
- Hill A., Waddell I. D., Hopwood D., Burchell A. The microsomal glucose-6-phosphatase enzyme of human gall-bladder. J Pathol. 1989 May;158(1):53–56. doi: 10.1002/path.1711580111. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Veech R. L. Glucose-6-phosphatase activity in brain. Science. 1986 Nov 28;234(4780):1128–1129. doi: 10.1126/science.3022380. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Veech R. L. Glucose-6-phosphatase activity in brain. Science. 1986 Nov 28;234(4780):1128–1129. doi: 10.1126/science.3022380. [DOI] [PubMed] [Google Scholar]
- Huang M., Veech R. L. The quantitative determination of the in vivo dephosphorylation of glucose 6-phosphate in rat brain. J Biol Chem. 1982 Oct 10;257(19):11358–11363. [PubMed] [Google Scholar]
- KIZER D. E., MCCOY T. A. Phosphomannose isomerase activity in a spectrum of normal and malignant rat tissues. Proc Soc Exp Biol Med. 1960 Apr;103:772–774. doi: 10.3181/00379727-103-25665. [DOI] [PubMed] [Google Scholar]
- Kasai M., Nunogaki K. Permeability of sarcoplasmic reticulum. Methods Enzymol. 1988;157:437–468. doi: 10.1016/0076-6879(88)57095-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liemans V., Malaisse-Lagae F., Willem R., Malaisse W. J. Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates; diastereotopic specificity, isotopic discrimination and intramolecular hydrogen transfer. Biochim Biophys Acta. 1989 Oct 5;998(2):111–117. doi: 10.1016/0167-4838(89)90261-6. [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Morrison J. H., Shoemaker W. J., Sapin V., Bloom F. E. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti P. J. Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabete Metab. 1988 May-Jun;14(3):237–246. [PubMed] [Google Scholar]
- Maxwell K., Berliner J. A., Cancilla P. A. Stimulation of glucose analogue uptake by cerebral microvessel endothelial cells by a product released by astrocytes. J Neuropathol Exp Neurol. 1989 Jan;48(1):69–80. doi: 10.1097/00005072-198901000-00006. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ionic permeability of isolated muscle sarcoplasmic reticulum and liver endoplasmic reticulum vesicles. Methods Enzymol. 1988;157:417–437. doi: 10.1016/0076-6879(88)57094-5. [DOI] [PubMed] [Google Scholar]
- Nelson T., Lucignani G., Atlas S., Crane A. M., Dienel G. A., Sokoloff L. Reexamination of glucose-6-phosphatase activity in the brain in vivo: no evidence for a futile cycle. Science. 1985 Jul 5;229(4708):60–62. doi: 10.1126/science.2990038. [DOI] [PubMed] [Google Scholar]
- Nelson T., Lucignani G., Goochee J., Crane A. M., Sokoloff L. Invalidity of criticisms of the deoxyglucose method based on alleged glucose-6-phosphatase activity in brain. J Neurochem. 1986 Mar;46(3):905–919. doi: 10.1111/j.1471-4159.1986.tb13057.x. [DOI] [PubMed] [Google Scholar]
- Pears J., Jung R. T., Jankowski J., Waddell I. D., Burchell A. Glucose-6-phosphatase in normal adult human intestinal mucosa. Clin Sci (Lond) 1992 Dec;83(6):683–687. doi: 10.1042/cs0830683. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B., Elmer K., Roggendorf W., Reinhart P. H., Hamprecht B. Immunohistochemical demonstration of glycogen phosphorylase in rat brain slices. Histochemistry. 1990;94(1):73–80. doi: 10.1007/BF00266792. [DOI] [PubMed] [Google Scholar]
- Phelps C. H. Barbiturate-induced glycogen accumulation in brain. An electron microscopic study. Brain Res. 1972 Apr 14;39(1):225–234. doi: 10.1016/0006-8993(72)90797-4. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., Mallat M. Astrocyte diversity. Ann N Y Acad Sci. 1988;540:52–63. doi: 10.1111/j.1749-6632.1988.tb27051.x. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Swanson R. A. The regional distribution of glycogen in rat brain fixed by microwave irradiation. Brain Res. 1987 Aug 4;417(1):172–174. doi: 10.1016/0006-8993(87)90195-8. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Lucignani G., Mori K., Jay T., Palombo E., Nelson T., Pettigrew K., Holden J. E., Sokoloff L. Refinement of the kinetic model of the 2-[14C]deoxyglucose method to incorporate effects of intracellular compartmentation in brain. J Cereb Blood Flow Metab. 1989 Jun;9(3):290–303. doi: 10.1038/jcbfm.1989.47. [DOI] [PubMed] [Google Scholar]
- Scott H. M., Burchell A. Pentamidine activates T1 the hepatic microsomal glucose 6-phosphate transport protein of the glucose-6-phosphatase system. Biochim Biophys Acta. 1991 Jul 26;1097(1):31–36. doi: 10.1016/0925-4439(91)90020-a. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sorg O., Magistretti P. J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991 Nov 1;563(1-2):227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- Spagnoli D., Dobrosielski-Vergona K., Widnell C. C. Effects of hormones on the activity of glucose-6-phosphatase in primary cultures of rat hepatocytes. Arch Biochem Biophys. 1983 Oct 1;226(1):182–189. doi: 10.1016/0003-9861(83)90283-7. [DOI] [PubMed] [Google Scholar]
- Stephens H. R., Sandborn E. B. Cytochemical localization of glucose-6-phosphatase activity in the central nervous system of the rat. Brain Res. 1976 Aug 20;113(1):127–146. doi: 10.1016/0006-8993(76)90011-1. [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990 Dec 17;536(1-2):220–226. doi: 10.1016/0006-8993(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M., Evêquoz-Mercier V., Perrottet P., Buchner E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8727–8731. doi: 10.1073/pnas.85.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]