Abstract
Introduction: Diabetic patients frequently experience a serious complication known as impaired wound healing, which increases the likelihood of foot infection and limb amputation. Investigators have been looking for novel methods to treat diabetic foot ulcers (DFUs) recently.
Case Report: A 75-year-old woman with type one diabetes mellitus (DM) has been accepted. There was a sizable (40 cm2 full-thickness cutaneous wound) in the plantar part of her right foot (Wagner Ulcer Grade Classification System: grade 3) which had not been treated by the usual treatment for DFUs. In this present case, we used amniotic fluid gel (AF gel) and photobiomodulation therapy (PBMT) (400 mW/cm2; 810 nm, once a week for 16 weeks) to treat and speed up the healing of a harsh DFU. The size of the ulcer area significantly decreased as combination therapy progressed, and within 16 weeks, the wound was healed and the pain was reduced.
Conclusion: This revealed contextual analysis demonstrated the useful effect of the mix of PBMT and AF gel on a serious DFU. To confirm the findings, we recommend conducting additional clinical trials in a clinical setting. In addition, it is recommended that additional research using preclinical models uncover the mechanism of action of the combination therapy.
Keywords: Amniotic fluid gel, Diabetic foot ulcer, Neuropathic pain, Photobiomodulation therapy, Stem cell
Introduction
The metabolic disorder called diabetes mellitus (DM) is a lifelong disease and this is a chronic metabolic disorder with negative consequences affecting the patient, their families, and society as a whole.1 Around 463 million people worldwide are estimated to have DM, and it is expected that this number will increase by 25% by 2030. Diabetic foot ulcer (DFU) is one important complication in diabetic patients that causes leg amputation.2
Diabetic neuropathic pain is a frequent issue of both type 1 and type 2 diabetes, affecting more than 90% of diabetics. Poorly managed hyperglycemia and side effects of DM can lead to peripheral neuropathy, hypoxia, inflammation, and ischemia, which can cause foot deformities and DFUs. DFUs are regarded as a major health issue.3
The wound healing process consists of an intricate and dynamic three-phase process, composed of inflammation regulation, cell proliferation, or tissue regeneration. The action of a variety of cytokines, chemokines, and growth factors is highly regulated by these processes. Delayed wound healing and scar formation occur due to the disruption of these processes. DM patients account for nearly 60% of all amputations of the whole limb.4 Leg amputations are almost preceded by an infected DFU. Infected DFUs are linked with Staphylococcus aureus. Misuse of antibiotics, particularly in patients with DFUs, has been linked to an increase in drug-resistant microorganisms.5
After the injury, stem cells undergo self-renewal, differentiate into multiple cell types, and affect paracrine signals in the wound environment, and all of these characteristics are essential for scarless tissue regeneration. As a promising new approach to regenerative medicine, stem cell-based therapies have emerged.6
The amniotic fluid (AF) envelops the fetus within the amniotic cavity, which is a transparent aqueous solution. It consists of heterogeneous and multipotent cell types, which are populations with different morphologies, in vitro specifics, in vivo potential, cell surface markers, and biochemical aspects.7
In human amniotic fluid (hAF), transforming growth factor (TGF)-α, TGF-β1, platelet-derived growth factor, and fibroblast growth factor seem to stimulate the cutaneous fibroblast proliferation.8 PBMT, which involves the modulation of the cell and molecular pathways, has been shown to reduce inflammation, stimulate wound healing, or improve pain in diabetic wounds. PBMT-treated ischemic tissues demonstrated enhanced angiogenesis and nitric oxide release as well as an increase in cells expressing vascular endothelial growth factor and hypoxia-inducible factor alpha (HIF-1).4,9,10
Therefore, the previous related studies have demonstrated that the simultaneous use of the two treatment modalities may be a valuable adjunct for the rehabilitation of serious diseases such as DFU. They can be additive and can be used simultaneously as an advanced treatment for DFU.11 In the current case study, we investigated the mixed impact of the AF gel with PBMT on the healing of a complicated case of DFU in a 75-year-old woman with type one DM, who was unresponsive to routine treatments and at risk of foot amputation.
Case Report
A 75-year-old woman with type one DM has been accepted to the ambulatory clinic. There was a sizable (40 cm2 full-thickness cutaneous wound) in the plantar part of her right foot (Wagner Ulcer Grade Classification System: grade 3) which had not been treated by the usual treatment for DFUs (irrigation and antibiotics) (Figure 1). Patient history: duration of diabetes disease: 30 years, no immunodeficiency disease, and metformin medication consumption. Significant colliquative necrosis of soft tissue and pus discharge from the ulcer were observed during a clinical examination (WBC: 21 × 109 cells per litter,FBS: 300 mg/dL, Hba1C: 11%). In one step, debridement was performed.
Figure 1.
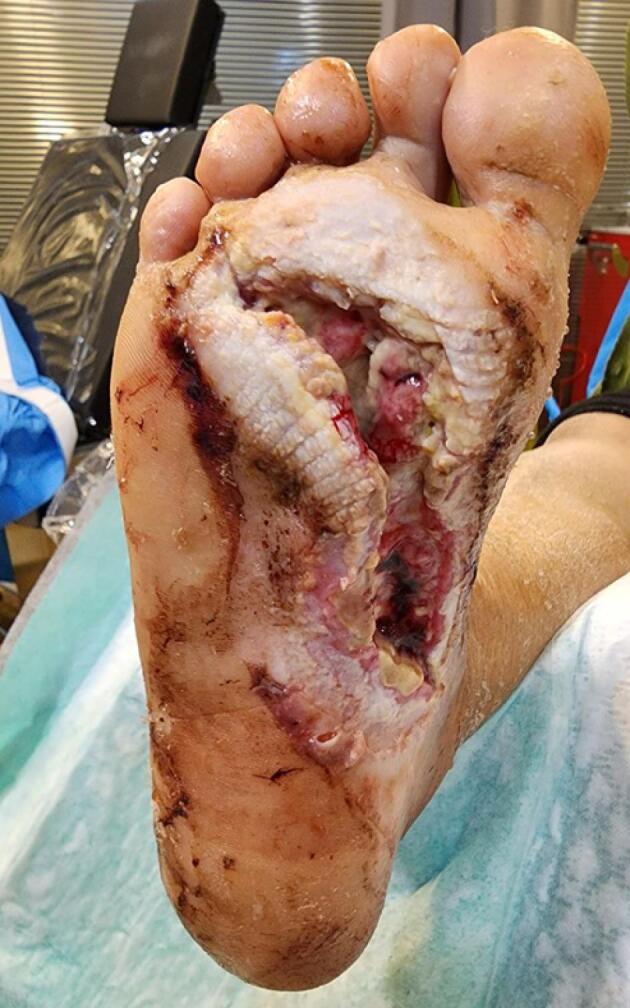
An Extensive Ulcer in the Right Plantar (Wagner Ulcer Grade Classification System: grade 3)
For 16 weeks, PBMT was administered once a week and 40 irradiations of the laser were performed over the ulcer surface and adjacent to normal skin in each session (Figure 2). PBMT was performed according to the guidelines of a previous study, listed in Table 1.
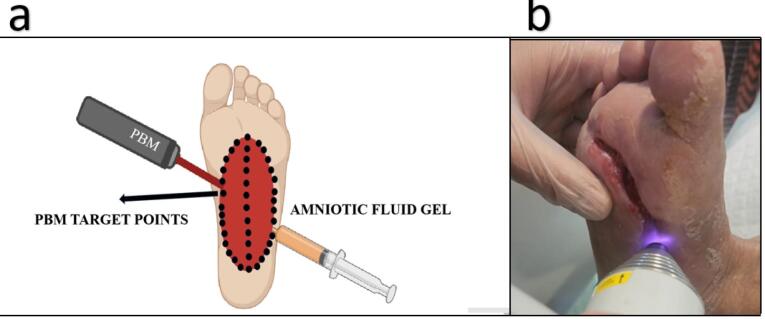
Figure 2.
(a) Graphical image: combination use of photobiomodulation therapy and amniotic fluid gel on a diabetic foot ulcer. (b) Administration of photobiomodulation therapy on the wound surface
Table 1. Parameters of Photobiomodulation Therapy of the Current Study .
| Mode | Pulsed |
| Peak power (mW) | 1000 |
| Average power (mW) | 400 |
| Energy density (J/cm2) | 3 |
| Frequency (number) | 10 000 |
| Wavelength (nm) | 870 |
| Irradiance of light (mW/cm2) | 400 |
| Exposure time per point (s) | 6 |
| Spot area (cm2) | 1 |
| Number of points | 40 |
After PBMT, AF gel was topically used and the ulcers were dressed up with Vaseline. Next, the offloading bandage was applied to reduce pressure from the weight-bearing portion of the foot, and it was changed weekly.
The patient’s clinical condition was assessed each week, and observations were made about a reduction of the ulcer size, pain, and inflammatory response, along with more specific tissue formation, hemostasis, and remarkable coagulation. In the 16th week of the follow-up, the absolute remission of lesions with high levels of healthy skin was the most beneficial aspect (Figure 3), and the visual analogue scale (VAS) was used to evaluate the pain (Table 2).
Figure 3.
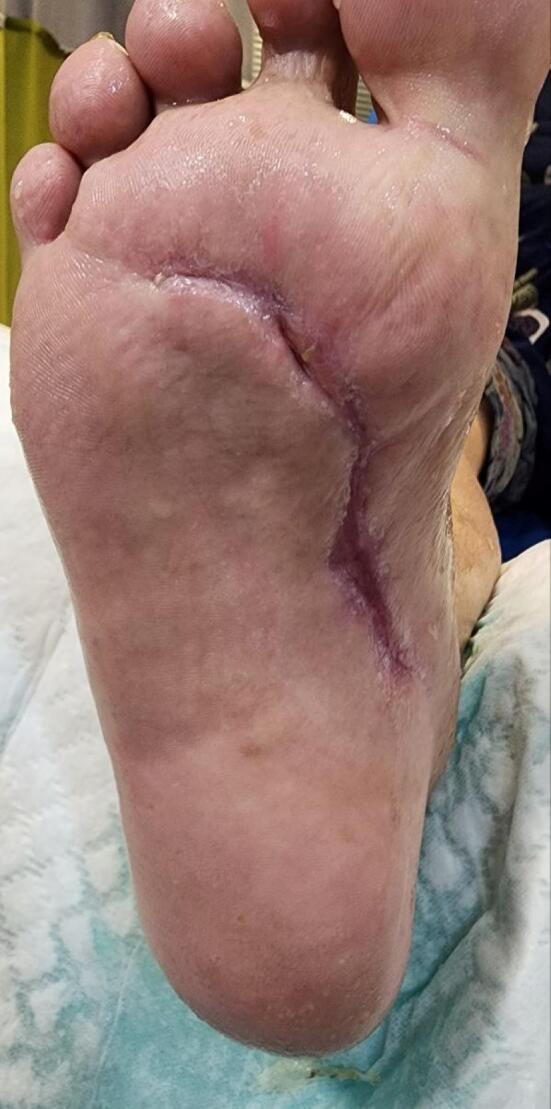
Wound Healing of Diabetic Foot Ulcer at 16th Week
Table 2. Assessment of the Level of Pain in the VAS During the Follow-up Period .
| Week 1 | Week 6 | Week 11 | Week 16 |
| 8 | 5 | 3 | 0 |
Discussion
The inflammatory phase of wound healing takes only a few days under normal conditions, and the healing phase progresses normally.However, the inflammatory phase lasts longer in DM, and the entire damaged skin does not heal, leading to slow healing.12 In patients with a DFU, there are a range of therapies available during their lives that are costly and can place heavy financial pressure on families, society, and insurance companies. The cost of chronic wound care has increased as the population ages and the number of diabetics increases. New treatment procedures that improve patients’ ability to cope with the condition will be extremely beneficial for the patients.13
The current case study reveals a special and complementing approach in which both modalities (AF gel and PBMT) were chosen to promote healing and provide patient comfort. This approach also reduced the healing time of ulcerated areas.
PBMT appears to be stimulating a variety of cell types and actions, particularly in the wound bed, and angiogenesis. PBMT is also a non-intrusive application that alleviates pain through analgesic and anti-inflammatory aspects. The release of neurochemical agents like endogenous endorphins (-endorphin), a reduction in C-fiber and bradykinin activity under the influence of PBMT, and a shift in pain threshold are thought to be some of the factors that contribute to the analgesic effect.14 During treatment, patients experienced reduced pain and were assessed by using VAS analysis in the previous and present studies. PBMT can also stimulate the growth of epithelial, endothelial, and mesenchymal cells while also increasing tissue oxygenation and microcirculation.14,15
The effects of AF gel on wound healing in DFUs have been evaluated by Niami et al. The patients were assigned to three intervention groups and one placebo group at random. The wound dressing for the three intervention groups was gauze impregnated with a gel containing an AF formulation that did not contain any topical agent, while the wound dressing for the control group consisted of only simple gauze. In terms of wound severity, color, and condition of tissues around the wound, statistical differences between these four groups were observed at the end of the study. For the treatment of chronic DFUs, AF gel is considered to be an effective and reliable option.16
In the repair of partial-thickness burns in rats, Amorim et al. appraised the effect of PBMT alone and in combination with the human amniotic membrane (hAM). In an experimental study, patients were randomized into four groups: Control, hAM, PBMT, and PBMT associated with hAM. Seven and 14 days after the burn, the histopathological aspects of the skin samples were evaluated. In all treatment groups related to the control group, histopathological analysis revealed a reduction in inflammation and increased proliferation of fibroblasts, most notably on day 7. Compared to other groups, greater efficacy of PBMT in association with hAM was observed for the accelerated healing process on day 14.17
In the present case study, similar to the above studies, the combination of PBMT and AFG reduced the size of the wound and reduced inflammation.
Limitations of the Study
The present study suffers from the lack of controls and the inability to distinguish the impact of PBMT as compared to AF.
Conclusion
The positive impact of the combination of PBMT and AF gel dressing was demonstrated in this present case report. To validate our results, we suggest further clinical trials. Moreover, it is recommended that additional research using preclinical models uncover the mechanism of action of the PBMT and AF gel. To reduce amputation rates and physical, psychological, social, and economic difficulties associated with DFUs, nurses and even all members of the health care team must take part in training programs aimed at teaching them how to manage and prevent DFUs.
Authors’ Contribution
Conceptualization: Elahe Motamedi Nasab, Joe DiDuro, Mohammad Bayat.
Data curation: Fatemeh Zare, Roya Derakhshan.
Formal analysis: Maryam Rahmannia, Ladan Arab Yaqoubi, Mohammad Javad Fredoni.
Investigation: Houssein Ahmadi, Babak Sabet, Mohammad Hossein Heidari.
Methodology: Houssein Ahmadi, Fatemeh Zare, Mohammad Bayat.
Project administration: Babak Sabet, Roya Derakhshan.
Supervision: Babak Sabet, Mohammad Hossein Heidari.
Writing–original draft: Elahe Motamedi Nasab, Houssein Ahmadi, Joe DiDuro.
Writing–review & editing: Elahe Motamedi Nasab, Houssein Ahmadi.
Competing Interests
No conflict of interest.
Ethical Approval
This study was approved by University of Zahedan (reference number: IR.ZAUMS.REC.1403.104). Written informed consent was obtained from the patient.
Funding
No funding was received by a third party or institution.
Please cite this article as follows: Motamedi Nasab E, DiDuro J, Bayat M, Zare F, Derakhshan R, Rahmannia M, et al. A case report: the effects of photobiomodulation therapy and amniotic fluid gel on a severe diabetic foot ulcer. J Lasers Med Sci. 2024;15:e25. doi:10.34172/ jlms.2024.25.
References
- 1.Mostafavinia A, Ahmadi H, Amini A, Roudafshani Z, Hamblin MR, Chien S, et al. The effect of photobiomodulation therapy on antioxidants and oxidative stress profiles of adipose derived mesenchymal stem cells in diabetic rats. Spectrochim Acta A Mol Biomol Spectrosc. 2021;262:120157. doi: 10.1016/j.saa.2021.120157. [DOI] [PubMed] [Google Scholar]
- 2.Mostafavinia A, Amini A, Sajadi E, Ahmadi H, Rezaei F, Ghoreishi SK, et al. Photobiomodulation therapy was more effective than photobiomodulation plus arginine on accelerating wound healing in an animal model of delayed healing wound. Lasers Med Sci. 2022;37(1):403–15. doi: 10.1007/s10103-021-03271-8. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi H, Amini A, Fadaei Fathabady F, Mostafavinia A, Zare F, Ebrahimpour-Malekshah R, et al. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res Ther. 2020;11(1):494. doi: 10.1186/s13287-020-01967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadi H, Bayat M, Amini A, Mostafavinia A, Ebrahimpour-Malekshah R, Gazor R, et al. Impact of preconditioned diabetic stem cells and photobiomodulation on quantity and degranulation of mast cells in a delayed healing wound simulation in type one diabetic rats. Lasers Med Sci. 2022;37(3):1593–604. doi: 10.1007/s10103-021-03408-9. [DOI] [PubMed] [Google Scholar]
- 5. Edmonds M. Diabetic foot ulcers. In: Type 2 Diabetes. CRC Press; 2016. p. 327-40.
- 6.Ebrahimpour-Malekshah R, Amini A, Mostafavinia A, Ahmadi H, Zare F, Safaju S, et al. The stereological, immunohistological, and gene expression studies in an infected ischemic wound in diabetic rats treated by human adipose-derived stem cells and photobiomodulation. Arch Dermatol Res. 2023;315(6):1717–34. doi: 10.1007/s00403-023-02563-z. [DOI] [PubMed] [Google Scholar]
- 7.Nyman E, Huss F, Nyman T, Junker J, Kratz G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: in vitro studies of re-epithelialisation in human skin wounds. J Plast Surg Hand Surg. 2013;47(2):89–92. doi: 10.3109/2000656x.2012.733169. [DOI] [PubMed] [Google Scholar]
- 8.Fukutake M, Ochiai D, Masuda H, Abe Y, Sato Y, Otani T, et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum Cell. 2019;32(1):51–63. doi: 10.1007/s13577-018-0222-1. [DOI] [PubMed] [Google Scholar]
- 9.Ardeshirzadeh A, Ahmadi H, Mirzaei M, Omidi H, Mostafavinia A, Amini A, et al. The combined use of photobiomodulation and curcumin-loaded iron oxide nanoparticles significantly improved wound healing in diabetic rats compared to either treatment alone. Lasers Med Sci. 2022;37(9):3601–11. doi: 10.1007/s10103-022-03639-4. [DOI] [PubMed] [Google Scholar]
- 10.Fallahi F, Mostafavinia A, Sharifi Z, Mohaghegh Shalmani L, Amini A, Ahmadi H, et al. Effects of photobiomodulation on mitochondrial function in diabetic adipose-derived stem cells in vitro. Spectrochim Acta A Mol Biomol Spectrosc. 2023;285:121835. doi: 10.1016/j.saa.2022.121835. [DOI] [PubMed] [Google Scholar]
- 11.Omidi H, Sohrabi K, Amini A, Fadaei Fathabady F, Mostafavinia A, Ahmadi H, et al. Application of combined photobiomodulation and curcumin-loaded iron oxide nanoparticles considerably enhanced repair in an infected, delayed-repair wound model in diabetic rats compared to either treatment alone. Photochem Photobiol Sci. 2023;22(8):1791–807. doi: 10.1007/s43630-023-00411-7. [DOI] [PubMed] [Google Scholar]
- 12.Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109–16. doi: 10.1016/j.trsl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med. 2021;13(585):eabe4839. doi: 10.1126/scitranslmed.abe4839. [DOI] [PubMed] [Google Scholar]
- 14.Derakhshan R, Ahmadi H, Bayat M, Mehboudi L, Pourhashemi E, Amini A, et al. The combined effects of a methacrylate powder dressing (Altrazeal powder) and photobiomodulation therapy on the healing of a severe diabetic foot ulcer in a diabetic patient: a case report. J Lasers Med Sci. 2022;13:e38. doi: 10.34172/jlms.2022.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo E, Signore A, Aicardi S, Zekiy A, Utyuzh A, Benedicenti S, et al. Experimental and clinical applications of red and near-infrared photobiomodulation on endothelial dysfunction: a review. Biomedicines. 2021;9(3):274. doi: 10.3390/biomedicines9030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niami F, Molavynejad S, Hemmati AA, Bijan Nejad D, Yazdanpanah L, Shakiba Maram N, et al. Evaluation of the effect of a gel made with amniotic fluid formulation on the healing of diabetic foot ulcers: a triple-blind clinical trial. Front Public Health. 2022;10:1025391. doi: 10.3389/fpubh.2022.1025391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amorim FC, Arisawa EÂ, Sant’Anna LB, Fonseca KM, Costa DR, Rodrigues AB, et al. Amniotic membrane applied to burns healing: pre-clinical study. Res Soc Dev. 2021;10(4):38110414286. doi: 10.33448/rsd-v10i4.14286. [DOI] [Google Scholar]