Abstract
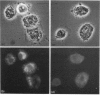
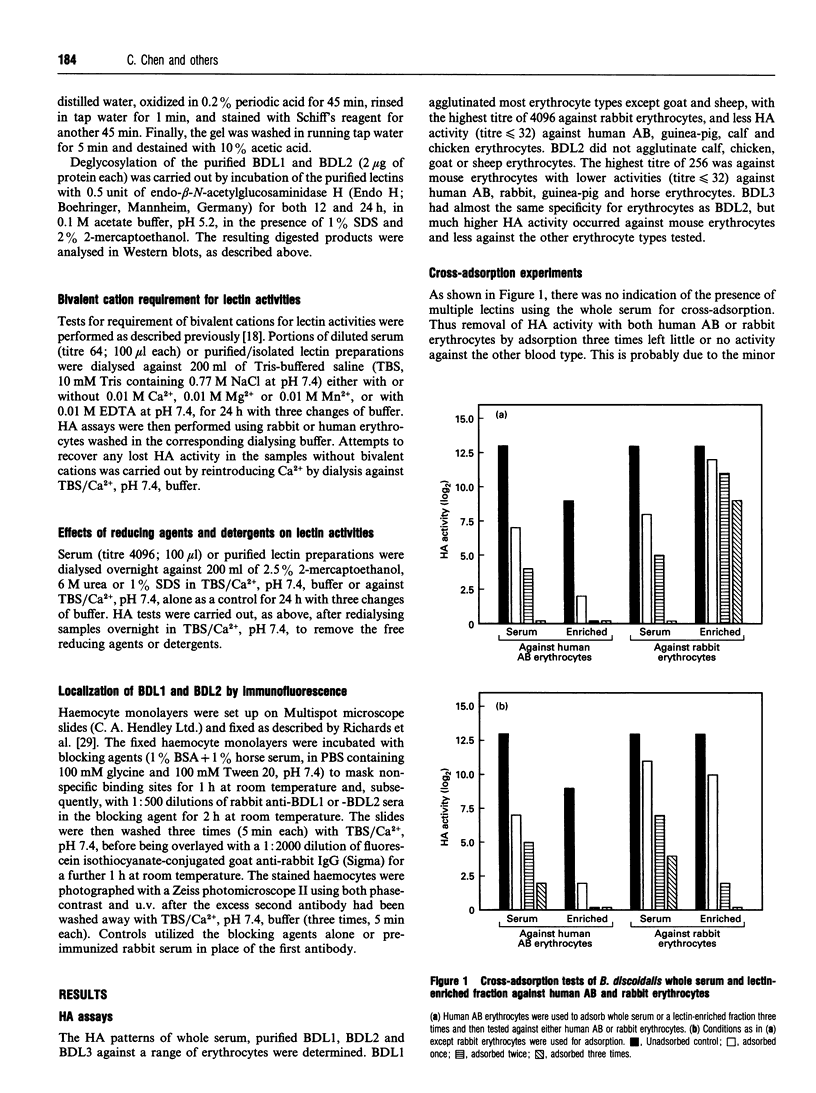
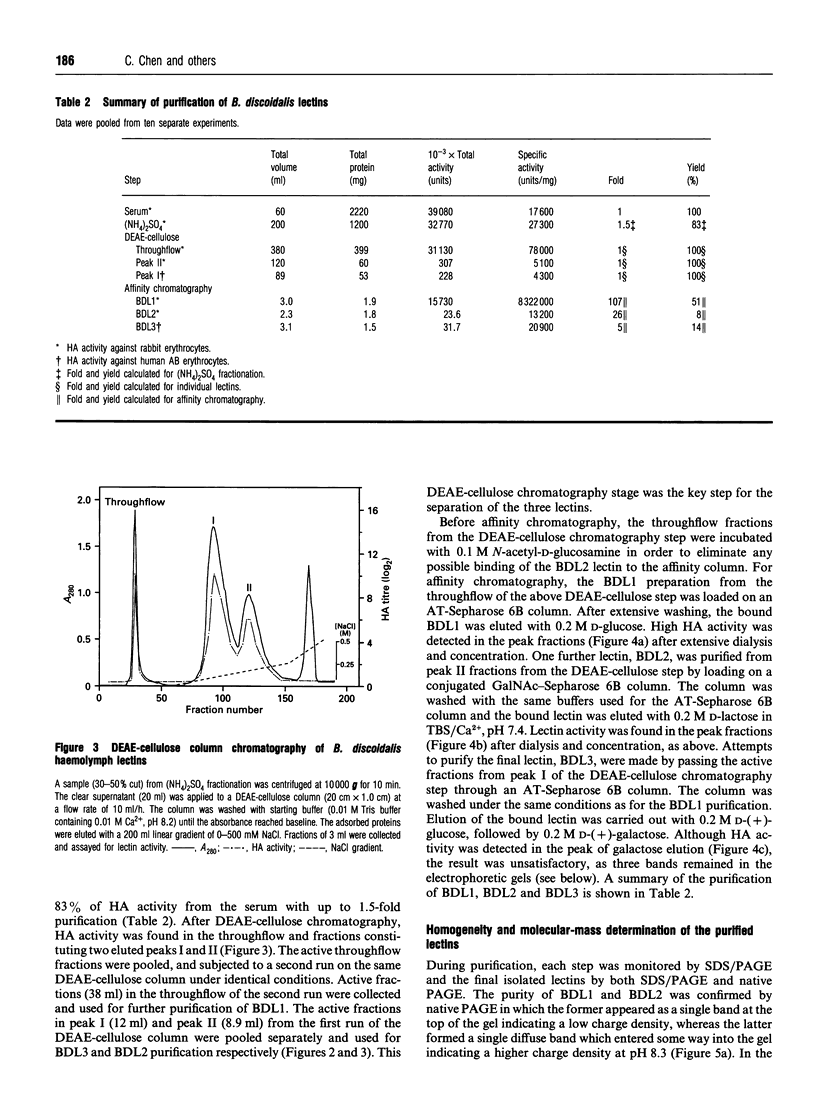
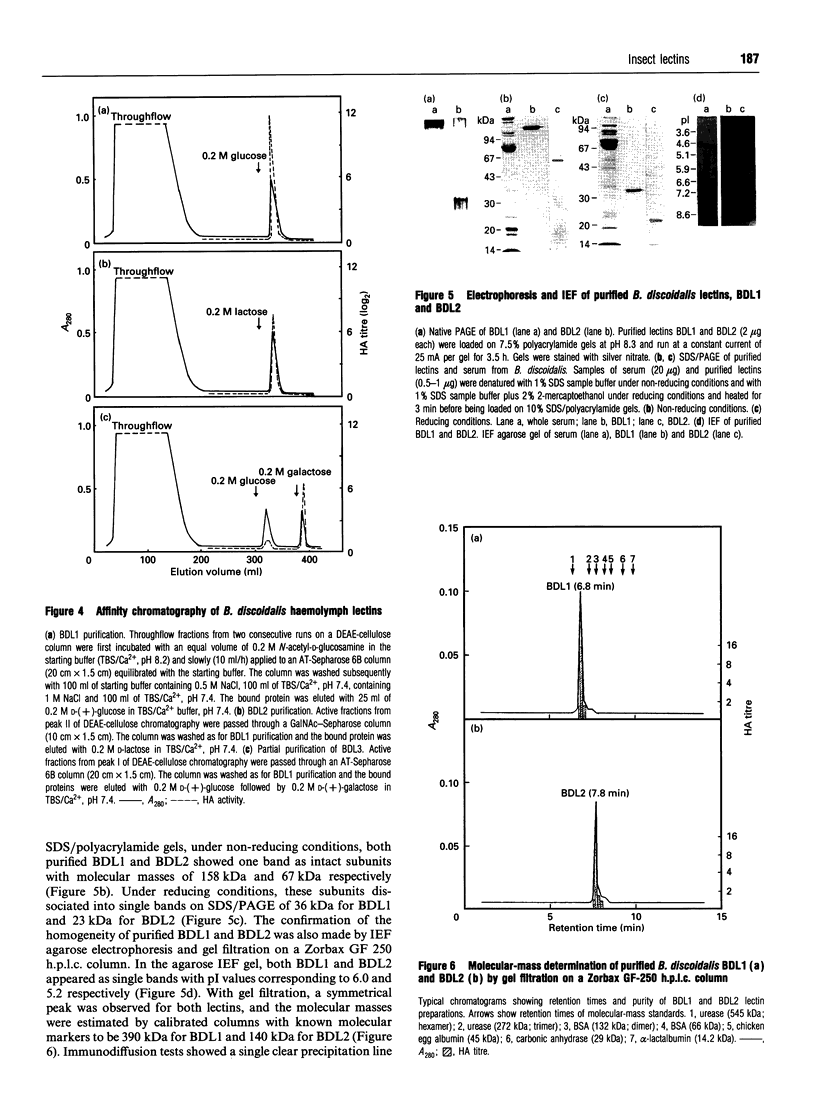
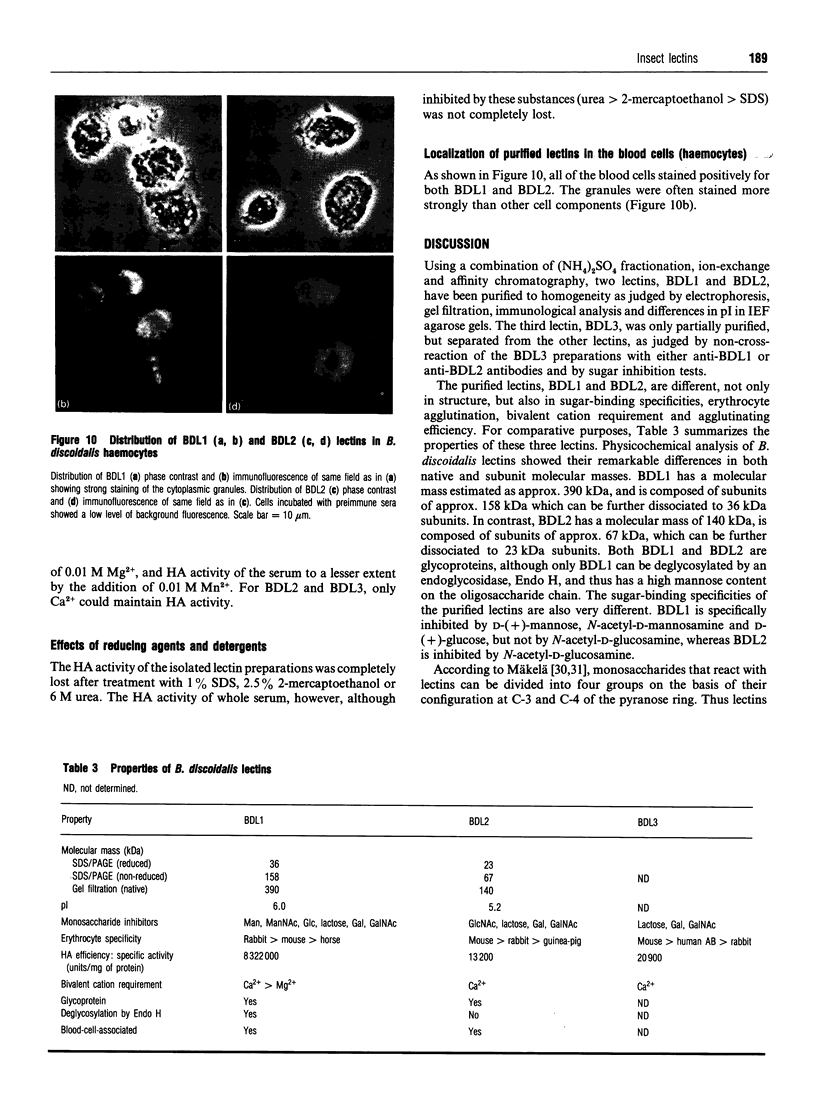
Three agglutinins (lectins), designated BDL1, BDL2 and BDL3, were identified in the haemolymph of the cockroach Blaberus discoidalis by erythrocyte cross-adsorption and sugar inhibition tests. With the use of (NH4)2SO4 fractionation, anion-exchange and affinity chromatography, BDL1 and BDL2 have been purified to homogeneity, and BDL3 has been partially purified to three bands on SDS/PAGE. BDL1 has a molecular-mass estimate of 390 kDa by gel filtration and approx. 158 kDa by SDS/PAGE under non-reducing conditions, further reduced to subunits of 36 kDa under reducing conditions. BDL2 has a molecular mass of approx. 140 kDa and is composed of subunits of 67 kDa which can be further reduced to identical subunits of 23 kDa. Isoelectric focusing in agarose gels revealed that BDL1 and BDL2 both focused as single bands at pH 6.0 and pH 5.2 respectively. The purified forms of BDL1 and BDL2 were stained by the periodic acid/Schiff's reagent showing that both lectins are glycoproteins. In addition, BDL1 was deglycosylated by endo-beta-N-acetylglucosaminidase H. Immunological tests showed that these three lectins are not structurally related. All three lectins bind galactose but have different specificities for binding other sugars and for a range of vertebrate erythrocytes. BDL1 is specifically inhibited by D-(+)-glucose, D-(+)-mannose and N-acetyl-D-mannosamine, but not by N-acetyl-D-glucosamine, and BDL2 is inhibited by N-acetyl-D-glucosamine, but not by D-(+)-glucose, D-(+)-mannose or N-acetyl-D-mannosamine. BDL3 is strongly inhibited by N-acetyl-D-galactosamine, but not by any of the other above-mentioned sugars. Erythrocyte specificities showed that BDL1 is more specific for rabbit than mouse erythrocytes, whereas BDL2 and BDL3 are more specific for mouse than rabbit erythrocytes. The haemagglutinating activities of both the serum and isolated lectins are Ca(2+)-dependent. Localization of BDL1 and BDL2 with fluorescein isothiocyanate-labelled antibodies showed that both lectins are associated with the granules and other areas of the cytoplasm of all blood cell types.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Karim F. W., Cohen R. E. Atypical stromal cells of lower female genital tract. Histopathology. 1990 Sep;17(3):249–253. doi: 10.1111/j.1365-2559.1990.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Wang J. L. Binding and functional properties of concanavalin A and its derivatives. III. Interactions with indoleacetic acid and other hydrophobic ligands. J Biol Chem. 1978 May 10;253(9):3016–3022. [PubMed] [Google Scholar]
- Ersson B., Aspberg K., Porath J. The phytohemagglutinin from sunn hemp seeds (Crotalaria juncea). Purification by biospecific affinity chromatography. Biochim Biophys Acta. 1973 Jun 15;310(2):446–452. doi: 10.1016/0005-2795(73)90128-1. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Sannoh T., Kawasaki N., Kawasaki T., Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987 Jun 5;262(16):7451–7454. [PubMed] [Google Scholar]
- Ingram G. A., East J., Molyneux D. H. Naturally occurring agglutinins against trypanosomatid flagellates in the haemolymph of insects. Parasitology. 1984 Dec;89(Pt 3):435–451. doi: 10.1017/s0031182000056687. [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J Biochem. 1989 Sep;106(3):483–489. doi: 10.1093/oxfordjournals.jbchem.a122878. [DOI] [PubMed] [Google Scholar]
- Komano H., Natori S. Participation of Sarcophaga peregrina humoral lectin in the lysis of sheep red blood cells injected into the abdominal cavity of larvae. Dev Comp Immunol. 1985 Winter;9(1):31–40. doi: 10.1016/0145-305x(85)90057-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAKELA O. Studies in hemagglutinins of leguminosae seeds. Ann Med Exp Biol Fenn. 1957;35(Suppl 11):1–133. [PubMed] [Google Scholar]
- Mauchamp B. Purification of an N-acetyl-D-glucosamine specific lectin (P.B.A.) from epidermal cell membranes of Pieris brassicae L. Biochimie. 1982 Nov-Dec;64(11-12):1001–1008. doi: 10.1016/s0300-9084(82)80380-5. [DOI] [PubMed] [Google Scholar]
- Millar D. A., Ratcliffe N. A. Activity and preliminary characterisation of a hemagglutinin from the hemichordate Saccoglossus ruber. Dev Comp Immunol. 1987 Spring;11(2):309–320. doi: 10.1016/0145-305x(87)90075-9. [DOI] [PubMed] [Google Scholar]
- Minnick M. F., Rupp R. A., Spence K. D. A bacterial-induced lectin which triggers hemocyte coagulation in Manduca sexta. Biochem Biophys Res Commun. 1986 Jun 13;137(2):729–735. doi: 10.1016/0006-291x(86)91139-3. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Andrade A. F., Ribeiro J. M. Lectins of distinct specificity in Rhodnius prolixus interact selectively with Trypanosoma cruzi. Science. 1981 Feb 6;211(4482):597–600. doi: 10.1126/science.7006082. [DOI] [PubMed] [Google Scholar]
- Richards E. H., Ratcliffe N. A. Direct binding and lectin-mediated binding of erythrocytes to haemocytes of the insect, Extatosoma tiaratum. Dev Comp Immunol. 1990 Summer;14(3):269–281. doi: 10.1016/0145-305x(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu K., Kosaka S., Suzuki T. Purification and characterization of a lectin from the beetle, Allomyrina dichotoma. J Biochem. 1984 Jan;95(1):239–245. doi: 10.1093/oxfordjournals.jbchem.a134590. [DOI] [PubMed] [Google Scholar]
- Vasta G. R., Hunt J. C., Marchalonis J. J., Fish W. W. Galactosyl-binding lectins from the tunicate Didemnum candidum. Purification and physicochemical characterization. J Biol Chem. 1986 Jul 15;261(20):9174–9181. [PubMed] [Google Scholar]
- Vasta G. R., Marchalonis J. J. Galactosyl-binding lectins from the tunicate Didemnum candidum. Carbohydrate specificity and characterization of the combining site. J Biol Chem. 1986 Jul 15;261(20):9182–9186. [PubMed] [Google Scholar]
- Yeaton R. W. Invertebrate lectins: I. Occurrence. Dev Comp Immunol. 1981 Summer;5(3):391–402. doi: 10.1016/s0145-305x(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Sawada H., Abe Y., Numakunai T., Ishii S. Galactose-specific lectin in the hemolymph of solitary ascidian, Halocynthia roretzi : isolation and characterization. Biochem Biophys Res Commun. 1982 Jul 30;107(2):451–457. doi: 10.1016/0006-291x(82)91512-1. [DOI] [PubMed] [Google Scholar]