TO THE EDITOR: In 2024, the Centers for Disease Control and Prevention (CDC) estimated that syphilis cases had risen by 79% between 2018 and 2022.1 CDC and Canadian guidelines for syphilis treatment recommend penicillin G, administered parenterally, for all stages of syphilis.2 A single 2-g oral dose of azithromycin was listed as an alternative regimen for those with penicillin allergy starting in 2002, until mutations conferring macrolide resistance were found in 53% of Treponema pallidum (T. pallidum) strains across the United States from 2007 through 20093; current CDC and Canadian guidelines state that azithromycin should not be used for syphilis.2 Given the global resurgence in syphilis and recurring shortages of the mainstay of treatment of uncomplicated syphilis, benzathine penicillin G, estimation of the current prevalence of azithromycin resistance provides context when strategies for syphilis treatment are being considered (alternative antibiotic choices are discussed in Part A in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Here we report that 599 of 604 T. pallidum strains (99.2%) that were sampled in North America from 2017 through 2023 were genotypically resistant to azithromycin (Fig. 1A).
Figure 1. Azithromycin Resistance in Tested Treponema pallidum Strains.
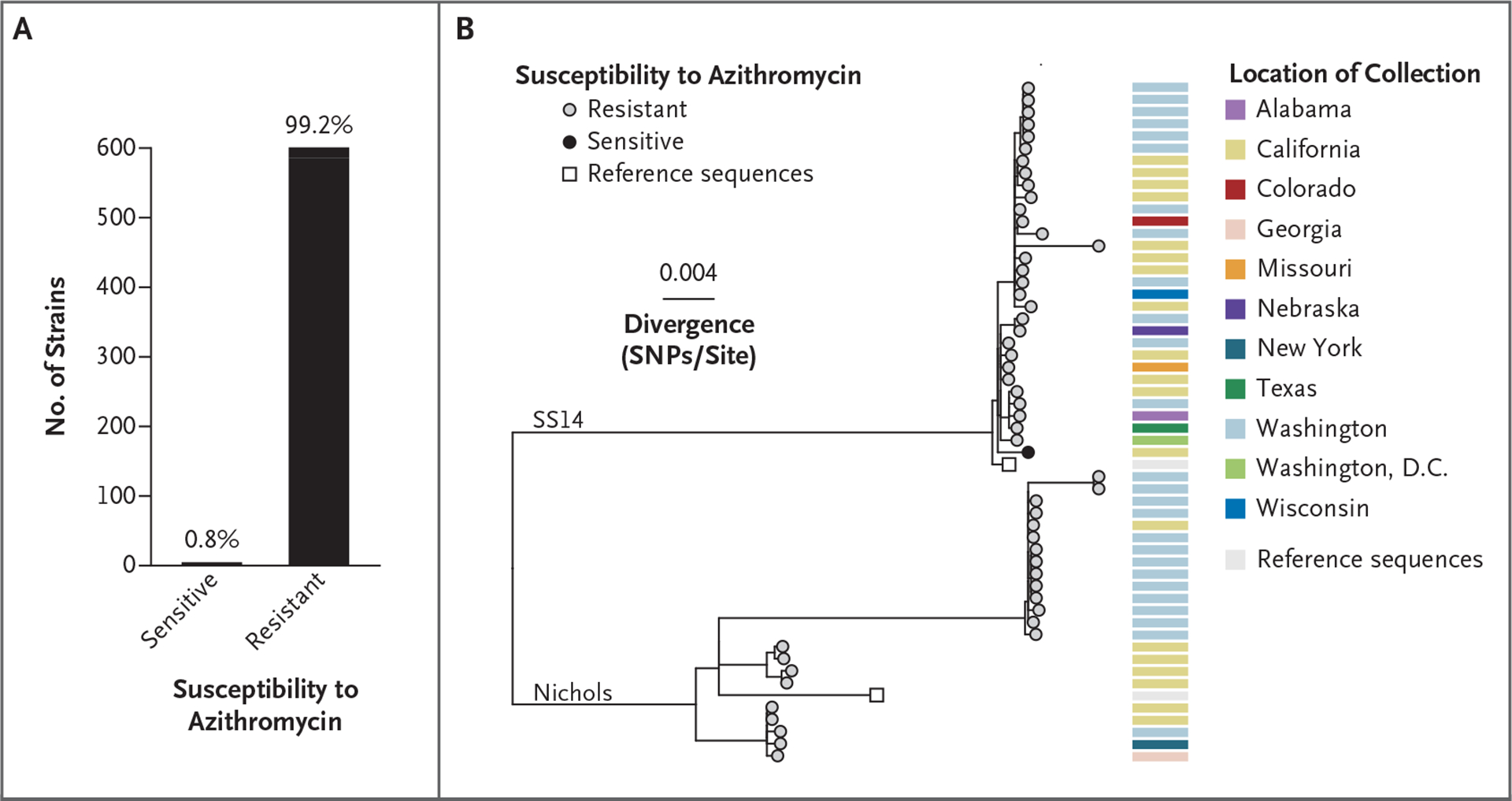
Of the 604 strains for which genotypic susceptibility to macrolides could be determined, 599 were resistant (584 through the A2058G mutation and 15 through the A2059G mutation) (Panel A). A single-nucleotide polymorphism (SNP)–only maximum-likelihood phylogenetic tree (Panel B) was constructed with the use of a subset of 54 strains with sufficient sequencing data. Tips with gray fill indicate resistance to azithromycin, and black fill indicates sensitivity. Nichols and SS14 reference sequences are included as white squares.
Samples were collected from patients in 13 U.S. states, Washington, D.C., and two Canadian provinces (Table S1 in the Supplementary Appendix; also see the Supplementary Methods). The median age of the patients was 33 years (range, 0 to 76). A total of 466 of 588 patients (79.3%) were male. Among male patients with sex-partner information available, 73 of 88 (83%) were men who have sex with men. The syphilis stage was documented for 115 patients, with secondary syphilis being the most prevalent (56 of 115 [48.7%]). Among strains with a near-complete genome obtained, 23 of 54 (43%) belonged to the Nichols-like lineage and 31 of 54 (57%) to the SS14-like lineage (Fig. 1B).
Of the 599 azithromycin-resistant strains, 584 (97.5%) were resistant through the A2058G mutation in the gene encoding the 23S ribosomal RNA subunit and 15 (2.5%) through the A2059G mutation. The resistance phenotype conferred by these mutations persists in the presence of doses of up to 64 times the minimum inhibitory concentration of azithromycin for T. pallidum (Part B in the Supplementary Appendix).4 Although resistance to azithromycin among women, as well as men who have sex with women, was present in only 8 of 57 specimens (14%) obtained from 2007 through 2009,3 resistance increased in these populations to 99.3% (136 of 137 specimens) from 2017 through 2023.
The patients included in this study were generally representative of patients with syphilis in North America (Table S2). Limitations of this study include sparse metadata, owing to our reliance on deidentified remnant clinical samples, and the use of convenience sampling, which resulted in over-representation of samples from the western United States and Canada. Our results are consistent with those of studies (Table S3) evaluating the prevalence of macrolide resistance among T. pallidum strains, including in a broader population sampled throughout England from 2012 through 2018 (90.3% of strains were macrolide resistant).5
Our data do not support the use of azithromycin as a treatment option for syphilis in the United States and Canada, even in the context of limited alternatives owing to shortages of benzathine penicillin G. If faced with shortages of benzathine penicillin G, clinicians should choose doxycycline or ceftriaxone, not azithromycin, in nonpregnant patients with syphilis.
Supplementary Material
Acknowledgments
Supported by grants (T32 AI07140 and UM 1AI148684, to Dr. Reid; U19 AI144133, to Drs. Reid, Cannon, Giacani, and Greninger; and R01 AI155442, to Dr. Golden) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, and by a contract (HHSN272201300012, to the Sexually Transmitted Infections Clinical Trials Group) from the NIAID.
Footnotes
The findings and conclusions in this letter are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Anna Berzkalns, Public Health — Seattle and King County, Seattle, WA
Stephanie E. Cohen, San Francisco Department of Public Health, San Francisco, CA
Lori M. Newman, National Institutes of Health, Rockville, MD
Sol Aldrete, Medical College of Wisconsin, Milwaukee, WI
Michael Kron, Medical College of Wisconsin, Milwaukee, WI
Olusegun O. Soge, University of Washington, Seattle, WA
Kimberly Workowski, Emory University, Atlanta, GA
Charlotte Perlowski, FHI 360, Durham, NC
Prenilla Naidu, Alberta Precision Laboratories, Edmonton, AB, Canada
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted infections surveillance, 2022. January 30, 2024. (https://www.cdc.gov/std/statistics/2022/default.htm).
- 2.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70: 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A2058G Prevalence Workgroup. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis 2012; 39: 794–8. [DOI] [PubMed] [Google Scholar]
- 4.Tantalo LC, Lieberman NAP, Pérez-Mañá C, et al. Antimicrobial susceptibility of Treponema pallidum subspecies pallidum: an in-vitro study. Lancet Microbe 2023; 4(12): e994–e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beale MA, Thorn L, Cole MJ, et al. Genomic epidemiology of syphilis in England: a population-based study. Lancet Microbe 2023; 4(10): e770–e780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


