Abstract
If we have any hope of achieving a cure for HIV infection, close attention to the cell types capable of getting infected with HIV is necessary. Of these cell types, astrocytes are the most ideal cell type for the formation of such a reservoir. These are long-lived cells with a very low turnover rate and are found in the brain and the gastrointestinal tract. Although astrocytes are evidently resistant to infection of cell-free HIV in vitro, these cells are efficiently infected via cell-to-cell contact by which immature HIV virions bud off lymphocytes and have the ability to directly bind to CXCR4, triggering the process of fusion in the absence of CD4. In this review, we closely examine the evidence for HIV infection of astrocytes in the brain and the mechanisms for viral entry and regulation in this cell type, and discuss an approach for controlling this viral reservoir.
Keywords: Astrocyte, CXCR4, endocytosis, HIV, latency, reservoir
INTRODUCTION
The current focus of HIV research has shifted towards the possible eradication of HIV reservoirs, prevention of their formation by early treatment or keeping them in a dormant state once they are formed. While much attention has been devoted to resting CD4 T cells as a reservoir, the role of other cell types as a reservoir has not been well studied. There is abundant evidence that HIV can enter the brain early in the course of infection and establish a reservoir in resident microglia and astrocytes. Unless we develop an understanding of the mechanism of HIV entry and replication in these cell types, we will never be able to develop therapies for controlling the reservoirs. The current review is focused on HIV infection of brain astrocytes; however, it is important to note that these cells can also be found in the gastrointestinal tract [1–3] which may be another site of HIV persistence.
HIV EARLY AND PERSISTENT INFECTION OF THE BRAIN
Early infection of the central nervous system (CNS) was reported in patients with primary HIV infection in the 1980s (4–6) and HIV was isolated from the cerebrospinal fluid (CSF) of these patients [4, 5]. This was further confirmed in two patients with iatrogenic HIV inoculation, in the brains of whom the viruses were recovered 15 days post-infection [7, 8]. A recent study showed that HIV RNA was detected in the CSF from 15 of 18 subjects as early as 8 days after estimated HIV transmission, with an average detection period of 15 days after exposure [9]. In the macaque model of simian immunodeficiency virus (SIV) infection, the virus could be isolated from CSF within one week and the proviral DNA/viral RNA was detected in brain tissues within 2 weeks post-intravenous inoculation [10–13]. Similarly, early infection of the CNS is also observed in the cats with feline immunodeficiency virus (FIV) [14]. Viral replication may be low or suppressed in the brain during the asymptomatic period after primary infection [4, 11]; however, the viral DNA remains stable from acute through asymptomatic infection [11, 15, 16]. Also, low levels of CSF HIV-1 RNA can even be detected in 17% (12 of 70) of the CSF samples taken after up to 10 years of suppressive antiretroviral therapy [17]. These findings strongly indicate that the brain is an important reservoir for HIV where the virus establishes residence soon after infection.
ASTROCYTE AS ONE OF HIV TARGET CELLS IN THE BRAIN
Major target cells for HIV infection in the brain are perivascular macrophages, microglia and astrocytes, and both microglia [18–20] and astrocytes [21] have an extremely low turnover rate. Cellular immune response to HIV infection in the CNS is inefficient and many antiretroviral drugs cannot efficiently penetrate through the blood-brain barrier [22]. No significant cytopathic effect (CPE) has ever been observed in HIV-infected astrocytes [23, 24] or in microglia at the asymptomatic stage of HIV infection. Both of these cells maintain persistent HIV infection or latency in the CNS. Therefore, as a critical sanctuary or reservoir of HIV, the CNS becomes an insurmountable obstacle for an HIV cure.
Astrocytes are the most abundant cell type in the brain that outnumber neurons by 5–10 fold [25, 26]. Even if HIV only infected a very small percentage of astrocytes, it would still significantly contribute to establishing a reservoir in the brain. HIV infection in astrocytes has been a controversial topic for many years. In part this may be attributed to the difficulty in detection of HIV infection in vivo due to low level of viral replication in these cells and the inefficient infection in astrocytic cell lines in vitro, properties of which are quite different from primary astrocytes. This review mainly focuses on the mechanisms by which HIV infects primary astrocytes.
IN VIVO EVIDENCE OF HIV INFECTION IN ASTROCYTES
Although numerous studies show that HIV infection in the brain mainly occurs in macrophages and microglia [27, 28], in vivo evidence indicates that astrocytes can also be infected by HIV [29]. A late 1980s study detected HIV-1 structural protein p25 in astrocytes from patients with AIDS dementia complex by immunohistochemistry [30]. However, since the authors used only morphological features to identify cell type instead of specific markers, it was not conclusively proven that the stained cells were indeed astrocytes. Stronger evidence was provided in subsequent investigations by applying immunohistochemical staining to detect both glial fibrillary acidic protein (GFAP) and HIV early/structural proteins or applying in situ hybridization (ISH)/PCR-ISH to detect HIV mRNA/proviral DNA as well [31–36]. These studies convincingly confirmed that HIV does infect astrocytes in vivo. Further evidence was added by detecting HIV DNA in astrocytes in brain tissues using immunohistochemistry, laser capture microdissection (LCM) and PCR techniques [16, 37, 38]. Churchill and colleagues showed that the frequency of HIV-infected astrocytes correlated with the severity of neuropathological changes and proximity to brain blood vessels and perivascular macrophages [38]. These findings are somewhat controversial since the possibility of contamination during nested/triple PCR or from surrounding cellular material cannot be excluded [39]. Nevertheless, detection of HIV DNA in astrocytes across all brain regions examined, even those distant from other HIV-susceptible cell types, strongly suggests that these cells can be infected under the right circumstances. In addition, the infection of astrocytes is also observed in SIV macaque models [40, 41]. SIV proviral DNA or early proteins Nef and Rev can be detected in the ex vivo cultures of astrocytes isolated from the macaques infected with SIV for 1 – 3 years [42]. Although it has been difficult to accurately estimate the HIV infection rate of astrocytes in vivo [29, 38], this ex vivo study detected SIV Nef and Rev in 0.05% to 0.1% of GFAP-positive cells [42]. In conclusion, HIV infection of astrocytes is strongly supported by cumulative in vivo and ex vivo evidence. The frequency of infection appears to be quite low overall but may be enhanced in certain conditions, as has been observed in post-mortem tissues from encephalitis and dementia patients.
CAPACITY OF ASTROCYTES TO SUPPORT PRODUCTIVE HIV INFECTION
Although there is little doubt that astrocytes are infected by HIV based on the in vivo evidence, the debate continues whether HIV generates productive or abortive infection after its entry into astrocytes [43]. Some studies show that HIV/SIV structural proteins are detected in the infected astrocytes [30, 32, 41]; however, most studies, both in vivo and in vitro, demonstrate that only viral early gene products (e.g., Nef) can be reliably detected [31, 36, 40] and productive infection is only transiently observed after the infection [44–46]. This indicates that astrocytes might have some intrinsic restriction mechanisms to limit HIV production. However, no obstacle is observed and persistent infection can be established very well when primary astrocytes are infected with VSV-G pseudotyped HIV [47, 48] or cell lines of astrocytic origin are transfected with HIV proviral plasmids [23, 49]. Productive infection with CPE can be extensively generated in the astrocytic cell lines that express CD4 and co-receptors CXCR4/CCR5 [50–52]. Our data also confirmed that persistent infection and stable viral production could be easily established in primary astrocytes when the cells were infected with VSV-G pseudotyped HIV or transfected with HIV proviral DNA, and similar results were further generated from primary astrocytes that were pre-transfected with CD4 plasmid [T4-pMV7 [53]] and then infected with cell-free HIV. Recent studies [48, 54] as well as our data showed that productive, persistent infection can be established in primary astrocytes when HIV particles or viral RNA are able to escape from degradation in endosomes/endolysosomes and get released into the cytosol in presence of lysosomotropic agents (e.g., chloroquine). Thus, it can be concluded that astrocytes do not have significant barriers to the intracellular process of the HIV life cycle.
RESTRICTION AND LATENCY OF HIV INFECTION IN ASTROCYTES
Several groups have identified post-entry blocks to productive HIV replication in astrocytes, focusing primarily on expression of cellular co-factors for viral proteins and host restriction factors. These include low expression of Sam68, a Src-associated protein that is required for Rev function [55, 56]; high expression of the Rev-interacting domain (Risp) protein family, which inhibit Rev function [57]; low levels of TRBP, an inhibitor of double-stranded RNA-dependent kinase PKR [58–61], as well as the Rev-interacting RNA helicases DDX1/DDX3 [62–64]; and abundant expression of TCF-4, the downstream effector of the canonical Wnt/β-catenin pathway [65–68]. However, it is worth noting that these studies were primarily conducted in in vitro culture systems with astrocytic cell lines or fetal cells and may not accurately represent characteristics of astrocytes in vivo. Indeed, few studies have shown that Rev function is unimpaired in human fetal astrocyte cultures [54, 69] and that Rev-dependent transcripts from transiently transfected plasmids are efficiently transported into the cytoplasm of astrocytes [70, 71]. Several groups reported persistent, productive HIV infection in astrocytes when entry restrictions were bypassed by transfection [54, 72] or pseudotyping of HIV [47, 54, 73]; these findings are supported by our own data. Therefore, while the majority of in vitro and ex vivo studies support restricted HIV infection in astrocytes, it is evident that these cells are capable of supporting productive replication. There is also significant evidence that viral replication can be significantly enhanced in nonproductively infected astrocytes under appropriate circumstances such as exposure to pro-inflammatory cytokines (TNFα, IFNγ, IL-1β) [29, 46, 66, 74–76] or lysosomotropic agents [48, 77]. Thus, HIV infection in astrocytes may be restricted by a variety of intracellular mechanisms when the infection is very low; however, the restrictions can be completely overcome when barrier of HIV entry is bypassed.
There is a convincing body of literature that indicates astrocytes support latent HIV infection that can be reactivated from dormancy to produce infectious virions [38, 74, 78–81]. In fact, latently infected astrocytes can transmit HIV to activated lymphocytes in co-culture [24, 78, 81]. Using a variety of latency-reversing agents (LRAs), Narasipura and colleagues recently reported that the HIV LTR in chronically infected progenitor-derived astrocytes is silenced by epigenetic changes, particularly histone modifications performed by class I histone deacetylases (HDACs) and the histone trimethyltransferase (HMT) SU(VAR)3–9 which trimethylates histone 3 at lysine 9 (H3K9) [81]. Interestingly, other LRAs such as 5-aza-2-deoxycytidine (5-aza-CdR), BIX-01294 (a specific inhibitor of G9a, an H3K9 dimethyltransferase) and UNC0628 (an inhibitor of both G9a and GLP, an H3K9 monomethyltransferase) had no effect on HIV expression in astrocytes. In contrast, 5-aza-CdR and BIX-01294 have been shown to reactivate latent HIV replication in T-cell (ACH-2, CEM) and monocytic (OM10.1) cell lines [82]. Additionally, BIX-01294 reactivated HIV in resting T cells isolated from HIV patients on HAART [83]. These findings are particularly interesting because they suggest the epigenetic modifications that regulate HIV latency are not uniform across cell types, which may be relevant to either future eradication strategies or enforced viral quiescence in the CNS. However, Chauhan and colleagues showed that latency-reversing agents actually had no effect on HIV reactivation in astrocytes persistently and productively infected with VSV-G pseudotyped HIV [84]. We also found no increase of HIV production by HDAC inhibitors in long-term, productively HIV-infected astrocytes, in which the infection was established with treatment of chloroquine (unpublished data). Therefore, HIV latency may exist in some circumstances in which the infection is extremely low.
RECEPTORS MEDIATING HIV INFECTION IN ASTROCYTES
Unlike microglia that express CD4 and chemokine coreceptors CCR5 and CCR3 for HIV infection [85], no detectable level of CD4 has been demonstrated in astrocytes [86, 87] and anti-CD4 antibodies or soluble CD4 (sCD4) cannot block HIV infection [46, 74, 88, 89]. While CXCR4 is well expressed on astrocytes [90–92] and can be upregulated by pro-inflammatory cytokines [93], CCR5 is only present under some circumstances. Therefore, other receptors or non-receptor mechanisms have been implicated in HIV entry into astrocytes [46]. These include a CC chemokine receptor, D6, which can function as a co-receptor for various primary dual-tropic strains [94]. An early study showed that antibodies against galactosyl ceramide (galactocerebroside, or GalC) could inhibit HIV-1 entry into a glioma cell line, U373-MG, suggesting that GalC might serve as a receptor for HIV infection in astrocytes [95]; however, this finding has not been confirmed so far. Two independent studies demonstrated that membrane proteins 260 kD or 65 kD in size on the surface of astrocytes were responsible for specific binding to HIV-1 gp120 [86, 96], but these proteins have not been further characterized. Later, another study identified human mannose receptor (hMR), a 165 kD protein, as a mediator of HIV entry into astrocytic cell line where HIV-1 viral particles bound to hMR via the abundant and highly mannosylated sugar moieties of glycoprotein gp120 [97]. Another study indicated that DC-SIGN as well as CCR5 mediates HIV endocytosis in astrocytes [98], however the mRNA of both receptors was barely detectable in primary fetal astrocytes in our study. Altogether, there is still no consensus on the primary receptors responsible for HIV infection in astrocytes.
CD4 mRNA can be detected in astrocytes though CD4 protein is undetectable on the membrane [99]. A residual level of CD4 may be still involved in the process of HIV entry into astrocytes in some circumstances because anti-CD4 antibody can partially inhibit the chloroquine-mediated infection of astrocytes (data not shown; further discussed below). This is also supported by the finding that pretreatment of HIV virions with sCD4 results in a decrease of HIV infection in astrocytes [96].
ENDOCYTOSIS OF HIV IN ASTROCYTES AND THE BARRIER TO VIRAL INFECTION
Viral entry mechanisms can be generally classified as endocytic or initiated by membrane fusion. Receptor-mediated endocytosis and viral escape from the endosome-lysosome pathway serve as a common mechanism for a variety of viruses to enter into specific target cells [100–102]. In the acidic environment of endosomes/lysosomes, low pH triggers a conformational change in the viral envelope that induces fusion of viral and endosomal/lysosomal membranes, resulting in release of viral DNA/RNA into the cytoplasm. Vesicular stomatitis virus (VSV) is one example of a virus that utilizes this mechanism to infect target cells [103]. In the circumstance of HIV infection, membranous fusion classically occurs as a result of receptor engagement, independent of pH [104, 105]. Particularly, the direct fusion of viral envelope with cell membrane is commonly seen in CD4 T lymphocytes with high expression of CD4 and co-receptors [106]. However, there is significant evidence that membrane fusion is not the only avenue of entry for HIV; endocytosis has also been described as a pathway of HIV infection [107, 108], especially in non-lymphocytic cells [109–111], and HIV endosomal fusion can be seen to follow after the endocytosis [111]. This indicates that the low pH may still facilitate HIV endosomal fusion in the cells that express low levels of CD4 or co-receptors [112] though HIV gp120 is not as sensitive to low pH as other viral envelopes such as VSV-G.
The exact mechanism of HIV infection in astrocytes remains elusive. Although endocytosis has been proposed as a pathway of HIV entry into astrocytes [48, 54, 89, 96, 97], the infection is extremely inefficient. This indicates that the majority of internalized viruses are trapped in endosomes/lysosomes and finally degraded there. In general, ligands or viruses internalized via receptor-mediated endocytosis are trafficked to early sorting endosomes, and then rapidly to late endosomes and endolysosomes for degradation [100, 113–115]. Therefore, very few HIV particles are able to escape from degradation in lysosomes and finally gain entry into the cytosol. We observed the full process of HIV endocytosis in astrocytes via electron microscopy and found that infection of astrocytes was significantly enhanced while the cells were simultaneously treated with chloroquine, a lysosomotropic agent that elevates the pH in the lumen of endosomes/lysosomes and therefore impairs the integrity of the endosomal/lysosomal membrane. Although a previous study [99] as well as our data (not yet published) demonstrated that astrocytes express CD4 mRNA and residual levels of its protein, the low pH is still not able to efficiently trigger endosomal fusion while CD4 is only expressed at a minimal level. Thus, the barrier of HIV infection in astrocytes is at entry and the release of viral RNA into the cytosol is blocked in endosomes/endolysosomes where the majority of viral particles are finally degraded.
EFFICIENT HIV INFECTION IN ASTROCYTES CAN OCCUR VIA CELL-TO-CELL CONTACT
Previous studies have shown that astrocytes can be infected with HIV by co-cultivation with HIV-infected lymphocytes [24, 116]. While primary astrocytes cultured with cell-free virus are largely resistant to infection [44–46], infectivity is significantly increased in co-cultures of astrocytes with HIV-infected lymphocytes [117, 118]. This is consistent with studies that show HIV infection by cell-to-cell transmission between lymphocytes is 3 – 4 orders of magnitude more efficient than using cell-free virus [119, 120]. However, the mechanism of cell-to-cell HIV transmission does not appear to be shared across all cell types. Although there are studies showing that cells expressing HIV co-receptors can promote the infection of CD4-negative cells by transcomplementation of receptor deficiency [121, 122], we recently showed that, in the transmission from lymphocytes to astrocytes, HIV may only infect astrocytes before the process of its maturation is completed [117]. Only immature virions enter astrocytes via this route, while entry of mature particles is blocked. This phenomenon can be specifically attributed to a variety of contacts and/or virological synapses formed between astrocytes and lymphocytes [117, 123]. This unique mechanism is CD4-independent but requires CXCR4 and the model of infection has been proposed (Fig. 1) [117]. Classical receptor-mediated fusion occurs via a stepwise process in which binding of gp120 on a mature virion to CD4 on a target cell induces a conformational change in gp120, exposing the CXCR4-interacting domain. The new model proposes that in immature/budding virions the gp120 molecule remains in an “open” conformation that allows interaction with CXCR4, resulting in occurrence of the fusion step in absence of CD4. Thus, cell-to-cell contact might be a possible mode by which HIV can infect astrocytes in vivo.
Fig. (1).
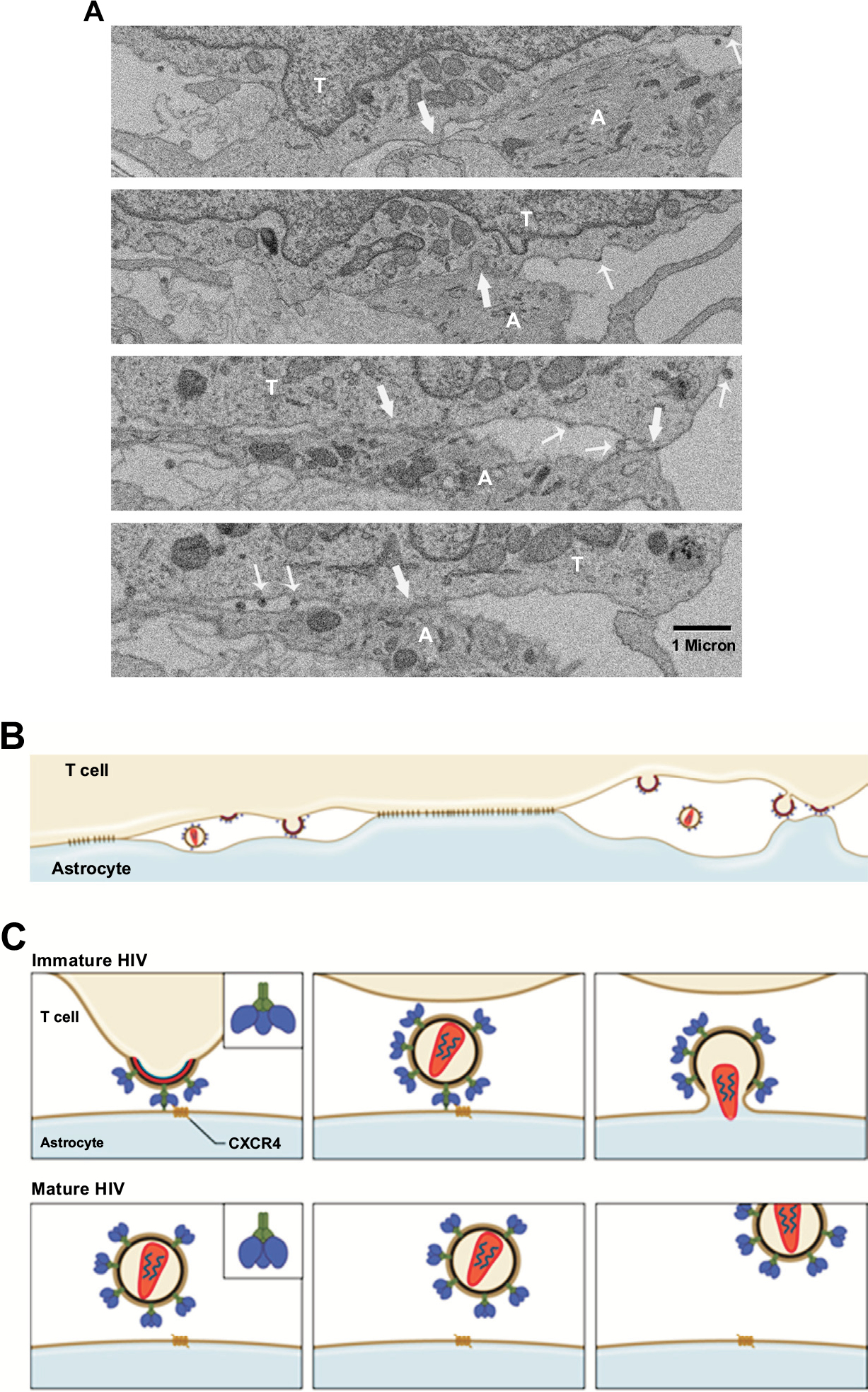
(A) Serial, discontinuous sections of EM were aligned. Multiple tight contacts (block arrow) are observed between HIV-infected JKT cell and astrocyte. The viruses from the infected cell (thin arrow) are seen budding into intercellular spaces or directly onto the membrane of astrocyte. (B) Diagram shows that HIV particles are budding into virological synapses between the areas of tight contacts or directly onto the membrane of astrocyte. The immature, budding viral particles make contact with an astrocyte. (C) A hypothesis is proposed based on the EM observations that CXCR4-binding sites on the surface of the envelope of budding or immature HIV is in an “open” state which allows the virus to directly bind to CXCR4 on the membrane of astrocytes, and hidden following a conformational change that is triggered during HIV maturation. However, the pre-bound virus would trigger the fusion process of HIV envelope with the astrocyte membrane while the maturation is completed, leading to HIV transmission from lymphocytes to astrocytes. This process cannot occur with cell-free mature HIV in astrocytes that lack CD4 expression since the CXCR4-binding sites are hidden in the envelope of mature HIV particles. T – T lymphocyte, A – astrocyte.
Based on these findings, it seems that only X4 or dualtropic R5X4 viruses are able to infect astrocytes via cell-to-cell contact because CCR5 is not detectable in cultured astrocytes [117]. One argument against this hypothesis is that most HIV strains isolated from the brain are R5-tropic [124]; however, some studies have reported that X4-tropic or R5X4 dual-tropic HIV-1 viruses are present in the brain or CSF of patients with HIV-associated dementia [124–127]. Additionally, there is evidence that suggests some R5X4 viruses preferentially use CXCR4 for entry [126, 128–130]. Combined antiretroviral therapy induces a switch in HIV tropism from R5 to X4 usage [131]. This switch may appear late in the CNS compartment compared to the periphery. Also, cell-cell fusion assays show that many bioinformatic prediction programs underestimate CXCR4 usage by R5X4 viruses in the brain [132]. Furthermore, studies show that lymphocytes actually migrate into the brain and the frequency of migration is significantly higher in asymptomatic carriers compared to patients with early or late stage AIDS [133, 134]. The migration of lymphocytes into the brain is also observed in SIV/FIV-infected animal models [135, 136]. HIV-infected and/or immune activated macrophages produce IL-1β that further induces secretion of SDF-1 from astrocytes [137]. Thus, SDF-1 can trigger HIV-infected lymphocytes to migrate through the blood-brain barrier (BBB) and contact with astrocytes leading to HIV infection of astrocytes. This is also supported by the finding that HIV infection of astrocytes occurs predominantly in perivascular regions [38].
PROSPECT
Although studies have shown that infection of astrocytes with cell-free HIV is inefficient due to poor viral entry, cell-to-cell contact can facilitate HIV transmission from lymphocytes to astrocytes leading to productive infection. Further, in patients with HIV-associated neurological disorders the inflammatory environment associated with activation of different factors (e.g., cytokines, chemokines) [93, 138–140] may upregulate expression of HIV receptor CD4 and coreceptors in astrocytes that make the cells permissive to HIV infection. Thus, we propose that HIV infection of astrocytes in the brain occurs by two major mechanisms as depicted in the diagram (Fig. 2). Therefore, in addition to infection by immature HIV virions, cell-free mature HIV may also be able to infect the astrocytes that have been induced to upregulate CD4/coreceptors in vivo.
Fig. (2).
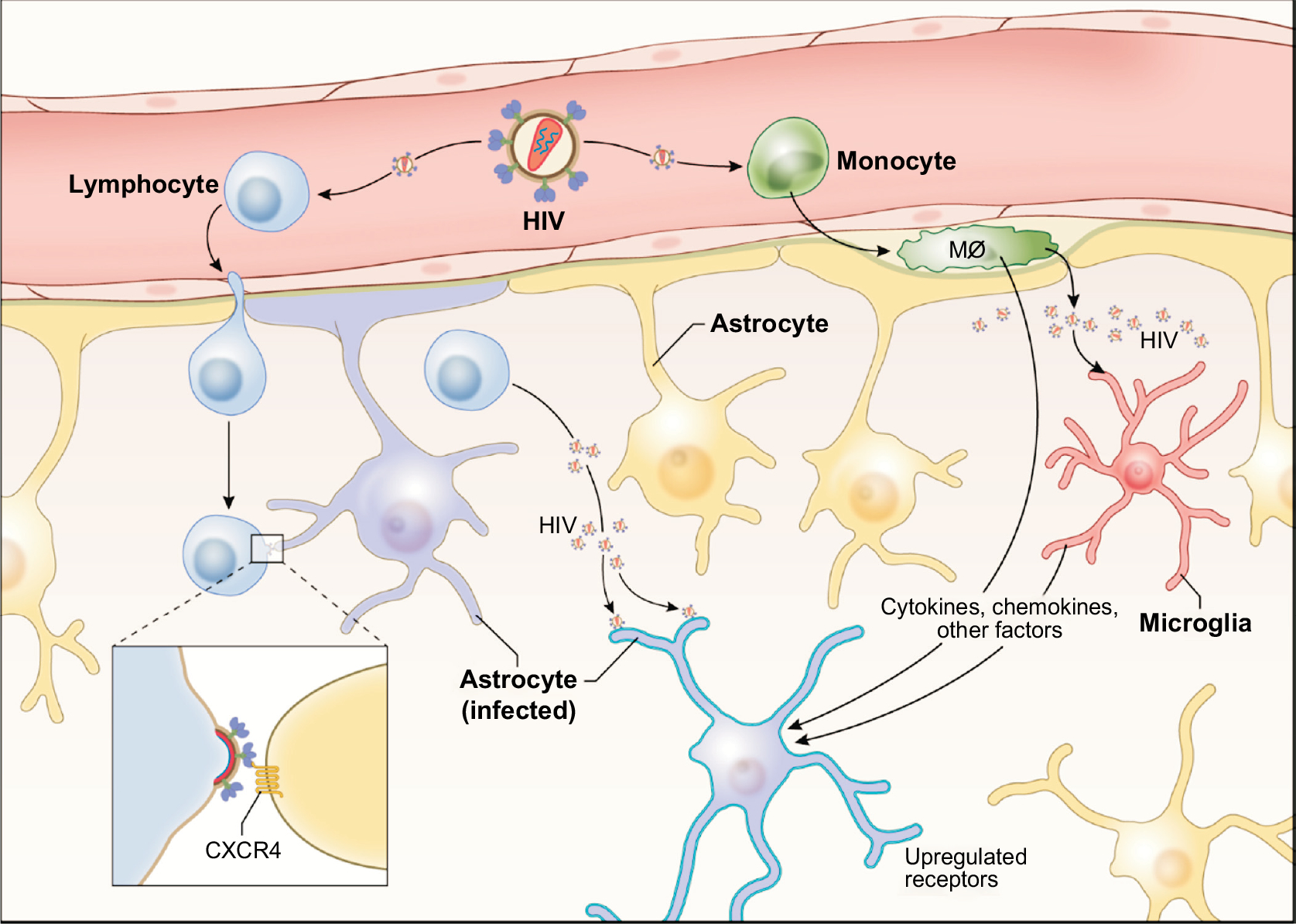
Two major mechanisms that might be involved in HIV infection of astrocytes in vivo are shown. One occurs via the cell-to-cell contact with HIV-infected lymphocytes, by which immature HIV particles are transmitted to astrocytes. The second may require inflammatory factors (e.g., cytokines, chemokines, etc.) produced from HIV-infected perivascular macrophages and/or microglia that up-regulate expression of HIV receptor and co-receptors in astrocytes making the cell (highlighted) permissive to HIV infection. Mø - macrophage
Astrocytes, by the very nature of being long-lived cells that do not undergo any CPE while persistently infected, are an important reservoir for HIV infection in the CNS. The ability of these cells to respond to cytokines with a burst of HIV replication suggests that, unless this reservoir is prevented from being formed, it will be able to reseed the periphery even if a cure is achieved in lymphocytes. Viral entry into astrocytes can be achieved by immature virons where it is capable of engaging co-receptor CXCR4 in the absence of CD4. Thus, blockers of this co-receptor have the potential for preventing the establishment of the astrocyte reservoir. This may be important for the brain as well as the gut since astrocytes are found in both organs.
Biography

Guan-Han Li
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980; 286(5774): 736–7. [DOI] [PubMed] [Google Scholar]
- [2].Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003; 41(1): 81–93. [DOI] [PubMed] [Google Scholar]
- [3].Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012; 9(11): 625–32. [DOI] [PubMed] [Google Scholar]
- [4].Ho DD, Sarngadharan MG, Resnick L, Dimarzoveronese F, Rota TR, Hirsch MS. Primary human T-lymphotropic virus type III infection. Annals of internal medicine. 1985; 103(6 ( Pt 1)): 880–3. [DOI] [PubMed] [Google Scholar]
- [5].Chiodi F, Albert J, Olausson E, et al. Isolation frequency of human immunodeficiency virus from cerebrospinal fluid and blood of patients with varying severity of HIV infection. AIDS research and human retroviruses. 1988; 4(5): 351–8. [DOI] [PubMed] [Google Scholar]
- [6].Brew BJ, Perdices M, Darveniza P, et al. The neurological features of early and ‘latent’ human immunodeficiency virus infection. Australian and New Zealand journal of medicine. 1989; 19(6): 700–5. [DOI] [PubMed] [Google Scholar]
- [7].Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992; 42(9): 1736–9. [DOI] [PubMed] [Google Scholar]
- [8].Palmer DL, Hjelle BL, Wiley CA, et al. HIV-1 infection despite immediate combination antiviral therapy after infusion of contaminated white cells. The American journal of medicine. 1994; 97(3): 289–95. [DOI] [PubMed] [Google Scholar]
- [9].Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. The Journal of infectious diseases. 2012; 206(2): 275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith MO, Heyes MP, Lackner AA. Early intrathecal events in rhesus macaques (Macaca mulatta) infected with pathogenic or nonpathogenic molecular clones of simian immunodeficiency virus. Laboratory investigation; a journal of technical methods and pathology. 1995; 72(5): 547–58. [PubMed] [Google Scholar]
- [11].Clements JE, Babas T, Mankowski JL, et al. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. The Journal of infectious diseases. 2002; 186(7): 905–13. [DOI] [PubMed] [Google Scholar]
- [12].Thompson KA, Varrone JJ, Jankovic-Karasoulos T, Wesselingh SL, McLean CA. Cell-specific temporal infection of the brain in a simian immunodeficiency virus model of human immunodeficiency virus encephalitis. Journal of neurovirology. 2009; 15(4): 300–11. [DOI] [PubMed] [Google Scholar]
- [13].Zaritsky LA, Dery A, Leong WY, Gama L, Clements JE. Tissue-specific interferon alpha subtype response to SIV infection in brain, spleen, and lung. J Interferon Cytokine Res. 2013; 33(1): 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu P, Hudson LC, Tompkins MB, et al. Cerebrospinal fluid is an efficient route for establishing brain infection with feline immunodeficiency virus and transfering infectious virus to the periphery. Journal of neurovirology. 2006; 12(4): 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sinclair E, Gray F, Ciardi A, Scaravilli F. Immunohistochemical changes and PCR detection of HIV provirus DNA in brains of asymptomatic HIV-positive patients. Journal of neuropathology and experimental neurology. 1994; 53(1): 43–50. [DOI] [PubMed] [Google Scholar]
- [16].Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. The American journal of pathology. 2011; 179(4): 1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS (London, England). 2014; 28(15): 2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993; 7(1): 19–24. [DOI] [PubMed] [Google Scholar]
- [19].Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. Journal of neuropathology and experimental neurology. 1993; 52(5): 460–70. [DOI] [PubMed] [Google Scholar]
- [20].Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001; 36(2): 118–24. [DOI] [PubMed] [Google Scholar]
- [21].McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. The Journal of comparative neurology. 1988; 271(4): 589–603. [DOI] [PubMed] [Google Scholar]
- [22].Eisfeld C, Reichelt D, Evers S, Husstedt I. CSF penetration by antiretroviral drugs. CNS Drugs. 2013; 27(1): 31–55. [DOI] [PubMed] [Google Scholar]
- [23].Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. Journal of virology. 1987; 61(12): 3774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brack-Werner R, Kleinschmidt A, Ludvigsen A, et al. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS (London, England). 1992; 6(3): 273–85. [PubMed] [Google Scholar]
- [25].Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. The American journal of physiology. 1992; 263(1 Pt 1): C1–16. [DOI] [PubMed] [Google Scholar]
- [26].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010; 119(1): 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dubois-Dalcq M, Altmeyer R, Chiron M, Wilt S. HIV interactions with cells of the nervous system. Current opinion in neurobiology. 1995; 5(5): 647–55. [DOI] [PubMed] [Google Scholar]
- [28].Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus research. 2005; 111(2): 194–213. [DOI] [PubMed] [Google Scholar]
- [29].Brack-Werner R Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS (London, England). 1999; 13(1): 1–22. [DOI] [PubMed] [Google Scholar]
- [30].Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Annals of neurology. 1987; 21(5): 490–6. [DOI] [PubMed] [Google Scholar]
- [31].Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994; 44(3 Pt 1): 481–7. [DOI] [PubMed] [Google Scholar]
- [32].Ranki A, Nyberg M, Ovod V, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS (London, England). 1995; 9(9): 1001–8. [DOI] [PubMed] [Google Scholar]
- [33].Nuovo GJ, Gallery F, MacConnell P, Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. The American journal of pathology. 1994; 144(4): 659–66. [PMC free article] [PubMed] [Google Scholar]
- [34].Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Annals of neurology. 1996; 39(6): 705–11. [DOI] [PubMed] [Google Scholar]
- [35].An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. Journal of neuropathology and experimental neurology. 1999; 58(11): 1156–62. [DOI] [PubMed] [Google Scholar]
- [36].Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, et al. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathology and applied neurobiology. 2003; 29(4): 378–88. [DOI] [PubMed] [Google Scholar]
- [37].Trillo-Pazos G, Diamanturos A, Rislove L, et al. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain pathology (Zurich, Switzerland). 2003; 13(2): 144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Annals of neurology. 2009; 66(2): 253–8. [DOI] [PubMed] [Google Scholar]
- [39].Wiley CA. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain pathology (Zurich, Switzerland). 2003; 13(3): 415; author reply −6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Overholser ED, Coleman GD, Bennett JL, et al. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. Journal of virology. 2003; 77(12): 6855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011; 31(26): 9456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guillemin G, Croitoru J, Le Grand RL, Franck-Duchenne M, Dormont D, Boussin FD. Simian immunodeficiency virus mac251 infection of astrocytes. Journal of neurovirology. 2000; 6(3): 173–86. [DOI] [PubMed] [Google Scholar]
- [43].Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain pathology (Zurich, Switzerland). 2004; 14(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McCarthy M, He J, Wood C. HIV-1 strain-associated variability in infection of primary neuroglia. Journal of neurovirology. 1998; 4(1): 80–9. [DOI] [PubMed] [Google Scholar]
- [45].Di Rienzo AM, Aloisi F, Santarcangelo AC, et al. Virological and molecular parameters of HIV-1 infection of human embryonic astrocytes. Archives of virology. 1998; 143(8): 1599–615. [DOI] [PubMed] [Google Scholar]
- [46].Sabri F, Tresoldi E Di Stefano, et al. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999; 264(2): 370–84. [DOI] [PubMed] [Google Scholar]
- [47].Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. Journal of virology. 2001; 75(17): 7925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vijaykumar TS, Nath A, Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008; 381(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shahabuddin M, Volsky B, Kim H, Sakai K, Volsky DJ. Regulated expression of human immunodeficiency virus type 1 in human glial cells: induction of dormant virus. Pathobiology : journal of immunopathology, molecular and cellular biology. 1992; 60(4): 195–205. [DOI] [PubMed] [Google Scholar]
- [50].Volsky B, Sakai K, Reddy MM, Volsky DJ. A system for the high efficiency replication of HIV-1 in neural cells and its application to anti-viral evaluation. Virology. 1992; 186(1): 303–8. [DOI] [PubMed] [Google Scholar]
- [51].Harrington RD, Geballe AP. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. Journal of virology. 1993; 67(10): 5939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vodicka MA, Goh WC, Wu LI, et al. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997; 233(1): 193–8. [DOI] [PubMed] [Google Scholar]
- [53].Kirschmeier PT, Housey GM, Johnson MD, Perkins AS, Weinstein IB. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988; 7(3): 219–25. [DOI] [PubMed] [Google Scholar]
- [54].Chauhan A, Mehla R, Vijayakumar TS, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology. 2014; 456–457: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li J, Liu Y, Kim BO, He JJ. Direct participation of Sam68, the 68-kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway. Journal of virology. 2002; 76(16): 8374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li J, Liu Y, Park IW, He JJ. Expression of exogenous Sam68, the 68-kilodalton SRC-associated protein in mitosis, is able to alleviate impaired Rev function in astrocytes. Journal of virology. 2002; 76(9): 4526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vincendeau M, Kramer S, Hadian K, et al. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp). AIDS (London, England). 2010; 24(16): 2433–42. [DOI] [PubMed] [Google Scholar]
- [58].Daher A, Laraki G, Singh M, et al. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Molecular and cellular biology. 2009; 29(1): 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gatignol A, Laine S, Clerzius G. Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology. 2005; 2: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bannwarth S, Gatignol A. HIV-1 TAR RNA: the target of molecular interactions between the virus and its host. Current HIV research. 2005; 3(1): 61–71. [DOI] [PubMed] [Google Scholar]
- [61].Sanghvi VR, Steel LF. The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. Journal of virology. 2011; 85(23): 12614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004; 119(3): 381–92. [DOI] [PubMed] [Google Scholar]
- [63].Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005; 336(2): 299–307. [DOI] [PubMed] [Google Scholar]
- [64].Ishaq M, Hu J, Wu X, et al. Knockdown of cellular RNA helicase DDX3 by short hairpin RNAs suppresses HIV-1 viral replication without inducing apoptosis. Molecular biotechnology. 2008; 39(3): 231–8. [DOI] [PubMed] [Google Scholar]
- [65].Rossi A, Mukerjee R, Ferrante P, Khalili K, Amini S, Sawaya BE. Human immunodeficiency virus type 1 Tat prevents dephosphorylation of Sp1 by TCF-4 in astrocytes. The Journal of general virology. 2006; 87(Pt 6): 1613–23. [DOI] [PubMed] [Google Scholar]
- [66].Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. Journal of virology. 2007; 81(11): 5864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F, Al-Harthi L. Role of beta-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. Journal of virology. 2012; 86(4): 1911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Henderson LJ, Narasipura SD, Adarichev V, Kashanchi F, Al-Harthi L. Identification of novel T cell factor 4 (TCF-4) binding sites on the HIV long terminal repeat which associate with TCF-4, beta-catenin, and SMAR1 to repress HIV transcription. Journal of virology. 2012; 86(17): 9495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chauhan A Unperturbed posttranscriptional regulatory Rev protein function and HIV-1 replication in astrocytes. PloS one. 2014; 9(9): e106910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. Journal of neurovirology. 1998; 4(4): 377–86. [DOI] [PubMed] [Google Scholar]
- [71].Gorry PR, Howard JL, Churchill MJ, et al. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. Journal of virology. 1999; 73(1): 352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. Journal of neurovirology. 1999; 5(2): 115–24. [DOI] [PubMed] [Google Scholar]
- [73].Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC neuroscience. 2007; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. Journal of virology. 1991; 65(11): 6094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. Journal of virology. 1994; 68(1): 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. Journal of virology. 2006; 80(1): 541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chauhan A, Tikoo A. The enigma of the clandestine association between chloroquine and HIV-1 infection. HIV medicine. 2015. [DOI] [PubMed] [Google Scholar]
- [78].Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. Infection of brain-derived cells with the human immunodeficiency virus. Journal of virology. 1987; 61(4): 1244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Atwood WJ, Tornatore CS, Traub R, Conant K, Drew PD, Major EO. Stimulation of HIV type 1 gene expression and induction of NF-kappa B (p50/p65)-binding activity in tumor necrosis factor alpha-treated human fetal glial cells. AIDS research and human retroviruses. 1994; 10(10): 1207–11. [DOI] [PubMed] [Google Scholar]
- [80].Messam CA, Major EO. Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue. Journal of neurovirology. 2000; 6 Suppl 1: S90–4. [PubMed] [Google Scholar]
- [81].Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. Journal of virology. 2014; 88(5): 3031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Imai K, Togami H, Okamoto T. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. The Journal of biological chemistry. 2010; 285(22): 16538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bouchat S, Gatot JS, Kabeya K, et al. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4(+) T cells from HIV-1-infected HAART-treated patients. AIDS (London, England). 2012; 26(12): 1473–82. [DOI] [PubMed] [Google Scholar]
- [84].Chauhan A, Tikoo A, Patel J, Abdullah AM. HIV-1 endocytosis in astrocytes: a kiss of death or survival of the fittest? Neurosci Res. 2014; 88: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997; 385(6617): 645–9. [DOI] [PubMed] [Google Scholar]
- [86].Ma M, Geiger JD, Nath A. Characterization of a novel binding site for the human immunodeficiency virus type 1 envelope protein gp120 on human fetal astrocytes. Journal of virology. 1994; 68(10): 6824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Peudenier S, Hery C, Ng KH, Tardieu M. HIV receptors within the brain: a study of CD4 and MHC-II on human neurons, astrocytes and microglial cells. Research in virology. 1991; 142(2–3): 145–9. [DOI] [PubMed] [Google Scholar]
- [88].Harouse JM, Kunsch C, Hartle HT, et al. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. Journal of virology. 1989; 63(6): 2527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hao HN, Chiu FC, Losev L, Weidenheim KM, Rashbaum WK, Lyman WD. HIV infection of human fetal neural cells is mediated by gp120 binding to a cell membrane-associated molecule that is not CD4 nor galactocerebroside. Brain research. 1997; 764(1–2): 149–57. [DOI] [PubMed] [Google Scholar]
- [90].Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. The American journal of pathology. 1997; 151(5): 1341–51. [PMC free article] [PubMed] [Google Scholar]
- [91].Reeves JD, Hibbitts S, Simmons G, et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. Journal of virology. 1999; 73(9): 7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Klein RS, Williams KC, Alvarez-Hernandez X, et al. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. Journal of immunology. 1999; 163(3): 1636–46. [PubMed] [Google Scholar]
- [93].Croitoru-Lamoury J, Guillemin GJ, Boussin FD, et al. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003; 41(4): 354–70. [DOI] [PubMed] [Google Scholar]
- [94].Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. Journal of virology. 2005; 79(15): 9618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Harouse JM, Bhat S, Spitalnik SL, et al. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science (New York, NY. 1991; 253(5017): 320–3. [DOI] [PubMed] [Google Scholar]
- [96].Hao HN, Lyman WD. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain research. 1999; 823(1–2): 24–32. [DOI] [PubMed] [Google Scholar]
- [97].Liu Y, Liu H, Kim BO, et al. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. Journal of virology. 2004; 78(8): 4120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Deiva K, Khiati A, Hery C, et al. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS research and human retroviruses. 2006; 22(11): 1152–61. [DOI] [PubMed] [Google Scholar]
- [99].Boutet A, Salim H, Taoufik Y, et al. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001; 34(3): 165–77. [PubMed] [Google Scholar]
- [100].Lozach PY, Huotari J, Helenius A. Late-penetrating viruses. Curr Opin Virol. 2011; 1(1): 35–43. [DOI] [PubMed] [Google Scholar]
- [101].Nour AM, Modis Y. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol. 2014; 24(8): 449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006; 124(4): 729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Boritz E, Gerlach J, Johnson JE, Rose JK. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. Journal of virology. 1999; 73(8): 6937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987; 49(5): 659–68. [DOI] [PubMed] [Google Scholar]
- [105].Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annual review of immunology. 1999; 17: 657–700. [DOI] [PubMed] [Google Scholar]
- [106].Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harbor perspectives in medicine. 2012; 2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002; 76(22): 11440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000; 10(16): 1005–8. [DOI] [PubMed] [Google Scholar]
- [109].Pritschet K, Donhauser N, Schuster P, Ries M, Haupt S, Kittan NA, et al. CD4- and dynamin-dependent endocytosis of HIV-1 into plasmacytoid dendritic cells. Virology. 2012; 423(2): 152–64. [DOI] [PubMed] [Google Scholar]
- [110].Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009; 137(3): 433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. Journal of virology. 2001; 75(22): 11166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Permanyer M, Ballana E, Este JA. Endocytosis of HIV: anything goes. Trends in microbiology. 2010; 18(12): 543–51. [DOI] [PubMed] [Google Scholar]
- [113].Marsh M, Pelchen-Matthews A. Endocytosis in viral replication. Traffic. 2000; 1(7): 525–32. [DOI] [PubMed] [Google Scholar]
- [114].Mellman I Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996; 12: 575–625. [DOI] [PubMed] [Google Scholar]
- [115].Riezman H, Woodman PG, van Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997; 91(6): 731–8. [DOI] [PubMed] [Google Scholar]
- [116].Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. Journal of neuropathology and experimental neurology. 1995; 54(3): 320–30. [DOI] [PubMed] [Google Scholar]
- [117].Li GH, Anderson C, Jaeger L, Do T, Major EO, Nath A. Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS (London, England). 2015; 29(7): 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Luo X, He JJ. Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. Journal of neurovirology. 2015; 21(1): 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. Journal of virology. 1993; 67(4): 2182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. Journal of virology. 2007; 81(2): 1000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mack M, Kleinschmidt A, Bruhl H, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000; 6(7): 769–75. [DOI] [PubMed] [Google Scholar]
- [122].Speck RF, Esser U, Penn ML, et al. A trans-receptor mechanism for infection of CD4-negative cells by human immunodeficiency virus type 1. Current biology : CB. 1999; 9(10): 547–50. [DOI] [PubMed] [Google Scholar]
- [123].Do T, Murphy G, Earl LA, et al. Three-dimensional imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. Journal of virology. 2014; 88(18): 10327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Dunfee R, Thomas ER, Gorry PR, Wang J, Ancuta P, Gabuzda D. Mechanisms of HIV-1 neurotropism. Current HIV research. 2006; 4(3): 267–78. [DOI] [PubMed] [Google Scholar]
- [125].Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, et al. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. Journal of virology. 2003; 77(22): 12336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. Journal of virology. 2001; 75(21): 10073–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gorry PR, Taylor J, Holm GH, et al. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. Journal of virology. 2002; 76(12): 6277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ghaffari G, Tuttle DL, Briggs D, et al. Complex determinants in human immunodeficiency virus type 1 envelope gp120 mediate CXCR4-dependent infection of macrophages. Journal of virology. 2005; 79(21): 13250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Simmons G, Reeves JD, McKnight A, et al. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. Journal of virology. 1998; 72(10): 8453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yi Y, Shaheen F, Collman RG. Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes. Journal of virology. 2005; 79(3): 1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Vissers M, Stelma FF, Koopmans PP. Could differential virological characteristics account for ongoing viral replication and insidious damage of the brain during HIV 1 infection of the central nervous system? Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010; 49(4): 231–8. [DOI] [PubMed] [Google Scholar]
- [132].Mefford ME, Gorry PR, Kunstman K, Wolinsky SM, Gabuzda D. Bioinformatic prediction programs underestimate the frequency of CXCR4 usage by R5X4 HIV type 1 in brain and other tissues. AIDS research and human retroviruses. 2008; 24(9): 1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kibayashi K, Mastri AR, Hirsch CS. Neuropathology of human immunodeficiency virus infection at different disease stages. Human pathology. 1996; 27(7): 637–42. [DOI] [PubMed] [Google Scholar]
- [134].Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. Journal of neurovirology. 2003; 9(1): 36–44. [DOI] [PubMed] [Google Scholar]
- [135].Czub S, Muller JG, Czub M, Muller-Hermelink HK. Nature and sequence of simian immunodeficiency virus-induced central nervous system lesions: a kinetic study. Acta neuropathologica. 1996; 92(5): 487–98. [DOI] [PubMed] [Google Scholar]
- [136].Ryan G, Grimes T, Brankin B, et al. Neuropathology associated with feline immunodeficiency virus infection highlights prominent lymphocyte trafficking through both the blood-brain and blood-choroid plexus barriers. Journal of neurovirology. 2005; 11(4): 337–45. [DOI] [PubMed] [Google Scholar]
- [137].Peng H, Erdmann N, Whitney N, et al. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006; 54(6): 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yao H, Bethel-Brown C, Li CZ, Buch SJ. HIV neuropathogenesis: a tight rope walk of innate immunity. J Neuroimmune Pharmacol. 2010; 5(4): 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Frontiers in bioscience : a journal and virtual library. 2004; 9: 1073–81. [DOI] [PubMed] [Google Scholar]
- [140].Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. Journal of neurovirology. 2000; 6 Suppl 1: S24–32. [PubMed] [Google Scholar]


