Abstract
Purple mangosteen scarfskin polysaccharide has many important physiological functions, but its preparation method, structure, and function need further exploration. A polysaccharide was obtained from mangosteen scarfskin by ultrasonic-assisted extraction and purified. On this basis, its structure and physicochemical properties were investigated. The Congo red experiment was used to determine whether it has a triple helix conformation. The structure of purple mangosteen scarfskin polysaccharide was further analyzed by infrared spectroscopy and nuclear magnetic analysis. The antioxidant activities of the above three polysaccharides were studied by related experiments. It was found that the monosaccharide composition of purple mangosteen scarfskin polysaccharide mainly contained a large amount of arabinose, a small amount of rhamnoose and a very small amount of galacturonic acid, and its core main chain was composed of 1,4-α-arabinose. It did not have this spatial configuration. After the acetylation of purple mangosteen scarfskin polysaccharide, the acetylated derivative with a degree of substitution of 0.33 was obtained. It was found that they had certain scavenging and inhibiting effects on hydroxyl radicals and lipid peroxidation, and their activities were related to the concentration of polysaccharides. Meanwhile, the antioxidant activity of the polysaccharide was significantly enhanced after the modified treatment of acetylation, which indicated that chemical modification could effectively improve some activities of polysaccharide. The above studies provided some reference value for the further research and development of purple mangosteen scarfskin polysaccharide.
Keywords: Ultrasonic-assisted extraction, Purple mangosteen scarfskin polysaccharide, Chemical modification, Antioxidant activity
1. Introduction
Purple mangosteen (Garcinia mangostana L.) is a small arboreal plant of Garcinia of Garciniaceae, which originated in Maluku and mainly distributed in tropical Asia and Africa in the world. For hundreds of years, mangosteen has not only been eaten as a fruit, but its scarfskin has even been used as a kind of Chinese medicine after being dried. For the medicinal value of mangosteen scarfskin, in recent years, more and more scholars have begun to conduct relevant studies and found that the extract of mangosteen scarfskin is rich in active components such as polysaccharides, polyphenols and anthocyanins. [1], [2], [3]. On this basis, some studies have shown that polysaccharide components in mangosteen scarfskin have antioxidant, antibacterial and immunomodulatory activities, etc. [1], [4], [5]. But even so, the research reports on purple mangosteen scarfskin polysaccharide (UAEE-PMSP) and its activities are still relatively few. In view of this, it is necessary to further study the polysaccharide from the scarfskin of purple mangosteen and explore its application potential.
Animals and plants contain abundant amounts of various biological macromolecules, such as proteins, nucleic acids, polysaccharides, etc., which are essential components of life forms [6], [7], [8], [9]. In addition, polysaccharides also have significant activity in fighting various biological diseases or negative effects. Polysaccharides and other natural substances are mostly extracted by traditional and simple hot water extraction method. However, in view of the low efficiency of this method, more emerging environmentally friendly and efficient methods such as ultrasound-assisted extraction, enzyme-assisted extraction and even ultrasonic/wave-assisted extraction have emerged and been widely used [10], [11], [12]. In order to improve the bioactivity of the extracted polysaccharide as much as possible, different substituting groups are often used to replace the hydroxyl group on the sugar ring of the polysaccharide, so as to carry out appropriate chemical modification. Now, the commonly used decoration methods are phosphorylation, carboxymethylation, acetylation, sulfation, selenation and so on [13]. Phosphorylation has been extensively studied and applied because it can significantly improve the activity of polysaccharides [14]. In contrast, there are few studies on other methods, but some studies have shown that polysaccharide can significantly improve some biological activities after acetylation [15], [16], [17]. In view of the scarcity of acetylated polysaccharide and the few related studies, and its important role in molecular modification gradually emerged, so it began to receive extensive attention from scholars.
Chemical modification is often the most effective and direct way to obtain highly active polysaccharides, but the differences in activity of different polysaccharides are usually attributed to the differences in structural characteristics. A large number of studies have confirmed that the high biological activity of polysaccharides is related with molecular weight, the content of some monosaccharides in the composition of monosaccharides, the number and type of substituent groups, and the difference of main chain or branch chain structure [18], [19], [20], [21]. At present, researches on the structure and chemical modification of purple mangosteen scarfskin polysaccharide are scarce. In this research, the structure and composition of the purified polysaccharide of purple mangosteen scarfskin were analyzed, and acetyl group was introduced through chemical modification, so as to explore the effect of this substituent on the antioxidant activity of polysaccharide of purple mangosteen scarfskin. Therefore, we hope that the above research can provide some positive value for the in-depth application of purple mangosteen scarfskin polysaccharide.
2. Materials and methods
2.1. Materials and reagents
The fresh mangosteen used in this experiment was produced in Thailand, and the scarfskins were peeled off and dried and crushed to get powder. DEAE-52 cellulose and Sephadex G-100 were purchased from Shanghai Bio-Technology Co., Ltd, and other related reagents were purchased through commercial channels, all of which were analytical purity.
2.2. Preparation of UAEE-PMSP and acetylated derivative (Ac-UAEE-PMSP)
2.2.1. Preparation of crude polysaccharide from purple mangosteen scarfskin
20 g dried mangosteen scarfskin powder was mixed with 1000 mL cellulase solution with a concentration of 4 %. Next, the extraction of polysaccharide was carried out in an ultrasonic instrument with a power of 180 w. After 40 min of extraction, the extraction mixture was filtered and the filtrate was collected, then concentrated to a total solution volume of 100 mL. Anhydrous ethanol is added to the concentrated solution until the ethanol concentration is 50 %, and the supernatant is collected centrifugally after 0.5 d to remove most of the gelatinous substances. After that, the supernatant was reduced pressure concentrated to remove the residual ethanol, and then anhydrous ethanol was added to the supernatant obtained after concentration in accordance with the volume ratio of 1:3 for a second alcohol precipitation, and the alcohol precipitation solids were collected by centrifugation after standing for 2 h. After the precipitate was redissolved by adding 50 ml of deionized water, 25 mL of sevag reagent (Vchloroform: Vn-butanol = 4:1) was added and the protein was removed by vigorous shaking for 20 min. Then it was left for 10 min, centrifuged to collect the supernatant, and the supernatant was deproteinized again. Finally, the supernatant collected after deproteinization was put into 3500 da dialysis bag, and dialysis was performed with flowing water for 1.5 d and then with deionized water for 1.5 d, and finally freeze-dried dialysate to get crude polysaccharide from purple mangosteen scarfskin
2.2.2. Separation and purification of UAEE-PMSP-1A
The crude polysaccharide from purple mangosteen scarfskin was purified in two steps by column chromatography and isolated to obtain UAEE-PMSP-1. Simply put, the first step was preliminary separation by Ion-exchange cellulose column chromatography, that is, 80 mg crude polysaccharide from purple mangosteen scarfskin was weighed and dissolved in 8 mL deionized water. Then the above solution was slowly injected into DEAE-52 cellulose column (2.5 × 30 cm) by needle tube, and then eluted by concentration gradient with deionized water, 0.1 mol·L−1 NaCl solution and 0.2 mol·L−1 NaCl solution (both 300 mL), respectively. The eluent flow rate for this chromatographic column was set to 1 mL/min. Elution fractions with different concentrations were determined by phenol–sulfuric acid method and eluted fractions containing polysaccharides were collected. Each fraction was named as UAEE-PMSP-0, UAEE-PMSP-1 and UAEE-PMSP-2 in the order of increasing concentration, and the second fraction was enriched and freeze-dried. The second step was secondary separation by Gel column chromatography, that is, 10 mg UAEE-PMSP-1 was redissolved in 5 ml 0.1 mol/mL NaCl solution. The impurities were then filtered by 0.45 µm filter membrane and slowly packed into Sephadex G-100 gel column (2 × 40 cm). Finally, 200 ml 0.1 mol/ml NaCl solution was used for elution. After elution, the polysaccharide was collected and named as UAEE-PMSP-1A.
2.2.3. Synthesis of Ac-UAEE-PMSP
Ac-UAEE-PMSP was prepared by introducing acetyl group into polysaccharide structure with acetic anhydride reagent [17]. The specific steps were as follows: by using 10 mL deionized water to fully dissolve 0.5 g freeze-dried purple mangosteen scarfskin crude polysaccharide powder, and then adding the concentration of 2 mol·L−1 NaOH solution, the pH value of polysaccharide solution was accurately regulated by pH meter to stabilize at about 9. Next, a total of 1 mL of acetic anhydride solution was put into the above solution by adding in batches, while maintaining the pH value of the entire reaction system between 9 and 10 by using sodium hydroxide solution in the process. After the above operation was completed, it was immediately transferred to the oil bath set at 25 ℃ for reaction 2 h. After the heating reaction was completed, the temperature of the reaction solution was naturally reduced to the equivalent of room temperature, and then the pH of the whole reaction system was adjusted to about neutral by using a concentration of 1 mol/L HCI solution. Finally, the small molecules inside the solution were completely removed by dialysis treatment. After two days of dialysis, the dialysate was properly concentrated and the required volume of anhydrous ethanol was added according to the ratio of dialysate to anhydrous ethanol by volume of 1:3. The alcohol-deposited solid was obtained by centrifugation the next day, and the solid was freeze-dried to get acetylated purple mangosteen scarfskin polysaccharide.
Acid-base titration method for determining the degree of substitution of acetyl groups [22]. That is, remove 10 mL of NaOH solution with a concentration of 0.01 mol·L−1 to fully dissolve 20 mg Ac-UAEE-PMSP. The above solution was then heated in a water bath at 50 °C. After that, saponification reaction was carried out for two hours, heating was stopped and cooled to room temperature. Phenolphthalein was added to the solution as an indicator and titrated with 0.01 mol·L−1 HCl solution until the pH value of the solution system was 7. Finally, the corresponding formula to calculate the degree of substitution of acetylated polysaccharides was DSAc = 132A / (4300 − 42A), where A is the content of acetyl group in polysaccharides, A = (VNaOHCNaOH − VHClCHCl) × 0.043 × 100/M, M is the mass of acetylated polysaccharide (g).
2.3. Determination of physicochemical properties of polysaccharides
For the determination of total sugar content, protein content, and glucuronic acid content of UAEE-PMSP, UAEE-PMSP-1A, and Ac-UAEE-PMSP, three methods were used: phenol–sulfuric acid method, Coomassie brilliant blue staining method and carbazole-sulfuric acid method, and the corresponding standard samples were glucose monohydrate, bovine serum albumin and galacturonic acid, respectively [23], [24], [25]. The linear regression equation of glucose standard curve was Y=1.44386X+0.06924 (R2 = 0.99942, linear range 0–1 mg/mL); the linear regression equation of bovine serum albumin standard curve was Y=0.461X+0.9464 (R2 = 0.99978, linear range 0–0.8 mg/mL); the linear regression equation of galacturonic acid standard curve was Y=5.06393X+0.12205 (R2 = 0.99938, linear range 0–0.4 mg/mL). The calculation formula of polysaccharide yield was Yield (%) = (M1/M2) × 100, where M1 and M2 were the quality of polysaccharide and raw materials, respectively.
2.4. Determination of monosaccharide component
Monosaccharide component of polysaccharide was determined by ion chromatography. The 5 mg of purified polysaccharide were weighed and placed in tube with stopper. 2 mol/L trifluoroacetic acid (TFA, 1 mL) was added. The polysaccharide was hydrolyzed at 121℃ with 2 h. After reaction was completed, the TFA was removed with nitrogen (N2). 10 mg/L solution was prepared with ultra-pure water and tested with pulsed amperometric detector and Dionex Carbopac PA20 column (150 mm × 3 mm) high performance anion exchange column.
2.5. Determination of molecular weight
5 mg of purified SDP was weighed, dissolved in 5 mL dimethyl sulfoxide (DMSO), and prepared into 1 mg/mL solution. Liquid phase system was U3000, difference detector was optilab T-rEX, and laser light scattering detector was DAWN HELEOS Ⅱ
2.6. Detection of structural characterization
2.6.1. Congo red experiment
The Congo Red experiment was carried out based on the following specific steps [26]. That is, according to the volume ratio of 2:1:1, 2 mL UAEE-PMSP-1A aqueous solution (2 mg·mL−1) and 1 mL Congo red solution (0.16 mg·mL−1) were mixed, then 1 mL NaOH solution (0, 0.2, 0.4, 0.6, 0.8, 1 mol/mL) was added to the above solution to obtain six groups of test solutions. After standing for 0.5 h, the ultraviolet spectrophotometer was used to detect and record the maximum absorbance of the mixture in the wavelength range of 400–600. Then, according to the above steps, the instrument was used to detect the blank control group and record the peak UV absorbance. Finally, a correlation curve was plotted based on the recorded data, with the vertical coordinate indicating the peak absorbance and the horizontal scale representing the concentration of NaOH solution.
2.6.2. FT-IR detection
The detection wavelength range was set to 4000–400 cm−1, and then 2 mg of dry KBr was pressed into a sheet and its transmittance was measured as a background. After this, the polysaccharide sample with a mass of 2 mg was mixed with 100 mg of dried KBr and pressed into a sheet. Finally, the corresponding transmittance of the sample was measured.
2.6.3. NMR detection
UAEE-PMSP and Ac-UAEE-PMSP were weighed at 30 mg each and added to 0.6 mL heavy water (D2O) with a purity of 99.99 % respectively. The two were analyzed by NMR after ultrasonic dissolution. The spectra determined with UAEE-PMSP-1A were categorized into 1D and 2D spectra, the former contains 1H NMR and 13C NMR, and the latter including 1H–1H COSY (1H–1H correlation spectroscopy), HSQC (heteronuclear single quantum coherence) and HMBC (heteronuclear multiple bond coherence). The spectrum of Ac-UAEE-PMSP was 1D spectrum, namely 13C NMR. In this study, the NMR spectrometer was purchased from Bruck, Switzerland, the machine model was AVANCE II, the machine frequency was 300 Hz, and the detection solvent was 99.99 % D2O.
2.7. Detection of antioxidant activity in vitro
2.7.1. Determination of hydroxyl radical scavenging ability
The specific steps of hydroxyl radical activity determination experiment were as follows [27]. That is, firstly, the polysaccharide was mixed with deionized water to prepare a polysaccharide solution with a total concentration of 2 mg·mL−1. Prepare a series of polysaccharide solutions with concentrations of 0.125 mg·mL−1, 0.25 mg·mL−1, 0.5 mg·mL−1, 1 mg·mL−1 and 2 mg·mL−1, respectively. After that, 1 mL of newly prepared FeSO4 solution (6 mmol·L−1), 1 mL of H2O2 solution (5 mmol·L−1) and 1 mL of salicylic acid solution (20 mmol·L−1) were added to the polysaccharide solutions at different concentrations. Finally, when the temperature of the water bath was stable at about 37 ℃, the above mixed solution was placed at the temperature for 30 min of reaction. After heating, UV scanning was performed in the range of 400–600 nm and the absorbance value at 510 nm was recorded. Deionized water was used as the blank group instead of polysaccharide solution, the experiment was repeated three times according to the above experimental steps and the absorbance was measured, and Vc was used as the positive control group instead of polysaccharide solution, the experimental steps were the same as the blank group and the absorbance was measured. The scavenging rate of polysaccharide on hydroxyl radical was calculated as E (%) = [(A0 − A1)/A0] × 100, where A0 and A1 in the formula are the absorbance of the system with or without reactants, respectively.
2.7.2. Determination of lipid peroxidation inhibition ability
Based on the actual situation, some adjustments were made to the experiment based on Liu et al. [28]. In simple terms, 2 mL of UAEE-PMSP, UAEE-PMSP-1A and Ac-UAEE-PMSP with different mass concentrations (0.125 mg·mL−1, 0.25 mg·mL−1, 0.5 mg·mL−1, 1 mg·mL−1 and 2 mg·mL−1) were taken into the test tube, and 1.8 mL of soybean lecithin solution and 0.4 mL of FeSO4 solution were added to them in turn (the concentrations were 1 mg/mL and 10 mmol/L, respectively). Next, when the temperature of the water bath was about 37℃, the above mixed solution was placed at this temperature for heating reaction for 20 min. After heating, 1 mL of trichloroacetic acid solution and thiobarbituric acid solution (the mass concentrations of the two solutions were 20 % and 0.8 %, respectively) were added in turn. Lastly, heat the upper clear liquid in a boiling water bath for 15 min and centrifuged after the reaction to remove a small amount of precipitate. The supernatant was scanned by UV in the range of 400–600 nm and the absorbance value at 535 nm was recorded. The polysaccharide solution was replaced by deionized water and Vc respectively. The former was used as a blank group and the latter as a positive control group. The absorbance of the blank group and the positive control group were measured for 3 times respectively, and the average value was taken. The formula for calculating the inhibition rate of lipid peroxidation was I (%) = [(A0 − Ai)/A0] × 100. Among them, A0 and Ai represented the absorbance of the system without and with the sample, respectively.
3. Results and discussion
3.1. Extraction and preliminary purification of UAEE-PMSP
The polysaccharide extract obtained by ultrasonic-assisted enzyme extraction was light brown as a whole. That is, after concentration under reduced pressure, the solution was treated with alcohol precipitation, and then centrifuged to obtain precipitation. The precipitation was redissolved, deproteinized, dialyzed and lyophilized. The light brown crude polysaccharide powder obtained was 0.696 g, and the calculated yield was 3.48 %. The total sugar content, protein content and uronic acid content calculated by phenol–sulfuric acid method, Coomassie brilliant blue staining method and carbazole-sulfuric acid method were 70.78 %, 4.71 % and 0.92 %, respectively. The sugar content of the crude polysaccharide of purple mangosteen scarfskin was ideal, probably because the first low concentration of alcohol precipitation removed most of the non-sugar substances, while the second high concentration of alcohol precipitation allowed more polysaccharides to be precipitated from the extract. The protein content was 4.71 %, indicating that after two deproteinization, most of the protein could be removed and the loss of polysaccharide could be reduced as much as possible. The concentration of uronic acid was less than 1 %, indicating that the crude polysaccharide had very few acidic components. In conclusion, it can be seen from the above that the main components of crude polysaccharide from purple mangosteen scarfskin were polysaccharides, but they also contained proteins, pigments and other substances, which need to be further purified to get pure polysaccharides with higher purity. The quantitative results of the composition analysis of UAEE-PMSP were shown in Table 1.
Table 1.
Quantitative results of composition analysis of UAEE-PMSP and UAEE-PMSP-1A.
| Polysaccharides | Total sugar content(%) | Protein content(%) | Uronic acid content(%) |
|---|---|---|---|
| UAEE-PMSP | 70.78 | 4.71 | 0.92 |
| UAEE-PMSP-1A | 91.39 | 0.24 | 0.72 |
3.2. Separation and purification of UAEE-PMSP-1A
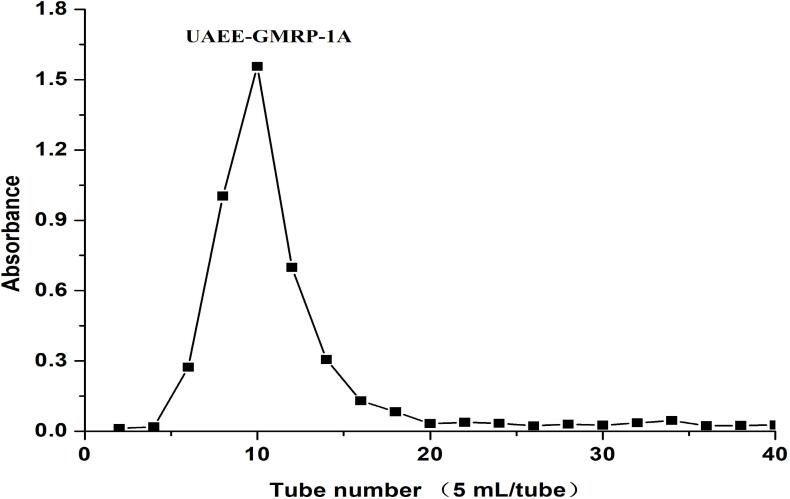
DEAE-52 cellulose column and Sephadex G-100 gel column were used to separate and purify the crude polysaccharide from purple mangosteen scarfskin, and the residual pigment in the polysaccharide was removed [29]. As shown in Fig. 1, three obvious peaks were obtained after cellulose column separation. Among them, the overall peak area of UAEE-PMSP-1 was the largest, indicating that it was the main component of purple mangosteen scarfskin polysaccharide, and it was easier to collect than the other two components. Therefore, UAEE-PMSP-1 was selected for the next step of separation and purification, and its yield was 27.71 %. Next, as shown in Fig. 2, secondary purification of uae-PMSP-1 was performed based on Sephadex G-100 gel column, and there was only a set of large and symmetrical single peaks in the elution curve, which indicated that the UAEE-PMSP-1A was a polysaccharide component, and the corresponding yield was 72.4 %. In addition, the total sugar content of UAEE-PMSP-1A was increased to 91.39 % by comparison with crude polysaccharide, which indicated that the chromatographic column method was able to enrich the polysaccharides of the same component to improve the purity. Besides, the protein content and uronic acid content of UAEE-PMSP-1A decreased to 0.24 % and 0.72 %, respectively, indicating that the chromatographic column method could further effectively separate polysaccharides and proteins, but may also cause certain losses to some polysaccharide components. The molecular weight of polysaccharide was 2.32 KDa. The quantitative results of the composition analysis of UAEE-PMSP-1A were shown in Table 1.
Fig. 1.
Elution diagram of DEAE-Cellulose 52 of UAEE-PMSP-1.
Fig. 2.
Elution diagram of Sephadex G-100 gel column of UAEE-PMSP-1A.
3.3. Preparation of Ac-UAEE-PMSP
Acetylation of UAEE-PMSP using acetic anhydride reagent gave Ac-UAEE-PMSP in 62.58 % yield and 57.47 % total sugar content. The reason for the above situation may be related to the conditions of the acetylation reaction. During the reaction, the pH value of the system needed to be maintained between 9 and 10 to fully promote the effective conduct of the nucleophilic substitution reaction. However, prolonged exposure to alkaline environments can also lead to the degradation of some polysaccharides, thereby affecting the yield and polysaccharide content of derivatives to varying degrees. According to the substitution degree formula, the corresponding substitution degree of Ac-UAEE-PMSP is 0.33, indicating that acetylation modification was successful. In addition, it was found that the degree of substitution of acetylated pumpkin polysaccharide could be effectively regulated by adding pyridine as a catalyst [30]. However, whether pyridine could be used to effectively improve the degree of substitution of Ac-UAEE-PMSP and other derived polysaccharides required more accurate and complete data support.
3.4. Results and analysis of structural characterization
3.4.1. Analysis of Congo red experiment
The spatial structure of polysaccharides was often presented in the form of triple helix structure, and this structure was also considered to be a key factor affecting the activities of polysaccharides [31].
In general, based on the Congo red experiment, that was, within the appropriate concentration range of sodium hydroxide, after mixing the Congo red reagent with the substance to be measured, if the complex was observed and accompanied by a more obvious red shift phenomenon, it could be judged that the substance had a triple helix structure. Fig. 3 shows that with the increase of sodium hydroxide concentration, the peak absorbance of the blank group (Congo red + water) was decreasing. In contrast, the peak absorbance of UAEE-PMSP-1A had a red shift in this range, but it was not obvious. Meanwhile, the peak absorbance of UAEE-PMSP-1A varied little throughout the concentration range. Thus, in this study, the purified polysaccharides from purple mangosteen scarfskin did not have a triple helix structure.
Fig. 3.
Congo red test results for UAEE-PMSP-1A. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4.2. Analysis of FT-IR
Infrared spectroscopy is widely used to identify the characteristic functional groups of organic compounds, and for polysaccharides, they have some typical characteristic absorption peaks in the range of 4000 to 500 cm−1. The FT-IR spectrum of UAEE-PMSP-1A was shown in the Fig. 4. The most prominent characteristic absorption peaks at 3278 cm−1 and 2930 cm−1 are attributed to the stretching vibration of hydroxyl groups in the sugar ring and the stretching vibration of C—H bonds of methyl or methylene groups, respectively [17]. For the absorption peaks at 1611 cm−1 and 1416 cm−1, the former belongs to the bound water in polysaccharides, while the latter belongs to the bending vibration of C—H bonds [32], [33]. In addition, a small weak peak was found at 1730 cm−1, which, combined with two strong peaks at 1611 cm-1 and 1416 cm−1, indicated that there was a certain amount of uronic acid in UAEE-PMSP-1A, which was also in good agreement with the 0.72 % of uronic acid content obtained by quantitative determination in Table 1. Typically, the presence of pyranose tended to result in three consecutive characteristic absorptions at 1200–1000 cm−1, while UAEE-PMSP-1A had only one absorption peak of 1039 cm−1 in this interval. In contrast, there was an obvious absorption peak in the characteristic range of furanose ring 846–763 cm−1. The above two cases indicated that the sugar ring of UAEE-PMSP-1A may be mainly composed of furanose ring [34]. Finally, a weak peak was found at 868 cm−1, suggesting an α-glycosidic bond in the polysaccharide structure [35].
Fig. 4.
Comparison of FT-IR spectra of UAEE-PMSP-1A, UAEE-PMSP and Ac-UAEE-PMSP.
FT-IR spectral comparison results of UAEE-PMSP and Ac-UAEE-PMSP were also shown in the Fig. 4. It can be found that the introduction of acetyl leads to the enhancement of the stretching vibration of the related C O bond, and then leads to the enhancement of two related absorption peaks of Ac-UAEE-PMSP, namely, two peaks located at 1621 cm−1 and 1732 cm−1 [36]. In addition, after acetylation treatment, the intensity of the peaks generated by the symmetrical deformation vibration of the methyl group at 1371 cm−1 and the stretching vibration of the carbonyl C-O-C at 1231 cm−1 were greatly enhanced, and their peak shapes became sharper [37]. Thus, it was known from the above findings that the acetylation of polysaccharide from purple mangosteen scarfskin was successful. At the same time, the absorption peaks at 3268 cm−1 and 3341 cm−1, 2929 cm−1 and 2941 cm−1, 1041 cm−1 and 1036 cm−1, 808 cm−1 and 787 cm−1 did not change significantly due to the introduction of acetyl group. This showed that the main skeleton of polysaccharide did not change significantly before and after acetylation treatment.
3.4.3. Analysis of NMR
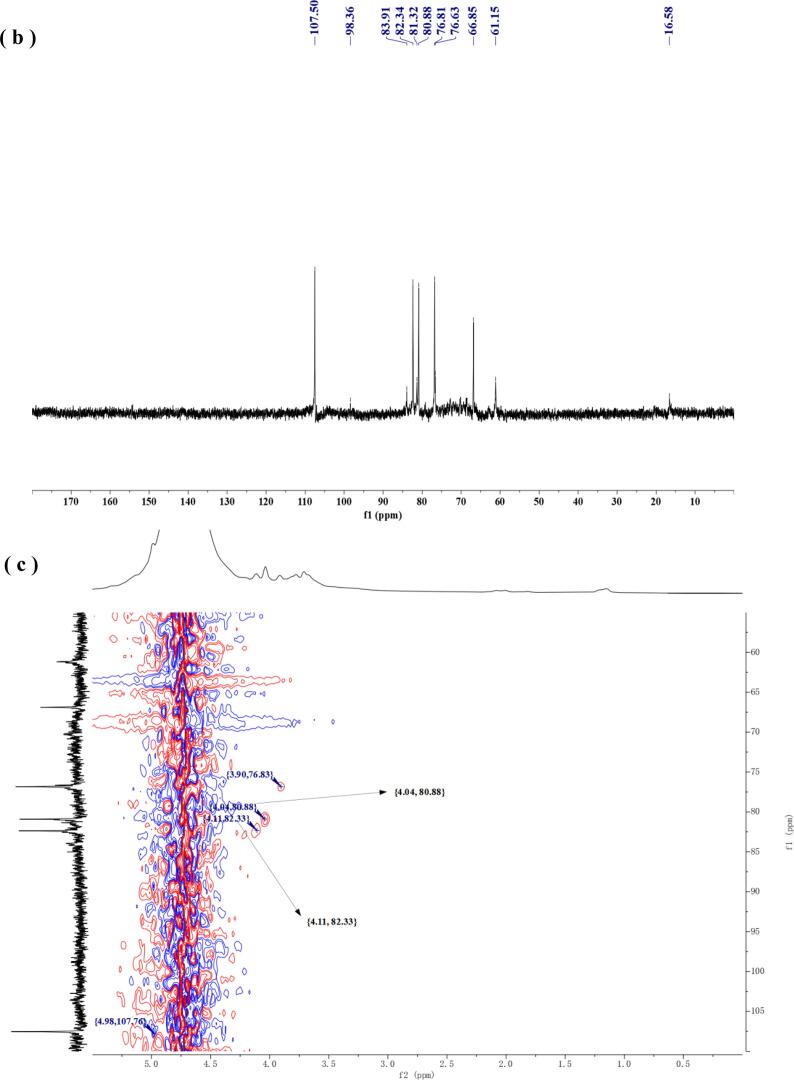
Using nuclear magnetic resonance (NMR) analysis technology, we can effectively obtain information about monosaccharide composition, allocephalic configuration, and connection order between glycosidic residues, which can help us to further understand the structure of polysaccharides. For the 1H NMR and 13C NMR of one-dimensional NMR, since the signals of the anomeric carbon atom (C1) and the anomeric hydrogen atom (H1) of the polysaccharide are usually more obviously distributed in the two regions of 90–110 ppm and 4.5–5.5 ppm, and this feature is often used to analyze the structure of the related monosaccharide residues and the α / β configuration. The 1H NMR of UAEE-PMSP-1A was shown in Fig. 5(a), among which there was a most obvious single peak at δ 4.73 with the largest peak area, which was caused by D2O and it belonged to the solvent peak. Although the reagent peak interfered to some extent with the identification of the other proton peaks, it was not difficult to find that there were still two more distinct telomeric proton resonance peaks at δ 5.00 and δ 5.14. Because the signal of the telomeric proton peak of the α-type glycoside residue was greater than or equal to δ 5.00, the above two glycoside residues were α-configuration, which was also consistent with the results of infrared spectrum analysis. Besides, the resonance peaks at δ1.15 and δ2.01 were attributed to the H6 signal and the H5 signal, respectively, with the former belonging to the hydrogen signal of rhamnose and the latter to arabinose [38]. The remaining signals in the region of 3.5–4.4 ppm were signals of hydrogen atoms of other bit numbers. The 13C NMR of UAEE-PMSP-1A was shown in Fig. 5(b), and its C2 ∼ C5 signals were mainly concentrated in the region of 60–90 ppm. Meanwhile, two C1 signal peaks (δ 107.50 and δ 98.36), one strong and the other weak, appeared in the signal region of the anomeric carbon atom. This further suggested that UAEE-PMSP-1A contained two monosaccharide residues, one of which, with its lower content, produced the corresponding resonance peak at δ 98.36. In addition, it had been reported that arabinose would produce related resonance peaks near δ 107 and δ 82, while C6 of rhamnose would produce resonance peaks near δ 16.50 [39], [40]. It could be seen that the resonance peaks at δ 107.50 and δ 82.34 were generated by α-arabinose, while the resonance peak at δ 16.58 was generated by α-rhamnose. To facilitate the subsequent attribution of C and H signals, the α-arabinose residue with strong C1 signaling was here named Ra. In contrast, another α-rhamnose residue with weak C1 signal was named Rb. Through the analysis of 1H NMR and 13C NMR, we obtained some C and H signals of Ra and Rb. The signal attribution of the remaining C and H atoms needed to be further analyzed in combination with relevant literature and two-dimensional spectrum.
Fig. 5.
NMR spectra of UAEE-PMSP-1A: (a) 1H NMR; (b) 13C NMR;(c)HSQC; (d) 1H-1H COSY; (e) HMBC.
In the HSQC spectrum of Fig. 5(c), we found that there were four cross-peak signals of δH 4.98 / δC 107.76, δH 4.11 / δC 82.33, δH 4.04 / δC 80.88, δH 3.90 / δC 76.83, which indicated that the H and C atoms represented by these signals were directly connected. Thus, from the above data and previous analyses, it was clear that the H1 and H5 signals of Ra were δ 5.00 and δ 2.01, and the H1 and H6 signals of Rb were δ 5.14 and δ 1.15. Next, in the 1H–1H COSY spectrum of Fig. 5(d), five obvious correlated cross-peak signals were found, which were δH 4.98 / δH 4.04, δH 4.04 / δH 4.11, δH 3.90 / δH 4.11, δH 1.15 / δH 3.69, and δH 3.78 / δH 3.69, respectively. It could be inferred that the H2 signal of Ra was δ 4.04, the H3 signal was δ 3.90 and the H4 signal was δ 4.11. Similarly, the H5 signal and H4 signal of Rb were δ 3.69 and δ 3.78, respectively. For H2 and H3 of Rb, because the content of rhamnose residues was less and the signal overlap of some proton peaks was more serious, their obvious signals were not found in the correlation spectrum, so it was not discussed. Next, we discussed the attribution of the residual C atom signals of Ra and Rb. In the previous HSQC spectrum, the signals of the directly connected C and H atoms were mentioned, and accordingly, together with the 13C NMR, we knew that the signals of C2 ∼ C4 of Ra were δ 80.88, δ 76.81 and δ 82.34 in that order. After the C1 to C4 atomic signals of Ra were assigned, for the C5 signal, the δ66.85 signal could be attributed to the C5 of Ra according to the literature on NMR signal assignment [41]. According to the above analysis, all the C atomic signals of Ra were deduced, and the values of C1 to C5 were δ 107.50, δ 80.88, δ 76.81, δ 82.34 and δ 66.85, respectively, which were basically consistent with the carbon atomic signals of 1, 5-α-arabinose [41]. In addition, the less pronounced signals at 83.91, 76.63, and 61.15 ppm were associated with terminal α-arabinofuranose fragments [41], but due to the low content of this monosaccharide residue, its C1 signal and other C and H atom related signals could not be well identified and identified. For the assignment of the remaining C atom signal of Rb, we could combine the HMBC spectrum for correlation attribution analysis. In Fig. 5(e), there were two cross-peak signals, δH 4.99 / δC 82.24 and δH 3.77 / δC 81.37. This indicated that the H and C atoms represented by these signals were separated by two to three covalent bonds. The cross-peak signal of the former further proved that δ 82.34 in the 13C NMR was a signal of C4 of Ra, while the latter indicated that δ 81.32 in the 13C NMR was a signal of C2 or C3 of Rb. Since rhamnose was a common six-membered pyranose, and the C6 and H6 signals of Rb mentioned above were δ 16.58 and δ 1.15, only δ 81.32 was the C2 signal of Rb could satisfy the condition that the C3 and C5 signals of pyranose were less than δ 80 [42], [43]. Moreover, because the correlation signal of C3 ∼ C5 of Rb was too low, it was not obvious in the correlation spectrum, so the signal attribution of the above three carbon atoms was not discussed in detail here. However, it was worth noting that some obvious carbon signals could be found in the subsequent 13C NMR spectrum of the UAEE-PMSP, and the corresponding carbon signals such as δ 98.41, δ 76.79, δ 69.34, δ 72.01, δ 69.34 and δ 16.58 could be well consistent with the C1 ∼ C6 signals of 1, 2-α-rhamnose. Other carbon atomic signals such as δ 68.55, δ 79.11, δ 70.27 were basically consistent with C3, C4, C5 atomic signals of 1,4-α-galacturonic acid. It could be inferred that α-rhamnopyranose in UAEE-PMSP-1A should also be linked by 1,2 glucoside bonds and contain very small amounts of α-galacturonic acid [40]. Finally, all the attribution results were collated and presented in Table 2. Combining all the above analyses and the signals of the C and H atoms of the two monosaccharide residues, it could be reasonably inferred that UAEE-PMSP-1A was mainly composed of a large amount of α-arabinose, a small amount of α-rhamnoose and a very small amount of α-galacturonic acid. Further through the above comprehensive analysis, it could be seen that the core main chain part of the pure polysaccharide was 1,5-α-arabinose, and this arabinose was connected to C4 of some rhamnoose.
Table 2.
Attribution of partial carbon and hydrogen of major monosaccharide residues of UAEE-PMSP-1A.
| Symbols | Monosaccharide residues | C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 |
|---|---|---|---|---|---|---|---|
| Ra | α-Ara | 107.50 | 80.88 | 76.81 | 82.34 | 66.85 | |
| 4.98 | 4.04 | 3.90 | 4.11 | 2.01 | |||
| Rb | α-Rha | 98.36 | 81.32 | – | – | – | 16.58 |
| 5.14 | – | – | 3.78 | 3.69 | 1.15 | ||
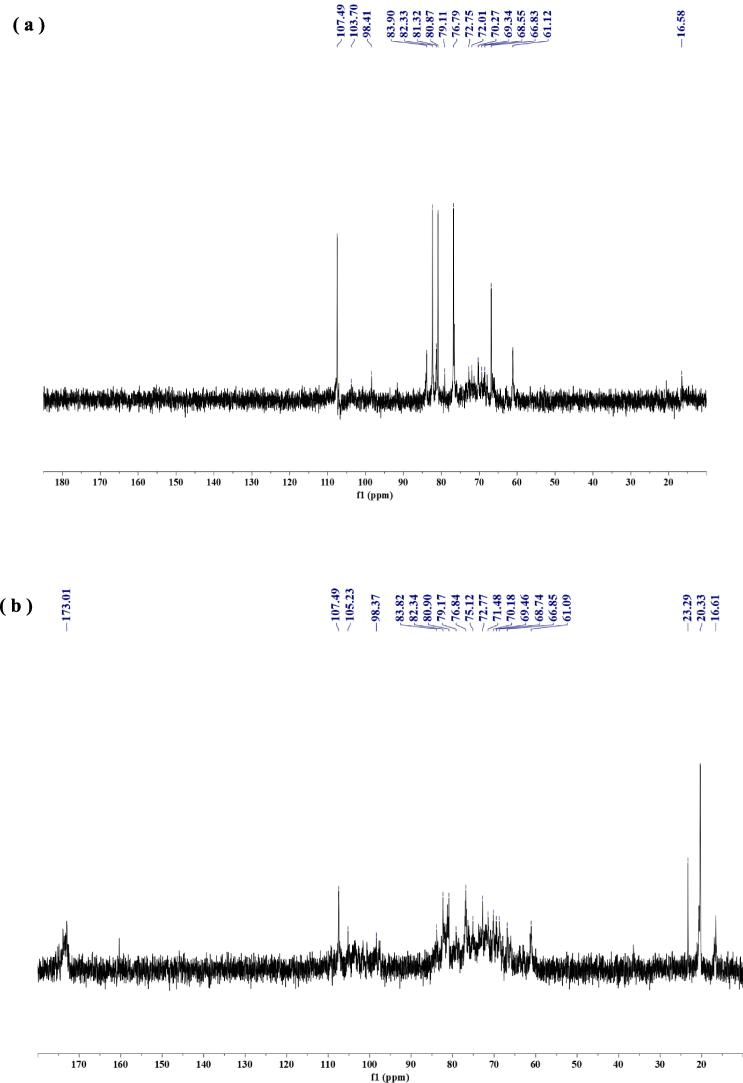
The 13C NMR of UAEE-PMSP and its acetylated derivatives were shown in Fig. 6 (a) and (b), respectively. In Fig. 6 (a), it can be seen that there is no resonance peak in the low field region of UAEE-PMSP within the range of 170–180 ppm, indicating a very low content of uronic acid, which was in good agreement with the analysis results of infrared spectroscopy. Furthermore, by comparing the two spectrum, we could find that Ac-UAEE-PMSP had three new resonance peaks related to acetyl groups, namely δ 173.01, δ 20.33 and δ 23.29. The new signal in the low field region was related to the introduced acyl carbon of the acetyl group, while the two new signals in the high field region were related to the methyl carbon atom on the acetyl group [36]. Besides, the resonance peak signals of some carbon atoms in the anomeric carbon signal region (90–110 ppm) and the C2 ∼ C6 signal region (50–90 ppm) of the two polysaccharides had a certain degree of chemical shift, however it did not lead to the lack of related resonance peaks. This indicated that acetylation modification was carried out without affecting the main structure of polysaccharides. In summary, based on the results of nuclear magnetic analysis, it was further suggested that the acetylation modification of UAEE-PMSP was successful.
Fig. 6.
13C NMR spectra: (a) UAEE-PMSP; (b) Ac-UAEE-PMSP.
3.5. In vitro antioxidant activity results and analysis
3.5.1. Analysis of hydroxyl radical scavenging ability
The strongly oxidizing reactive oxygen species are recognized to be closely related to human aging and the occurrence of certain diseases, and hydroxyl radical is one of these substances. In this study, the in vitro scavenging activities of three polysaccharides on hydroxyl radicals were tested using the Fenton reaction system, and the related scavenging rates were used as evaluation indexes. As shown in Fig. 7, a significant positive correlation between the clearance of all three polysaccharides and their concentrations could be clearly seen. The clearance rate of UAEE-PMSP and UAEE-PMSP-1A reached half of the control group after reaching the highest concentration of 2 mg·mL−1. At the same time, although the growth trend of the clearance rate of the above two polysaccharides decreased compared with the previous trend after the polysaccharide concentration reached 1 mg·mL−1, it showed an increasing trend. According to this, it could be predicted that further increasing their concentration could significantly improve the scavenging capacity. In addition, the scavenging rates of UAEE-PMSP and UAEE-PMSP-1A were close at at corresponding concentrations, suggesting that the scavenging activity of both of them for hydroxyl radicals mainly depended on the polysaccharide components. In the presence of Ac-UAEE-PMSP, the clearance rates of samples with different concentrations were higher than those of the other two polysaccharides with the same concentration. In particular, when its concentration was increased to the concentration of 2 mg·mL−1, the clearance rate had reached more than half of the control group, and it could be found that its clearance rate still had a good increasing trend. The reason for this may be that the introduction of acetyl changed the spatial structure of the polysaccharide, thereby enhancing the hydrogen supply capacity of the polysaccharide and exposing more reaction sites [17], [44]. It could be seen that the above three polysaccharides had certain scavenging activity on hydroxyl radicals, but Ac-UAEE-PMSP had significantly stronger scavenging ability, which indicated that acetylation could be used as an effective chemical modification method to improve the scavenging ability of hydroxyl radicals of polysaccharides.
Fig. 7.
Hydroxyl radical scavenging rates of UAEE-PMSP, Ac-UAEE-PMSP and UAEE-PMSP-1A.
The scavenging ability of hydroxyl radicals is one of the indexes to evaluate the antioxidant activity of natural active polysaccharides. The clearance rates of both UAEE-PMSP-1A and UAEE-PMSP were close at all concentrations. When the maximum concentration was 2 mg·mL−1, the clearance rates of both were 49.85 % and 46.86 % respectively. The above two data were relatively ideal. For example, at the concentration of 2 mg·mL−1, the scavenging rate of the acidic polysaccharide from Calocybe indica on hydroxyl radicals was 42.89 %, while the values of UAEE-PMSP-1A and UAEE-PMSP were slightly higher than those of the above polysaccharide [45]. For another example, it was found that the hydroxyl radical scavenging rate of polysaccharide from Morchella importuna reached 89.39 % when the maximum concentration was 4 mg/mL, while the polysaccharide from purple mangosteen scarfskin may also approach this value at the same concentration [46]. In conclusion, compared with other natural active polysaccharides, the non-chemically modified purple mangosteen scarfskin polysaccharide also has a certain degree of antioxidant potential. As for the polysaccharide from purple mangosteen scarfskin after acetylation, it was clear from the previous data analysis that its hydroxyl radical scavenging ability had been improved, which was also consistent with the conclusion that polysaccharides after acetylation had stronger hydroxyl radical scavenging ability as mentioned in another study [47]. At the same time, a study showed that the polysaccharides from Cyclocarya paliurus leaves after acetylation had stronger immunomodulatory activity [48], which further proved that acetylation modification was a powerful method to effectively enhance various activities of natural active polysaccharides, including antioxidant activity, immunomodulatory activity and so on.
3.5.2. Analysis of lipid peroxidation inhibition ability
The lipid peroxidation inhibition ability of the three polysaccharides was shown in Fig. 8. It indicated that their inhibition ability was Vc > Ac-UAEE-PMSP>UAEE-PMSP ≈ UAEE-PMSP-1A. Among them, in the concentration range of 0.5 mg/mL∼2 mg/mL, the anti-lipid peroxidation ability of UAEE-PMSP and UAEE-PMSP-1A was more obvious than that of low concentration range, and there was still a good growth trend after reaching the maximum concentration. In the activity assay, the two polysaccharides showed similar lipid peroxidation inhibitory ability at different polysaccharide concentrations, suggesting that the polysaccharide component is still the main active ingredient in the activity assay. However, overall, the inhibitory effects of UAEE-PMSP and UAEE-PMSP-1A on lipid peroxidation were still somewhat gap from those of the control group. For Ac-UAEE-PMSP, after acetylation modification, the inhibitory effect of polysaccharides was significantly improved, exceeding the inhibitory ability of the control group by more than half at the highest concentration. As with other antioxidant experiments, the reasons for the above changes may be diversified, which may be closely related to molecular weights, monosaccharide compositions, number and location of substituents, and chain conformations [49], [50]. In general, it can be seen from the above exploration experiments that the three polysaccharides also show certain application value in the inhibition of lipid peroxidation, and the antioxidant activity of polysaccharides is significantly improved after acetylation.
Fig. 8.
Lipid peroxidation scavenging rate of UAEE-PMSP, Ac-UAEE-PMSP and UAEE-PMSP-1A.
Similarly, in addition to the ability of hydroxyl radical scavenging, the ability to inhibit lipid peroxidation is also one of the indexes to investigate the antioxidant capacity of natural active polysaccharides. In this study, the corresponding clearance rates of the three polysaccharides at the maximum concentration were 42.9 %, 31.74 % and 31.93 %, respectively, which were basically equivalent to the clearance rates of another natural active polysaccharide, namely Hypsizygus ulmarius polysaccharide and its acetylated derivatives, and there was still a possibility of further improvement from the trend of change [47]. Therefore, they also have the potential for practical application, which also further indicate that the effect of acetylation modification is indeed ideal.
4. Conclusion
In summary, the crude polysaccharide from purple mangosteen scarfskin was successfully obtained by ultrasonic-assisted extraction, and then a polysaccharide (UAEE-PMSP-1A) was purified and isolated by column chromatography. It was determined by Congo red experiment that the pure polysaccharide had no triple helix structure, and it was mainly composed of a large amount of arabinose, a small amount of rhamnoose and a very small amount of galacturonic acid by infrared spectroscopy and nuclear magnetic analysis, and 1,4-α-arabinose was its core skeleton. In addition, α-arabinose constitutes its core backbone. Meanwhile, different in vitro antioxidant assays showed that Ac-UAEE-PMSP, UAEE-PMSP and UAEE-PMSP-1A all exhibited some degree of scavenging activity and inhibition of hydroxyl radicals and lipid peroxidation. Among them, the acetylated polysaccharides showed stronger antioxidant activities, which further indicated that acetylation could effectively improve the polysaccharide-related activities and provide some theoretical references for the further development of purple mangosteen scarfskin polysaccharide. Meanwhile, we still have some work to do in the structural analysis of purple mangosteen scarfskin polysaccharide. Therefore, it is necessary to further analyze the primary and advanced structure of purple mangosteen scarfskin polysaccharide and its derivatives in the future, so as to explore more content about the structure–activity relationship of this polysaccharide.
CRediT authorship contribution statement
Zhenjie Tang: Wrote the manuscript. Gangliang Huang: Reviewed & edited the manuscript. Hualiang Huang: Reviewed & edited the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Gangliang Huang, Email: huangdoctor226@163.com.
Hualiang Huang, Email: hlhuang@wit.edu.cn.
References
- 1.Chanarat P., Chanarat N., Fujihara M., Nagumo T. Immunopharmacological activity of polysaccharide from the pericarb of mangosteen garcinia: phagocytic intracellular killing activities. J. Med. Assoc. Thai. 1997;80(Suppl 1):S149–S154. [PubMed] [Google Scholar]
- 2.Gutierrez-Orozco F., Failla M.L. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. 2013;5(8):3163–3183. doi: 10.3390/nu5083163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nawawi N.I.M., Ijod G., Abas F., Ramli N.S., Mohd Adzahan N., Mohamad A.E. Influence of Different Drying Methods on Anthocyanins Composition and Antioxidant Activities of Mangosteen (Garcinia mangostana L.) Pericarps and LC-MS Analysis of the Active Extract. Foods. 2023;12(12):2351. doi: 10.3390/foods12122351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Huang G., Huang H. Ultrasonic/enzymatic extraction, characteristics and comparison of leechee peel polysaccharide. Ultrason. Sonochem. 2024;108 doi: 10.1016/j.ultsonch.2024.106948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., An L., Li Z., Wang H., Shi L., Zhang J., Li Y., Jin D.Q., Tuerhong M., Ohizumi Y., Shuai L., Xu J., Guo Y. An active heteropolysaccharide from the rinds of Garcinia mangostana Linn.: Structural characterization and immunomodulation activity evaluation. Carbohydr. Polym. 2020;235 doi: 10.1016/j.carbpol.2020.115929. [DOI] [PubMed] [Google Scholar]
- 6.Dohouonan D., Brice O.E.J., Julien G.K., Yao T. Effectiveness of Calotropis procera (Ait. R. Br.) and Cassia siamea (Lamk.) leave powders in the control of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) Int. J. Agri Biosci. 2022;11(2):84–89. [Google Scholar]
- 7.Soma D.M., Pouya B.M., Zongo N.A., Kiba D.I., Gnankambary Z., Sedogo P.M. Potential of Biogas and Organic Fertilizers Production Through Anaerobic Digestion of Slaughterhouse Waste in Ouagadougou, Burkina Faso. Int. J. Agri. Biosci. 2023;12(1):27–30. [Google Scholar]
- 8.Zhou S., Huang G., Huang H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. 2022;388:133000. doi: 10.1016/j.foodchem.2022.133000. [DOI] [PubMed] [Google Scholar]
- 9.Lin B., Huang G. An important polysaccharide from fermentum. Food Chem X. 2022;15:100388. doi: 10.1016/j.fochx.2022.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong X., Yang W., Huang G., Huang H. Ultrasonic-assisted extraction, characteristics and activity of Ipomoea batatas polysaccharide. Ultrason Sonochem. 2023;96:106420. doi: 10.1016/j.ultsonch.2023.106420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z., Xiong X., Huang G. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason Sonochem. 2023;95:106416. doi: 10.1016/j.ultsonch.2023.106416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabi B.G., Mukhtar K., Ansar S., Hassan S.A., Hafeez M.A., Bhat Z.F., Khaneghah A.M., Haq A.U., Aadil R.M. Application of ultrasound technology for the effective management of waste from fruit and vegetable. Ultrason. Sonochem. 2024;102 doi: 10.1016/j.ultsonch.2023.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Huang G. Extraction, purification, structural modification, activities and application of polysaccharides from different parts of mulberry. Food Funct. 2024;15(8):3939–3958. doi: 10.1039/d3fo05747j. [DOI] [PubMed] [Google Scholar]
- 14.Chen F., Huang G., Huang H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021;252 doi: 10.1016/j.carbpol.2020.117179. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Xie J., Jia S., Huang L., Wang Z., Li C., Xie M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017;98:576–581. doi: 10.1016/j.ijbiomac.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Li H., Zhao H., Gao Z., Song X., Wang W., Yuan F., Feng Y., Zhang Y., Zhang J., Zhang S., Jia L. The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor. Molecules. 2019;24(15):2698. doi: 10.3390/molecules24152698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J.H., Zhang F., Wang Z.J., Shen M.Y., Nie S.P., Xie M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015;133:596–604. doi: 10.1016/j.carbpol.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Yang W., Huang G. Extraction methods and activities of natural glucans. Trends Food Sci. Technol. 2021;112:50–57. [Google Scholar]
- 19.Cui C., Lu J., Sun-Waterhouse D., Mu L., Sun W., Zhao M., Zhao H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT Food Sci. Technol. 2016;73:602–608. [Google Scholar]
- 20.Jin W., Zhang W., Liu G., Yao J., Shan T., Sun C., Zhang Q. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. Int. J. Biol. Macromol. 2017;105(Pt 1):686–692. doi: 10.1016/j.ijbiomac.2017.07.089. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Huang G. Extraction and derivatisation of active polysaccharides. J. Enzyme Inhib. Med. Chem. 2019;34(1):1690–1696. doi: 10.1080/14756366.2019.1660654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin B., Fan Y., Huang G. Preparation, analysis and properties of shaddock ped polysaccharide and its derivatives. Carbohydr. Res. 2023;533 doi: 10.1016/j.carres.2023.108932. [DOI] [PubMed] [Google Scholar]
- 23.Huang G., Lin B. Preparation for shaddock skin polysaccharide derivatives by response surface method. Sci. Rep. 2024;14(1):14054. doi: 10.1038/s41598-024-63851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Bitter T. A modified uronic acid carbazole reaction. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W., Xiang Q., Zhao J., Mao G., Feng W., Chen Y., Li Q., Wu X., Yang L., Zhao T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L (okra) flowers. Int. J. Biol. Macromol. 2020;155:740–750. doi: 10.1016/j.ijbiomac.2020.03.235. [DOI] [PubMed] [Google Scholar]
- 27.Chen F., Huang G., Yang Z., Hou Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019;138:673–680. doi: 10.1016/j.ijbiomac.2019.07.129. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Huang G. The derivatization and antioxidant activities of yeast mannan. Int. J. Biol. Macromol. 2018;107(Pt A):755–761. doi: 10.1016/j.ijbiomac.2017.09.055. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Shi H., Yu J., Lei Y., Huang G., Huang H. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Ind. Crop. Prod. 2022;189 [Google Scholar]
- 30.Song Y., Yang Y., Zhang Y., Duan L., Zhou C., Ni Y., Liao X., Li Q., Hu X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva) Carbohydr. Polym. 2013;98(1):686–691. doi: 10.1016/j.carbpol.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 31.Shi H.M., Li J.C., Yu J., Li H., Huang G.L., Zhang T. Extraction, purification and antioxidant activity of polysaccharides from different parts of Hibiscus manihot L. J. Mol. Struct. 2024;1295 [Google Scholar]
- 32.Luo Q.L., Tang Z.H., Zhang X.F., Zhong Y.H., Yao S.Z., Wang L.S., Lin C.W., Luo X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016;89:219–227. doi: 10.1016/j.ijbiomac.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.T., You Y.X., Li Y.W. Characterization of carboxymethylated polysaccharides from Catathelasma ventricosum and their antioxidant and antibacterial activities. J. Funct. Foods. 2017;38(PA):355–362 [Google Scholar]
- 34.Yi P., Li N., Wan J.B., Zhang D., Li M., Yan C. Structural characterization and antioxidant activity of a heteropolysaccharide from Ganoderma capense. Carbohydr. Polym. 2015;121:183–189. doi: 10.1016/j.carbpol.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Vasilieva T., Sigarev A., Kosyakov D., Ul'yanovskii N., Anikeenko E., Chuhchin D., Ladesov A., Hein A.M., Miasnikov V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017;163:54–61. doi: 10.1016/j.carbpol.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Chen Z., Shi H., Yu J., Huang G., Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrasonics Sonochem. 2023;93 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Z., Wang Y., Huang G., Huang H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023;97 doi: 10.1016/j.ultsonch.2023.106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu W., Xue X., Zhang Z. Structural, physicochemical, antioxidant and antitumor property of an acidic polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2017;96:494–500. doi: 10.1016/j.ijbiomac.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Zhang X., Huo D., Cao C., Li Y., Liang Y., Li B., Li L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019;215:307–315. doi: 10.1016/j.carbpol.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J., Zhang F., Liu X., St Ange K., Zhang A., Li Q., Linhardt R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017;163:330–336. doi: 10.1016/j.carbpol.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 41.Cordeiro L.M.C., de Fátima V., Reinhardt B.C.H., et al. Arabinan and arabinan-rich pectic polysaccharides from quinoa (Chenopodium quinoa) seeds: Structure and gastroprotective activity. Food Chem. 2012;130(4):937–944. [Google Scholar]
- 42.Ahrazem O., Prieto A., Leal J., Jiménez-Barbero J., Bernabé M. Fungal cell wall galactomannan isolated from Apodus deciduus. Carbohydr. Res. 2002;337(16):1503–1506. doi: 10.1016/s0008-6215(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 43.Bergström N., Nair G.B., Weintraub A., Jansson P.E. Structure of the O-polysaccharide from the lipopolysaccharide from Vibrio cholerae O6. Carbohydr. Res. 2002;337(9):813–817. doi: 10.1016/s0008-6215(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z., Wang X., Zhao M., Qi H. O-acetylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Int. J. Biol. Macromol. 2014;69:39–45. doi: 10.1016/j.ijbiomac.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 45.Nataraj A., Govindan S., Rajendran A., Ramani P., Subbaiah K.A., Munekata P.E.S., Pateiro M., Lorenzo J.M. Effects of Carboxymethyl Modification on the Acidic Polysaccharides from Calocybe indica: Physicochemical Properties, Antioxidant, Antitumor and Anticoagulant Activities. Antioxidants (Basel). 2022;12(1):105. doi: 10.3390/antiox12010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian J., Zhang Z., Shang Y., Zheng Y. Extraction, structure and antioxidant activity of the polysaccharides from morels (Morchella spp.): A review. Int. J. Biol. Macromol. 2024;264(Pt 2) doi: 10.1016/j.ijbiomac.2024.130656. [DOI] [PubMed] [Google Scholar]
- 47.Thimmaraju A., Govindan S., Rajendran A., Ramani P., Pateiro M., Lorenzo J.M. Enhancement of physicochemical properties, antioxidant, antitumor, and anticoagulant activities via acetylation of Hypsizygus ulmarius polysaccharide. Int. J. Food Sci. Tech. 2023;58(6):3478–3487. [Google Scholar]
- 48.Chen F., Huang G. Preparation and immunological activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;112:211–216. doi: 10.1016/j.ijbiomac.2018.01.169. [DOI] [PubMed] [Google Scholar]
- 49.Tang Q., Huang G., Zhao F., Zhou L., Huang S., Li H. The antioxidant activities of six (1→3)-β-D-glucan derivatives prepared from yeast cell wall. Int J Biol Macromol. 2017;98:216–221. doi: 10.1016/j.ijbiomac.2017.01.132. [DOI] [PubMed] [Google Scholar]
- 50.Huang G., Mei X., Hu J. The antioxidant activities of natural polysaccharides. Curr. Drug Targets. 2017;18:1296–1300. doi: 10.2174/1389450118666170123145357. [DOI] [PubMed] [Google Scholar]