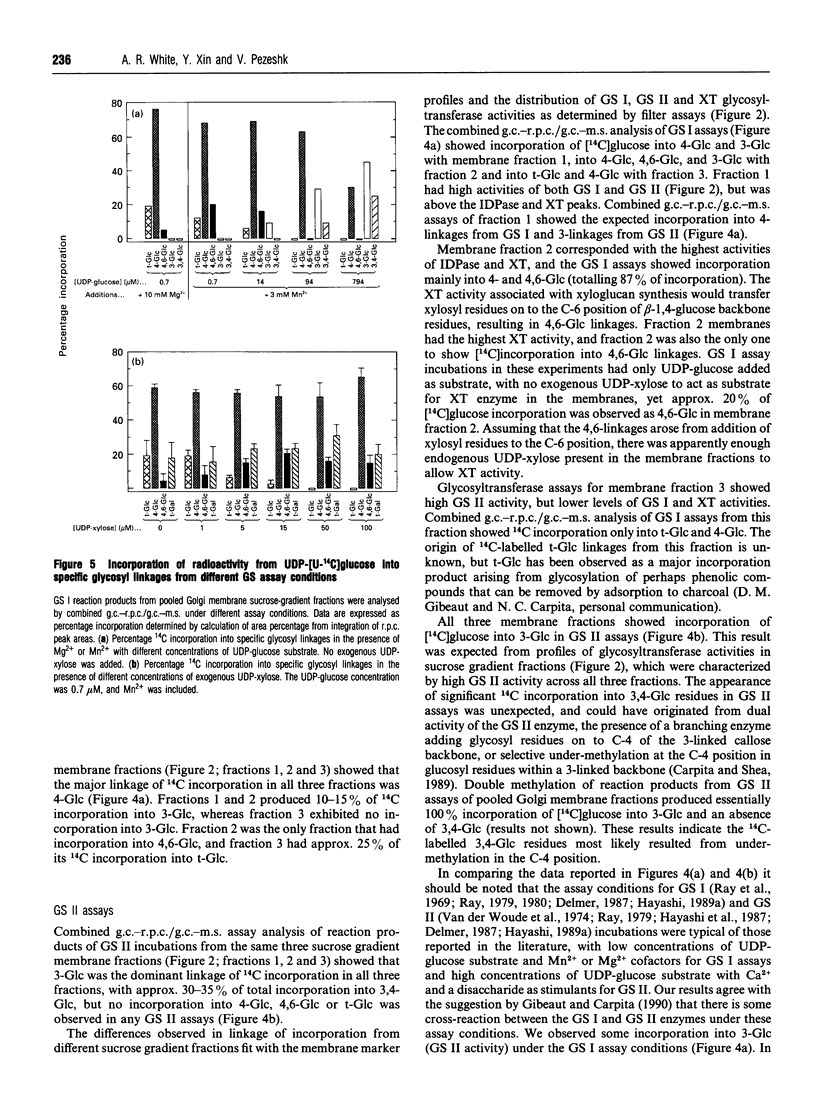
Abstract
Cell membranes from etiolated Pisum sativum (pea) tissues were separated by ultracentrifugation on linear sucrose density gradients and assayed for membrane marker and glycosyltransferase activity. Membrane fractions were shown to incorporate glucose from UDP-D-[14C]glucose into polysaccharides with glycosyl linkages consistent with synthesis of xyloglucan. A combined assay using g.c., radiogas proportional counting and m.s. was employed to determine the identities of 14C-labelled glycosyl residues and the glycosyl linkages between them. In glucan synthase I assays, membrane fractions enriched for Golgi membranes showed 14C incorporation into 4- and 4,6-glucose residues, with minor incorporation into 3-glucose residues. In glucan synthase II assays, all 14C incorporation was into 3- and 3,4-glucose. There was a shift in glycosyl linkage of 14C incorporation from predominantly 4-glucose at low UDP-glucose concentration to predominantly 3- and 3,4-glucose at high UDP-glucose concentrations. Mn2+ stimulated incorporation of radioactivity into 4,6-glucose residues characteristic of xyloglucan polysaccharides. Addition of exogenous UDP-xylose to assay mixtures stimulated incorporation into 4,6-glucose, with a maximum at 15 microM UDP-xylose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baydoun E. A., Waldron K. W., Brett C. T. The interaction of xylosyltransferase and glucuronyltransferase involved in glucuronoxylan synthesis in pea (Pisum sativum) epicotyls. Biochem J. 1989 Feb 1;257(3):853–858. doi: 10.1042/bj2570853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. E., Brett C. T., Hillman J. R. A xylosyltransferase involved in the synthesis of a protein-associated xyloglucan in suspension-cultured dwarf-French-bean (Phaseolus vulgaris) cells and its interaction with a glucosyltransferase. Biochem J. 1988 Aug 1;253(3):795–800. doi: 10.1042/bj2530795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D. M., Carpita N. C. Tracing cell wall biogenesis in intact cells and plants : selective turnover and alteration of soluble and cell wall polysaccharides in grasses. Plant Physiol. 1991 Oct;97(2):551–561. doi: 10.1104/pp.97.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R., Maclachlan G. Incorporation of UDP-[C]Glucose into Xyloglucan by Pea Membranes. Plant Physiol. 1989 Sep;91(1):373–378. doi: 10.1104/pp.91.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffing L. R. Comparisons of Golgi structure and dynamics in plant and animal cells. J Electron Microsc Tech. 1991 Feb;17(2):179–199. doi: 10.1002/jemt.1060170206. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. Occurrence and some properties of xyloglucan 4-beta-D-glucosyltransferase and 6-alpha-D-xylosyltransferase. J Biol Chem. 1981 Nov 10;256(21):11117–11122. [PubMed] [Google Scholar]
- Hayashi T., Read S. M., Bussell J., Thelen M., Lin F. C., Brown R. M., Delmer D. P. UDP-Glucose: (1-->3)-beta-Glucan Synthases from Mung Bean and Cotton: Differential Effects of Ca and Mg on Enzyme Properties and on Macromolecular Structure of the Glucan Product. Plant Physiol. 1987 Apr;83(4):1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Staehelin L. A. Synthesis, assembly and function of plant cell wall macromolecules. Curr Opin Cell Biol. 1992 Oct;4(5):856–862. doi: 10.1016/0955-0674(92)90111-o. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Auxin and Fusicoccin Enhancement of beta-Glucan Synthase in Peas : An Intracellular Enzyme Activity Apparently Modulated by Proton Extrusion. Plant Physiol. 1985 Jul;78(3):466–472. doi: 10.1104/pp.78.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Cooperative action of beta-glucan synthetase and UDP-xylose xylosyl transferase of Golgi membranes in the synthesis of xyloglucan-like polysaccharide. Biochim Biophys Acta. 1980 May 22;629(3):431–444. doi: 10.1016/0304-4165(80)90149-x. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers M. W., Bolwell G. P. Partial purification of Golgi-bound arabinosyltransferase and two isoforms of xylosyltransferase from French bean (Phaseolus vulgaris L.). Biochem J. 1992 Dec 15;288(Pt 3):817–822. doi: 10.1042/bj2880817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott L. D., Ray P. M. Molecular size and separability features of pea cell wall polysaccharides : implications for models of primary wall structure. Plant Physiol. 1992 Jan;98(1):357–368. doi: 10.1104/pp.98.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]