Abstract
Background
Cystic fibrosis (CF) is a multi-organ disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Individuals with CF often have gastrointestinal (GI) dysbiosis due to chronic inflammation and antibiotic use. Previous studies suggested a role for vitamin D in reversing the GI dysbiosis found in CF.
Objective
To explore the potential role of a combination of high-dose oral cholecalciferol (vitamin D3) and fermentable dietary fiber, inulin, to impact bacterial composition, richness, and diversity of intestinal and airway microbiota in adults with CF.
Methods
This was a 2 × 2 factorial, double-blinded, placebo-controlled, randomized, pilot clinical trial in which adults with CF received oral cholecalciferol (vitamin D3) (50,000 IU/week) and/or inulin (12 g/day) for 12 weeks. Thus, there were 4 study groups (n = 10 subjects per group); 1) placebo 2) vitamin D3 3) inulin 4) vitamin D3 plus inulin. Stool and sputum samples were collected at baseline (just before) and after the intervention and were analysed using 16S ribosomal RNA gene sequencing for gut and airway microbiota composition. Statistical analyses assessed alpha and beta diversity to evaluate microbial community changes.
Results
Of a total of 254 screened participants, 40 eligible participants were randomized to one of the 4 treatment arms. Participants receiving vitamin D3 plus inulin exhibited greater changes in microbiome indexes in both intestinal and airway relative to those in the other study groups. Specific taxonomic changes supported the potential beneficial influence of this combination to mitigate both intestinal and airway dysbiosis in adults with CF.
Conclusion
This pilot study established that the combination of oral vitamin D3 and the prebiotic inulin was well tolerated over 12 weeks in adults with CF and altered gut and airway bacterial communities. Future research appear warranted to define clinical outcomes and the role of microbiota changes therein with this approach.
Keywords: Vitamin D, vitamin D deficiency, Cystic fibrosis, Microbiome, Prebiotics, Inulin, Cholecalciferol
Introduction
Cystic fibrosis (CF) is a multi-organ disease caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR), which is responsible for chloride transport across epithelial cells. In addition to the respiratory system, CF also impacts the gastrointestinal (GI) system by causing fat malabsorption, chronic GI inflammation, and altered GI microbiome, termed GI dysbiosis [[1], [2], [3], [4], [5]]. The intestinal microbiome in CF is further altered by chronic systemic inflammation and frequent antibiotic use [3], [4], [5]. The severity of the CFTR mutation is also associated with the degree of intestinal dysbiosis [6]. Moreover, there is a strong association between GI dysbiosis and CF morbidities, including nutrient malabsorption [7], GI tract inflammation [7], decreased pulmonary function [8], and increased need for intravenous antibiotics [8]. Therefore, correcting GI dysbiosis may potentially lead to better clinical outcomes in patients with CF.
In rodent models of CF and in clinical studies of people with CF, vitamin D deficiency has been associated with GI dysbiosis [9]. There is evidence to suggest that vitamin D3 supplementation can have a positive impact on GI microbiota in individuals with CF with and without vitamin D deficiency. Specifically, vitamin D3 supplementation has been associated with a decreased abundance of potentially pathogenic bacteria and an enrichment of less pathogenic bacteria [10]. Additionally, high-dose vitamin D3 supplementation in adults with CF during an acute pulmonary exacerbation has been shown to alter metabolic pathways potentially related to intestinal microbial metabolism in a high-resolution plasma metabolomics study [11]. The mechanism by which vitamin D is protective may relate to its role in reducing proinflammatory cytokines and maintaining epithelial surface integrity [10]. These findings suggest that vitamin D3 may be an important factor in maintaining a healthy GI microbiome.
In addition to vitamin D, probiotics, a selection of potentially beneficial bacteria, have shown some promise in improving CF-related GI dysbiosis [12], [13], but the safety of probiotics is a concern in people with CF who may have supressed immunity [14], [15]. Prebiotics, are non-digestible dietary fibers that are metabolized by intestinal bacteria, thereby promoting the growth of benficial bacteria and increasing levels of fermentation products, both of which may potentially promote health with less risk of bacteremia relative to probiotics [16], [17]. Some randomized controlled trials (RCTs) have shown that prebiotics can improve intestinal microbiota composition and reduce respiratory infections in children without CF [18], [19], [20], but more research is needed to determine their efficacy in people with CF.
The objective of this pilot study was to evaluate the feasibility and impact of high-dose oral vitamin D3 in combination with prebiotic inulin on the bacterial composition, richness, and diversity of GI microbiota in stable outpatient adults with CF. Our hypothesis was that the combination of vitamin D3 and inulin would alleviate GI dysbiosis commonly found in CF. To test our hypothesis, we designed a 2 × 2 factorial, double-blinded, placebo-controlled, randomized, pilot clinical trial examining vitamin D3 and inulin administered over 12 weeks in adults with CF to explore the feasibility and impact of these interventions on the intestinal and airway microbiome.
Methods
Study procedures
The clinical study was approved by the Emory University Institutional Review Board (IRB) and registered at clinicaltrials.gov (NCT04118010). Participant enrollment began in October 2020 and follow-up for the last participant was completed in December 2022. Subject enrollment and allocation are outlined in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Fig. 1). Our study methods and procedures were published in detail [21].
Fig. 1.
CONSORT diagram of completed Vitamin D3 and Prebiotics for Intestinal Health in Cystic Fibrosis (Pre-D) clinical trial.
Participant eligibility
Potential study participants were screened for eligibility at routine clinical outpatient visits at the Emory University Cystic Fibrosis Center by reviewing the electronic medical record for eligibility criteria. As previously published, the inclusion criteria included the following: 1) male and female patients (18 years old or older) with confirmed CF by genetic mutation and/or sweat chloride testing, 2) not currently on oral or systemic antibiotics for pulmonary exacerbation for any other reason, including chronic use of oral azithromycin (or macrolide therapy) within four weeks prior to starting study (chronically inhaled antibiotics were allowed as long as the participants had not started the inhaled antibiotic less than four weeks from study enrollment), 3) use of CFTR modulator therapy was allowed. The exclusion criteria included 1) active GI disease, abdominal pain and/or diarrhea, 2) chronic kidney disease worse than stage 3 (eGFR < ml/min per 1.73 m2), 3) any vitamin D supplement or vitamin D analogue use greater than 2,000 IU daily or its equivalent weekly 4) use of immunosuppressants or history of organ transplantation, or 5) current use of prebiotics or probiotics [21].
Study participants who were taking a vitamin D supplement containing more than 2,000 IU of vitamin D could be re-screened for eligibility if they decreased their vitamin D supplement to less than 2,000 IU of vitamin D for 6 weeks. The amount of vitamin D contained in CF-specific multivitamins was not used to determine screening eligibility but was calculated as part of the participant’s baseline daily vitamin D intake.
Intervention
The participants were randomized in blocks of four by the Emory University Hospital Investigational Drug Service. The 4 intervention groups included oral vitamin D3 (50,000 IU) weekly plus oral corn-derived maltodextrin daily (12 g) as the oral prebiotic placebo; oral chicory-derived inulin (12 g) prebiotic daily plus oral placebo vitamin D3 weekly; combined oral vitamin D3 weekly and oral inulin daily; and oral placebo vitamin D3 weekly and oral placebo prebiotic daily. The Emory University Hospital Investigational Dug Service stored and dispensed the study medication at baseline as 1 pill weekly (50,000 IU of vitamin D3 or placebo) and 1 powder satchet packet daily (12 g of inulin or placebo) for 12 weeks. The vitamin D3 capsules were 120 % of the stated label as a confirmed from by independent laboratory (ARL Bio Pharma, Oklahoma City, OK). The dose of vitamin D3 was selected from our previously conducted study in adults with cystic fibrosis [10]. The dose of the inulin was selected based on a previously conducted study of healthy individuals with constipation [19].
The study medication, along with instructions, was mailed to the participants' residences. Participants were advised to take a capsule once a week and a sachet of powder dissolved in a liquid beverage of their choice daily. The study team monitored adherence to the study protocol and recorded potential adverse events by calling the study participants every two weeks. To detect potential symptoms of hypercalcemia caused by excessive vitamin D intake, participant questionnaires were administered serially by phone during the study and at its end, assessing excessive thirst, frequent urination, constipation, and confusion. At the end of the study, a patient questionnaire was used to evaluate study medication adherence and tolerability. The treatment assignment was kept blind from the study investigators, coordinators, research technicians, clinicians, and participants until the trial's completion.
Collection of stool and sputum samples
For characterization of intestinal and airway microbiota, participants were asked to provide a stool and sputum sample via return express mail at baseline and endpoint (12-week). Stool and sputum were collected prior to starting and after completing study interventions. Participants were provided two stool collection kits (OMR-200, OMNIgene Gut sample collection kit, Ottawa, Ontario, Canada) and two sputum collection kits (standard 50 mL conical tube) on the baseline visit day to mail directly to the lab on ambient temperature within 48 h after each collection. The OMR-200 kit provides microbiome stability at room temperature within 60 days of collection [22]. Non-induced expectorated sputum was produced and collected by participants upon a deep cough. After return receipt of study specimens in the laboratory, study technicians pipetted the stool and sputum samples into 0.5 to 1.0 ml aliquots that were stored at −80 °C.
Analysis of microbiota composition
Generation of 16S Ribosomal RNA gene sequencing data
DNA was extracted and purified from the frozen stool and sputum samples using DNeasy 96 PowerSoil Pro QIAcube HT kit, supplemented with PowerBead Pro Plates (Qiagen). V3-V4 region of 16S rRNA genes were amplified using the following primers: 341F 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′; 805R 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. PCR products of each sample were purified using Ampure XP magnetic purification beads. An index PCR was performed to attach dual barcodes and Illumina sequencing adapters using Nextera XT Index kit (Illumina). Final PCR products was verified on 1.5 % DNA agarose gel, purified again using Ampure XP magnetic purification beads and quantified using Pico dsDNA assay (Invitrogen). An equal molar of each sample was then combined as the library. The library was diluted and spiked with 5 % PhiX control (Illumina) and sequenced by Illumina MiSeq Sequencing System (2 × 250 bp). Demultiplexed fastq files were generated on instrument.
Analysis of 16S sequencing data
Feces: 65 stool samples from 34 subjects were assayed as described above. One sample was excluded because of low DNA after amplicon amplification. After quality filtering (DADA2 plugin in Qiime2), a range of 55,174–293,372 reads per sample was obtained. For alpha and beta diversity analysis, reads were rarefied to 55,174 reads per sample. For PCoA plot, all samples were included (n = 64). For all other analyses, only subjects with samples at both timepoints were included (n = 60 from 30 subjects), resulting in a sample size of placebo (subjects = 8), vitamin D (subjects = 8), inulin (subjects = 7), vitamin D & inulin (subjects = 7). Taxonomy was assigned using weighted Silva 138.1 classifier (human-stool).
Sputum: 54 sputum samples from 33 subjects were received for 16 s sequencing. Five samples were excluded before of low DNA concentration after amplicon amplification. After quality filtering (DADA2 plugin in Qiime2), 515–31,094 reads per sample were obtained. For alpha and beta diversity analysis, reads were rarefied to 2,892 reads per sample (two samples were excluded after rarefaction). For PCoA plot, all samples were included (n = 47). For all other analyses, only subjects with samples at both timepoints were included (n = 34 from 17 subjects), resulting a sample size of placebo (subjects = 4), vitamin D (subjects = 6), inulin (subjects = 3), vitamin D & inulin (subjects = 4). Taxonomy was assigned using weighted Silva 138.1 classifier (human-oral).
Statistical analysis
For alpha diversity, significance was determined by two-tailed paired Wilcoxon test (before vs after for each subject). PERMANOVA was done in Qiime2. The effect of vitamin D and inulin on Bray-Curtis distance between two samples of each patient was tested by two-way ANOVA. In case of a significant interaction between vitamin D and inulin (p<0.05), two-tailed unpaired Welch’s t-tests were done to determine differences between groups. Taxonomies with abundance less than 0.01 % was removed from analysis. Two methods were used to identify taxa that differed between time-points and groups: 1) Two-tailed paired t-tests (before vs after for each subject) was performed to identify taxonomy that was changed after each intervention. 2) One-way ANOVA with multiple pairwise comparisons adjusted by Tukey was performed to identify taxonomy that the delta abundance of which (“before” as the baseline for each subject) was different between intervention groups. Statistical Significance was reported if p ≤ 0.05. All statistical tests were done in R version 4.2.3.
Results
Study participants
Out of 254 potential study participants screened, a total of 56 participants were deemed eligible and provided consent for participation in the study (Fig. 1: CONSORT diagram). Of the 56 subjects who provided consent, 16 subjects were excluded due to failure to return a baseline stool or sputum sample. The 40 remaining eligible participants were randomized into 4 treatment groups (n = 10 subjects per group); 1) placebo 2) vitamin D3 3) inulin 4) vitamin D3 & inulin. Thirty-one of the participants completed the study (Fig. 1) and were included in the final analysis. The baseline characteristics of the 31 participants who completed the study were equally matched for age, weight, height, BMI, sex and race and shownin Table 1.
Table 1.
Demographics for participants randomized into each interventional arm who completed the study (n = 31).
| Treatment branches | Total | Vitamin D | Inulin | Vitamin D & Inulin | Placebo | p-value |
|---|---|---|---|---|---|---|
| 31 | 8 (25.81 %) | 7 (22.58 %) | 7 (22.58 %) | 9 (29.03 %) | ||
| Age (years) | 33.1 ± 11.9 | 29.3 ± 7.9 | 39.6 ± 15.6 | 29.3 ± 5.4 | 34.4 ± 14.2 | 0.30 |
| Weight (kg) | 67.9 ± 14.1 | 63.6 ± 14.8 | 68.7 ± 20.8 | 70.6 ± 11.5 | 68.9 ± 10.5 | 0.81 |
| Height (cm) | 166.1 ± 10.2 | 162.3 ± 11.9 | 167.7 ± 10.1 | 166.9 ± 10.2 | 167.6 ± 9.5 | 0.69 |
| BMI (kg/m2) | 24.5 ± 3.8 | 23.9 ± 3.5 | 24.3 ± 5.9 | 25.2 ± 1.8 | 24.6 ± 3.7 | 0.94 |
| Sex (female) | 13 (41.94 %) | 3 (37.5 %) | 5 (71.43 %) | 1 (14.29 %) | 4 (44.44 %) | 0.20 |
| Race (Caucasian) | 29 (93.55 %) | 8 (100 %) | 7 (100 %) | 7 (100 %) | 7 (77.78 %) | 0.077 |
| CFTR gene mutation | ||||||
| Homozygous delta F508 | 4 (50 %) |
0 | 5 (71.43 %) | 4 (44.44 %) | ||
| Heterozygous delta F508 | 3 (37.5 %) | 3 (42.86 %) | 2 (28.57 %) | 0 | ||
| Other | 1 (12.5 %) | 4 (57.14 %) | 0 | 5 (55.56 %) | ||
| Pancreatic enzyme replacement therapy | 6 (75 %) | 5 (71.43 %) | 7 (100 %) | 9 (100 %) | ||
| CFTR modulators | ||||||
| Elexacaftor/tezacaftor/ivacaftor | 8 (100 %) | 4 (57.14 %) | 7 (100 %) | 8 (88.89 %) | ||
| Ivacaftor | 0 | 2 (28.57 %) | 0 | 0 | ||
| No | 0 | 1 (14.29 %) | 0 | 1 (11.11 %) |
*Sex and race: frequency (percentage); applied fisher exact test.
*age, weight, height, BMI: mean ± std; applied ANOVA.
Intestinal microbiota composition
16S sequencing data was analyzed to determine the impact the change in microbiota composition over the course of the intervention or placebo; i.e. viewing each subject as their own control. This approach found that alpha diversity (Shannon index, a quantitative measure of community richness) of intestinal microbiota was decreased in the placebo group (p = 0.02), suggesting an inherent progression of GI dysbiosis in these CF patients. Such decreases in Shannon diversity were not observed in other intervention groups (Fig. S1). PCoA plot of Bray-Curtis distance (a quantitative measure of community dissimilarity) reveals slightly better clustering of intervention groups after the intervention (Fig. 2A and B) suggesting the interventions had impacted microbiota composition, albeit modestly. Despite of the significant overall difference by PERMANOVA (p = 0.02), no significant pairwise difference was determined between groups before the intervention. However, some significant differences were observed post intervention; adjusted p-values = 0.052, 0.052, and 0.052 for placebo vs vitamin D, vitamin D vs inulin, and inulin vs vitamin D & inulin, respectively. Relative changes in Bray-Curtis distance of each subject between the pre- and post-intervention timepoints are shown in Fig. 2C. This analysis indicated that, as predicted, the intestinal microbiotas of subjects administered both vitamin D and inulin exhibited the greatest change in Bray-Curtis distance over the course of the intervention. In contrast to our prediction, those consuming only inulin or vitamin D exhibited less changes than the placebo group. One possible explanation for this result is the placebo group displayed changes in microbiota composition that reflected worsening dysbiosis. To identify individual bacterial taxa whose abundance changed post-intervention, two-tailed paired t-tests were performed. We observed post-intervention altered abundance of 4, 2, 2, and 11 taxa in the placebo, vitamin D, inulin, and vitamin D & inulin groups (Supplement Table 1 and Fig. S2). To identify individual bacterial taxa that the delta abundance of which (“before” as the baseline for each subject) was different between intervention groups, multiple pairwise-comparison, adjusted by Tukey following one-way ANOVA was performed. The delta abundance of 1 taxa was identified different between inulin vs placebo; 2 for placebo vs vitamin D & inulin; 7 for vitamin D vs vitamin D & inulin; 2 for inulin vs vitamin D & inulin (Supplement Table 2 and Fig. S3). Genus Bifidobacterium has been widely acknowledged as a biomarker to be increased after inulin dietary intervention. In this study, we did not find the abundance of Bifidobacterium was significantly increased after inulin intervention (p = 0.11, paired t-tests), but the delta abundance was greater after inulin intervention than that after placebo (p = 0.04, one-tailed unpaired t-test, Fig. S4).
Fig. 2.
Beta diversity of intestinal microbiota. PCoA plot of Bray-Curtis distance before (A) and after (B) the intervention. PERMANOVA was done to determine the difference between groups; p-values were p = 0.02 (A) and p = 0.01 (B). The adjusted p-values of multiple comparisons were as follows: (A) all pairs not significant; (B) p = 0.052 for Placebo vs Vitamin D; p = 0.052 for Vitamin D vs Inulin; p = 0.052 for Inulin vs (Vitamin D& Inulin), the other pairs not significant. (C) Box plot of bray-Curtis distance of each subject between time points. Two-tailed unpaired Welch’s t-test was done to determine difference between groups (p < 0.05).
Fig. 3.
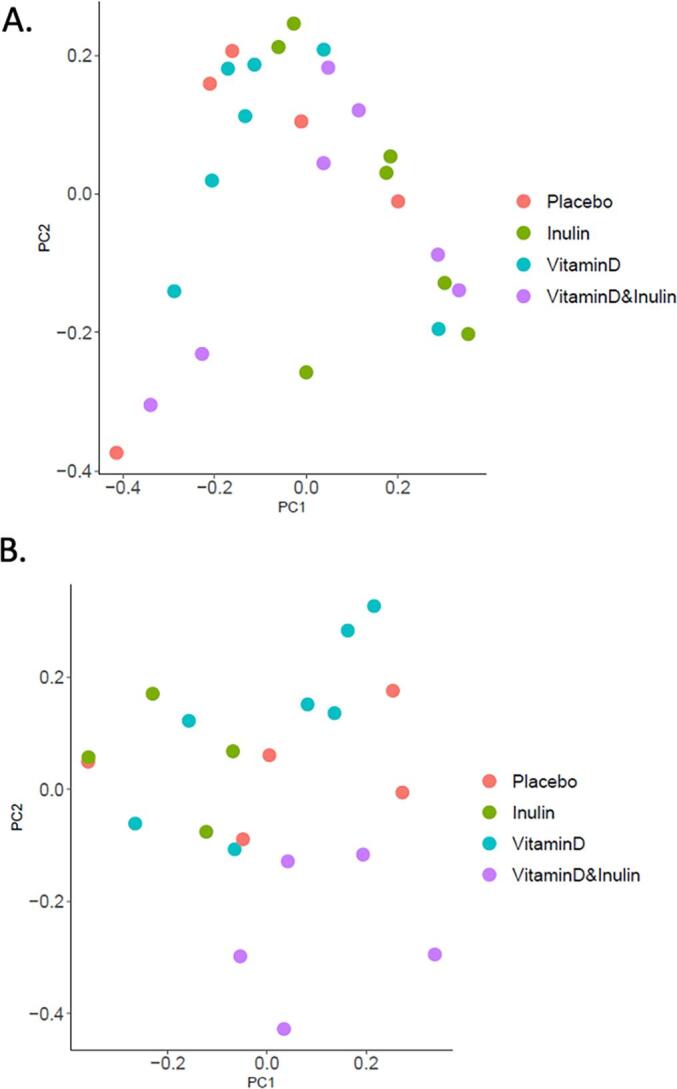
Beta diversity of airway microbiota. PCoA plot of Bray-Curtis distance before (A) and after (B) the intervention. PERMANOVA was done to determine the difference between groups; p-values were p = 0.37 (A) and p = 0.06 (B). The adjusted p-values of multiple comparisons were as follows: (A) all pairs not significant; (B) p = 0.02 for VitaminD vs (Vitamin D& Inulin), the other pairs not significant. C) Box plot of bray-Curtis distance of each subject between time points. Two-tailed unpaired Welch’s t-test was done to determine difference between groups (p < 0.05).
Airway microbiota composition
Assesment of relative changes in alpha diversity of airway microbiota in each subject did not reveal a clear pattern of impacts in any of the groups although a trend of slightly decrease Shannon diversity was seen in the vitamin D & inulin group (Fig. S5). PCoA of Bray-Curtis distance revealed strong clustering of groups after intervention (Fig. 3B, PERMANOVA, p = 0.06), especially clear separation between vitamin D vs vitamin D & inulin groups (adjusted p-value = 0.02). Relative changes in Bray-Curtis distance of each subject between the pre- and post-intervention timepoints are shown in Fig. 3C. This analysis indicated that, oral administration of inulin or vitamin D alone did not result in greater community changes of airway microbiota compared to the placebo group, but the vitamin D & inulin group did (p = 0.004, compared to placebo), suggesting additive effects of these interventions. To identify individual bacterial taxonomy that the abundance was changed after each intervention, two-tailed paired t-tests were performed. We observed altered abundance, post-intervention, of 0, 1, 6, and 4 taxa in the placebo, vitamin D, inulin, and vitamin D & inulin groups. respectively (Supplement Table 3 and Fig. S6). To identify individual bacteria that the delta abundance of which (“before” as the baseline for each subject) was different among intervention groups, multiple pairwise-comparison adjusted by Tukey following one-way ANOVA was performed. The delta abundance of 3, 1,1, and 5 taxa were identified as being different between placebo vs vitamin D, placebo vs vitamin D & inulin, vitamin D vs vitamin D & inulin, and inulin vs vitamin D & inulin groups (Supplement Table 3 and Fig. S7).
Tolerability of the study medications
A clinical trial tolerability rating score and number of adverse effects including symptoms such as excessive thirst, increased urination, constipation, memory loss, loose stools, bloating, and stomach pain and participant’s adherence were collected from a total of 29 participants who provided both pre-intervention and post-intervention stool samples [Supplementary Table 5]. The participants reported a mean score of 4.06 out of 5, indicating that the study medications were moderately acceptable [Supplementary Table 5A]. Participants who received placebo fiber reported more loose stools compared to those who received inulin. Partcipants who received both vitamin D and inulin reported less abdominal pain compared to participants who received inulin alone [Supplementary Table 5B]. In term of adherence, most participants reported missing 1–2 doses of the daily study medication (inulin or placebo) (48.3 %) and the majority reported not missing any of the weekly study medication (vitamin D or placebo) (62.1 %) [Supplementary Table 5C].
Discussion
This randomized, 2 × 2 factorial, prospective, clinical pilot and feasibility study found the combination of vitamin D3 and the prebiotic inulin over a 12-week period resulted in greater microbial diversity in the intestinal and airway of adults with cystic fibrosis compared to vitamin D alone, inulin alone, or placebo. The effect of the combined administration of inulin and vitamin D3 altered both the intestinal and airway microbiomes. These findings comport with our previously conducted study of vitamin D alone in adults with cystic fibrosis in which 23 adults were randomized to oral vitamin D3 (cholecalciferol) 50,000 IU once a week or placebo for 12 weeks [10]. The group assigned to vitamin D3 had a shift in intestinal microbiota population clustering compared to the group assigned to placebo [10]. However, in our present study we only found differences in intestinal microbiome populations when vitamin D3 was combined with inulin and not with the administration of vitamin D3 or inulin alone [10].
Several clinical studies evaluating the impact of vitamin D on intestinal microbiome composition have demonstrated mixed results. A study conducted in older Australian adults without known GI dysbiosis found no changes in intestinal microbiome diversity indices or differential clustering of bacterial communities when randomized to 60,000 IU of oral vitamin D3 monthly or placebo for 5 years [23]. A study conducted in pregnant women showed no impact of vitamin D3 on intestinal microbiome diversity [24]. In contrast, studies conducted in adults with Clostridioides difficile infection [25] and adults with colon cancer demonstrate changes in diversity and bacterial populations in response to vitamin D supplementation [26]. Studies in healthy adults with vitamin D deficiency have been mixed with one study showing alterations in bacterial populations [27] and another showing no changes in response to vitamin D supplementation [28]. The results of these published studies suggest that vitamin D intervention may be more effective in people with pre-existing gastrointestinal dysbiosis and are at risk for vitamin D deficiency.
People with CF have airway and intestinal dysbiosis due to chronic inflammation, poor nutrition, recurrent infections, impaired immune responses, and use of frequent use of systemic antibiotics [29], [30], [31], [32], [33]. The bacterial populations often found to be enriched the intestinal and airway are typically those associated with increased pro-inflammatory response [32]. Intestinal dysbiosis in CF occurs early in childhood and is associated with markers of poor growth and lung function [34]. There are limited studies evaluating the use of prebiotics or probiotics to reverse airway and intestinal dysbiosis in CF [35], [36]. Early studies suggest CFTR modulator therapy may result in improved bacterial diversity and decrease in inflammatory bacteria in the airway and intestinal [37]. It remains unclear which interventions are effective in reversing the airway and intestinal dysbiosis found in CF and are associated with improved clinical outcomes.
Vitamin D is thought to play a key role in maintaining host-microbiota homeostasis leading to the hypothesis that alleviating vitamin D deficiency may ameliorate airway and intestinal dysbiosis observed in CF. Studies conducted in mice models suggest that vitamin D supplementation may alter the intestinal microbiome via reduction of pro-inflammatory cytokines in the intestine [38]. It is unlikely that vitamin acts directly upon intestinal bacteria as few bacteria possess the vitamin D receptor (VDR). Rather, vitamin D may mitigate airway and intestinal dysbiosis in adults with CF by decreasing local inflammation, reducing epithelial permeability, and/or enhancing the innate immune system [39], [40], [41]. Some bacteria such as Streptomyces griseolus also may convert parent vitamin D to the biologically active form of vitamin D, 1,25-dihydroxyvitamin D, to act locally on the epithelial surfaces [42]. Our findings are in accord with the notion that vitamin D, given in combination with the prebiotic inulin in adults with CF, may ameliorate GI dysbiosis.
As a pilot study, this trial was designed primarily to test the feasibility of the combination of a prebiotic and vitamin D3 for a larger trial. Thus, a weaknesses of the study was the small sample size, which limits the statistical power to detect significance. The participants in the trial tolerated the combination well without any severe adverse reactions to the intervention. In addition, for taxa identification due to the limited sample size, we did not adjust p-values at the family level. The study was conducted during the COVID19 global pandemic which limited our in-person visits. Thus, stool samples had to be returned via express mail and blood could not be collected due to our institution’s restriction on in-person clinical studies. These restrictions precluded us from confirming by blood samples that vitamin D status improved in the vitamin D intervention arms; however, based on our past study, our vitamin D regimen improves 25-hydroxyvitamin D status well into the sufficient range (>30 ng/mL). Also due to the global COVID19 pandemic, collection of stool and sputum were performed by the participants at home and not under direct observation. Another potential limitation was that there were no specialized sputum collection kits for the sputum samples (discontinued by manufacturer) as there were for the stool samples as conducted in our previous study [10]. Its unclear if sputum samples sent on ambient temperature significantly altered the bacterial communities. In addition, we did not randomize the screened participants who failed to return the initial baseline sample and greater than 75 % of the participants returned their final sample.
In summary, results of this pilot and feasibility study demonstrated that the combined treatment of vitamin D3 and inulin was well tolerated over a 12-week period in adults with cystic fibrosis. Furthermore, our results indicate that vitamin D3 and inulin have additive ability to mitigate intestinal and airway microbiota in adults with cystic fibrosis. Future research is warranted to define clinical outcomes of these interventions and the role of microbiota changes therein.
CRediT authorship contribution statement
Pichatorn Suppakitjanusant: Writing – review & editing, Writing – original draft, Project administration, Investigation. Yanling Wang: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Funding acquisition, Formal analysis. Alisa K. Sivapiromrat: Writing – review & editing, Writing – original draft, Project administration, Investigation. Chengcheng Hu: Writing – review & editing, Writing – original draft, Software, Formal analysis, Data curation. Jose Binongo: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. William R. Hunt: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology. Samuel Weinstein: Writing – review & editing, Writing – original draft, Methodology, Investigation. Ishaan Jathal: Writing – review & editing, Writing – original draft, Methodology, Investigation. Jessica A. Alvarez: Writing – review & editing, Writing – original draft, Methodology, Investigation. Benoit Chassaing: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Thomas R. Ziegler: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation, Funding acquisition, Conceptualization. Andrew T. Gewirtz: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Vin Tangpricha: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Vin Tangpricha reports financial support was provided by Cystic Fibrosis Foundation. Vin Tangpricha reports financial support was provided by National Institutes of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
VT, JAA, TRZ, WRH and AG designed research; PS, SW, IJ and AKS conducted research; PS, AKS, CH, YW, JB, and AG analyzed data; and PS, AKS, YW, AG and VT wrote the paper. VT had primary responsibility for final content. All authors read and approved the final manuscript. The study investigators would like to acknowledge the CF care team at the Emory Adult Cystic Fibrosis and the research study coordinators for making this study feasible. This study was supported by Cystic Fibrosis Foundation Awards TANGPR19A0 and CC002-AD (United States) and NIH awards P30DK125013 and 3UL1TR002378-05S2 (United States).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2024.100362.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Crites K.S., Morin G., Orlando V., Patey N., Cantin C., Martel J., et al. CFTR Knockdown induces proinflammatory changes in intestinal epithelial cells. J Inflamm (London, England) 2015;12:62. doi: 10.1186/s12950-015-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin G., Orlando V., St-Martin Crites K., Patey N., Mailhot G. Vitamin D attenuates inflammation in CFTR knockdown intestinal epithelial cells but has no effect in cells with intact CFTR. Am J Physiol Gastrointest Liver Physiol. 2016;310:G539–G549. doi: 10.1152/ajpgi.00060.2015. [DOI] [PubMed] [Google Scholar]
- 3.de Freitas M.B., Moreira E.A.M., Tomio C., Moreno Y.M.F., Daltoe F.P., Barbosa E., et al. Altered intestinal microbiota composition, antibiotic therapy and intestinal inflammation in children and adolescents with cystic fibrosis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan J.C. Neonatal gastrointestinal and respiratory microbiome in cystic fibrosis: potential interactions and implications for systemic health. Clin Ther. 2016;38(4):740–746. doi: 10.1016/j.clinthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munck A. Cystic fibrosis: evidence for gut inflammation. Int J Biochem Cell Biol. 2014;52:180–183. doi: 10.1016/j.biocel.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S., Needham B., Leach S.T., Day A.S., Jaffe A., Thomas T., et al. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci Rep. 2016;4(6) doi: 10.1038/srep24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matamouros S., Hayden H.S., Hager K.R., Brittnacher M.J., Lachance K., Weiss E.J., et al. Adaptation of commensal proliferating Escherichia coli to the intestinal tract of young children with cystic fibrosis. Proc Natl Acad Sci USA. 2018;115(7):1605–1610. doi: 10.1073/pnas.1714373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke D.G., Fouhy F., Harrison M.J., Rea M.C., Cotter P.D., O'Sullivan O., et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017;17(1):58. doi: 10.1186/s12866-017-0968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanhere M., Chassaing B., Gewirtz A.T., Tangpricha V. Role of vitamin D on gut microbiota in cystic fibrosis. J Steroid Biochem Mol Biol. 2018;175:82–87. doi: 10.1016/j.jsbmb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanhere M., He J., Chassaing B., Ziegler T.R., Alvarez J.A., Ivie E.A., et al. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2018;103(2):564–574. doi: 10.1210/jc.2017-01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez J.A., Chong E.Y., Walker D.I., Chandler J.D., Michalski E.S., Grossmann R.E., et al. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration. Metabolism. 2017;70:31–41. doi: 10.1016/j.metabol.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Campo R., Garriga M., Pérez-Aragón A., Guallarte P., Lamas A., Máiz L., et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: a double blind prospective study. J Cyst Fibros. 2014;13(6):716–722. doi: 10.1016/j.jcf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzese E., Callegari M.L., Raia V., Viscovo S., Scotto R., Ferrari S., et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghighat L., Crum-Cianflone N.F. The potential risks of probiotics among HIV-infected persons: bacteraemia due to Lactobacillus acidophilus and review of the literature. Int J STD AIDS. 2016;27(13):1223–1230. doi: 10.1177/0956462415590725. [DOI] [PubMed] [Google Scholar]
- 15.Singhi S.C., Kumar S. Probiotics in critically ill children. F1000Res. 2016;5 doi: 10.12688/f1000research.7630.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas D.W., Greer F.R. American academy of pediatrics committee on nutrition; American academy of pediatrics section on gastroenterology, hepatology, and nutrition. Pediatrics. 2010;126(6):1217–1231. [Google Scholar]
- 17.Dror T., Dickstein Y., Dubourg G., Paul M. Microbiota manipulation for weight change. Microb Pathog. 2017;106:146–161. doi: 10.1016/j.micpath.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Drabińska N., Jarocka-Cyrta E., Markiewicz L.H., Krupa-Kozak U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: results of a randomized, placebo-controlled trial. Nutrients. 2018;10(2) doi: 10.3390/nu10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandeputte D., Falony G., Vieira-Silva S., Wang J., Sailer M., Theis S., et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolucci A.C., Hume M.P., Martínez I., Mayengbam S., Walter J., Reimer R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153(3):711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Sivapiromrat A.K., Suppakitjanusant P., Wang Y., Hu C., Binongo J., Hunt W.R., Weinstein S., Jathal I., Alvarez J.A., Chassaing B., Ziegler T.R., Gewirtz A.T., Tangpricha V. Vitamin D and prebiotics for intestinal health in cystic fibrosis: Rationale and design for a randomized, placebo-controlled, double-blind, 2 x 2 trial of administration of prebiotics and cholecalciferol (vitamin D3) (Pre-D trial) in adults with cystic fibrosis. Contemporary clinical trials communications. 2024;38:101278. doi: 10.1016/j.conctc.2024.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macklaim J, Mikalauskas A, Cunningham L, Egloff C, Doukhanine E. OMNIgeneTMGUT provides ambient temperature stabilization of pediatric fecal samples for microbiome profiling. PD-WP-00057. DNA Genotek Inc.
- 23.Pham H., Waterhouse M., Rahman S., Baxter C., Duarte Romero B., McLeod D.S.A., et al. The effect of vitamin D supplementation on the gut microbiome in older Australians – results from analyses of the D-Health Trial. Gut Microbes. 2023;15(1) doi: 10.1080/19490976.2023.2221429. PMID: 37287399; PMCID: PMC10251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aparicio A., Gold D.R., Weiss S.T., Litonjua A.A., Lee-Sarwar K., Liu Y.Y. Association of vitamin D level and maternal gut microbiome during pregnancy: findings from a randomized controlled trial of antenatal vitamin D supplementation. Nutrients. 2023;15(9) doi: 10.3390/nu15092059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.H., Park H.K., Kang C.D., Choi D.H., Park S.C., Park J.M., et al. High dose intramuscular vitamin D3 supplementation impacts the gut microbiota of patients with Clostridioides Difficile infection. Front Cell Infect Microbiol. 2022;6(12) doi: 10.3389/fcimb.2022.904987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellerba F., Serrano D., Johansson H., Pozzi C., Segata N., NabiNejad A., et al. Colorectal cancer, Vitamin D and microbiota: a double-blind Phase II randomized trial (ColoViD) in colorectal cancer patients. Neoplasia. 2022;34 doi: 10.1016/j.neo.2022.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charoenngam N., Shirvani A., Kalajian T.A., Song A., Holick M.F. The effect of various doses of oral vitamin d3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551–556. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 28.Shieh A., Lee S.M., Lagishetty V., Gottleib C., Jacobs J.P., Adams J.S. Pilot trial of vitamin D3 and calcifediol in healthy vitamin D deficient adults: does it change the fecal microbiome? J Clin Endocrinol Metab. 2021;106(12):3464–3476. doi: 10.1210/clinem/dgab573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scanlan P.D., Buckling A., Kong W., Wild Y., Lynch S.V., Harrison F. Gut dysbiosis in cystic fibrosis. J Cyst Fibr. 2012;11(5):454–455. doi: 10.1016/j.jcf.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Cox M.J., Allgaier M., Taylor B., Baek M.S., Huang Y.J., Daly R.A., et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosispatients. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011044. e11044.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan J.C., Koestler D.C., Stanton B.A., Davidson L., Moulton L.A., Housman M.L., et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3(4) doi: 10.1128/mBio.00251-12. e00251–12.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caley L.R., White H., de Goffau M.C., Floto R.A., Parkhill J., Marsland B., et al. Cystic fibrosis-related gut dysbiosis: a systematic review. Digestive Dis. Sci. 2023;68(5):1797–1814. doi: 10.1007/s10620-022-07812-1. [DOI] [PubMed] [Google Scholar]
- 33.Viteri-Echeverría J., Calvo-Lerma J., Ferriz-Jordán M., Garriga M., García-Hernández J., Heredia A., et al. Association between dietary intake and faecal microbiota in children with cystic fibrosis. Nutrients. 2023;15(24):5013. doi: 10.3390/nu15245013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffey M.J., Nielsen S., Wemheuer B., Kaakoush N.O., Garg M., et al. Gut microbiota in children with cystic fibrosis: a taxonomic and functional dysbiosis. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-55028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffey M.J., Garg M., Homaira N., Jaffe A., Ooi C.Y. Probiotics for people with cystic fibrosis. Cochrane Database Syst. Rev. 2020;1(1) doi: 10.1002/14651858.CD012949.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dorst J.M., Tam R.Y., Ooi C.Y. What do we know about the microbiome in cystic fibrosis? Is there a role for probiotics and prebiotics. Nutrients. 2022;14(3):480. doi: 10.3390/nu14030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lussac-Sorton F., Charpentier É., Imbert S., Lefranc M., Bui S., Fayon M., et al. The gut-lung axis in the CFTR modulator era. Front. Cell Infect Microbiol. 2023;15(13) doi: 10.3389/fcimb.2023.1271117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia P.M., Moore J., Kahan D., Hong M.Y. Effects of vitamin D supplementation on inflammation, colonic cell kinetics, and microbiota in colitis: a review. Molecules (Basel, Switzerland) 2020;25(10):2300. doi: 10.3390/molecules25102300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun R.F., Liu P.T., Modlin R.L., Adams J.S. Hewison MImpact of vitamin D on immune function: lessons learned from genome-wide analysis. Front. Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assa A., Vong L., Pinnell L.J., Rautava J., Avitzur N., Johnson-Henry K.C. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm. Bowel Dis. 2015;21(2):297–306. doi: 10.1097/MIB.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 41.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 42.Takita T., Sakuma H., Ohashi R., Nilouyal S., Nemoto S., Wada M., et al. Comparison of the stability of CYP105A1 and its variants engineered for production of active forms of vitamin D. Biosci. Biotech. Biochem. 2022;86(4):444–454. doi: 10.1093/bbb/zbac019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






