Abstract
There is currently no licensed vaccine for respiratory syncytial virus (RSV). Here, we assess the effect of RSV fusion protein (F) conformation on B cell responses in a post-hoc comparison of samples from the DS-Cav1 (prefusion, pre-F) and MEDI7510 (postfusion, post-F) vaccine clinical trials. We compared the magnitude and quality of the serological and B cell responses across timepoints and vaccines. We measured RSV A and B neutralization, F-binding IgG titers, and competition assays at week 0 (pre-vaccination) and week 4 (post-vaccination) to evaluate antibody specificity and potency. To compare B cell specificity and activation, we used pre-F and post-F probes in tandem with a 17-color immunophenotyping flow cytometry panel at week 0 (pre-vaccination) and week 1 (post-vaccination). Our data demonstrate that both DS-Cav1 and MEDI7510 vaccination robustly elicit F-specific antibodies and B cells, but DS-Cav1 elicited antibodies that more potently neutralized both RSV A and B. The superior potency was mediated by antibodies that bind antigenic sites on the apex of pre-F that are not present on post-F. In the memory (CD27+) B cell compartment, vaccination with DS-Cav1 or MEDI7510 elicited B cells with different epitope specificities. B cells preferentially binding the pre-F probe were activated in DS-Cav1-vaccinated participants, but not in MEDI7510-vaccinated participants. Our findings emphasize the importance of using pre-F as an immunogen in humans due to its deterministic role in eliciting highly potent neutralizing antibodies and memory B cells.
One Sentence Summary:
A respiratory syncytial virus prefusion subunit vaccine elicited higher potency neutralizing antibodies than a postfusion subunit vaccine in humans.
Editor’s Summary:
Function Follows Form. Respiratory syncytial virus (RSV) can cause severe infection in infants and older adults; however, no vaccine for RSV is currently approved. Candidate vaccines that target the RSV fusion (F) protein have been developed. Older vaccines use the postfusion, or Post-F, version of the protein, whereas new vaccines employ a prefusion stabilized, or Pre-F, immunogen. Here, Chang and Phung et al. directly compared immune responses elicited by a Post-F vaccine, MEDI7510, and a Pre-F vaccine, DS-Cav1. The authors found that DS-Cav1 elicited antibodies that more potently neutralized RSV A and B than MEDI7510. This enhanced response was mediated by the presence of antibodies that could bind sites on the F protein that were only present in the Pre-F state. Together, these data highlight the value of structure-based vaccine design.
INTRODUCTION
Respiratory syncytial virus (RSV) is an orthopneumovirus in the Pneumoviridae family that is responsible for millions of severe respiratory illness cases in infants, older adults, and the immunocompromised worldwide (2–8). Almost all infants are infected with RSV by the age of two, with repeated infections throughout life (9). Despite the ubiquitous nature of RSV infection and magnitude of its impact, no licensed vaccine is currently available, indicating a major gap in global health. Clinical evaluation of a formalin-inactivated whole virus RSV vaccine adjuvanted with aluminum salts (FI-RSV) in infant cohorts during the 1960s resulted in enhanced respiratory disease in vaccinated infants, hampering the momentum of RSV vaccine development for several decades (10, 11). The majority of subsequent RSV vaccines have been based on the fusion (F) protein, an attractive target due to its high degree of antigenic and genetic conservation (11). The clinical success of palivizumab, an anti-F monoclonal antibody, illustrates the potential for F-directed, neutralizing antibodies to prevent severe disease. Subsequent studies have also demonstrated that a high concentration of neutralizing activity in the serum correlates with protection (12–18).
The F glycoprotein exists in two major conformations on the surface of RSV: the active conformation, prefusion F (pre-F), and the inactive conformation, postfusion F (post-F). Pre-F displays six major antigenic sites (Ø, I, II, III, IV, V), whereas only four of these sites (I, II, III, IV) are retained following transition to post-F (11). Prior to the stabilization of pre-F, many F-based vaccine candidates in clinical testing used post-F as the vaccine antigen, given its high stability; however no post-F vaccine candidates have advanced to licensure (19, 20). The stabilization and structural characterization of pre-F led to the identification of highly neutralization-sensitive epitopes (sites Ø and V) exclusive to the pre-F surface, motivating efforts to use pre-F as a vaccine antigen capable of eliciting high titers of neutralizing antibody (11, 12, 21–26). A pre-F-exclusive monoclonal antibody targeting site Ø, nirsevimab (MEDI8897), has also demonstrated efficacy in preterm, late preterm, and term infants (27, 28). Although both conformations of F have been represented as vaccine candidates in the pipeline for clinical evaluation, direct comparison of immunity elicited by pre- and post-F vaccines has not been done in humans.
Serum samples and peripheral blood mononuclear cells (PBMCs) were collected from a phase 1 clinical trial evaluating a pre-F (DS-Cav1) vaccine antigen as well as two independent phase 1 and 2 clinical trials evaluating a post-F vaccine antigen (MEDI7510). DS-Cav1 is a stabilized pre-F vaccine, recently evaluated in phase 1 clinical testing (NCT03049488), and administered to healthy adult volunteers (ages 18 to 50) in 50 μg, 150 μg, or 500 μg doses with or without aluminum hydrogel (alum) adjuvant (29–32). MEDI7510 is a vaccine composed of 120 μg of soluble post-F (sF) adjuvanted with 5 μg of glucopyranosyl lipid adjuvant (GLA) in a 2% stable emulsion (SE), which was administered to older adults (≥60 years) and was evaluated in phase 1 (NCT02115815, NCT02289820) and phase 2b (NCT02508194) clinical trials (33–38). In this substudy, we compare antibody and B cell responses elicited by DS-Cav1 and MEDI7510 to elucidate differences in the serological profile and B cell phenotype of the participants and better understand the advantages and disadvantages of each conformation as a vaccine immunogen, informing future RSV vaccine design.
RESULTS
Pre-F vaccination in humans elicited a greater magnitude of potently neutralizing, pre-F-exclusive antibodies than post-F vaccination.
The neutralization capacity of serum samples from the pre-vaccination (W0) and 4 weeks post-vaccination (W4) timepoints for all participants was evaluated against both the RSV A and B subtypes (Fig. 1 A to C). At W4, DS-Cav1 elicited an increase in RSV A and B neutralizing antibodies by 8.0-fold (P < 0.0001) and 6.8-fold (P < 0.0001), respectively, whereas MEDI7510 elicited a 1.5-fold increase in both RSV A (P = 0.0316) and B (P = 0.0062) neutralizing antibodies. To evaluate neutralization mediated by antibodies binding to the pre-F-exclusive antigenic sites Ø and V, serum samples were diluted in excess post-F to adsorb dual-binding and post-F-exclusive antibodies (Fig. 1B). By this metric, pre-F-exclusive neutralizing activity against RSV A increased 6.9-fold (P < 0.0001) in DS-Cav1 between W0 and W4. Overall, the increase in W4 neutralization of RSV A and B and pre-F-exclusive RSV A neutralization in DS-Cav1-vaccinated individuals was significantly greater than in MEDI7510-vaccinated participants (RSV A: P < 0.0001; RSV B: P < 0.0001; pre-F exclusive RSV A: P < 0.0001; Fig. 1D to F), and as expected, post-F vaccination did not elicit antibodies to antigenic sites only present on pre-F. There was no increase in pre-F-exclusive neutralization for MEDI7510 vaccinated individuals (Fig. 1E).
Figure 1. Pre-F vaccination elicits a greater magnitude of pre-F-exclusive and highly neutralizing antibodies than post-F vaccination.
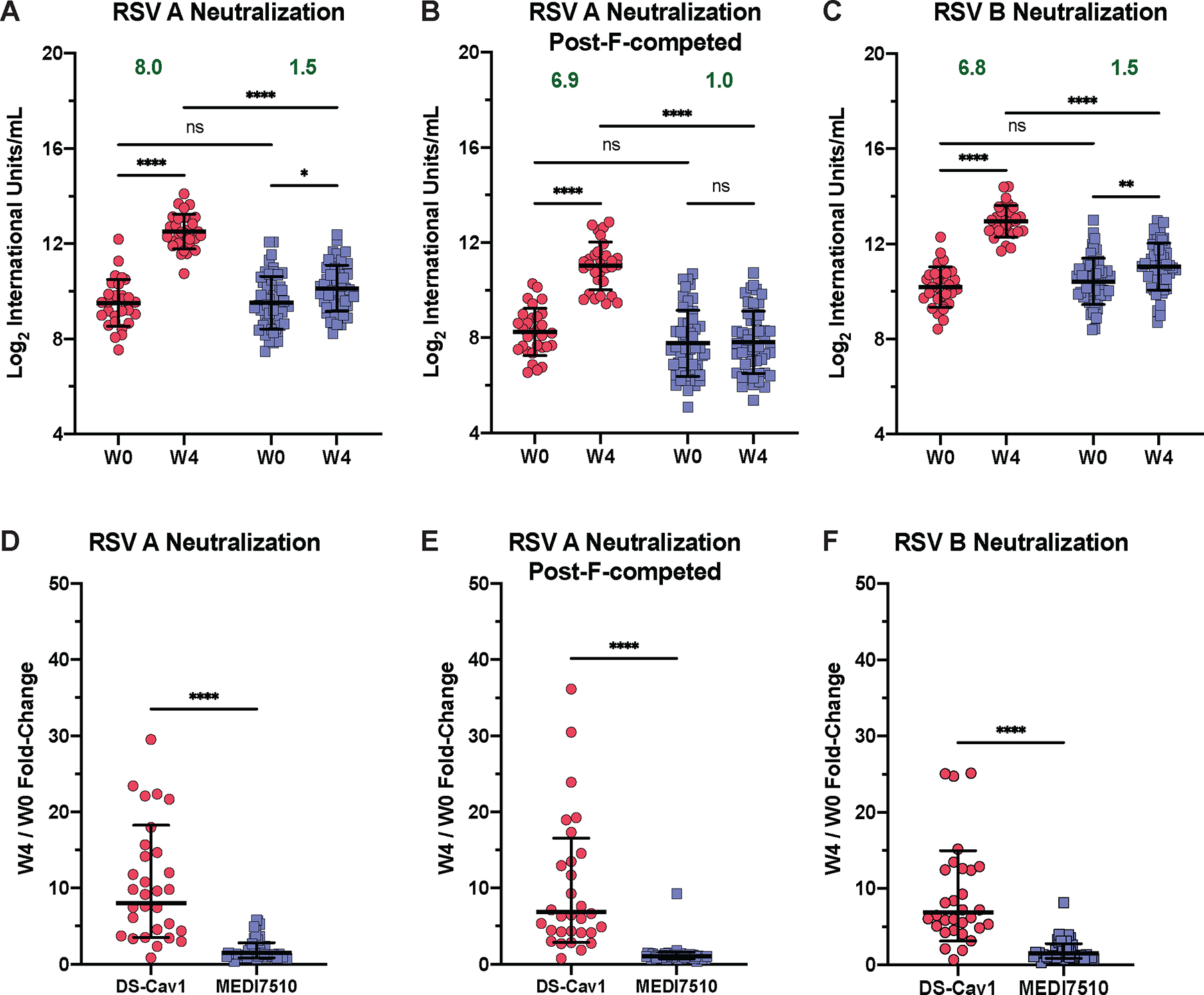
(A to C) Conformation-specific neutralization capacity of serum obtained at week 0 (W0) and week 4 (W4) after vaccination with DS-Cav1 (pre-F, N = 30) or MEDI7510 (post-F, N = 52) was measured for (A) RSV subtype A2 without post-F competition or (B) with post-F competition, and (C) subtype B. In (B), excess post-F was used to adsorb post-F-exclusive and dual-binding antibodies to assess the neutralization capacity of pre-F exclusive antibodies in serum. (D to F) Fold-change in neutralization capacity at W4 after vaccination with either DS-Cav1 or MEDI7510 was calculated for RSV subtype A2 (D) without or (E) with post-F competition, and (F) subtype B. Bars represent mean ± standard deviation (SD) of log2 data. Dotted lines indicate the limit of detection (LOD) for each assay. For all graphs, asterisks denote the P-value, with ****P < 0.0001, ***P = 0.0001 to 0.001, **P = 0.001 to 0.01, *P = 0.01 to 0.05, and ns = not significant as determined using Brown-Forsythe and Welch ANOVA tests (A to C), Kruskal-Wallis tests (D and E), or Mann-Whitney tests (F). Red circles denote participants who received DS-Cav1, blue squares denote participants who received MEDI7510. Geometric mean W4/W0 fold-change is shown in green text.
Pre-F vaccination elicits pre-F-exclusive binding antibodies with higher average potency.
Pre-F-specific binding IgG titers were measured in W0 and W4 serum samples from DS-Cav1 and MEDI7510 vaccinated participants (Fig. 2A and B). Similar to neutralization, excess post-F was used to adsorb dual-binding and post-F-exclusive antibodies for quantification of pre-F-exclusive binding antibodies. We observed an 8.3-fold (P < 0.0001) increase in pre-F binding and a 11.0-fold (P < 0.0001) increase in pre-F-exclusive binding for DS-Cav1 vaccinated participants at W4 (Fig. 2A, fig. S1A). In contrast, although MEDI7510 vaccination elicited a 4.2-fold increase (P < 0.0001) in pre-F binding, there was no increase in pre-F-exclusive binding (Fig. 2B, fig. S1B). Both vaccines had comparable induction of high post-F binding IgG titers, with a 7.7-fold (P < 0.0001) and 8.4-fold (P < 0.0001) increase in titer for DS-Cav1- and MEDI7510-vaccinated participants, respectively (Fig. 2C). MEDI7510 vaccination did not elicit higher increases in post-F binding IgG titers compared to DS-Cav1 (fig. S1C).
Figure 2. Pre-F vaccination elicits pre-F-exclusive binding antibody and higher neutralization potency.
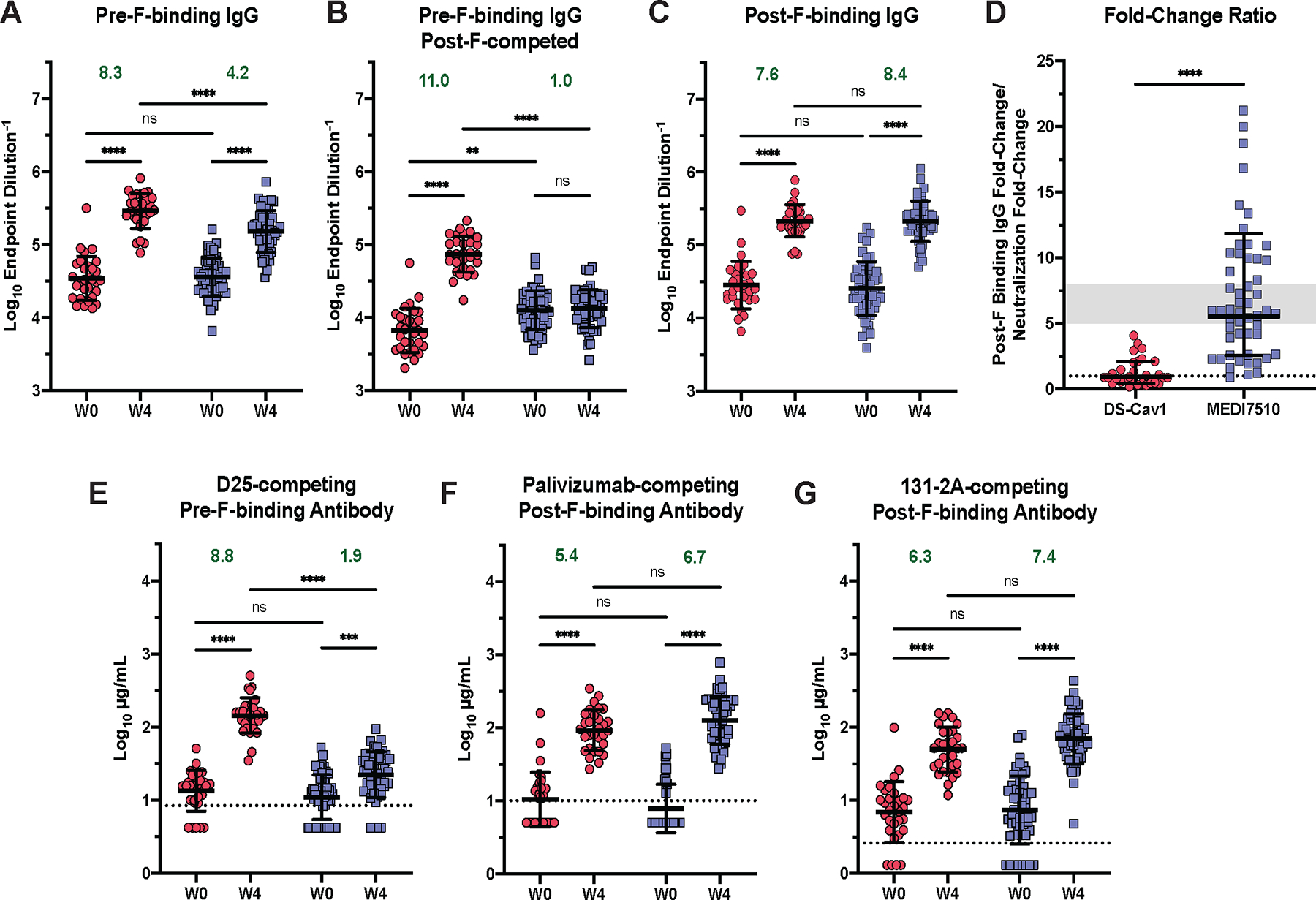
(A) W0 and W4 pre-F binding IgG endpoint titers are shown for serum samples from participants after vaccination with DS-Cav1 (pre-F, N = 30) or MEDI7510 (post-F, N = 52). (B) Pre-F binding IgG endpoint titers are shown as in (A), except excess post-F was used to adsorb post-F-exclusive and dual-binding antibodies from serum to leave pre-F-exclusive binding antibodies. W0 and W4 (C) post-F binding IgG endpoint titers are shown. (D) The fold-change ratio of post-F binding to neutralization is shown for each participant. Fold-change ratio = (W4/W0 fold-change, post-F IgG binding)/(W4/W0 fold-change, RSV A neutralization). A ratio below 1 indicates the increase in neutralization capacity exceeded the increase in binding capacity, demonstrating the elicitation of highly potent neutralizing antibodies. The gray area represents the historical range of this ratio observed in previous clinical trials of post-F subunit vaccines (fold-change ratio = 5 to 8). (E to G) Concentrations of (E) pre-F-binding D25-competing antibodies (DCA), (F) post-F-binding palivizumab-competing antibodies (PCA), and (G) post-F-binding 131–2A-competing antibodies (1CA) in serum are shown for samples collected before (W0) or 4 weeks after (W4) vaccination with DS-Cav1 or MEDI7510. Bars represent mean ± SD of log10 data. Dotted lines indicate the lower limit of quantitation (LLOQ) for (E to G), with undetectable values arbitrarily set to ½ LLOQ. Fold-change and statistics were calculated using values above the LLOQ. For all graphs, asterisks denote the P-value, with ****P < 0.0001, ***P = 0.0001 to 0.001, **P = 0.001 to 0.01, *P = 0.01 to 0.05, and ns = not significant as determined using Brown-Forsythe and Welch ANOVA tests (A to C, G), Mann-Whitney tests (D), or Kruskal-Wallis tests (E and F). Red circles denote participants who received DS-Cav1, blue squares denote participants who received MEDI7510. Geometric mean W4/W0 fold-change is shown in green text.
To evaluate the average potency of an F antibody elicited by DS-Cav1 or MEDI7510 vaccination, the fold-change ratio, defined as the W4/W0 fold-change in post-F IgG binding divided by the W4/W0 fold-change in RSV A neutralization, was calculated for all participants (Fig. 2D). A ratio below 1 denotes the average potency of an F antibody elicited by vaccination is high, due to the increase in neutralization surpassing the increase in binding. A ratio above 1 denotes a lower average potency (30). Within the investigatory subset of participants, 17 of 30 DS-Cav1 vaccinated participants had a ratio below 1, compared to only 1 of 52 MEDI7510 participants. The mean fold-change ratio was 0.95 for DS-Cav1 vaccinated participants, demonstrating robust elicitation of potent, neutralizing antibodies with DS-Cav1 vaccination. Contrastingly, the mean fold-change ratio in MEDI7510 vaccinated individuals was 5.5. This is within the historical range for post-F or structurally undefined F subunit vaccines, where the elicitation of binding antibodies with low neutralization potency surpassed the elicitation of highly neutralizing antibodies and fall within a fold-change ratio range of 5 to 10 (39, 40).
Pre-F vaccination elicits greater magnitude of apex-binding antibodies and similar amounts of side-binding antibodies compared to post-F vaccination.
To further dissect the site-specificity of the serologic response, the concentration of antibodies in serum competing with D25 (site Ø) for pre-F binding, palivizumab (site II) for post-F binding, or 131–2A (site I) for post-F binding was evaluated (Fig. 2E to G, fig. S1D to F). D25-competing antibodies (DCA) and palivizumab-competing antibodies (PCA) can be more broadly classified as apex- or side-binding antibodies since antibodies targeting adjacent antigenic sites confound competition assay readouts (41). 131–2A, a canonical non-neutralizing, post-F-preferring site I antibody, was used to develop an assay for the quantification of antibodies that compete with 131–2A for binding to site I and its adjacent sites on post-F (1CA).
DS-Cav1 vaccination elicited an 8.8-fold (P < 0.0001) increase in DCA (apex-binding antibodies), whereas MEDI7510 vaccination elicited a 1.9-fold (P = 0.0002) increase between W0 and W4. These results are expected, given the absence of apex epitopes (site Ø and site V) on post-F (Fig. 2E). The minor increase in DCA in the MEDI7510 individuals is likely attributable to antibodies binding to adjacent antigenic sites, such as site II, which is present on both pre-F and post-F (42, 43). Both DS-Cav1 and MEDI7510 vaccination resulted in similar concentrations of PCA at W4, however PCA was undetectable in a majority of participants at W0 for both vaccination regimens (Fig. 2F). Individuals above the limit of detection had a 5.4-fold (P < 0.0001) and 6.7-fold (P < 0.0001) increase in PCA for DS-Cav1 and MEDI7510 vaccinated participants, respectively, demonstrating that both vaccine antigens were able to elicit similar amounts of antibodies binding to shared site II epitopes (Fig. 2F). Additionally, DS-Cav1 and MEDI7510 vaccination elicited a 6.2-fold (P < 0.0001) and 7.4-fold (P < 0.0001) increase in 1CA, respectively. The increase in 1CA antibodies with DS-Cav1 vaccination was unexpected. Antigenic site I is slightly reconfigured on the pre-F conformation, making it unlikely to elicit large quantities of site I-directed antibodies. Further examination using a panel of 39 site-specific monoclonal antibodies demonstrated that site III and IV antibodies were able to compete with 131–2A, demonstrating that antibodies binding adjacent sites can contribute to the rise in 1CA after vaccination (fig. S2) (44). Overall, DS-Cav1 vaccination elicited robust apex- and side-binding antibodies. MEDI7510 vaccination elicited antibodies targeting side epitopes, and higher titers of antibodies that competed with 131–2A. A majority of site I antibodies are poorly neutralizing, which provides a possible explanation for the low neutralizing activity in MEDI7510-vaccinated participants. The distinct serological profiles elicited from both vaccine antigens directly translate to the superior potency of neutralizing antibodies elicited from pre-F vaccination.
Pre-F and post-F vaccination elicit different specificities of memory B cells.
Next, we evaluated the memory B cell populations elicited by vaccination with DS-Cav1 or MEDI7510. Both DS-Cav1 and MEDI7510 vaccination robustly mobilized plasmablasts at W1 post-vaccination, with a 5.2-fold (P < 0.0001) increase at W1 compared to W0 and at similar frequencies (fig. S3). To investigate the antigen specificity and activation status of F-specific memory B cells, fluorescently labeled pre- and post-F probes were used to compare the vaccine-elicited memory B cell response in DS-Cav1- and MEDI7510-vaccinated participants using a 17-color flow cytometry panel (45). Cells were gated on CD19+/CD38− and CD20+/IgD− to identify memory B cells. Then the pre- and post-F probes were used to further delineate class-switched IgG+ and IgA+ CD27+ memory B cells into three compartments: pre-F-preferring, dual-binding, or post-F-preferring (Fig. 3A and B). Before vaccination, all individuals had low but detectable frequencies of F-specific memory B cells. At W1 post-vaccination, DS-Cav1 elicited an enrichment of pre-F-preferring and dual-binding B cells, whereas MEDI7510 resulted in an enrichment of post-F-preferring and dual-binding IgG+ and IgA+ B cells (Fig. 3C and D). There was also an increase in the frequency of post-F-preferring B cells following DS-Cav1 vaccination, likely due to imprecision in gating between cells that only bind post-F and cells with a strong preference for post-F that can also weakly bind pre-F (Fig. 3A and B). Although there were significantly higher (P < 0.0001) frequencies of pre-F-preferring IgG+ B cells at W1 after DS-Cav1 vaccination compared to MEDI7510 vaccination, both vaccines elicited similar frequencies of dual-binding B cells (Fig. 3C). Similar patterns were observed in IgA+ B cells (Fig. 3D). These data demonstrate that DS-Cav1 and MEDI7510 vaccination elicited distinct patterns of B cell specificities, mirroring the response measured in the serum.
Figure 3. Pre-F vaccination elicits pre-F-preferring and dual-binding memory B cells.
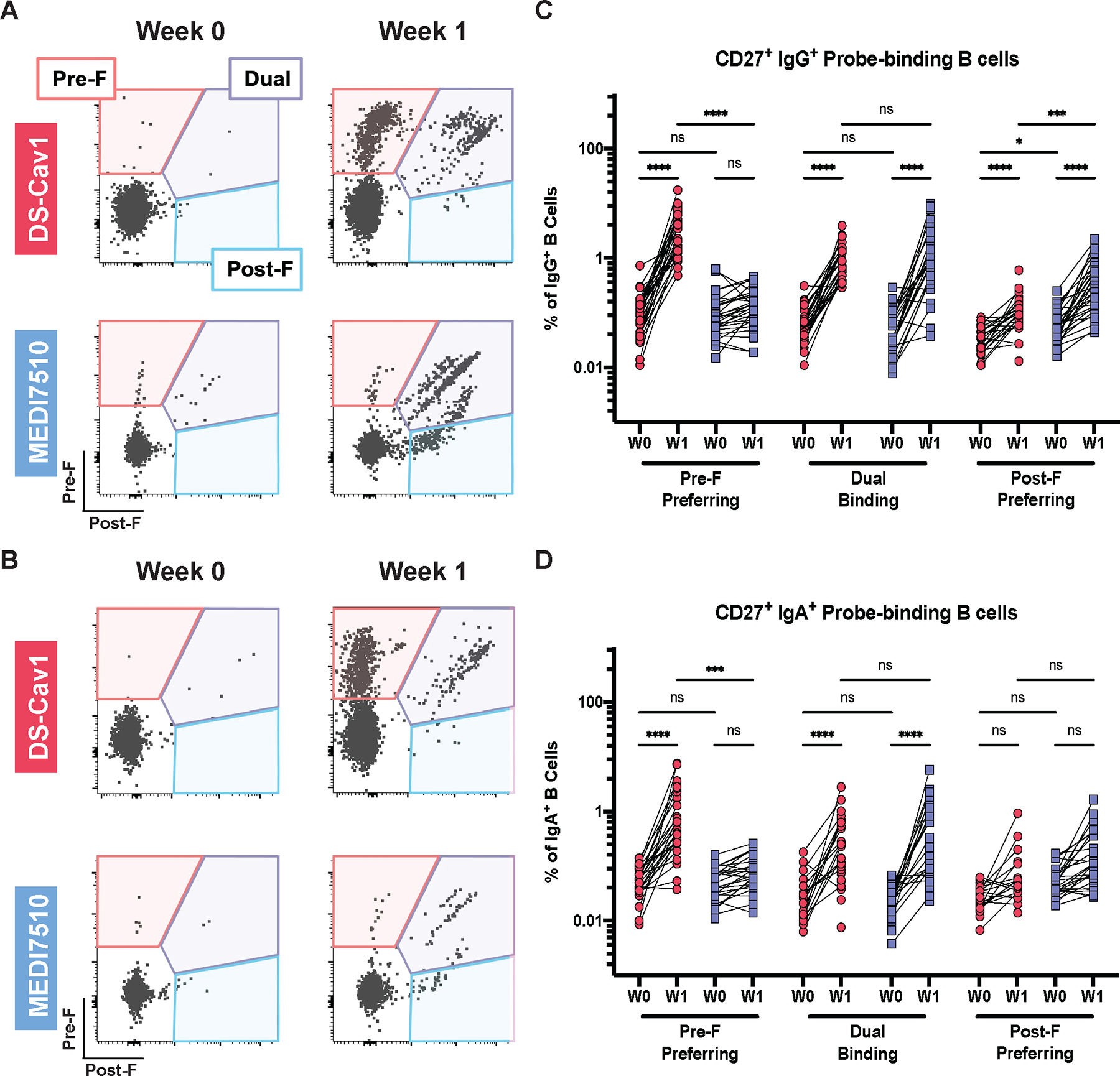
Representative pre-F and post-F probe-binding profiles from a single participant vaccinated with DS-Cav1 (pre-F, N = 30) or MEDI7510 (post-F, N = 30) at W0 and W1 are shown. Fluorescently labeled pre-F or post-F probes were used to separate class-switched memory B cells into three compartments (pre-F-preferring, dual-binding, or post-F-preferring). IgG+ (A) representative binding profile and (B) quantification of probe-binding B cells are shown. IgA+ (C) representative binding profile and (D) quantification of probe-binding B cells are shown. For all graphs, asterisks denote the P-value, with ****P < 0.0001, ***P = 0.0001 to 0.001, **P = 0.001 to 0.01, *P = 0.01 to 0.05, and ns = not significant as determined using Brown-Forsythe and Welch ANOVA tests (B) or Kruskal-Wallis tests (D). Red circles denote participants who received DS-Cav1, blue squares denote participants who received MEDI7510.
Activated memory B cells (ABCs) can differentiate into long-lived plasma cells, antibody secreting cells, or travel to the germinal center for additional rounds of affinity maturation (46–48). To determine whether DS-Cav1 or MEDI7510 vaccination activated F-specific memory B cells, the probe-binding cells were further analyzed for downregulation of CD21, a common surface marker used to identify ABCs, and the expression of CD71, a surface marker for proliferating B cells (46–48). Prior to DS-Cav1 or MEDI7510 vaccination, 65 to 88% of probe-binding IgG+ B cells were predominantly CD21+CD27+ across all F-specificities, indicating a resting memory phenotype (Fig. 4A and B). These phenotypes and probe-binding profiles were observed in the IgA+ B cell compartment as well (Fig. 4C and D). One week after DS-Cav1 vaccination, 94.9% of pre-F-preferring and 93.3% of dual-binding IgG+ B cells had diminished surface expression of CD21 and increased CD71 expression, with activation of 81.9% post-F-preferring IgG+ B cells (Fig. 4B). Similarly, DS-Cav1 activated 91.2% of pre-F-preferring, 91.1% of dual-binding, and 61.2% of post-F-preferring IgA+ B cells at W1 (Fig. 4D). In contrast, predominantly dual-binding and post-F-preferring IgG+ B cells were activated following MEDI7510 vaccination, 88.1% and 81.9% respectively, with just 24.7% of pre-F-preferring B cells exhibiting an activated phenotype (Fig. 4B). Activated phenotypes were observed in 88.9% of dual-binding, 65.9% of post-F-preferring, and 43.3% of pre-F-preferring IgA+ B cells at W1 following MEDI7510 vaccination (Fig. 4D). Collectively, these results demonstrate that DS-Cav1 vaccination promoted the activation and proliferation of memory B cells that target the most neutralization-sensitive sites, further emphasizing the importance of F conformation in RSV vaccine design and supporting the production of a pre-F vaccine.
Figure 4. Activation phenotypes of memory B cells following pre-F or post-F vaccination parallels F probe-binding profiles.
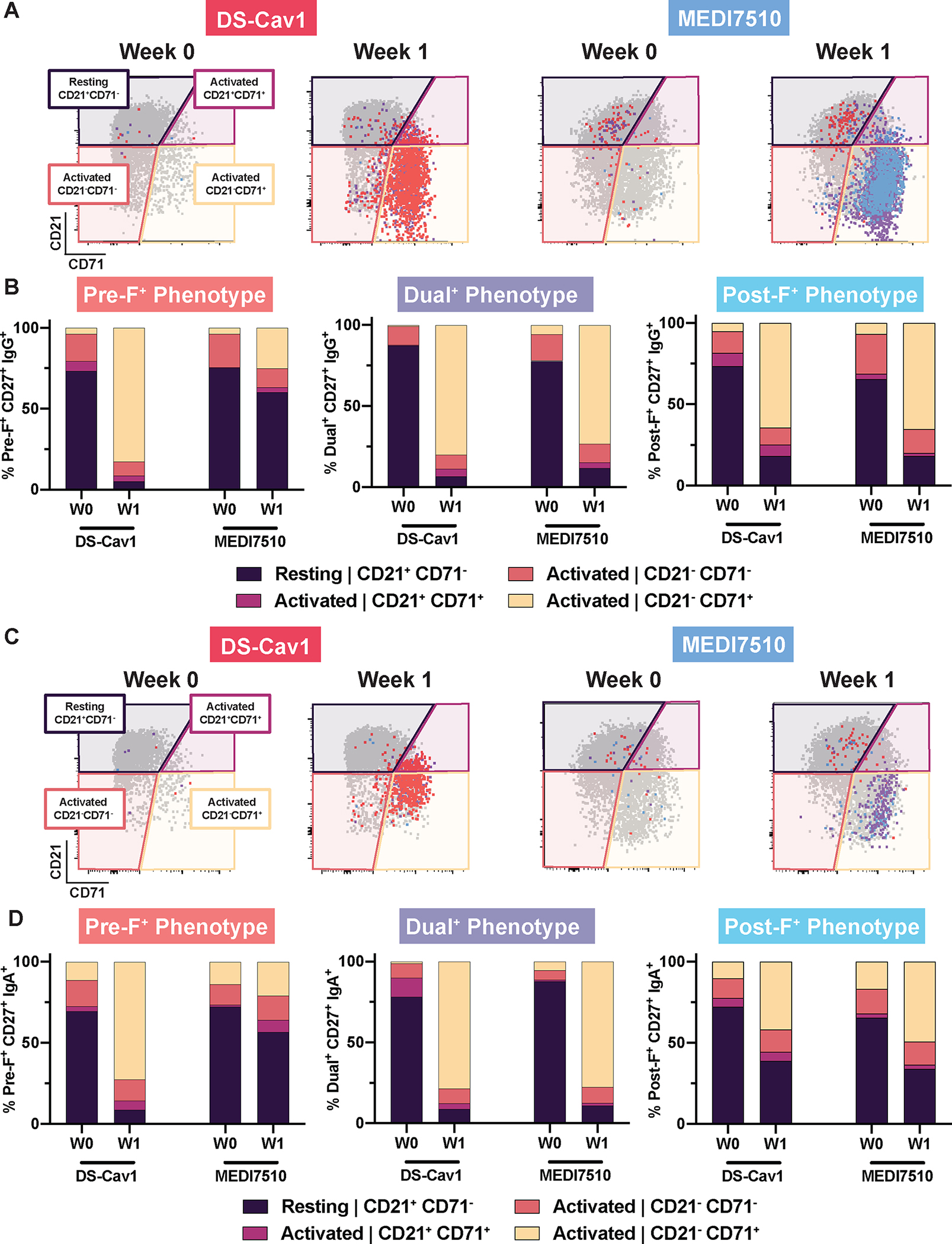
Representative pre- and post-F probe-binding profiles are shown for a single participant vaccinated with DS-Cav1 (pre-F, N = 30) or MEDI7510 (post-F, N = 30) at W0 and W1. CD27+ B cells were delineated based on expression of surface markers CD27 and CD21 as activated (CD21+CD71+, CD21−CD71−, CD21−CD71+) or resting (CD21+CD71−). F probe-binding B cells were overlaid for each compartment. IgG+ (A) representative activation profiles and (B) activation phenotype by F probe-binding specificity are shown. IgA+ (C) representative activation profiles and (D) activation phenotype by F probe-binding specificity are shown.
DISCUSSION
Interrogating the differences in antibody and B cell specificity elicited from vaccination with a pre-F vaccine compared to a post-F vaccine in humans highlights the importance of F conformation in RSV vaccine development. In this study, we demonstrated several conformation-dependent differences in the serological and B cell probe-binding profiles of participants vaccinated with one of two RSV F vaccine candidates: DS-Cav1 (pre-F) or MEDI7510 (post-F). Both vaccines elicit antibodies, induce plasmablasts at similar frequencies, and reactivate memory B cells specific to the conformation of the vaccine as well as to both conformations of F through shared epitopes. Our data demonstrate that DS-Cav1 vaccination elicited a more favorable serological profile and B cell phenotype due to the presence of highly neutralization-sensitive sites Ø and V in the vaccine, sites that are exclusively found on pre-F.
Post-F competition studies evaluating conformation-specific binding and neutralization confirmed that MEDI7510 vaccination elicited antibodies to antigenic sites shared by pre- and post-F. Pre-F-competed neutralization was not performed since previous investigation demonstrated pre-F adsorption abrogated greater than 90% of neutralizing activity in serum (12). Furthermore, through monoclonal antibody competition assays, we observed a higher magnitude of post-F binding antibodies that competed with palivizumab and 131–2A for binding to sites I and II, respectively. In contrast, DS-Cav1 vaccination elicited a greater increase in binding and neutralizing antibodies that target shared and pre-F-exclusive sites, and more antibodies that competed with D25 (sites Ø and V) than did MEDI7510 vaccination. These discrepancies likely contributed to the observed differences in vaccine-elicited antibody potency: MEDI7510-vaccinated participants had a greater increase in titers of binding antibodies compared to titers of neutralizing antibodies and thus had a lower average neutralizing potency compared to DS-Cav1-vaccinated participants.
We observed an increase in DCA for MEDI7510-vaccinated participants and in 1CA for DS-Cav1-vaccinated individuals. As demonstrated by Phung et al. and in fig. S2, although competition assays provide some degree of antigenic site-specificity, antibody binding to adjacent sites can confound readings due to the size of antibodies relative to the RSV F protein (41). This is especially relevant in polyclonal serum responses, since antibodies to all sites are elicited upon F vaccination or infection (11, 25, 31, 44). As such, we speculate that the modest increase in pre-F-binding DCA in MEDI7510-vaccinated participants is in part due to the robust elicitation of side-binding antibodies, with some binding more proximally to the apex region and competing with D25, and not through bona fide elicitation of antibodies to site Ø and V as seen after pre-F vaccination (42, 43). This phenomena has been documented before - binding of CR9501 (site V) is substantially reduced when motavizumab (site II) is bound to pre-F first, providing evidence that side-binding antibodies can sterically interfere with the binding of apex-directed antibodies (49). Similarly, the increase in post-F-binding 1CA we observed in DS-Cav1-vaccinated participants may be attributed to binding of antibodies to the adjacent sites III and IV, which contributed to the 1CA readout during our assessment with a panel of 39 site-specific monoclonal antibodies. Given that antibodies to sites III and IV have the capacity to be potently neutralizing, the 1CA readout therefore should not be interpreted as a measure of non-functional, non-neutralizing antibody, although it captures predominantly site I antibodies (25, 44, 50).
Probe-binding profiles of class-switched IgG+ and IgA+ B cells closely mirrored the observed serological profiles. DS-Cav1 vaccination activated pre-F-preferring and dual-binding B cells, whereas MEDI7510 vaccination activated post-F-preferring and dual-binding B cells. The activation of pre-F-preferring memory B cell populations by DS-Cav1 vaccination demonstrates the ability of a pre-F vaccine to recruit memory B cells specific to pre-F-exclusive sites that are not induced by a post-F immunogen, which likely contribute to the potent neutralizing activity observed in the serum of DS-Cav1 vaccinated participants. The differences in B cell specificities elicited from DS-Cav1 and MEDI7510 vaccination further highlights the importance of using pre-F for RSV vaccination.
Our study has numerous strengths. Whereas previous studies comparing the two major conformations of F as a vaccine immunogen were done in animals, our study evaluates the conformation-dependent humoral response in human samples with matched timepoints. Further, although samples were obtained from three independent clinical trials, the same methodology was applied to all sets of clinical samples, decreasing variability across clinical trials and allowing for direct in vitro comparison of pre- versus post-F vaccine responses.
The limitations of our study must also be taken into consideration, such as disparities in participant demographics and vaccine formulation. DS-Cav1 was evaluated in healthy adults 18 to 50 years of age, whereas MEDI7510 was evaluated in older adults 60 years of age and above. Immunosenescence and the increased frequency of comorbidities with age can affect the magnitude of vaccine-elicited responses (1, 51, 52). Nevertheless, the fold-increase in neutralization and binding titers for MEDI7510 participants remained consistent with other post-F vaccine trials conducted in healthy adults, and the conformational specificity of antibodies or B cells was not impacted by participant age in our own studies (38, 39). Furthermore, it has been shown that vaccination with RSV pre-F elicits a similarly vigorous neutralizing response in participants 50 to 85 years of age as in individuals between the ages of 18 and 49, supporting the idea that the disparity in the response magnitude between the pre-F and post-F vaccine trials used in our study is tied to F conformation rather than participant age (53, 54). Another major difference that may affect our direct comparison of the two vaccines is the formulation. For both serological and B cell studies, dosage was approximately matched but adjuvant was not. DS-Cav1 was administered with or without alum, whereas MEDI7510 contains GLA-SE, a Th1 biasing agent. As demonstrated by Ruckwardt et al. for DS-Cav1, there was no adjuvant effect whereas Falloon et al. showed some effect for inclusion of adjuvant for MEDI7510 in an older adult population (32, 37). However, a recent phase 1/2 study of a pre-F subunit vaccine demonstrated that neither alum nor CpG/alum adjuvant enhanced neutralizing responses in older adults compared to protein alone (53). Therefore, adjuvant differences are unlikely to drive the qualitative differences in the serological and probe-binding profiles of participants. Additional studies addressing a possible impact of adjuvants on the specificity and function of the humoral response to RSV or other pathogens are warranted. Finally, we did not examine T cell responses in this study. Both pre-F and post-F immunization have been shown to elicit F-specific T helper 1 (Th1) CD4+ T cell responses and as anticipated, vaccination with DS-Cav1 did not increase the frequency of F-specific CD8+ T cells above baseline (30, 36, 37). Evaluation of F-specific follicular helper T cell responses (Tfh) might offer additional insight into how germinal center formation and maintenance may be facilitated by this specialized subset of antigen-experienced CD4+ T cells (55).
The main objective of an RSV vaccine is prevention of disease caused by RSV, which is thought to require generation of a high and durable neutralizing antibody response and activation of antigen-specific memory B cells. By comparing and contrasting the humoral response to DS-Cav1 and MEDI7510, our findings collectively suggest that pre-F vaccination is more advantageous than post-F vaccination. Neutralizing activity is likely a correlate of protection against RSV infection, and DS-Cav1 vaccination elicited a five-fold greater increase in neutralizing antibodies than MEDI7510 at 4 weeks post-vaccination. Most of this increase can be attributed to antibodies directed to the neutralization-sensitive, pre-F-exclusive sites Ø and V. These responses are closely mirrored in the memory B cell phenotype, demonstrating that DS-Cav1 vaccination elicited activated memory B cells that recognize pre-F-exclusive and shared sites. In contrast, MEDI7510 vaccination recruited and activated memory B cells that recognize shared and post-F-exclusive antigenic sites. Whether or not the high average potency of F-specific antibody elicited by pre-F vaccination will translate to protection will be determined by phase 2 and 3 trials of pre-F-based vaccines evaluating efficacy. In summary, these data demonstrate a comparison of humoral and B cell responses between pre-F and post-F vaccination in humans, emphasizing the importance of RSV F conformation in vaccine design. These data indicate that using pre-F as the vaccine antigen can drive the production of potent, neutralizing antibodies in the serum and generate a favorable memory B cell phenotype that may contribute to long-lasting immunity.
MATERIALS AND METHODS
Study design
Clinical study design, vaccines, and study procedures for VRC 317 (NCT03049488), MedImmune D4420C00005 (NCT02508194), and MedImmune D4420C00004 (NCT02289820) are as previously described (30, 35, 37). Full primary clinical trial results have been reported for all three trials. The studies detailed herein were to address post-hoc exploratory endpoints, namely, to compare RSV F-specific serological and B cell responses between a pre- and post-F vaccine.
Human serum samples and peripheral blood mononuclear cell samples
Peripheral blood mononuclear cells (PBMC) and serum samples from 30 participants who received the 150 μg dose of DS-Cav1 with and without alum adjuvant in VRC 317 were used for this study to align with the 120 μg dosage of MEDI7510 in MedImmune D4420C00005 and MedImmune D4420C00004, respectively. A subset of serum from MedImmune D4420C00005 and PBMC from MedImmune D4420C00004 were provided to the Vaccine Research Center (National Institutes of Health) by AstraZeneca for analyses. For the scope of this study, pre-vaccination (week 0, W0) and 1 week post-vaccination (week 1, W1), or pre-vaccination and 4 weeks post-vaccination (week 4, W4) time points were used for B cell or serological analyses, respectively.
Pre-F and post-F proteins for serological assays and flow cytometry probes
Pre-F (DS-Cav1) and post-F (RSV FΔFP) proteins used in all serological assays were produced using transient transfection of previously characterized constructs, containing a C-terminal 6xHis and Strep-tag, in Expi293T cells, as described previously (30). In brief, proteins were purified from cell supernatant by affinity chromatography through Ni-nitrilotriacetic acid (NTA) resin (GE) then Strep-Tactin resin (IBA Biosciences) to eliminate proteins without the C-terminal tags, followed by a fast protein liquid chromatography Superose column (Cytiva) for further purification and selection of trimers. Proteins used as B cell capture probes in flow cytometry had Avi and 6xHis tags and were purified using only NTA resin.
RSV neutralization and competition neutralization assays
Neutralization capacity of serum collected from all participants before vaccination and at 4 weeks after vaccination was determined using a fluorescence plate reader-based neutralization assay run on an automation system as previously described for both RSV A and B subtypes (32). Serum samples were initially diluted 1:10 then serially diluted 1:4 for a total of 8 dilutions and incubated with an equal volume of recombinant mKate-RSV for 1 hour at 37 °C before addition of the serum-virus mixture to NCI-H28 human mesothelioma cells (CRL-5820, the American Type Culture Collection) seeded in a 384-well black optical bottom plate. The plates were spectrophotometrically analyzed at 588 nm excitation and 635 nm emission at 24 hours after addition of serum-virus mixture to cells. To assess pre-F-exclusive neutralization, serum was diluted in excess post-F to adsorb dual-specific antibodies before incubation with the virus. The reciprocal half-maximal inhibitory concentration (IC50) dilutions were standardized to the 1st International Standard for Antiserum to Respiratory Syncytial Virus (IS) (NIBSC code: 16/284). Technical duplicates were performed for each sample.
RSV F binding and post-F competed enzyme-linked immunosorbent assays (ELISAs)
Pre-F binding, post-F-competed pre-F binding, and post-F IgG binding ELISAs were run on serum collected from all participants at W0 and W4 after vaccination as previously described (30). 96-well plates were coated with pre- or post-F protein in phosphate-buffered saline (PBS) and incubated overnight at 4 °C, then washed three times using 1X PBS with 0.05% Tween 20 (PBS-T) and blocked with 5% milk in PBS for 1 hour at room temperature. Serum samples were initially diluted 1:400 in 5% milk in PBS then serially diluted 1:4 for a total of 7 dilutions and added to protein-coated plates and incubated for 2 hours at room temperature. To assess pre-F-exclusive IgG binding in the post-F-competed pre-F binding ELISA, excess post-F was added to diluted serum to adsorb dual-specific antibodies as detailed previously before addition to protein-coated plates. Plates were incubated with a secondary antibody (SouthernBiotech, goat anti-human IgG-horseradish peroxidase) diluted in 5% milk in PBS for 1 hour at room temperature. To develop, plates were incubated with KPL SureBlue (SeraCare) for 7 minutes followed by an equivolume addition of sulfuric acid (0.501M) to stop the reaction (Fluka), then read at 450 nm and 650 nm. Technical duplicates were performed for each sample.
Monoclonal antibody competition ELISAs
D25-competed pre-F binding and palivizumab-competed post-F binding ELISAs were run on all serum samples using the previously described conditions (30, 32, 41). Briefly, 96-well plates were coated, blocked, and washed as described for the RSV F binding ELISAs. Serum samples were initially diluted 1:5 then serially diluted 1:2 for a total of 11 dilutions, then added to protein-coated plates and incubated at room temperature for 15 minutes. The analogous antibody (unbiotinylated) was used to generate a standard curve for each assay. An equivolume amount of biotinylated antibody was added on top of the diluted serum in plates for a total volume of 100 μL and incubated at room temperature for 1 hour. Plates were incubated with Streptavidin conjugated to horseradish peroxidase diluted in 5% milk in PBS for 1 hour at room temperature, then developed and read as described for the RSV F binding assays. For the 131–2A-competed post-F binding ELISA, 131–2A was biotinylated using the EZ-Link Sulfo-NHS-LC-Biotinylation Kit (Thermo Fisher Scientific, Cat. No. 21435) according to the manufacturer’s instructions. Experimental procedures for the 131–2A-competed post-F binding ELISA are as described for the D25- and palivizumab-competing monoclonal antibody competition ELISAs, but with the following modifications in coating, biotinylated antibody, and unbiotinylated antibody concentrations, respectively: 20 ng/mL of post-F in PBS, 20 ng/mL of biotinylated 131–2A in blocking buffer (5% skim milk in PBS), and 25 μg/mL of 131–2A in blocking buffer. Technical duplicates were performed for each sample.
Generation of RSV pre-F and post-F probes for flow cytometry and B cell phenotyping
Avi-tagged pre-F and post-F proteins for the identification of F-specific B cells were biotinylated using a Bir-A biotinylation it (Avidity). The proteins were then conjugated to allophycocyanin (APC)-labeled streptavidin and brilliant violet (BV) 421-labeled streptavidin, respectively, to generate fluorescent RSV F probes for flow cytometry as previously described (30–32, 45). Frozen human PBMC samples from VRC 317 and MedImmune Study D4420C00004 collected at the pre-vaccination and 1 week post-vaccination timepoints were thawed in R10 (RPMI-1640 containing 10% fetal bovine serum, 100 U/mL of penicillin/streptomycin, and 1X GlutaMAX; all from Gibco) media and immediately stained for flow cytometry using a previously described B cell phenotyping panel (30, 45). Samples were run on a Symphony A5 flow cytometer (BD Biosciences) and data were analyzed using FlowJo version 10.7. The gating strategy is as described previously (45). One sample per timepoint per participant was assessed.
Statistical analysis
Raw, individual-level data for palivizumab competition ELISAs are presented in data file S1. All data were analyzed using GraphPad Prism version 9.4.1. Simultaneous statistical comparisons within and across both groups pre- and post-vaccination were performed using one-way analysis of variance (ANOVA). Comparisons that passed the Anderson-Darling normality test were assessed by Brown-Forsythe and Welch ANOVA tests with Dunnett T3 correction for multiple comparisons. Comparisons that did not pass the Anderson-Darling normality test were assessed by non-parametric Kruskal-Wallis tests with Dunn’s correction for multiple comparisons. Comparisons of two groups were assessed by unpaired t-test for comparisons that passed the Anderson-Darling normality test or by Mann-Whitney test for comparisons that included non-parametric populations. Pearson correlation analysis and simple linear regression were used for figures with correlation plots. Details of statistical tests applied are described in the figure legends.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Azad Kumar for RSV pre-F and post-F protein production, as well as the VRC 317 Study Team, VRC Clinical Trials Program, and AstraZeneca for providing clinical samples.
Funding:
This work and NCT03049488 were supported by intramural funding from the National Institute of Allergy and Infectious Diseases: 1ZIAAI005129-06 (to B.S.G.) and 1ZIAAI0003782-39 (to B.S.G.). NCT02508194 and NCT02289820 were supported by AstraZeneca.
Footnotes
Competing interests: B.S.G. is on the scientific advisory board for Icosavax, Greenlight Biosciences, Vaccine Company, Inc, Foundry, and Afrigen WHO mRNA Biohub and is an ad hoc consultant for GSK, Pfizer, Janssen, AstraZeneca, Merck, and Sanofi. He is an inventor on patents for the stabilization of the RSV F protein (WO2014160463A1, Prefusion RSV F proteins and their use). M.T.E., T.V., J.F., and F.D. were all employees at AstraZeneca when the MEDI7510 clinical trial was performed and may own stock in AstraZeneca. All other authors declare no competing interests.
Data and materials availability:
All data associated with this study are in the paper or supplementary materials. Reagents and materials associated with this study are available and may also be requested from the corresponding author (T.J.R.) after completion of a material transfer agreement.
REFERENCES AND NOTES
- 1.Aiello A et al. , Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 10, 2247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beem M, Wright FH, Hamre D, Egerer R, Oehme M, Association of the chimpanzee coryza agent with acute respiratory disease in children. N. Engl. J. Med. 263, 523–530 (1960). [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Simőes EAF, Anderson LJ, in Challenges and Opportunities for Respiratory Syncytial Virus Vaccines, Anderson LJ, Graham BS, Eds. (Springer Berlin Heidelberg, Berlin, Heidelberg, 2013), pp. 39–57. [Google Scholar]
- 4.Hall CB, The burgeoning burden of respiratory syncytial virus among children. Infect. Disord. Drug Targets 12, 92–97 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Shi T et al. , Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CB et al. , Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315, 77–81 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Falsey AR, Walsh EE, Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13, 371–384 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rima B et al. , ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 98, 2912–2913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glezen WP, Taber LH, Frank AL, Kasel JA, Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140, 543–546 (1986). [DOI] [PubMed] [Google Scholar]
- 10.Kim HW et al. , Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89, 422–434 (1969). [DOI] [PubMed] [Google Scholar]
- 11.Ruckwardt TJ, Morabito KM, Graham BS, Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 51, 429–442 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Ngwuta JO et al. , Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7, 309ra162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang B et al. , Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J. Virol. 89, 9499–9510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capella C et al. , Prefusion F, Postfusion F, G Antibodies, and Disease Severity in Infants and Young Children With Acute Respiratory Syncytial Virus Infection. J. Infect. Dis. 216, 1398–1406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koivisto K et al. , Respiratory Syncytial Virus (RSV)-Specific Antibodies in Pregnant Women and Subsequent Risk of RSV Hospitalization in Young Infants. J. Infect. Dis. 225, 1189–1196 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taleb SA et al. , Level of maternal respiratory syncytial virus (RSV) F antibodies in hospitalized children and correlates of protection. Int. J. Infect. Dis. 109, 56–62 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Buchwald AG et al. , Respiratory Syncytial Virus (RSV) Neutralizing Antibodies at Birth Predict Protection from RSV Illness in Infants in the First 3 Months of Life. Clin. Infect. Dis. 73, e4421–e4427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Walsh EE, Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J. Infect. Dis. 177, 463–466 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Mazur NI et al. , The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 18, e295–e311 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Mazur NI et al. , Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect. Dis, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart-Jones GBE et al. , A Cysteine Zipper Stabilizes a Pre-Fusion F Glycoprotein Vaccine for Respiratory Syncytial Virus. PLoS One 10, e0128779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyington JC et al. , Structure-Based Design of Head-Only Fusion Glycoprotein Immunogens for Respiratory Syncytial Virus. PLoS One 11, e0159709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce MG et al. , Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat. Struct. Mol. Biol. 23, 811–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang B et al. , Improved Prefusion Stability, Optimized Codon Usage, and Augmented Virion Packaging Enhance the Immunogenicity of Respiratory Syncytial Virus Fusion Protein in a Vectored-Vaccine Candidate. J. Virol. 91, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin E et al. , Infants Infected with Respiratory Syncytial Virus Generate Potent Neutralizing Antibodies that Lack Somatic Hypermutation. Immunity 48, 339–349.e335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLellan JS et al. , Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin MP et al. , Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 383, 415–425 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Hammitt LL et al. , Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 386, 837–846 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Sastry M et al. , Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One 12, e0186854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crank MC et al. , A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365, 505–509 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Mukhamedova M et al. , Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses. Immunity 54, 769–780.e766 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruckwardt TJ et al. , Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir Med 9, 1111–1120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert SL et al. , A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models. PLoS One 10, e0119509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton K et al. , Enhanced immunogenicity of a respiratory syncytial virus (RSV) F subunit vaccine formulated with the adjuvant GLA-SE in cynomolgus macaques. Vaccine 33, 4472–4478 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Falloon J et al. , A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 34, 2847–2854 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Falloon J et al. , Dose Selection for an Adjuvanted Respiratory Syncytial Virus F Protein Vaccine for Older Adults Based on Humoral and Cellular Immune Responses. Clin. Vaccine Immunol. 24, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falloon J et al. , An Adjuvanted, Postfusion F Protein–Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J. Infect. Dis. 216, 1362–1370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg A et al. , Antibody and B cell responses to an investigational adjuvanted RSV vaccine for older adults. Hum. Vaccin. Immunother. 15, 2466–2474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leroux-Roels G et al. , Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 37, 2694–2703 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Madhi SA et al. , Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 383, 426–439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phung E et al. , Epitope-Specific Serological Assays for RSV: Conformation Matters. Vaccines (Basel) 7, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mousa JJ et al. , Structural basis for nonneutralizing antibody competition at antigenic site II of the respiratory syncytial virus fusion protein. Proc. Natl. Acad. Sci. U. S. A. 113, E6849–E6858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mousa JJ, Kose N, Matta P, Gilchuk P, Crowe JE Jr., A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein. Nat Microbiol 2, 16271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilman MSA et al. , Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phung E et al. , Elicitation of pneumovirus-specific B cell responses by a prefusion-stabilized respiratory syncytial virus F subunit vaccine. Sci. Transl. Med. 14, eabo5032 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palm A-KE, Henry C, Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 10, 1787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellebedy AH et al. , Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 17, 1226–1234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau D et al. , Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Science Immunology 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilman MSA et al. , Transient opening of trimeric prefusion RSV F proteins. Nat. Commun. 10, 2105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang A et al. , A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 10, 4153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh EE, Peterson DR, Falsey AR, Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 189, 233–238 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Mehta J, Walsh EE, Mahadevia PJ, Falsey AR, Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD 10, 293–299 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Baber J et al. , A Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine With and Without Adjuvant in Healthy Older Adults. J. Infect. Dis, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falsey Walsh, Scott, others, Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults With Concomitant …. The Journal of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juno JA, Hill DL, T follicular helper cells and their impact on humoral responses during pathogen and vaccine challenge. Curr. Opin. Immunol. 74, 112–117 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are in the paper or supplementary materials. Reagents and materials associated with this study are available and may also be requested from the corresponding author (T.J.R.) after completion of a material transfer agreement.


