Abstract
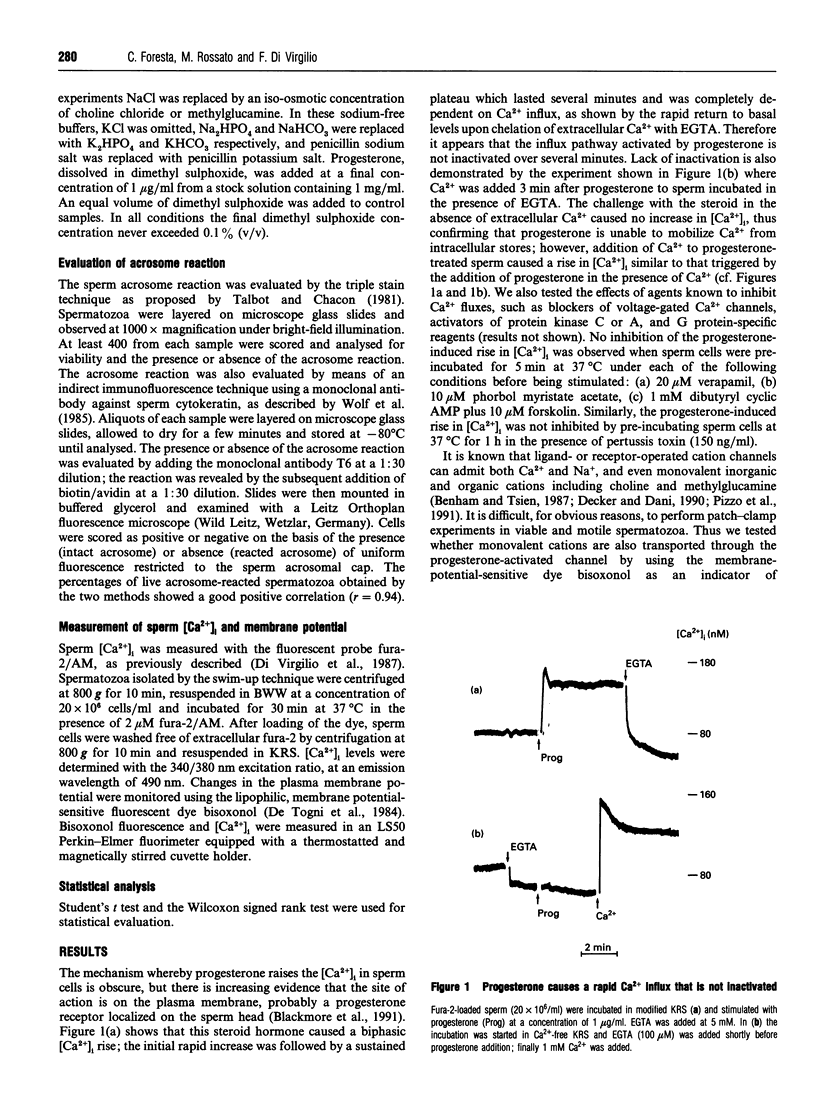
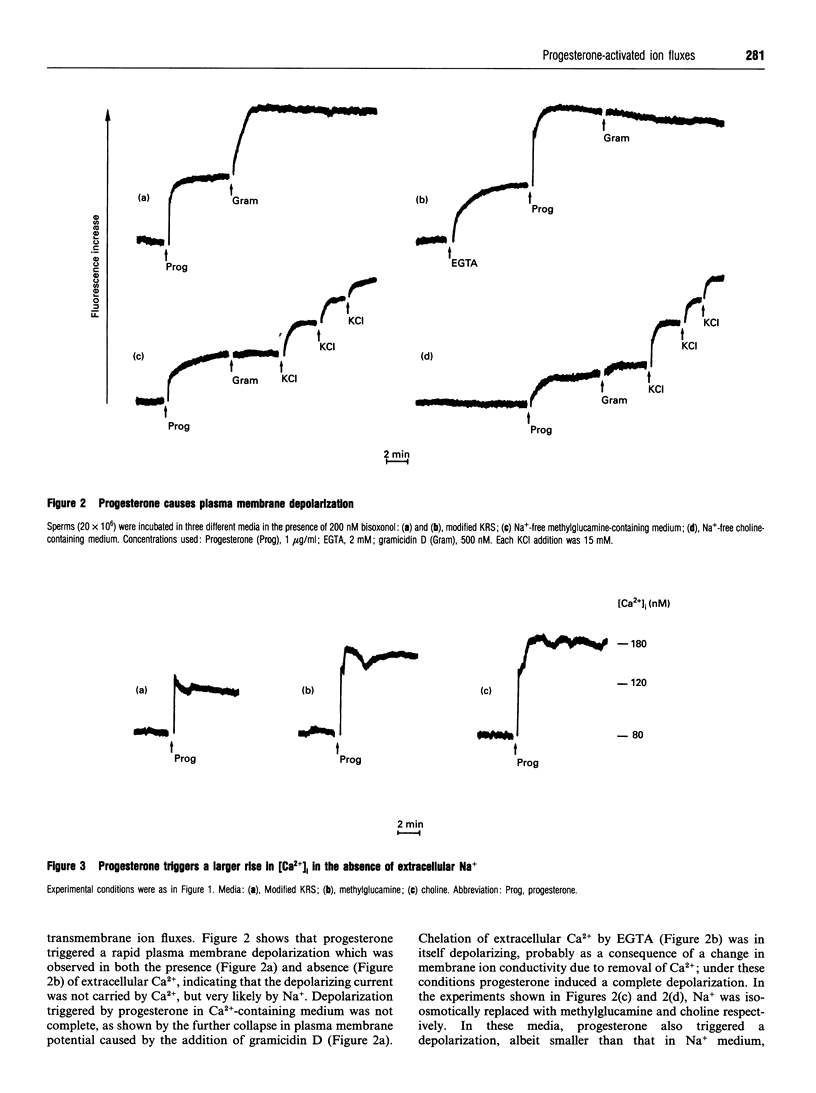
We have characterized ionic changes triggered by progesterone in human spermatozoa. This steroid, which is a fast-acting stimulator of the acrosome reaction, triggered a rapid increase in the cytoplasmic Ca2+ concentration ([Ca2+]i) which was entirely due to influx across the plasma membrane, as it was obliterated by chelation of extracellular Ca2+. Ca2+ fluxes were insensitive to verapamil and pertussis toxin, thus suggesting that they did not occur via voltage-gated channels and did not involve a pertussis toxin-sensitive G protein, and were potentiated in Na(+)-free, choline-containing or methylglucamine-containing medium. Progesterone also caused a depolarization of the plasma membrane in Na(+)-containing as well as in choline- or methyl-glucamine-containing saline; depolarization was larger in the absence of extracellular Ca2+, suggesting that Na+ and Ca2+ fluxes occurred through the same channel. Progesterone was able to trigger the acrosome reaction in the three media investigated (Na+, choline and methylglucamine), provided that extracellular Ca2+ was also present. We conclude that progesterone activates a membrane ion channel that is permeable to monovalent cations as well as to Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Beebe S. J., Danforth D. R., Alexander N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem. 1990 Jan 25;265(3):1376–1380. [PubMed] [Google Scholar]
- Blackmore P. F., Neulen J., Lattanzio F., Beebe S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991 Oct 5;266(28):18655–18659. [PubMed] [Google Scholar]
- De Togni P., Cabrini G., Di Virgilio F. Cyclic AMP inhibition of fMet-Leu-Phe-dependent metabolic responses in human neutrophils is not due to its effects on cytosolic Ca2+. Biochem J. 1984 Dec 1;224(2):629–635. doi: 10.1042/bj2240629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Milani D., Leon A., Meldolesi J., Pozzan T. Voltage-dependent activation and inactivation of calcium channels in PC12 cells. Correlation with neurotransmitter release. J Biol Chem. 1987 Jul 5;262(19):9189–9195. [PubMed] [Google Scholar]
- Duval D., Durant S., Homo-Delarche F. Non-genomic effects of steroids. Interactions of steroid molecules with membrane structures and functions. Biochim Biophys Acta. 1983 Aug 11;737(3-4):409–442. doi: 10.1016/0304-4157(83)90008-4. [DOI] [PubMed] [Google Scholar]
- Foresta C., Rossato M., Di Virgilio F. Extracellular ATP is a trigger for the acrosome reaction in human spermatozoa. J Biol Chem. 1992 Sep 25;267(27):19443–19447. [PubMed] [Google Scholar]
- Foresta C., Rossato M., Mioni R., Zorzi M. Progesterone induces capacitation in human spermatozoa. Andrologia. 1992 Jan-Feb;24(1):33–35. doi: 10.1111/j.1439-0272.1992.tb02605.x. [DOI] [PubMed] [Google Scholar]
- Lindner C., Lichtenberg V., Westhof G., Braendle W., Bettendorf G. Endocrine parameters of human follicular fluid and fertilization capacity of oocytes. Horm Metab Res. 1988 Apr;20(4):243–246. doi: 10.1055/s-2007-1010803. [DOI] [PubMed] [Google Scholar]
- Osman R. A., Andria M. L., Jones A. D., Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem Biophys Res Commun. 1989 Apr 28;160(2):828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- Pizzo P., Zanovello P., Bronte V., Di Virgilio F. Extracellular ATP causes lysis of mouse thymocytes and activates a plasma membrane ion channel. Biochem J. 1991 Feb 15;274(Pt 1):139–144. doi: 10.1042/bj2740139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrino G., Lorenzini E. C., Ferioli M. E. Polyamines and mammalian hormones. Part I: Biosynthesis, interconversion and hormone effects. Mol Cell Endocrinol. 1991 May;77(1-3):1–35. doi: 10.1016/0303-7207(91)90056-x. [DOI] [PubMed] [Google Scholar]
- Talbot P., Chacon R. S. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool. 1981 Feb;215(2):201–208. doi: 10.1002/jez.1402150210. [DOI] [PubMed] [Google Scholar]
- Thomas P., Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989 Dec 1;264(2):539–546. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. Early events in mammalian fertilization. Annu Rev Cell Biol. 1987;3:109–142. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- Wolf D. P., Boldt J., Byrd W., Bechtol K. B. Acrosomal status evaluation in human ejaculated sperm with monoclonal antibodies. Biol Reprod. 1985 Jun;32(5):1157–1162. doi: 10.1095/biolreprod32.5.1157. [DOI] [PubMed] [Google Scholar]



