Abstract
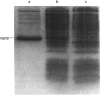
The oxoglutarate carrier, an intrinsic membrane-transport protein of the inner membranes of bovine-heart mitochondria, has been expressed at an abundant level in Escherichia coli. It accumulates in the bacterium as inclusion bodies, and none of the protein was detected in the bacterial inner membrane. The mitochondrial ADP/ATP carrier, a member of the same super-family of transport proteins as the oxoglutarate carrier, has also been expressed in E. coli. However, the expression of the ADP/ATP carrier in bacteria retards their growth, and so the levels of expression that were attained were lower than those of the oxoglutarate carrier. The oxoglutarate carrier inclusion bodies have been disaggregated with the detergent N-dodecanoyl-sarcosine, and the protein has been incorporated into liposomes. In its ability to transport oxoglutarate and malate and other known substrates of the carrier in mitochondria, and in its inhibition characteristics by a wide range of non-competitive and competitive inhibitors, this reconstituted oxoglutarate carrier is similar to the natural protein in the inner membranes of mitochondria, and to the carrier that has been purified from mitochondria and reconstituted in liposomes. These experiments remove significant obstacles to crystallization trials and to site-directed mutagenesis of the oxoglutarate carrier.
Full text
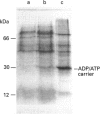
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquila H., Link T. A., Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 1985 Sep;4(9):2369–2376. doi: 10.1002/j.1460-2075.1985.tb03941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila H., Misra D., Eulitz M., Klingenberg M. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):345–349. [PubMed] [Google Scholar]
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaccia F., Indiveri C., Palmieri F. Purification and reconstitution of two anion carriers from rat liver mitochondria: the dicarboxylate and the 2-oxoglutarate carrier. Biochim Biophys Acta. 1988 Apr 22;933(2):229–240. doi: 10.1016/0005-2728(88)90030-8. [DOI] [PubMed] [Google Scholar]
- Bisaccia F., Indiveri C., Palmieri F. Purification of reconstitutively active alpha-oxoglutarate carrier from pig heart mitochondria. Biochim Biophys Acta. 1985 Dec 16;810(3):362–369. doi: 10.1016/0005-2728(85)90222-1. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Runswick M. J., Walker J. E. A homologue of the nuclear coded 49 kd subunit of bovine mitochondrial NADH-ubiquinone reductase is coded in chloroplast DNA. EMBO J. 1989 Mar;8(3):665–672. doi: 10.1002/j.1460-2075.1989.tb03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Pedersen P. L. Overexpression of higher eukaryotic membrane proteins in bacteria. Novel insights obtained with the liver mitochondrial proton/phosphate symporter. J Biol Chem. 1992 Mar 15;267(8):5460–5466. [PubMed] [Google Scholar]
- Indiveri C., Dierks T., Krämer R., Palmieri F. Reaction mechanism of the reconstituted oxoglutarate carrier from bovine heart mitochondria. Eur J Biochem. 1991 Jun 1;198(2):339–347. doi: 10.1111/j.1432-1033.1991.tb16021.x. [DOI] [PubMed] [Google Scholar]
- Indiveri C., Krämer R., Palmieri F. Reconstitution of the malate/aspartate shuttle from mitochondria. J Biol Chem. 1987 Nov 25;262(33):15979–15983. [PubMed] [Google Scholar]
- Indiveri C., Palmieri F., Bisaccia F., Krämer R. Kinetics of the reconstituted 2-oxoglutarate carrier from bovine heart mitochondria. Biochim Biophys Acta. 1987 Mar 4;890(3):310–318. doi: 10.1016/0005-2728(87)90158-7. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Haug A., McGroarty E. J. Relationship of growth temperature and thermotropic lipid phase changes in cytoplasmic and outer membranes from Escherichia coli K12. Biochim Biophys Acta. 1979 Jul 19;555(1):56–66. doi: 10.1016/0005-2736(79)90071-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., Van Dam K. The metabolic significance of anion transport in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):213–244. doi: 10.1016/0304-4173(74)90001-9. [DOI] [PubMed] [Google Scholar]
- Miroux B., Casteilla L., Klaus S., Raimbault S., Grandin S., Clément J. M., Ricquier D., Bouillaud F. Antibodies selected from whole antiserum by fusion proteins as tools for the study of the topology of mitochondrial membrane proteins. Evidence that the N-terminal extremity of the sixth alpha-helix of the uncoupling protein is facing the matrix. J Biol Chem. 1992 Jul 5;267(19):13603–13609. [PubMed] [Google Scholar]
- Palmieri F., Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979;56:279–301. doi: 10.1016/0076-6879(79)56029-7. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Quagliariello E., Klingenberger M. Kinetics and specificity of the oxoglutarate carrier in rat-liver mitochondria. Eur J Biochem. 1972 Sep 25;29(3):408–416. doi: 10.1111/j.1432-1033.1972.tb02003.x. [DOI] [PubMed] [Google Scholar]
- Passarella S., Palmieri F., Quagliariello E. The role of metal ions in the transport of substrates in mitochondria. FEBS Lett. 1973 Dec 15;38(1):91–95. doi: 10.1016/0014-5793(73)80521-6. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Medd S. M., Runswick M. J., Walker J. E. Two bovine genes for mitochondrial ADP/ATP translocase expressed differences in various tissues. Biochemistry. 1989 Jan 24;28(2):866–873. doi: 10.1021/bi00428a069. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Powell S. J., Nyren P., Walker J. E. Sequence of the bovine mitochondrial phosphate carrier protein: structural relationship to ADP/ATP translocase and the brown fat mitochondria uncoupling protein. EMBO J. 1987 May;6(5):1367–1373. doi: 10.1002/j.1460-2075.1987.tb02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick M. J., Walker J. E., Bisaccia F., Iacobazzi V., Palmieri F. Sequence of the bovine 2-oxoglutarate/malate carrier protein: structural relationship to other mitochondrial transport proteins. Biochemistry. 1990 Dec 18;29(50):11033–11040. doi: 10.1021/bi00502a004. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Walker J. E. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 1982 Aug 2;144(2):250–254. doi: 10.1016/0014-5793(82)80648-0. [DOI] [PubMed] [Google Scholar]
- Strosberg A. D. Biotechnology of beta-adrenergic receptors. Mol Neurobiol. 1990 Fall-Winter;4(3-4):211–250. doi: 10.1007/BF02780342. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin M. B., Smith K. M., Siegel F. L. Translocation of nascent non-signal sequence protein in heated Escherichia coli. J Biol Chem. 1986 Jun 15;261(17):8070–8075. [PubMed] [Google Scholar]