Guselkumab is a fully human monoclonal antibody that binds to the p19 subunit of IL-23, preventing binding to and activation of the IL-23 receptor and release of proinflammatory cytokines. The phase 3 QUASAR maintenance study evaluated the safety and efficacy of guselkumab subcutaneous (SC) maintenance treatment in patients with moderately to severely active UC who responded to induction treatment with intravenous (IV) guselkumab.1-3 Patients who had demonstrated a response in the phase 2b or phase 3 QUASAR induction study were enrolled.1,3 Prior to entering the QUA- SAR induction study, eligible patients had a baseline modified Mayo score of 5 to 9, with a rectal bleeding subscore of 1 or greater, and a Mayo endoscopic subscore of at least 2 based on central review. The phase 2b induction study randomized 313 patients evenly across 3 arms to receive guselkumab 400 mg IV, guselkumab 200 mg IV, or placebo, administered every 4 weeks for a total of 3 doses. The phase 3 QUASAR induction study enrolled 701 patients who were randomized 3:2 to receive IV guselkumab (200 mg, every 4 weeks) vs placebo at weeks 0, 4, and 8. Patients who responded to guselkumab induc- tion therapy and entered the QUA- SAR maintenance study were evenly randomized into 3 arms. Patients in Arm 1 received guselkumab SC (200 mg, every 4 weeks); patients in Arm 2 received guselkumab SC (100 mg, every 8 weeks); and patients in Arm 3 received placebo. Patients in Arm 3 experienced guselkumab withdrawal. The study continued through week 44. Tapering of corticosteroid therapy was mandatory during the maintenance portion of the study.
The QUASAR maintenance study enrolled 568 patients. Characteristics at induction baseline were well bal- anced across the 3 arms in the main- tenance study.2 The median age was 40.7±13.75 years, and 55% of patients were male. Sixty-four percent of patients had a modified Mayo score of 7, 8, or 9, and 66% had a Mayo endo- scopic subscore of 3, reflecting severe disease in the majority of patients prior to induction therapy. The median level of C-reactive protein (CRP) was 3.9 mg/L (interquartile range [IQR], 1.5-9.2 mg/L), and the median level of fecal calprotectin was 1605.0 mg/ kg (IQR, 669.0-3337.0 mg/kg). Forty percent of patients were using corti- costeroids, and 42% of patients had a history of inadequate response or intolerance to biologic and/or Janus kinase (JAK) inhibitor therapy.
Prior to entering the QUASAR maintenance study, 34% of the 568 patients were in clinical remission, 39% exhibited endoscopic improve- ment, and 22% were in endoscopic remission. The mean modified Mayo score was 2.5±1.53. Levels of inflam- matory markers had decreased from the induction baseline: the mean level of CRP was 1.5 mg/L (IQR, 0.6-3.8 mg/L), and the mean level of fecal calprotectin was 303.5 mg/kg (IQR, 79.5-1194.0 mg/kg). Through week 44, the rate of discontinuation was low, at 12.0% (range, 10.6%-13.7%). The majority of patients who discon- tinued study therapy did so owing to an adverse event (AE; 5.1%).
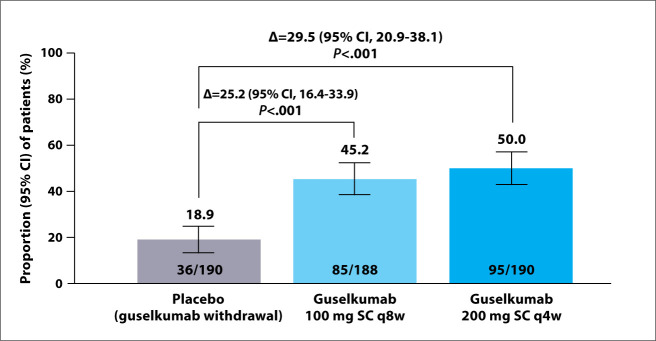
The QUASAR maintenance study met its primary endpoint, demonstrat- ing clinical remission rates at week 44 of 50.0% with the higher dose of gusel- kumab (P<.001 vs placebo), 45.2% with the lower dose of guselkumab (P<.001 vs placebo), and 18.9% with placebo (Figure 1). Sixty-nine percent of patients in Arm 1 or Arm 2 were also in endoscopic remission. The study also met its major secondary endpoints with statistical significance at week 44 for both Arm 1 and Arm 2 vs Arm 3, including corticosteroid-free clinical remission (P<.001 for both), main- tenance of clinical remission (P<.005 for both), maintenance of clinical response (P<.001 for both), and symp- tomatic remission (P<.001 for both). The trial met its secondary endoscopic and histologic endpoints, including endoscopic improvement, histologic- endoscopic mucosal improvement, and endoscopic remission (P<.001 for each comparison of Arm 1 or Arm 2 vs Arm 3). Patient-reported endpoints were also significantly improved with maintenance guselkumab compared with placebo. Guselkumab treatment was generally well tolerated, with no new safety signals.
Figure 1.
Primary endpoint of clinical remission at week 44. Clinical remission was defined as a Mayo stool frequency subscore of 0 or 1 and not increased from baseline,
a Mayo rectal bleeding subscore of 0, and Mayo endoscopic subscore of 0 or 1 with no friability present. Randomized full analysis set. 69% of guselkumab-treated patients in clinical remission were also in endoscopic remission (Mayo endoscopic subscore=0).
q4w, every 4 weeks; q8w, every 8 weeks; SC, subcutaneous.
Adapted from Rubin DT et al. DDW abstract 759. Gastroenterology. 2024;166(6)(suppl 1).2
Overall, 50% of patients with moderately to severely active UC receiving guselkumab SC 200 mg every 4 weeks and 45.2% of patients receiving guselkumab SC 100 mg every 8 weeks achieved the primary endpoint of clinical remission at week 44 compared with placebo (18.9%). In additional analyses of patients in clinical remission, 67% and 71%, respectively, were also in endoscopic remission at week 44. This study highlights the potential of guselkumab to enable patients with moderately to severely active UC to achieve durable, clinical remission and achieve important clinical endpoints, such as endoscopic remission to the point of normalization and histologic remission, which represents the level of progress needed in new treatments for IBD.
– Gary R. Lichtenstein, MD
References
- Peyrin-Biroulet L, Allegretti JR, Rubin DT et al. Guselkumab in patients with moderately to severely active ulcerative colitis: QUASAR phase 2b induction study. Gastroenterology. 2023;165(6):1443–1457. doi: 10.1053/j.gastro.2023.08.038. [DOI] [PubMed] [Google Scholar]
- Rubin DT, Allegretti JR, Panés J et al. The efficacy and safety of guselkumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 QUASAR maintenance study [DDW abstract 759]. Gastroenterology. 2024;166(6) suppl 1. [Google Scholar]
- Allegretti JR, Peyrin-Biroulet L, Feagan BG et al. The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 QUASAR induction study [DDW abstract 913b]. Gastroenterology. 2023;164(6):S-1572. suppl. [PMC free article] [PubMed] [Google Scholar]