Abstract
BACKGROUND
Biliary tract cancers (BTCs) are a heterogeneous group of tumors with high malignancy, poor prognosis, and limited treatment options.
AIM
To explore the efficacy and safety of nab-paclitaxel plus capecitabine as first-line treatment for advanced and metastatic BTCs.
METHODS
This open-label, non-randomized, double-center, phase II clinical trial recruited systemic therapy-naive patients with unresectable or metastatic BTCs between April 2019 and June 2022 at Beijing Cancer Hospital and the First Hospital of China Medical University. Eligible patients were administered nab-paclitaxel (150 mg/m2, day 1) and capecitabine (2000 mg/m2, twice daily, days 1-7) in 14-day cycles until experiencing intolerable toxicity or disease progression. The primary outcome was the objective response rate (ORR). The secondary outcomes included the disease control rate (DCR), overall survival (OS), progression-free survival (PFS), and safety.
RESULTS
A total of 44 patients successfully completed the trial, with a median age of 64.00 years (interquartile range, 35.00-76.00), and 26 (59.09%) were females. Tumor response assessment was impeded for one patient due to premature demise from tumor hemorrhage. Among the remaining 43 patients undergoing at least one imaging assessment, the ORR was 23.26% [95% confidence interval (CI): 11.80%-38.60%], and the DCR was 69.77% (95%CI: 53.90%-82.80%). The median OS was 14.1 months (95%CI: 8.3-19.9), and the median PFS was 4.4 months (95%CI: 2.5-6.3). A total of 41 patients (93.18%) experienced at least one adverse event (AE), with 10 patients (22.73%) encountering grade ≥ 3 AEs, and the most frequent AEs of any grade were alopecia (79.50%), leukopenia (54.55%), neutropenia (52.27%), and liver dysfunction (40.91%), and no treatment-related deaths were documented.
CONCLUSION
Nab-paclitaxel plus capecitabine may be an effective and safe first-line treatment strategy for patients with advanced or metastatic BTCs.
Keywords: Nab-paclitaxel, Capecitabine, Biliary tract cancer, Objective response rate, Phase II clinical trial
Core Tip: Our study investigates the efficacy and safety of nab-paclitaxel plus capecitabine as first-line treatment for advanced and metastatic biliary tract cancers (BTCs). Through an open-label, non-randomized, double-center, phase II clinical trial, we enrolled systemic therapy-naive patients. Our findings demonstrate that nab-paclitaxel plus capecitabine demonstrates both acceptable efficacy and safety in patients with advanced and metastatic BTCs. This regimen shows promise as a potential novel treatment option for this patient population and warrants further validation in future trials.
INTRODUCTION
Biliary tract cancer (BTCs) refers to a heterogeneous group of aggressive adenocarcinomas originating from biliary epithelial cells, accounting for about 3% of all gastrointestinal malignancies[1-3]. According to their anatomical origin, BTCs can be divided into gallbladder cancer and cholangiocarcinoma, which can be further divided into intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma, and distal cholangiocarcinoma[4]. Although BTCs are infrequent in numerous regions, their global incidence displays variations, with lower rates observed in developed countries such as Europe and the United States, whereas relatively higher rates in Asian countries[5,6]. The prognosis of BTCs varies according to their anatomical origin, but it generally has a poor prognosis[7,8]. The heterogeneity of BTC poses a particular challenge for treatment[9-11], and more effective treatment strategies are necessary.
BTCs are typically diagnosed at an advanced stage[12,13], with systemic therapy serving as the primary treatment approach[9-11]. For patients with unresectable BTCs, the TOPAZ-1 study established durvalumab plus cisplatin and gemcitabine as the reference regimen internationally, based on an improved overall survival (OS) compared with gemcitabine and cisplatin (GC) regimen[14]. Recently, pembrolizumab plus GC was proven to be a new treatment option[15]. Although combined therapy improved survival with extended survival times of about one month, chemotherapy remains the backbone of treatment for BTCs because most BTCs have an immune-suppressed microenvironment[16]. The efficacy of the gemcitabine plus cisplatin regimen and capecitabine plus oxaliplatin regimen had already been confirmed through previous research[17,18].
Nab-paclitaxel combination therapies are emerging as potentially effective systemic therapy against BTCs, including gemcitabine plus nab-paclitaxel[19] and gemcitabine plus cisplatin plus nab-paclitaxel[20], and those regimens are endorsed by guidelines[9]. Still, there is limited available evidence regarding nab-paclitaxel in BTCs. Potential synergism of nab-paclitaxel and gemcitabine was observed in a mouse model of pancreatic cancer[21] and was investigated in a phase II clinical trial[19]. Although the trial did not meet its primary endpoint, the results indicated that nab-paclitaxel plus gemcitabine may be an alternative option, with an objective response rate (ORR) of 30%. The addition of nab-paclitaxel to GAP demonstrated promising efficacy as a neoadjuvant treatment for previously untreated unresectable or metastatic BTCs[22,23]. Moreover, one study showed that first-line nab-paclitaxel plus cisplatin and gemcitabine had a higher ORR, but the toxicity was non-negligible[24]. There have been no prospective studies evaluating the combination of capecitabine and nab-paclitaxel in patients with advanced or metastatic BTCs. Therefore, this study aimed to explore the efficacy and safety of nab-paclitaxel plus capecitabine as first-line treatment for advanced and metastatic BTCs.
MATERIALS AND METHODS
Study design and patients
This is an open-label, non-randomized, double-center, phase II clinical trial enrolled systemic therapy-naive patients with unresectable or metastatic BTCs between April 2019 and June 2022 at Beijing Cancer Hospital and the First Hospital of China Medical University. The inclusion criteria were: (1) > 18 years old; (2) Histopathologically or cytologically confirmed as BTCs; (3) Diagnosed as locally advanced unresectable or metastatic BTCs; (4) No prior systematic therapy (adjuvant systemic therapy was permitted if received > 6 months before initiating trial medication); (5) With measurable lesions as per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1[25]; (6) Adequate hematologic (absolute neutrophil count ≥ 1.5 × 103/μL, platelets ≥ 100 × 103/μL, and hemoglobin > 90 g/L), hepatic (for patients without liver metastasis, the total bilirubin, aspartate aminotransferase, and alanine aminotransferase should be ≤ 3 times the upper limit of the institutional normal value. For patients with liver metastasis, they should be ≤ 5 times the upper limit of the institutional normal value), and renal function (glomerular filtration rate ≥ 60 mL/min/1.73 m2); and (7) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. Patients suffering from any other concurrent uncontrolled medical condition were excluded. The study was approved by the ethics committee of Beijing Cancer Hospital and the First Hospital of China Medical University (approval number: 2018YJZ70-ZY02). The trial adhered to the Declaration of Helsinki and the Good Clinical Practice. Written informed consent was obtained from all patients.
Procedure
Patients were administered intravenous nab-paclitaxel at an initial dosage of 150 mg/m2 on day 1, succeeded by oral capecitabine at a standardized dose of 2000 mg/m2 twice daily from days 1 to 7 within each 14-day cycle. The infusion of nab-paclitaxel was uniformly administered to all patients over a 30-minute period. In the event of patients experiencing adverse events (AEs), an associate chief physician in the Department of Digestive Oncology assessed the causal relationship between the toxicity and the study drugs, with the authority to reduce the dose of the relevant drug by 20%-25%. Discontinuation of treatment was determined if the patient remained unable to tolerate the regimen after two dose reductions, if disease progression was evident, or intolerable toxicity manifested, or the patient declined further medication.
Computed tomography or magnetic resonance imaging was performed at baseline and every 6 weeks. The response to treatment was assessed based on RECIST v1.1[25], including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The carbohydrate antigen 199 (CA199) and carcinoembryonic antigen levels were assessed on day 1 of each cycle.
Outcomes
The primary endpoint was ORR, defined as the proportion of patients with a confirmed CR or PR (as per RECIST 1.1). The secondary outcomes included the disease control rate (DCR: CR + PR + SD), OS, progression-free survival (PFS), and safety. Survival was measured from the first dose of trial medication. OS was calculated from the date of treatment initiation until the date of death. PFS was measured from treatment initiation until the date of documented disease progression or death. All AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs, version 5.0.
Sample size calculation
The response rate was expected to be approximately 45%[19,20]. Using a two-sided α level of 0.05 and 80% power, a sample size of 41 was calculated. Considering a 10% dropout rate due to protocol deviations, 46 patients were recruited to ensure that at least 41 patients were evaluable. The sample size calculation was performed using PASS 13.0.
Statistical analysis
The statistical analysis was performed using SPSS 26.0 (IBM, Armonk, NY, United States) and GraphPad Prism 9.5.0 (GraphPad Software Inc., San Diego, CA, United States). The continuous data with a normal distribution were presented as means ± SD; otherwise, they were presented as medians interquartile range (IQR). The categorical data were described as n (%). PFS and OS were analyzed using the Kaplan-Meier method. Two-sided P values < 0.05 were considered statistically significant.
RESULTS
Demographic characteristics
A total of 46 patients were initially enrolled in this study; however, one patient withdrew consent, and another experienced the first treatment delay due to coronavirus disease 2019 (COVID-19). Consequently, 44 patients successfully completed the trial (Figure 1). The median age of the patients was 64.00 years (IQR, 35.00-76.00), with 26 (59.09%) being female. Additionally, 25 (56.82%) patients scored an ECOG performance status of 0. Among the patients, 20 (45.45%) were diagnosed with gallbladder carcinoma (GBC), 11 (25.00%) with ICC, and 13 (29.55%) with extrahepatic cholangiocarcinoma (ECC). Multiple metastatic sites were observed in 20 patients (45.45%), with 20 (45.45%) experiencing liver metastases, 5 (11.36%) lung metastases, and 9 (20.5%) peritoneum metastases. Notably, 26 patients (59.09%) had undergone radical resection, while 7 (15.91%) had received adjuvant systemic therapy. The majority were at stage IV (90.91%), and 28 (63.64%) had CA199 levels exceeding 37 U/mL (Table 1).
Figure 1.
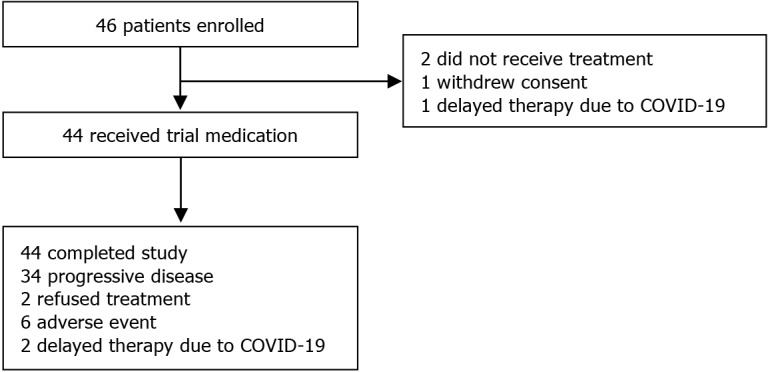
Patient flowchart. COVID-19: Coronavirus disease 2019.
Table 1.
Basic demographic characteristics, n (%)
|
Characteristic
|
Total (n = 44)
|
| Age, years (median, IQR) | 64.00 (35.00, 76.00) |
| Sex | |
| Male | 18 (40.91) |
| Female | 26 (59.09) |
| ECOG performance | |
| 0 | 25 (56.82) |
| 1 | 19 (43.18) |
| Tumor subtype | |
| Gallbladder cancer | 20 (45.45) |
| Intrahepatic cholangiocarcinoma | 11 (25.00) |
| Extrahepatic cholangiocarcinoma | 13 (29.55) |
| Number of metastatic sites | |
| No metastasis | 3 (6.82) |
| Single | 21 (47.73) |
| Multiple | 20 (45.45) |
| Metastatic sites | |
| Liver | 20 (45.45) |
| Lung | 5 (11.36) |
| Peritoneum | 9 (20.45) |
| Previous antitumor therapy | |
| Radical resection | 26 (59.09) |
| Adjuvant systemic therapy | 7 (15.91) |
| Stage | |
| III | 1 (2.27) |
| IV | 40 (90.91) |
| Uncertain | 3 (6.82) |
| Baseline CA199 | |
| < 37 U/mL | 16 (36.36) |
| ≥ 37 U/mL | 28 (63.64) |
ECOG: Eastern Cooperative Oncology Group; CA199: Carbohydrate antigen 199; IQR: Interquartile range.
Response to treatment
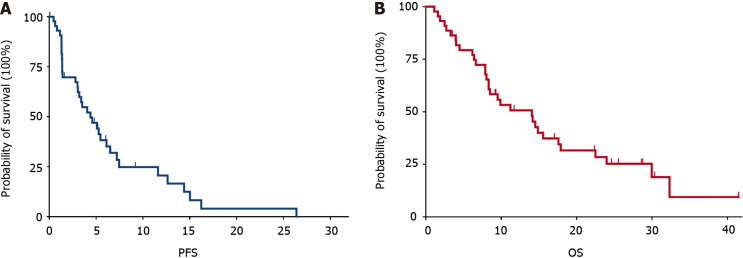
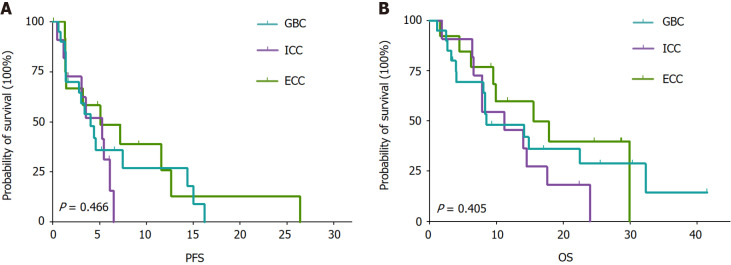
The assessment of tumor response was impeded for one participant due to premature demise resulting from tumor hemorrhage before the initial evaluation. Among the remaining 43 patients who underwent at least one imaging assessment, the outcomes were as follows: CR was observed in 2 patients (4.65%); PR was noted in 8 patients (18.60%); SD was evident in 20 patients (46.51%); PD manifested in 13 patients (30.23%). Consequently, an ORR of 23.26% (95%CI: 11.80%-38.60%) and a DCR of 69.77% (95%CI: 53.90%-82.80%) were determined (Table 2). The waterfall plot of the best response of the patients of GBC, ICC, and ECC was presented in Figure 2. The median OS was 14.1 months (95%CI: 8.3-19.9), and the median PFS was 4.4 months (95%CI: 2.5-6.3) (Figure 3). The median OS for patients with GBC, ICC, and ECC was 8.5 months (95%CI: 2.3-14.8), 11.2 months (95%CI: 5.2-17.3), and 15.6 months (95%CI: 3.9-27.2), respectively (P = 0.405). Additionally, the median PFS for patients with GBC, ICC, and ECC was 4.1 months (95%CI: 2.3-5.9), 5.3 months (95%CI: 2.0-8.5), and 5.1 months (95%CI: 0-11.0), respectively (P = 0.466) (Figure 4; Table 3). The ORRs for patients with GBC, ICC, and ECC were 25.00%, 18.18%, and 25.00%, respectively, while the DCRs for patients with GBC, ICC, and ECC were 70.00%, 72.73%, and 66.67%, respectively (Table 3). We have also analyzed the relationships between other disease characteristics and treatment responses, and found no significant differences in the ORR across patients with different ages, genders, ECOG performance status, number of metastatic sites, metastatic sites, stages, and baseline CA199 levels.
Table 2.
Treatment response in patients, n (%)
|
Therapeutic response assessment
|
Evaluable patients (n = 43)
|
| Objective response rate (95%CI) | 10 (23.26) (11.80-38.60) |
| Complete response | 2 (4.65) |
| Partial response | 8 (18.60) |
| Stable disease | 20 (46.51) |
| Progressive disease | 13 (30.23) |
| Disease control rate (95%CI) | 30 (69.77) (53.90-82.80) |
| Progression-free survival (95%CI, months) | 4.4 (2.5-6.3) |
| Overall survival (95%CI, months) | 14.1 (8.3-19.9) |
CI: Confidence interval.
Figure 2.
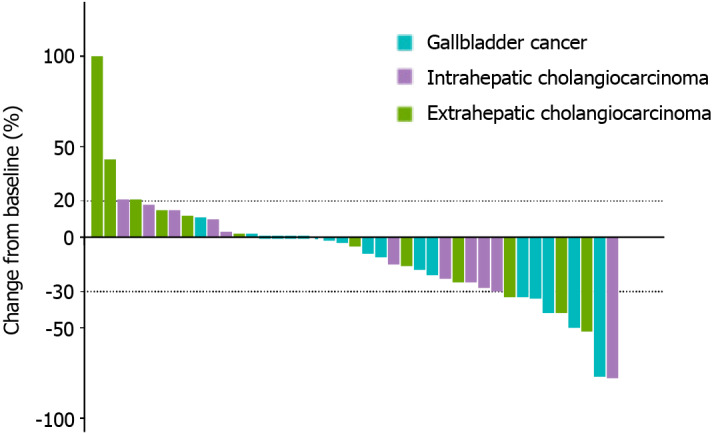
Waterfall plot of the best response of the patients with gallbladder cancer, intrahepatic cholangiocarcinoma, and extrahepatic cholangiocarcinoma, as per response evaluation criteria in solid tumors (RECIST 1.1).
Figure 3.
Kaplan-Meier curves for patients receiving nab-paclitaxel plus capecitabine. A: Progression-free survival; B: Overall survival. PFS: Progression-free survival; OS: Overall survival.
Figure 4.
Kaplan-Meier curves for patients with gallbladder cancer, intrahepatic cholangiocarcinoma, and extrahepatic cholangiocarcinoma. A: Progression-free survival; B: Overall survival. PFS: Progression-free survival; OS: Overall survival; GBC: Gallbladder cancer; ICC: Intrahepatic cholangiocarcinoma; ECC: Extrahepatic cholangiocarcinoma.
Table 3.
Treatment response in patients with gallbladder cancer, intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma
|
|
ORR
|
DCR
|
OS (95%CI)
|
PFS (95%CI)
|
| GBC | 25.00 (11.19-46.87) | 70.00 (48.10-85.45) | 8.5 (2.3-14.8) | 4.1 (2.1-5.9) |
| ICC | 18.18 (5.14-47.70) | 72.73 (43.44-90.25) | 11.2 (5.2-17.3) | 5.3 (2.0-8.5) |
| ECC | 25.00 (8.89-53.23) | 66.67 (39.06-86.19) | 15.6 (3.9-27.2) | 5.1 (0.0-11.0) |
GBC: Gallbladder cancer; ICC: Intrahepatic cholangiocarcinoma; ECC: Extrahepatic cholangiocarcinoma; ORR: Objective response rate; DCR: Disease control rate; OS: Overall survival; PFS: Progression-free survival; CI: Confidence interval.
Safety evaluation
A total of 41 (93.18%) patients experienced at least one AE, with 10 patients (22.73%) encountering grade ≥ 3 AEs. The most frequently observed AEs of any grade included alopecia (35, 79.50%), leukopenia (24, 54.55%), neutropenia (23, 52.27%), and liver dysfunction (18, 40.91%). Among the grade ≥ 3 AEs, the most common were neutropenia (4, 9.09%). Importantly, no treatment-related deaths were documented (Table 4). For common grade 3 and higher AEs such as leukopenia, neutropenia, and liver dysfunction, we administered recombinant human granulocyte colony-stimulating factor and hepatoprotective agents as symptomatic treatments. In cases of grade 4 Leukopenia and thrombocytopenia, grade 3 or higher anemia, or grade 2 or higher peripheral neuropathy and gastrointestinal toxicity, we reduced the drug dosage by 20%-25% to alleviate the AEs experienced by the patients.
Table 4.
Adverse events occurring in ≥ 10% of the patients, n (%)
|
Adverse event
|
Grade 1-2
|
Grade 3-4
|
All grades
|
| Alopecia | 35 (79.50) | 0 (0.00) | 35 (79.50) |
| Leukopenia | 22 (50.00) | 2 (4.55) | 24 (54.55) |
| Neutropenia | 19 (43.18) | 4 (9.09) | 23 (52.27) |
| Liver dysfunction | 18 (40.91) | 0 (0.00) | 18 (40.91) |
| Hyperbilirubinemia | 16 (36.36) | 2 (4.55) | 18 (40.91) |
| Anemia | 14 (31.81) | 2 (4.55) | 16 (36.36) |
| Neurotoxicity | 10 (22.71) | 2 (4.55) | 12 (27.27) |
| ST-T abnormal change | 9 (20.45) | 0 (0.00) | 9 (20.45) |
| Sinus tachycardia | 6 (13.64) | 0 (0.00) | 6 (13.64) |
| Anorexia | 5 (11.36) | 0 (0.00) | 5 (11.36) |
DISCUSSION
This study revealed an ORR of 23.26%, a DCR of 69.77%, and a median OS and PFS of 14.1 months and 4.4 months, respectively. A well-tolerated safety profile in patients with advanced or metastatic BTCs treated with nab-paclitaxel plus capecitabine suggests that it may be an effective and safe first-line treatment strategy for such patients.
In this study, the ORR of nab-paclitaxel plus capecitabine was 23.3% and the DCR was 69.8%; the ORR observed here was similar to those observed with capecitabine plus oxaliplatin (ORR = 26.4%) in patients with advanced BTCs[18] and gemcitabine plus nab-paclitaxel (ORR = 30.0%) in patients with advanced or metastatic cholangiocarcinoma[19], highlighting the need for more effective regimens. In addition, the ABC-02 trial reported ORRs of 26.1% for cisplatin plus gemcitabine and 15.5% for gemcitabine alone in patients with BTCs, and the CR rates were lower than 1.0% in each group[17]. Previous studies reported an ORR of 52.4% (all PRs) in patients with advanced, unresectable gallbladder cancer treated with nab-paclitaxel as a third-line treatment after first-line gemcitabine/platinum-based systemic therapy and second-line FOLFOX4[26], 45% (all PRs) in patients with BTC treated with first-line gemcitabine plus cisplatin plus nab-paclitaxel[20], 34% with nab-paclitaxel plus gemcitabine plus cisplatin[23], and 33.3% for advanced cholangiocellular carcinoma treated with second- or third-line nab-paclitaxel-based systemic therapy[27]. In the present study of treatment-naive patients with BTCs, the ORR of capecitabine plus nab-paclitaxel was 23.26%, but 4.65% of the patients achieved CR. The NAP-CAPABIL pilot study showed that 10 patients with advanced BTC treated with capecitabine and nab-paclitaxel had a DCR of 80% (8/10) after a median treatment duration of 4.3 months[28]. Nevertheless, those previous studies and the present one cannot be directly compared because of the differences in patient selection and treatment regimens (including lines of therapy), but capecitabine plus nab-paclitaxel appears promising for patients with advanced or metastatic BTCs. The differences among the above trials can be due to a number of factors besides the study treatments, including patient selection, previous treatments, and drug combinations.
Previous studies demonstrated that adding nab-paclitaxel to conventional systemic therapy and giving nab-paclitaxel after conventional therapy could result in better OS and PFS than conventional therapies[20,23,26-28]. The median OS of capecitabine plus nab-paclitaxel was 14.1 months in the present study, but the median PFS was only 4.4 months. The shorter PFS could be due to the complex challenge of 2-week treatment cycles in the context of the COVID-19 pandemic, with constraints due to lockdowns, restricted transportation, hospital overcrowding, and fear of COVID-19.
In the present study, capecitabine plus nab-paclitaxel was well tolerated, with the most common grade 3-4 AEs being neutropenia, leukopenia, anemia, neurotoxicity, and hyperbilirubinemia, which were all manageable after drug dose reduction, and without treatment-related deaths, similar to the NAP-CAPABIL pilot study in patients with advanced BTC (90% with any-grade AEs and 40% with grade > 3 AEs)[28] and in patients with other solid tumors[29,30]. Compared with the gemcitabine plus cisplatin regimen in the ABC-02 trial[17], the rate of severe AEs was relatively low (22.73% of patients with grade ≥ 3 AEs in the present study vs 70.7 % in ABC-02). In patients with BTCs treated with first-line gemcitabine plus nab-paclitaxel, grade ≥ 3 neutropenia and fatigue were observed in 43% and 14%, respectively[19]. Gemcitabine plus cisplatin plus nab-paclitaxel displayed a 58% rate of grade ≥ 3 AEs. Gemcitabine plus cisplatin plus nab-paclitaxel (SEOG 1815 trial) also showed a rate of grade ≥ 3 hematological AEs of 60%. Many previous trials included cisplatin in their regimens, and platinum salts are recognized to have high rates of hematological AEs in patients with solid tumors[31]. On the other hand, capecitabine is an oral drug, and the dose can be reduced dynamically, which might contribute to the lower occurrence of severe AEs. Therefore, capecitabine plus nab-paclitaxel could have the advantages of lower toxicity due to fewer drugs being used and a more dynamic dose adjustment.
This study had several limitations. It employed a single-arm, non-randomized design with a relatively small sample size, lacking comparator regimens and hindering reliable subgroup or multivariable analyses. Despite these constraints, the observed regimen demonstrated promising efficacy. To substantiate these findings, conducting a randomized controlled study with a larger sample size is imperative. Still, it should be noted that in the light of recent efficacy data on chemotherapy combined with immunotherapy trials (TOPAZ-1 and KEYNOTE-966 trials)[14,15], a regimen based on nab-paclitaxel and capecitabine without immunotherapy might not be enough as first-line therapy. On the other hand, for patients with intolerance to platinum, nab-paclitaxel plus capecitabine could be an alternative option. Future studies should also examine the role of immunotherapy with nab-paclitaxel.
CONCLUSION
In conclusion, nab-paclitaxel plus capecitabine demonstrates both acceptable efficacy and safety in patients with advanced and metastatic BTCs. This regimen shows promise as a potential novel treatment option for this patient population and warrants further validation in future trials.
ACKNOWLEDGEMENTS
The authors acknowledge the help of the CSPC Ouyi Pharmaceutical Co., Ltd., patients and their families in participating in the study and all the investigators and research staff who are enrolling patients in this trial.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Peking University Cancer Hospital and Institute (approval number: 2018YJZ70-ZY02).
Clinical trial registration statement: This study has been registered at https://www.chictr.org.cn (ID: ChiCTR1900025004).
Informed consent statement: All the individuals who participated in this study provided their written informed consent prior to study enrolment.
Conflict-of-interest statement: The authors declare that they have no competing interests.
CONSORT 2010 statement: The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Rodrigues-Pinto E; Tzang BS S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
Contributor Information
Ling-Xiao Xu, Department of Gastrointestinal Oncology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital and Institute, Beijing 100142, China.
Jia-Jia Yuan, Department of Gastrointestinal Oncology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital and Institute, Beijing 100142, China.
Ran Xue, Department of Gastrointestinal Oncology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital and Institute, Beijing 100142, China.
Jun Zhou, Department of Gastrointestinal Oncology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital and Institute, Beijing 100142, China. joelbmu@126.com.
Data sharing statement
No additional data are available.
References
- 1.Benavides M, Antón A, Gallego J, Gómez MA, Jiménez-Gordo A, La Casta A, Laquente B, Macarulla T, Rodríguez-Mowbray JR, Maurel J. Biliary tract cancers: SEOM clinical guidelines. Clin Transl Oncol. 2015;17:982–987. doi: 10.1007/s12094-015-1436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 6.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 7.Kang MJ, Lim J, Han SS, Park HM, Kim SW, Lee WJ, Woo SM, Kim TH, Won YJ, Park SJ. Distinct prognosis of biliary tract cancer according to tumor location, stage, and treatment: a population-based study. Sci Rep. 2022;12:10206. doi: 10.1038/s41598-022-13605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BW, Oh CM, Choi HY, Park JW, Cho H, Ki M. Incidence and Overall Survival of Biliary Tract Cancers in South Korea from 2006 to 2015: Using the National Health Information Database. Gut Liver. 2019;13:104–113. doi: 10.5009/gnl18105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiorean EG, Chiaro MD, Tempero MA, Malafa MP, Benson AB, Cardin DB, Christensen JA, Chung V, Czito B, Dillhoff M, Donahue TR, Dotan E, Fountzilas C, Glazer ES, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Masood A, Moravek C, Nakakura EK, Narang AK, Nardo L, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Truty MJ, Vollmer C, Wolff RA, Wolpin BM, Rn BM, Lubin S, Darlow SD. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:753–782. doi: 10.6004/jnccn.2023.0034. [DOI] [PubMed] [Google Scholar]
- 10.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 11.Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, Ebata T, Konishi M, Sano K, Shimada K, Shimizu H, Higuchi R, Wakai T, Isayama H, Okusaka T, Tsuyuguchi T, Hirooka Y, Furuse J, Maguchi H, Suzuki K, Yamazaki H, Kijima H, Yanagisawa A, Yoshida M, Yokoyama Y, Mizuno T, Endo I. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28:26–54. doi: 10.1002/jhbp.870. [DOI] [PubMed] [Google Scholar]
- 12.Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99:315–335. doi: 10.1016/j.suc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99:337–355. doi: 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, Nam AR, Oh KS, Kim JM, Lee Y, Guthrie V, McCoon P, Li W, Wu S, Zhang Q, Rebelatto MC, Kim JW. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7:522–532. doi: 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 16.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, Elarouci N, Ayadi M, Adam R, Azoulay D, Castaing D, Vibert E, Cherqui D, Samuel D, Sa Cuhna A, Marchio A, Pineau P, Guettier C, de Reyniès A, Faivre J. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 18.Kim ST, Kang JH, Lee J, Lee HW, Oh SY, Jang JS, Lee MA, Sohn BS, Yoon SY, Choi HJ, Hong JH, Kim MJ, Kim S, Park YS, Park JO, Lim HY. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann Oncol. 2019;30:788–795. doi: 10.1093/annonc/mdz058. [DOI] [PubMed] [Google Scholar]
- 19.Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, Munshi HG, Benson AB 3rd, O'Dwyer PJ. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:1707–1712. doi: 10.1001/jamaoncol.2018.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maithel SK, Keilson JM, Cao HST, Rupji M, Mahipal A, Lin BS, Javle MM, Cleary SP, Akce M, Switchenko JM, Rocha FG. NEO-GAP: A Single-Arm, Phase II Feasibility Trial of Neoadjuvant Gemcitabine, Cisplatin, and Nab-Paclitaxel for Resectable, High-Risk Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2023;30:6558–6566. doi: 10.1245/s10434-023-13809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, Mahipal A, Kalyan A, Javle MM, Aghajanian C, Tan BR, Cheema PS, Patel AK, Iyer RV, Kelley RK, Thumar JR, El-khoueiry AB, Chiorean EG, Hochster HS, Philip PA. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41:LBA490–LBA490. [Google Scholar]
- 24.Gedela S, Munot P, Vaidyanathan A, Joarder R, Chaugule D, Parulekar M, Nashikkar C, Ghadi A, Vadodaria D, Goel M, Patkar S, Mandavkar S, Ramaswamy A, Bhargava P, Srinivas S, Ostwal V. Gemcitabine, Cisplatin, and Nab-Paclitaxel as a First-Line Therapy for Advanced Biliary Tract Cancers. J Gastrointest Cancer. 2024;55:263–269. doi: 10.1007/s12029-023-00946-z. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Talwar V, Raina S, Goel V, Dash P, Doval DC. Nab-paclitaxel: An effective third-line chemotherapy in patients with advanced, unresectable gallbladder cancer. Indian J Med Res. 2020;152:475–481. doi: 10.4103/ijmr.IJMR_930_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unseld M, Scheithauer W, Weigl R, Kornek G, Stranzl N, Bianconi D, Brunauer G, Steger G, Zielinski CC, Prager GW. Nab-paclitaxel as alternative treatment regimen in advanced cholangiocellular carcinoma. J Gastrointest Oncol. 2016;7:588–594. doi: 10.21037/jgo.2016.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodford R, Brungs D, Leighton C, Grimison P, Sjoquist KM, Becker T, Robinson S, Gebski V, Wilson K, Chantrill L, Aghmesheh M. Combination chemotherapy with NAB(®) -paclitaxel and capecitabine for patients with advanced biliary tract cancer (NAP-CAPABIL Pilot Study) Asia Pac J Clin Oncol. 2022;18:e220–e226. doi: 10.1111/ajco.13599. [DOI] [PubMed] [Google Scholar]
- 29.Scheithauer W, Kornek G, Prager G, Stranzl N, Laengle F, Schindl M, Friedl J, Klech J, Roethlin S, Zielinski C. Phase II trial of capecitabine plus nab-paclitaxel in patients with metastatic pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7:234–238. doi: 10.3978/j.issn.2078-6891.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheithauer W, Ramanathan RK, Moore M, Macarulla T, Goldstein D, Hammel P, Kunzmann V, Liu H, McGovern D, Romano A, Von Hoff DD. Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial. J Gastrointest Oncol. 2016;7:469–478. doi: 10.21037/jgo.2016.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Q, Peng Y, Sun J, Liu J. Platinum-Based Chemotherapy and Immunotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis and Indirect Treatment Comparison. Front Oncol. 2021;11:693542. doi: 10.3389/fonc.2021.693542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.