Abstract
BACKGROUND
Inflammatory bowel disease, particularly Crohn’s disease (CD), has been associated with alterations in mesenteric adipose tissue (MAT) and the phenomenon termed “creeping fat”. Histopathological evaluations showed that MAT and intestinal tissues were significantly altered in patients with CD, with these tissues characterized by inflammation and fibrosis.
AIM
To evaluate the complex interplay among MAT, creeping fat, inflammation, and gut microbiota in CD.
METHODS
Intestinal tissue and MAT were collected from 12 patients with CD. Histological manifestations and protein expression levels were analyzed to determine lesion characteristics. Fecal samples were collected from five recently treated CD patients and five control subjects and transplanted into mice. The intestinal and mesenteric lesions in these mice, as well as their systemic inflammatory status, were assessed and compared in mice transplanted with fecal samples from CD patients and control subjects.
RESULTS
Pathological examination of MAT showed significant differences between CD-affected and unaffected colons, including significant differences in gut microbiota structure. Fetal microbiota transplantation (FMT) from clinically healthy donors into mice with 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced CD ameliorated CD symptoms, whereas FMT from CD patients into these mice exacerbated CD symptoms. Notably, FMT influenced intestinal permeability, barrier function, and levels of proinflammatory factors and adipokines. Furthermore, FMT from CD patients intensified fibrotic changes in the colon tissues of mice with TNBS-induced CD.
CONCLUSION
Gut microbiota play a critical role in the histopathology of CD. Targeting MAT and creeping fat may therefore have potential in the treatment of patients with CD.
Keywords: Mesenteric adipose tissue, Crohn’s disease, Fecal microbiota transplantation, Intestinal fibrosis, Intestinal barrier
Core Tip: The present study evaluated the complex interplay among creeping fat, inflammation, and intestinal microbiota involved in the pathogenesis of Crohn’s disease (CD). The intestinal microbiota was found to play a multifaceted role, mediating the properties of creeping fat while affecting the inflammatory and fibrotic phenotypes associated with CD.
INTRODUCTION
Inflammatory bowel disease (IBD) primarily includes Crohn’s disease (CD) and ulcerative colitis (UC). CD, first identified as a distinct pathological entity in 1932, is characterized by chronic inflammation of the gastrointestinal tract[1]. The IBD incidence and prevalence of IBD have been increasing worldwide, with CD significantly contributing to these trends[2]. Factors contributing to the pathogenesis of CD include genetic predisposition, environmental triggers, and intestinal microbiota[3]. Although dysbiosis in the gut microbiota has been detected in many patients with CD, the roles of the gut microbiota and metabolic disorders in its pathogenesis remain unclear. The absence of diagnostic and therapeutic approaches targeting the gut microbiota has been found to significantly affect patient prognosis and quality of life. Studies evaluating the roles of the gut microbiota in the pathogenesis and progression of CD may therefore identify new therapeutic approaches.
Creeping fat and mesenteric adipose tissue (MAT) have been associated with the pathogenesis of CD, with intraabdominal fat-to-total abdominal fat ratios being higher in patients with CD than in normal controls. Furthermore, a higher percentage of visceral fat is associated with a higher rate of postoperative disease recurrence[4,5]. This pathologically altered fat, known as “creeping fat”, is often found around inflamed intestinal regions and contributes to disease severity[6]. The inflammatory profile of MAT in patients with CD includes increased concentrations of cytokines, such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6, and of adipokines, such as leptin, resistin, chemerin, and visfatin[7,8]. These increases contribute to inflammation of the intestinal tract, whereas the downregulation of the beneficial adipokine adiponectin contributes to the pathogenesis of CD[7,8]. Creeping fat has distinctive features, including larger size and greater immune cell infiltration, differentiating it from normal mesenteric fat[9]. Furthermore, the selective enlargement of fat depots around diseased areas in CD patients indicates a causal correlation between MAT and mucosal alterations[10]. Mesenteric fat is also a source of C-reactive protein and a target of bacterial translocation, providing further evidence for the complex interactions between adipose tissues and inflammatory responses in CD[11]. The identification of this multifaceted relationship has broadened understanding of the pathogenesis of CD, highlighting the importance of MAT and creeping fat in inflammation, immune responses, and disease progression[12,13].
Gut microbial communities may be present in creeping fat. The composition of the gut microbiota in patients with CD is closely associated with the location of the disease, with the microbiota differing in patients with colonic and ileal CD. In addition to differences in their gut microbiota populations, patients with isolated ileal and colonic CD exhibit significant differences in disease behaviors, biomarkers, and responses to immunotherapy. In contrast, patients with colonic CD and colonic UC have similar gut microbiota compositions and disease behaviors.
Bacteria were found to persist in creeping fat within surgically excised specimens, suggesting that peritoneal creeping fat around the intestines prevented the dissemination of bacteria from the affected site into the bloodstream, thereby delaying disease progression. As the amount of creeping fat increases, however, these intestinal bacteria remain trapped in adipose tissue, which continues to migrate and proliferate towards the lesion site. The bacteria that enter the mesentery following intestinal barrier damage may be the root cause of the formation of creeping fat. The disruption of the intestinal barrier may play a role in the pathogenesis of CD and contribute to unfavorable patient prognosis. However, the temporal relationship between intestinal barrier disruption and gut microbiota dysbiosis remains unclear.
Inflammation-induced barrier dysfunction and intestinal microbiota dysbiosis have emerged as critical factors in CD pathogenesis[14,15]. Intestinal permeability has been linked to visceral adiposity[2], and the bacteria and bacterial DNA in MAT have been detected in patients with metabolic complications of obesity[16]. A microbiotic signature has been identified in MATs from CD patients, indicating that MAT-associated microbiota are involved in CD progression[14]. More importantly, the translocation of viable microbiota within human MAT was found to polarize macrophages, leading to adipogenesis, and promoting creeping fat formation in patients with CD[15]. These findings therefore suggested that gut microbiota might mediate the roles of MAT and creeping fat in CD.
Weakening of the intestinal barrier in CD may lead to the translocation of dysregulated gut microbiota into the MAT, triggering local and even systemic disturbances in fat metabolism and exacerbating intestinal inflammation and epithelial fibrosis in CD. The present study therefore evaluated the differences in histopathological characteristics and the levels of cytokines and adipokines in collected clinical mesenteric and intestinal tissues from CD patients and clinically healthy controls. Intestinal bacteria from these individuals were subsequently transplanted into C57BL/6 mice with 2,4,6-trinitrobenzene sulfonic acid (TNBS)-triggered CD. Markers of intestinal permeability and intestinal barrier function were evaluated, as were the blood concentrations of cytokines, adipokines and inflammatory factors, as well as histopathological alterations. Together, these findings suggested that targeting MAT and creeping fat may offer promise for therapeutic interventions in patients with CD.
MATERIALS AND METHODS
Collection of clinical specimens
Twelve patients definitively diagnosed with CD at the Second Xiangya Hospital were prospectively selected. Anthropometric parameters were measured and clinical phenotypes determined. Subjects were excluded if they were unable or refused to provide body tissue samples, had a history of abdominal surgery, had used antibiotics in the previous 2 weeks, had surgical contraindications, had an acute digestive tract infection or perforation, experienced an identified bleeding illness, or had been diagnosed with end-stage cancer.
Colon and MAT specimens were harvested from the mesentery of the colon corresponding to the lesions in CD patients. Control colon and MAT specimens were harvested from the mesentery of lesion-free colon areas in these CD patients. Lesion-free areas were defined as colonic mucosae without mucosal lesions, as determined by pathological diagnosis, and located > 5 cm from visible lesions. MAT and colon tissue samples were fixed in formalin or stored at -80 °C. All patients signed written informed consent forms, stating that they understood the main content and purpose of the study. The study protocol was approved by the Institutional Review Board of The Second Xiangya Hospital, CSU (approval No. 2022-333).
Histopathological examinations by HE and Masson staining
Human and mouse MATs and intestinal tissues were dehydrated using an alcohol series, incubated for 15 minutes with a 1:1 mixture of alcohol and xylene, and incubated for 15 minutes with xylene until the samples seemed to be transparent. The tissue samples were subsequently embedded in paraffin, sliced and stained with hematoxylin-eosin (HE) or Masson stain (Solarbio, Beijing, China). The samples were subsequently viewed and photographed using an inverted microscope (Olympus, Tokyo, Japan).
Immunohistochemical staining
Human intestinal tissue slices were stained with antibody to alpha-smooth muscle actin (α-SMA) using standard immunohistochemical staining (IHC) staining procedures, as previously described[17]. Briefly, tissue samples were deparaffinized and rehydrated. Endogenous peroxidases were blocked by incubation with 3% H2O2 for 10 minutes, and non-specific binding sites were blocked by incubation with 5% BSA for 2 hours. The samples were incubated with primary anti-α-SMA antibody (dilution 1:1000, 80008-1-RR, Proteintech, Wuhan, Hubei Province, China) overnight at 4 °C, washed, and incubated with poly-IgG-HRP-conjugated secondary antibody (Boster, Wuhan, Hubei Province, China) for 30 minutes at 37 °C. After washing, the samples were incubated with 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, United States) for 5-10 minutes at room temperature. The samples were viewed under a microscope, and the expression of α-SMA was quantified using Image J software (NIH, Bethesda, MD, United States).
Real-time reverse transcriptase-polymerase chain reaction
Total RNAs were extracted from tissue samples using Trizol reagent, followed by the synthesis of cDNA using StarScript II first strand cDNA Synthesis Mix (GenStar, China). Gene expression was determined by real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) using SYBR®Green PCR Master Mix (Qiagen GmbH), specific primer sequences (Supplementary Table 1) and an ABI7500 thermal cycler (Applied Biosystems; United States). The relative expression of target genes was calculated using the 2-ΔΔCt method, with GAPDH utilized as an internal reference.
Experimental animals
All animal experiments were strictly reviewed and approved by the Animal Care and Use Committee of Central South University. Twenty-four male C57BL/6 mice, aged 6 weeks, were obtained from the Hunan SJA Laboratory Animal Co., Ltd (SLAC) experimental animal center (Changsha, China). The mice were maintained at an ambient temperature of 22 °C ± 2 °C and a relative humidity of 50% ± 5% with a 12-hours light-dark cycle (light on at 7: 00 am).
Mouse model of antibiotics-induced bacterial depletion
Eighteen mice were administered an aqueous solution containing a mixture of the antibiotics (Abx) ampicillin, neomycin, metronidazole and vancomycin for 2 weeks to deplete bacteria[18]. Bacterial depletion was confirmed by colony-forming unit (CFU) assays. Briefly, mouse feces were collected into 1 mL sterile phosphate-buffered saline (PBS) in a sterilizing tube, and the homogenate was diluted 1000-fold. A 40 μL aliquot of this solution was applied to a TSA medium plate. The plates were incubated overnight at 37 °C, and the CFUs on each plate were counted.
Bacterial depletion was also confirmed by broad-range 16S PCR. Briefly, DNA was isolated from these fecal samples using TIANamp Stool DNA kits (Tiangen, Beijing, China) and subjected to qPCR to quantify 16S rDNA genes.
TNBS-induced CD in mice
The 18 Abx mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium. A catheter was inserted about 3 cm into the anus of each mouse, followed by the administration of 150 μL 1% TNBS in PBS. Control mice were administered an equal volume of PBS. The catheter was withdrawn, and the mice were fixed in a handstand posture for 2 minutes. Mice showed evidence consistent with CD 4 days later. For histopathological verification, inflammation in mouse colon samples was scored by researchers blinded to allocation using a score developed for DSS colitis[19].
Fecal microbiota transplantation
Fecal transplantation was performed as described[20]. In brief, stool samples were obtained five clinically healthy individuals, with no symptoms or family history of gastrointestinal disorders and who passed blood screening tests[21] and five CD patients who had not used antibiotics or probiotics within 8 weeks before sampling. The stool samples were weighed and homogenized with sterile silica beads in 1 mL of PBS at 45 Hz for 1 minutes, filtered with a 100-μm strainer, and centrifuged at 6000 g for 15 minutes. Each sample was resuspended in PBS containing 10% (v/v) glycerol and frozen at -80 °C. Before transplantation, the fecal samples were thawed, centrifuged and resuspended in PBS. Each mouse with TNBS-induced CD was administered 20 mg of fecal sample in 100 μL PBS containing 5 × 109 CFUs by oral gavage once daily for 10 days, with six mice each administered fecal samples from CD patients and normal controls and six control mice and six with TNBS-induced CD administered PBS by oral gavage. All mice were subsequently sacrificed and their blood, colon tissues and MAT collected for further investigation.
DNA extraction from stool and quantification of harmful gut bacteria by qPCR
DNA was isolated from stool samples of healthy donors and CD patients using Qiagen QIAamp DNA Stool Mini Kit. DNA quantity and quality were checked by Nanodrop, with the ratio of absorption at 260 nm/280 nm > 1.8 considered adequate. The abundances of adherent-invasive Escherichia coli (AIEC)[22], Clostridium difficile (C. difficile)[21], enterotoxigenic Bacteroides fragilis (ETBF)[23], and Fusobacterium nucleatum (F. nucleatum)[24] in stool samples was measured by qPCR as described above, using 20 ng DNA and specific primers for each bacterial species (Supplementary Table 2). The relative amounts of AIEC, C. difficile, ETBF, and F. nucleatum DNA in each sample were determined using a comparative cycle threshold method, normalized to the quantity of 16S RNA.
Intestinal permeability
intestinal permeability was determined using fluorescein isothiocyanate conjugated dextran (FITC-dextran) assay. Mice were fasted overnight, followed by oral administration of FITC-dextran (Sigma-Aldrich, 60 mg/100 g body weight) 4 hours prior to blood collection. FITC concentrations were determined using fluorescence spectrophotometers, with an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
Serum concentrations of cytokines and adipokines
Blood samples obtained from each mouse were allowed to clot, and serum was obtained by centrifugation. A 100 μL aliquot of serum was added to each well of an ELISA plate included in ELISA kits measuring serum concentrations of endotoxin (CSB-E13066m, CUSABIO, Wuhan, Hubei Province, China), IL-6 (CSB-E04639m, CUSABIO), IL-1β (Ab197742, Abcam, Cambridge, United Kingdom), TNF-α (CSB-E04741m, CUSABIO), MCP-1 (Ab208979, Abcam), leptin (ab100718, Abcam), and adiponectin (CSB-E07272m, CUSABIO). The plates were incubated for 2 hours at 37 °C, the liquid was removed, and 100 μL biotinylated targeted antibody were added to each well. The plates were incubated for 1 hour at 37 °C; each well was washed three times; 100 μL HRP-avidin were added to each well; and the plates were incubated for 1 hour at 37 °C. After washing the wells five times, 90 μL TMB substrate were added to each, and the plates were incubated for 30 minutes at 37 °C in the dark. A 50 μL aliquot of Stop solution was added to each well, and the absorption of each at 450 nm was measured spectrophotometrically. The concentrations of endotoxin, IL-1β, TNF-α, MCP-1, leptin and adiponectin were calculated relative to standard curves for each.
Immunofluorescent staining
Tissue slices embedded in paraffin were stained for occludin using standard immunofluorescence staining methods[25]. Briefly, the samples were deparaffinized, rehydrated and blocked, as described for IHC staining. The samples were incubated with primary anti-occludin antibody (dilution 1:1000, 27260-1-AP, Proteintech) overnight at 4 °C, followed by incubation with FITC-labeled goat anti-rabbit antibody (dilution 1:200, A0562, Beyotime) for 2 hours at room temperature in the dark. The samples were subsequently incubated with 4′,6-diamino-2-phenyl indole (Sigma-Aldrich) for 5 minutes at room temperature in the dark. Fluorescence was visualized using a fluorescence microscope (Zeiss, Jena, Germany) and quantified using Image J software.
Immunoblotting
Protein samples were extracted from mouse intestinal tissues and quantified using bicinchoninic acid protein assay kits (Beyotime Inst. Biotech). Supernatants containing 30 μg protein were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the separated proteins were electroblotted onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, United States). The membranes were fixed for 1 hour using 5% nonfat milk in Tris-buffered saline with 0.1% Tween (TBS-T), rinsed three times with TBS-T, and incubated overnight at 4 °C with primary antibodies against α-SMA, vimentin, occluding, ZO-1, E-cadherin, cannabinoid receptor 1 (CB1), CB2, and GAPDH (dilution 1:1000, Proteintech, Wuhan, Hubei Province, China). The membranes were washed three times with TBS-T for 15 minutes each, and incubated for 1 hour at room temperature (RT) with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:2000; Santa Cruz Technologies, CA, United States). The blots were washed three times in TBS-T for 5 minutes each, and protein binding was visualized using an enhanced chemiluminescence (ECL) kit (Applygen Inst. Biotech, Beijing, China).
Statistical analysis
All data were expressed as mean ± SD. Results in three or more groups were compared using one-way analysis of variance followed by Tukey’s post hoc tests. Results in two groups were compared using two-tailed unpaired or paired Student’s t-tests. P values < 0.05 were defined as statistically significant.
RESULTS
Clinical characteristics of the 12 included CD patients
Colon tissue and MAT specimens were collected from colonic areas with and without lesions of 12 CD patients. These 12 patients included three (25.0%) females and nine (75.0%) males. All 12 patients were aged > 16 years, with eight (66.7%) aged 17-40 years, and four (33.3%) aged > 40 years. One patient had a Disease duration was < 1 year in one patient (8.3%), 1-5 years in seven (58.3%) patients, and > 5 years in four (33.3%) patients. All 12 (100.0%) patients had recurrent CD and had received surgical treatment. Preoperative white blood cell counts were below 10 × 109/L in 10 patients (83.3%) and above 10 × 109/L in two patients (16.7%). Hemoglobin concentrations were < 100 g/L in three patients (25.0%) and ≥ 100 g/L in nine patients (75.0%). Serum albumin concentration was < 30 g/L in only one patient (8.3%) and ≥ 30 g/L in the remaining 11 (91.7%). Preoperative body mass index (BMI) was < 18.5 kg/m2 in four patients (33.3%) and ≥ 18.5 kg/m2 in eight patients (66.7%) (Table 1).
Table 1.
clinical data of 12 Crohn’s disease patients were collected in this study, n (%)
|
Patient characteristics
|
Quantity (percentage)
|
| Sex | |
| Female | 3 (25.0) |
| Male | 9 (75.0) |
| Age (years old) | |
| ≤ 16 | 0 (0.0) |
| 17-40 | 8 (66.7) |
| > 40 | 4 (33.3) |
| Course of the disease (years) | |
| < 1 | 1 (8.3) |
| 1-5 | 7 (58.3) |
| > 5 | 4 (33.3) |
| Onset or recurrence of diseases | |
| Onset | 0 (0.0) |
| Recurrence | 12 (100.0) |
| WBC count (× 109) | |
| < 10.0 | 10 (83.3) |
| ≥ 10.0 | 2 (16.7) |
| HGB (g/L) | |
| < 100.0 | 3 (25.0) |
| ≥ 100.0 | 9 (75.0) |
| ALB (g/L) | |
| < 30.0 | 1 (8.3) |
| ≥ 30.0 | 11 (91.7) |
| BMI (kg/m2) | |
| < 18.5 | 4 (33.3) |
| ≥ 18.5 | 8 (66.7) |
This table succinctly presents the demographic and clinical characteristics of the 12 participants diagnosed with Crohn’s disease in the study. Among the cohort, 9 individuals (75.0%) were male, and 3 (25.0%) were female. The age distribution revealed that all participants were above 17 years old, with 8 patients (66.7%) falling between 17 and 40 years old, 4 patients (33.3%) aged over 40 years, and 1 patient (8.3%) having a disease duration within 1 year. In terms of disease duration, 7 patients (58.3%) had a duration between 1 and 5 years, while 4 patients (33.3%) had a disease duration exceeding 5 years. All 12 patients experienced disease recurrence. Preoperative test results indicated that 10 individuals (83.3%) exhibited white blood cell counts below 10.0 × 109, and 2 individuals (16.7%) had counts reaching 10.0 × 109. Hemoglobin levels below 100 g/L were observed in 3 patients (25.0%), with the remaining 9 patients (75.0%) reaching levels of 100 g/L. Serum albumin levels were below 30 g/L in only 1 patient (8.3%), while the remaining 11 patients (91.7%) achieved a serum albumin level of 30 g/L or higher. Preoperative measurements of height and weight demonstrated that 4 patients (33.3%) had a body mass index (BMI) value below 18.5 kg/m2, and the remaining 8 patients (66.7%) had a BMI value equal to or exceeding 18.5 kg/m2. WBC: White blood cell; HGB: Hemoglobin; ALB: Albumin; BMI: Body mass index.
Histopathological alterations in mesenteric adipose and intestinal tissues of CD patients
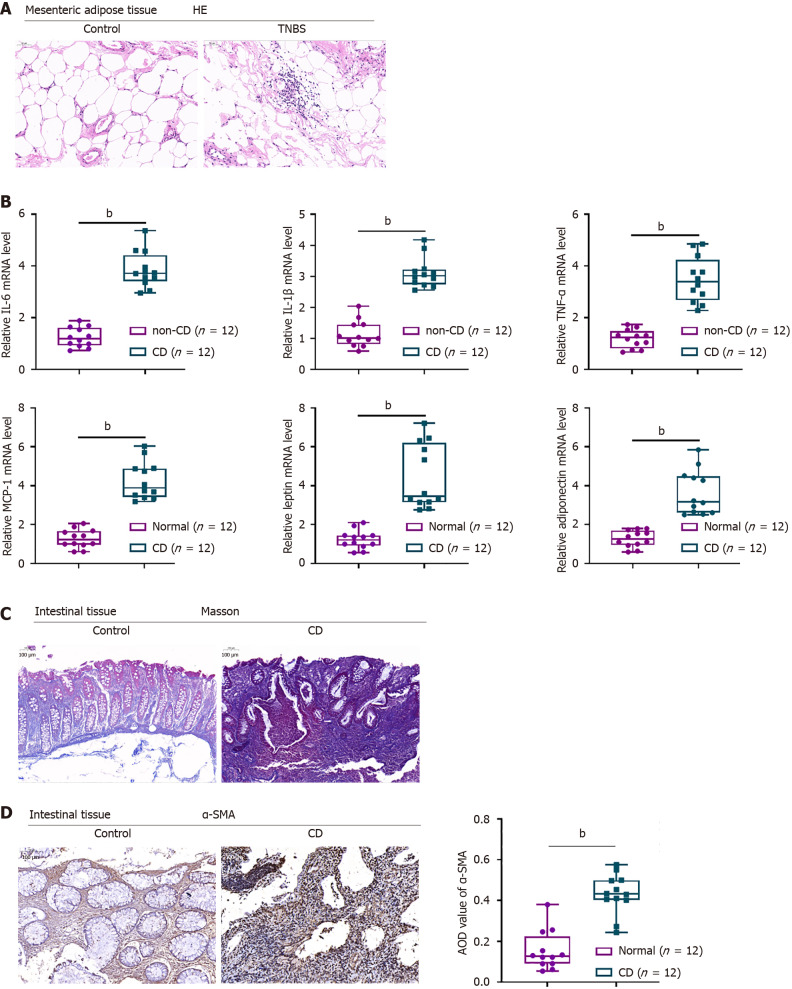
Histopathological examination revealed that MAT structure was more disorganized in diseased than in non-diseased colonic areas of CD patients, with an inflected neutrophil infiltration (Figure 1A). IL-6, IL-1β, TNF-α, MCP-1, leptin and adiponectin mRNA levels were significantly higher in diseased than in non-diseased MAT of CD patients (Figure 1B). Masson staining showed that areas of fibrosis were larger in diseased than in non-diseased colon tissue of CD patients (Figure 1C). Moreover, immunohistochemical staining showed that α-SMA levels were significantly higher in diseased than in non-diseased colon tissues, further confirming the fibrotic changes in the former (Figure 1D).
Figure 1.
Histopathological alterations in mesenteric adipose and intestinal tissues of patients with Crohn’s disease. A: Mesenteric adipose tissues from Crohn’s disease (CD) patients and non-CD subjects stained with hematoxylin-eosin to assess histopathological alterations (× 150); B: Real-time reverse transcriptase-polymerase chain reaction assays of the levels of expression of interleukin-6 (IL-6), IL-1β, tumour necrosis factor alpha, MCP-1, leptin, and adiponectin mRNAs; C: Intestinal tissues from CD patients and non-CD subjects stained with Masson stain to evaluate fibrotic alterations (× 150); D: Immunohistochemical staining of alpha-smooth muscle actin in intestinal tissues from CD patients and non-CD subjects (× 150). Average optical density was quantified using ImageJ software (right panel). n = 12, bP < 0.01 or n = 3, bP < 0.01 by paired t-tests. HE: Hematoxylin and eosin; TNBS: 2,4,6-trinitrobenzene sulfonic acid; CD: Crohn’s disease; TNF-α: Tumour necrosis factor alpha; IL: Interleukin; α-SMA: Alpha-smooth muscle actin; AOD: Average optical density.
Clinical characteristics of the fecal donors
Fecal samples were obtained from five patients with CD, one female (20.0%) and four males (80.0%). All five were aged > 16 years, including two (40.0%) aged 17-40 years and three (60.0%) aged > 40 years. Disease duration was < 1 year in one patient (20.0%), 1-5 years in three patients (60.0%), and > 5 years in one patient (20.0%). All five patients (100.0%) had recurrent CD. Two patients (40.0%) had white blood cell counts < 10 × 109/L and three (60.0%) had white blood cell counts > 10 × 109/L. All five patients (100.0%) had hemoglobin concentrations ≥ 100 g/L (100.0%); three (60.0%) had serum albumin concentrations < 30 g/L, and two (40.0%) had serum albumin concentrations ≥ 30 g/L. BMI was < 18.5 kg/m2 in two patients (40.0%) and ≥ 18.5 kg/m2 in three patients (60.0%) (Table 2).
Table 2.
Clinical data of 5 Crohn’s disease patients who served as stool specimen donors, n (%)
|
Patient characteristics
|
Quantity (percentage)
|
| Sex | |
| Female | 1 (20.0) |
| Male | 4 (80.0) |
| Age (years old) | |
| ≤ 16 | 0 (0.0) |
| 17-40 | 2 (40.0) |
| > 40 | 3 (60.0) |
| Course of the disease (years) | |
| < 1 | 1 (20.0) |
| 1-5 | 3 (60.0) |
| > 5 | 1 (20.0) |
| Onset or recurrence of diseases | |
| Onset | 0 (0.0) |
| Recurrence | 5 (100.0) |
| WBC count (× 109) | |
| < 10.0 | 2 (40.0) |
| ≥ 10.0 | 3 (60.0) |
| HGB (g/L) | |
| < 100.0 | 0 (0.0) |
| ≥ 100.0 | 5 (100.0) |
| ALB (g/L) | |
| < 30.0 | 3 (60.0) |
| ≥ 30.0 | 2 (40.0) |
| BMI (kg/m2) | |
| < 18.5 | 2 (40.0) |
| ≥ 18.5 | 3 (60.0) |
This table succinctly presents the demographic and clinical characteristics of the 5 stool specimen donor patients included in this study. Among them, 4 were male (80.0%) and 1 was female (20.0%). Age distribution showed that all patients were over 17 years old, 2 patients (40.0%) were between 17 and 40 years old, and the remaining 3 patients (60.0%) were over 40 years old. In terms of disease course, only 1 patient (20.0%) had a disease course within 1 year, 3 patients (60.0%) had a disease course between 1 and 5 years, and 1 patient (20.0%) had a disease course exceeding 5 years. All 12 patients had recurrent disease. Laboratory test results showed that the white blood cell (WBC) count of 2 people (40.0%) was lower than 10.0 × 109, and the WBC count of 3 people (60.0%) reached 10.0 × 109 and above. All 5 patients (100.0%) had hemoglobin levels of 100 g/L and above. Three patients (60.0%) had serum ALB levels below 30 g/L, and the remaining 2 patients (40.0%) had serum albumin levels of 30 g/L or higher. Height and weight measurements showed that 2 patients (40.0%) had a body mass index (BMI) value lower than 18.5 kg/m2, and the remaining 3 patients (60.0%) had a BMI value equal to or exceeding 18.5 kg/m2. WBC: White blood cell; HGB: Hemoglobin; ALB: Albumin; BMI: Body mass index.
Fetal microbiota transplantation in mice with TNBS-induced CD
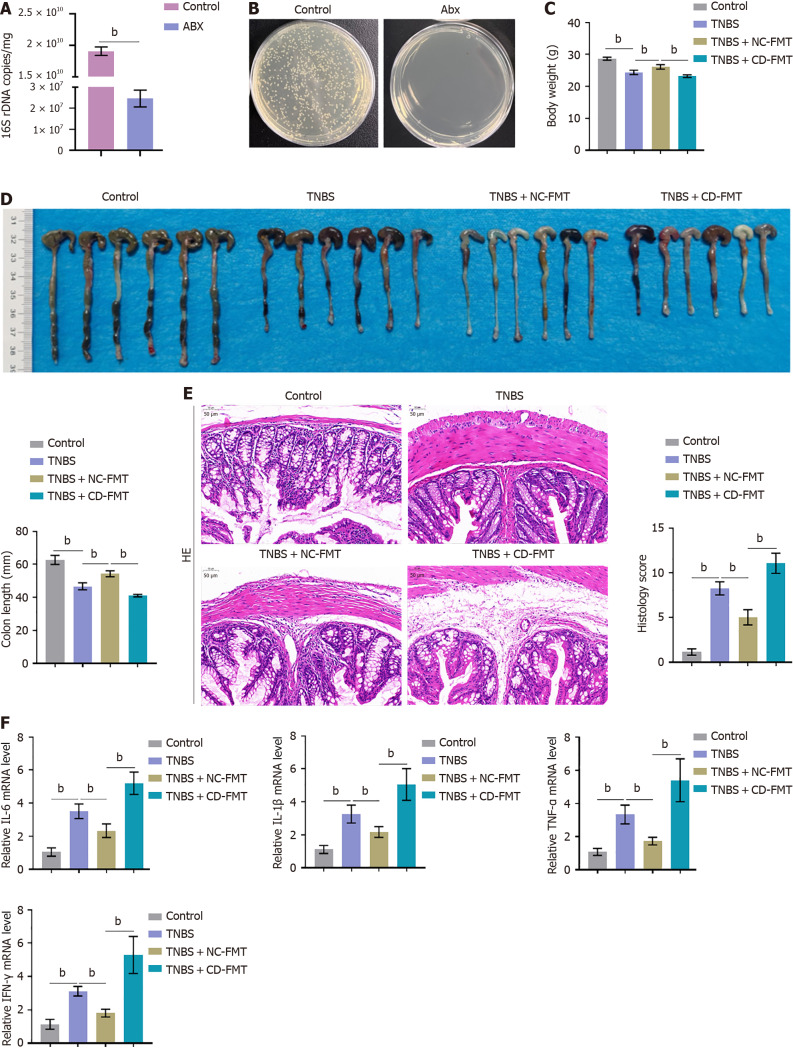
The presence of harmful bacteria associated with CD was assessed in fecal samples collected from five CD patients and five healthy control subjects by qPCR assays of 16S DNA. The relative abundances of AIEC, C. difficile, ETBF, and F. nucleatum were found to be significantly higher in fecal samples from CD patients than from normal controls (Supplementary Figure 1), suggesting that gut bacteria populations differ in CD patients and healthy donors. Before fetal microbiota transplantation (FMT), mice were subjected to antibiotic cleaning to achieve a pseudo germ-free intestinal environment. Bacterial culture measuring CFUs and PCR measuring 16S rDNA showed that antibiotic treatment drastically reduced the number of bacteria (Figure 2A and B). The mice were subsequently treated with TNBS to induce a CD-like condition, followed by FMT using samples from CD patients or clinically healthy donors. The body weights and colon lengths were much lower in mice with TNBS-induced CD than in control mice, with FMT of samples from healthy donors (NC-FMT) partially reversing these reductions in body weight and colon lengths (Figure 2C and D). In contrast, the body weights and colon lengths were significantly lower in TNBS-treated mice that had undergone FMT of samples from CD patients (CD-FMT) than from healthy donors (NC-FMT) (Figure 2C and D). Evaluations of histopathological characteristics showed that histology scores were significantly higher in TNBS-treated than in control mice, that these scores in TNBS-treated mice were somewhat reduced by NC-FMT, and that scores in TNBS-treated mice were higher following CD-FMT than NC-FMT (Figure 2E). IL-6, IL-1β, TNF-α, and interferon-gamma (IFN-γ) mRNA levels were found to be significantly higher in colon samples from TNBS-treated than from control mice, but were downregulated in the former by NC-FMT (Figure 2F). IL-6, IL-1β, TNF-α, and IFN-γ mRNA levels, however, were significantly higher in TNBS-treated mice following CD-FMT than NC-FMT (Figure 2F).
Figure 2.
Fecal microbiota transplantation into mice with 2,4,6-trinitrobenzene sulfonic acid-induced Crohn’s disease. A and B: Mice were subjected to antibiotic cleaning to achieve a pseudo germ-free intestinal environment, with the results verified by 16S rRNA sequencing. n = 6. bP < 0.01 by unpaired t-tests; C and D: 2,4,6-trinitrobenzene sulfonic acid (TNBS)-treated mice underwent fetal microbiota transplantation (FMT) from clinically healthy donors or Crohn’s disease patients, and their body weights and colon lengths were monitored; E: Evaluation of histopathological alterations in colon samples by hematoxylin-eosin staining and evaluation of histology scores (× 150); F: Real-time reverse transcriptase-polymerase chain reaction assays of interleukin-6 (IL-6), IL-1β, tumour necrosis factor alpha, and interferon-gamma mRNA expression levels in colon tissue samples. n = 6, bP < 0.01 vs control; bP < 0.01 vs TNBS alone; bP < 0.01 vs TNBS + healthy donor (NC)-FMT, by one-way ANOVA with post-hoc Tukey HSD test. Abx: Antibiotics; TNBS: 2,4,6-trinitrobenzene sulfonic acid; CD: Crohn’s disease; NC: Normal control; FMT: Fetal microbiota transplantation; IFN-γ: Interferon-gamma; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
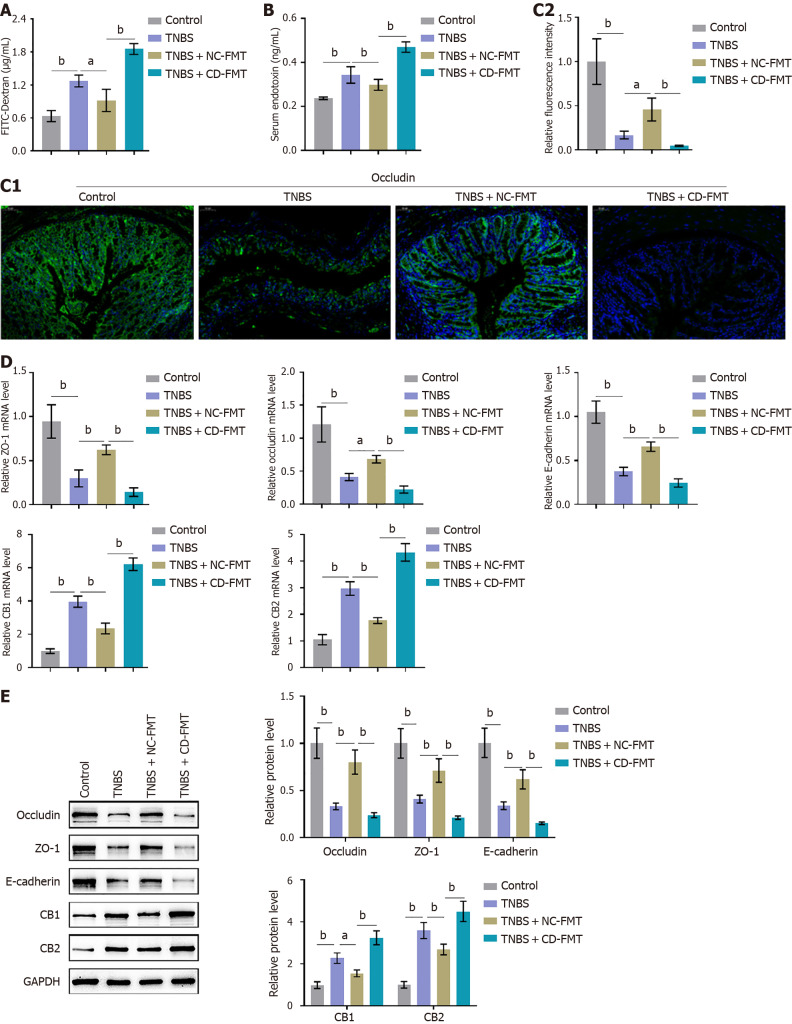
Effects of CD-FMT on intestinal penetration in mice with TNBS induced CD
FITC-dextran assays showed that TNBS significantly increased intestinal permeability in mice, an effect partially decreased by NC-FMT (Figure 3A). Moreover, intestinal permeability was greater in TNBS-treated mice following CD-FMT than NC-FMT (Figure 3A). Levels of serum endotoxin showed similar trends, with TNBS significantly increasing and NC-FMT reducing endotoxin levels and endotoxin levels being higher in TNBS-treated mice following CD-FMT than NC-FMT (Figure 3B). TNBS treatment significantly reduced the levels of occludin, a marker of intestinal barrier function, in colon tissues, an effect partially reversed by NC-FMT, with occludin levels being lower following CD-FMT than NC-FMT (Figure 3C). TNBS treatment reduced occludin, ZO-1, and E-cadherin protein levels, while increasing CB1 and CB2 protein levels, in mice; these alterations were partially reversed by NC-FMT, but enhanced by CD-FMT (Figure 3D and E).
Figure 3.
Effects of gut microbiota from Crohn’s disease patients on intestinal permeability in mice with 2,4,6-trinitrobenzene sulfonic acid-induced Crohn’s disease. A: Fluorescein isothiocyanate conjugated-dextran assays of intestinal permeability; B: ELISA assays of serum concentrations of lipopolysaccharide-binding protein to determine serum endotoxin levels; C: Immunofluorescent staining of colon tissues to determine the levels of occludin expression (× 150). Average fluorescence density was quantified using ImageJ software (right panel); D and E: Real-time reverse transcriptase-polymerase chain reaction and immunoblotting assays showing the levels of occludin, ZO-1, E-cadherin, CB1, and CB2 mRNA and protein in colon tissues. n = 6 or 3, bP < 0.01 vs control; aP < 0.05, bP < 0.01 vs 2,4,6-trinitrobenzene sulfonic acid (TNBS) alone; bP < 0.01 vs TNBS + NC-fetal microbiota transplantation, by one-way ANOVA with post-hoc Tukey HSD test. TNBS: 2,4,6-trinitrobenzene sulfonic acid; CD: Crohn’s disease; NC: Normal control; FMT: Fetal microbiota transplantation.
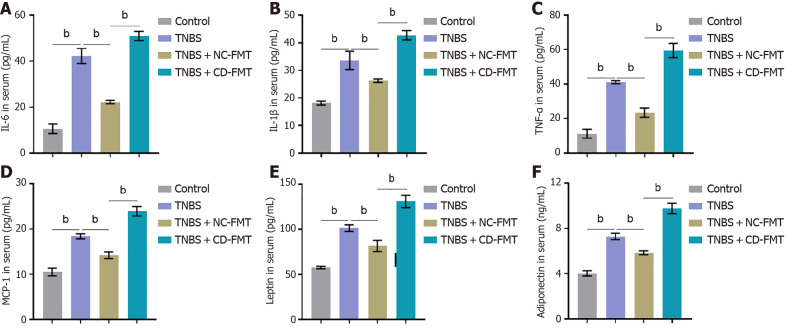
Compared with untreated mice, treatment with TNBS markedly increased the serum concentrations of cytokines and adipokine, including IL-6, IL-1β, TNF-α, MCP-1, leptin, and adiponectin (Figure 4). These changes were partially revered by NC-FMT, but were enhanced by CD-FMT (Figure 4).
Figure 4.
ELISA determinations of serum concentrations of cytokines and adipokines. A: Interleukin (IL)-6; B: IL-1β; C: Tumour necrosis factor alpha; D: MCP-1; E: Leptin; F: Adiponectin. n = 6, bP < 0.01 vs control; aP < 0.05, bP < 0.01 vs 2,4,6-trinitrobenzene sulfonic acid (TNBS) alone; bP < 0.01 vs TNBS + NC-fetal microbiota transplantation, by one-way ANOVA with post-hoc Tukey HSD test. TNBS: 2,4,6-trinitrobenzene sulfonic acid; CD: Crohn’s disease; NC: Normal control; FMT: Fetal microbiota transplantation.
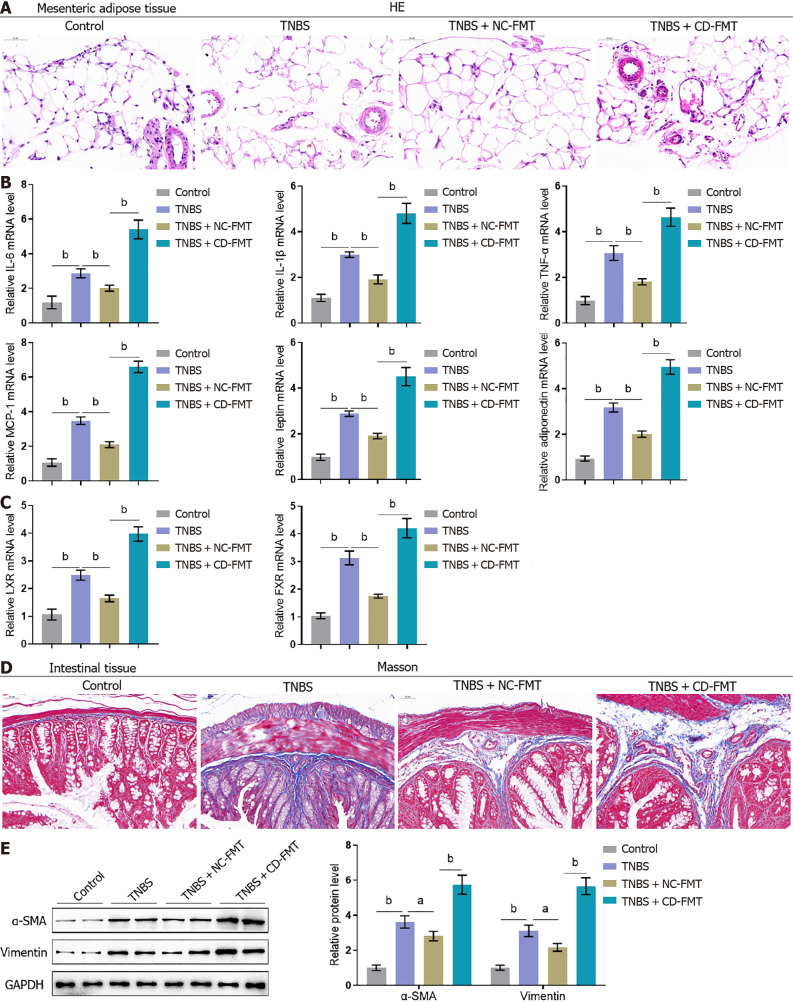
Effects of gut microbiota from CD patients on histopathological alterations in mesenteric adipose and intestinal tissues of mice with TNBS-induced CD
Histological examination of MAT from mice with TNBS-induced CD showed extensive small venous congestion, marginated neutrophils, perivascular accumulation of neutrophils, and hematomas, suggesting that TNBS induced inflammatory changes in the colonic mucosa (Figure 5A). These alterations were partially reversed by NC-FMT, but were exacerbated by CD-FMT (Figure 5A). Because of the mutual inhibition of LXR and FXR induced by pro-inflammatory mediators in IBDs[26-28], the levels of LXR and FXR in these mice were analyzed to assess whether they act as nuclear receptors involved in MAT functions in mice with CD. TNBS treatment was found to increase the levels of IL-6, IL-1β, TNF-α, MCP-1, leptin, adiponectin, LXR, and FXR mRNAs (Figure 5B and C). These effects were partially abolished by NC-FMT, but were further enhanced by CD-FMT (Figure 5B and C).
Figure 5.
Effects of gut microbiota from Crohn’s disease patients on the histopathological characteristics of mesenteric adipose and intestinal tissues from mice with 2,4,6-trinitrobenzene sulfonic acid-induced Crohn’s disease. A: Mesenteric adipose tissues collected from mice were stained with hematoxylin-eosin to assess histopathological alterations (× 150); B and C: Real-time reverse transcriptase-polymerase chain reaction assays of interleukin-6 (IL-6), IL-1β, tumour necrosis factor alpha, MCP-1, leptin, adiponectin, LXR, and FXR mRNA levels in mouse tissues; D: Intestinal tissues from mice stained with Masson stain to evaluate fibrotic alterations (× 150); E: Immunoblotting assays of alpha-smooth muscle actin and vimentin protein levels in mouse intestinal tissues. n = 6 or 3, bP < 0.01 vs control; aP < 0.05, bP < 0.01 vs 2,4,6-trinitrobenzene sulfonic acid (TNBS) alone; bP < 0.01 vs TNBS + NC-fetal microbiota transplantation, by one-way ANOVA with post-hoc Tukey HSD test. TNBS: 2,4,6-trinitrobenzene sulfonic acid; CD: Crohn’s disease; NC: Normal control; FMT: Fetal microbiota transplantation; IFN-γ: Interferon-gamma; TNF-α: Tumour necrosis factor alpha; IL: Interleukin; HE: Hematoxylin and eosin; α-SMA: Alpha-smooth muscle actin.
Masson staining showed that areas of intestinal fibrosis were larger in TNBS-treated than in control mice; that fibrosis was partially reduced by NC-FMT; and that fibrotic areas were further increased by CD-FMT (Figure 5D). These results were supported by assessing α-SMA and vimentin levels in intestinal tissue samples, with both being more highly expressed in TNBS-treated than in control mice, partially reduced by NC-FMT, and further enhanced by CD-FMT (Figure 5E).
DISCUSSION
The complex relationships involving MAT, creeping fat, inflammation, and gut microbiota in CD are topics of growing interest. The gut microbiota were shown to be involved in many physiological processes, including energy homeostasis, metabolism, intestinal epithelial repair, immune activity, and neurobehavioral development[29]. Several types of gut microbiota have been found to play significant roles in human pathological processes. Because alterations in gut microbiota among CD patients with lesions at different sites are inconsistent, their underlying mechanisms remain unclear. It is uncertain whether pathological alterations observed in CD patients, such as intestinal fibrosis and narrowing and weakened intestinal barrier function, lead to significant changes in gut microbiota populations, or if these changes in gut microbiota populations precede these pathological alterations. Alternatively, alterations in gut microbiota populations and histopathological changes in CD may mutually promote each other, forming a positive feedback system that drives the progression of CD. Moreover, it is unclear whether changes in gut microbiota are direct causes of CD. The present study explored the relationships among gut microbiota, MAT hyperplasia and hypertrophy, and intestinal fibrosis through FMT, providing new insights for assessing the pathogenesis of CD and its treatment.
This study showed that significant histopathological alterations occurred in creeping fat and intestinal tissues of CD patients, including structural disorganization, inflammatory infiltrates, and fibrotic changes. Moreover, the levels of expression of pro-inflammatory cytokines, such as TNF-α, IL-1β, MCP-1, and IL-6, and adipokines, such as leptin and adiponectin, were significantly elevated in creeping fat, suggesting that the local colonic mesentery of CD patients is influenced by a pathogenic factor likely associated with infection or immunity. Microorganisms present in the creeping fat of CD patients may originate from the gut microbiota. Compared with CD-unaffected colonic tissues, CD-affected colonic tissues showed marked higher levels of fibrosis, along with significantly higher expression of the fibrosis marker α-SMA. Creeping fat and intestinal stricture in CD patients often occur simultaneously in the same intestinal segments[30]. Moreover, intestinal segments with obstruction are often accompanied by significantly hypertrophied MAT. These findings suggest that the gut microbiota, MAT hypertrophy, and intestinal fibrosis may mutually promote each other in the pathogenesis of CD. However, but the upstream-downstream relationships among these three factors in the pathogenesis of CD remain undetermined.
The interrelationships among gut microbiota, MAT hyperplasia, and intestinal fibrosis in the pathogenesis of CD were investigated using a mouse model of TNBS-induced CD. FMT from clinically healthy donors had beneficial effects on body weights, colon lengths, and histopathological alterations in TNBS-treated mice, whereas FMT from CD patients had the opposite effects. Furthermore, FMT from clinically healthy donors partially improved, whereas FMT from CD patients exacerbated, intestinal permeability, barrier function, and serum cytokine and adipokine levels, indicating that gut microbiota might mediate the effects of MAT and creeping fat on CD progression. These findings provide a rationale for further studies targeting MAT and creeping fat, as they may provide a promising avenue for therapeutic intervention in patients with CD.
The histopathological alterations observed in MAT from CD patients may enhance understanding of the mechanisms underlying the development of CD. The structural disorganization and congested neutrophilic inflammatory infiltrate in MAT reflect an active inflammatory response, consistent with findings showing that MAT mediates inflammation in CD[9]. The significant increases in proinflammatory factors, including IL-6, IL-1β, and TNF-α, along with chemokines like MCP-1, provide further evidence for the occurrence of active inflammatory responses in MAT from patients with CD. These factors contribute to immune cell recruitment and activation within the inflamed intestinal regions[31-33]. Additionally, the increased expression of adipokines, including leptin and adiponectin, in MAT from CD patients further underscores the complex interplay between adipose tissue and inflammation, as these adipokines have been implicated in modulating immune responses and inflammation in CD[34]. Furthermore, collagen deposition and α-SMA expression, both indicators of fibrosis[35], were found to be higher in intestinal tissues from CD patients than from normal controls, suggesting the occurrence of intestinal fibrosis in patients with CD. Intestinal fibrosis is among the most common complications of CD, often linked to the progression of disorder and a stricturing phenotype[36]. Collectively, these findings enhance understanding of active inflammatory responses in MAT and intestinal tissues from patients with CD, suggesting that MAT and creeping fat may play a role in inflammation and the promotion of CD progression.
Although dysbiosis of the intestinal microbiota has been found to affect CD development, the specific mechanisms by which intestinal microbiota might mediate the roles and effects of MAT and creeping fat in CD remain unclear[37]. Analysis of the fecal microbiota showed that the relative abundances of AIEC, C. difficile, ETBF, and F. nucleatum were higher in feces from CD patients rom from controls. These bacteria, especially AIEC, C. difficile, and ETBF, are well-documented pathogens in IBD, and F. nucleatum has been associated with the pathogenesis of colorectal cancer, a complication of IBD, suggesting that the altered gut microbiota in CD patients might promote inflammatory responses[38-41]. FMT from healthy donors (NC-FMT) partially ameliorated these increases, whereas FMT from CD patients (CD-FMT) resulted in further deterioration in tissue congestion and neutrophil infiltration in mice with TNBS-induced CD. These findings suggest that NC-FMT may improve key features of CD, such as inflammation and intestinal permeability[42]. Intestinal permeability was found to be significantly greater in mice with TNBS-induced CD than in control mice, as evidenced by the results of FITC-dextran assays and increased serum endotoxin levels, along with elevated expression of barrier function markers, such as occludin, ZO-1, and E-cadherin[43,44]. Interestingly, these barrier dysfunctions were partially improved by NC-FMT, but were exacerbated by CD-FMT, leading to higher levels of proinflammatory factors (IL-6, IL-1β, TNF-α, and IFN-γ) and adipokines (leptin and adiponectin) within both colon tissues and serum. Bariatric surgery and duodenojejunal bypass have been reported to reduce colitis in animals with chemically induced IBD[45]. Taken together, these findings suggest that NC-FMT could improve, and CD-FMT could exacerbate, TNBS-induced CD by acting on MAT and inflammation. The histopathological alterations observed in MAT from CD patients, including venous congestion, neutrophil accumulation, and hematoma, further support the hypothesis, that intestinal microbiota might mediate the effects of MAT on CD progression in mouse models.
Fibrosis in CD is a complex multifactorial complication with a high morbidity rate[46]. MAT has been shown to exhibit a pro-fibrotic phenotype[47], with increased levels of expression of fibrotic markers, including α-SMA and vimentin, which are key to the activation of myofibroblasts and subsequent fibrotic responses[48]. The gut microbiota play a complex and multifaceted role in CD, influencing both the local intestinal environment and systemic immune responses[49]. Dysbiosis, which has been consistently observed in patients with CD, may accelerate chronic inflammation and disease progression[50,51]. Studies assessing interactions between gut microbiota and MAT have shown that certain bacterial species can influence adipose tissue function, including its role in fibrosis[52]. For example, translocation of viable microbiota within human MAT was found to polarize macrophages, leading to adipogenesis, and promoting creeping fat formation in patients with CD[53]. In the present study, CD-FMC further elevated the levels of fibrotic markers, including α-SMA and vimentin, within colon tissues of mice with TNBS-induced CD, emphasizing the multifaceted abilities of intestinal microbiota to shape the inflammatory and fibrotic landscape in CD.
CONCLUSION
The findings of this study illuminated the complex interplay among MAT, creeping fat, inflammation, and intestinal microbiota in CD. MAT and creeping fat may play a potential role in CD progression, offering novel insights and promising avenues for therapeutic interventions. FMT from healthy donors may have therapeutic effects in patients with CD, whereas FMT from CD patients may exacerbate the symptoms of CD. These findings provide evidence for the multifaceted effects of intestinal microbiota on the roles of MAT and creeping fat in shaping inflammatory and fibrotic phenotypes in CD. Further research is needed to translate these findings into effective CD management strategies.
ACKNOWLEDGEMENTS
The authors thank the CD patients who provided tissue samples and the patients and volunteers who provided stool samples.
Footnotes
Institutional review board statement: The study protocol was approved by the Institutional Review Board of The Second Xiangya Hospital, CSU (approval No. 2022-333).
Institutional animal care and use committee statement: The animal use protocol listed below has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), The Second Xiangya Hospital, Central South University (approval No. 2022560).
Conflict-of-interest statement: The authors have no conflicts of interest and financial disclosures.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Amodeo G; Vorobjova T S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
Contributor Information
Qiang Wu, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Lian-Wen Yuan, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China. yuanlianwen@csu.edu.cn.
Li-Chao Yang, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Ya-Wei Zhang, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Heng-Chang Yao, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Liang-Xin Peng, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Bao-Jia Yao, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Zhi-Xian Jiang, Geriatric Surgery of Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Data sharing statement
All data analyzed in this paper have been provided in the main manuscript and in supplementary documents, and it is permissible to contact the corresponding author to try to obtain it if necessary.
References
- 1.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67:263–268. [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 4.Kredel LI, Batra A, Stroh T, Kühl AA, Zeitz M, Erben U, Siegmund B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn's disease. Gut. 2013;62:852–862. doi: 10.1136/gutjnl-2011-301424. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Liu G, Zhang Y, Yao B, Wu Q, Peng L, Wang X, Yuan L. Quantitative analysis of adipose tissue for predicting Crohn's disease postoperative endoscopic recurrence and anastomotic ulcer. Int J Colorectal Dis. 2023;38:170. doi: 10.1007/s00384-023-04456-z. [DOI] [PubMed] [Google Scholar]
- 6.Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, Rieder F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn's Disease. Inflamm Bowel Dis. 2019;25:421–426. doi: 10.1093/ibd/izy331. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, Tamura S, Matsuzawa Y, Shimomura I, Shinomura Y. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019;9 doi: 10.3390/biom9120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westcott ED, Mattacks CA, Windsor AC, Knight SC, Pond CM. Perinodal adipose tissue and fatty acid composition of lymphoid tissues in patients with and without Crohn's disease and their implications for the etiology and treatment of CD. Ann N Y Acad Sci. 2006;1072:395–400. doi: 10.1196/annals.1326.034. [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol. 2013;64:143–155. [PubMed] [Google Scholar]
- 13.Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- 14.Kujawska-Luczak M, Szulinska M, Skrypnik D, Musialik K, Swora-Cwynar E, Kregielska-Narozna M, Markuszewski L, Grzymislawska M, Bogdanski P. The influence of orlistat, metformin and diet on serum levels of insulin-like growth factor-1 in obeses women with and without insulin resistance. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.5.08. [DOI] [PubMed] [Google Scholar]
- 15.Zubrzycki A, Cierpka-Kmiec K, Kmiec Z, Wronska A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.5.02. [DOI] [PubMed] [Google Scholar]
- 16.Ho SM, Lewis JD, Mayer EA, Plevy SE, Chuang E, Rappaport SM, Croitoru K, Korzenik JR, Krischer J, Hyams JS, Judson R, Kellis M, Jerrett M, Miller GW, Grant ML, Shtraizent N, Honig G, Hurtado-Lorenzo A, Wu GD. Challenges in IBD Research: Environmental Triggers. Inflamm Bowel Dis. 2019;25:S13–S23. doi: 10.1093/ibd/izz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Wu G, Wu Q, Peng L, Yuan L. METTL3 overexpression aggravates LPS-induced cellular inflammation in mouse intestinal epithelial cells and DSS-induced IBD in mice. Cell Death Discov. 2022;8:62. doi: 10.1038/s41420-022-00849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleich A, Mähler M, Most C, Leiter EH, Liebler-Tenorio E, Elson CO, Hedrich HJ, Schlegelberger B, Sundberg JP. Refined histopathologic scoring system improves power to detect colitis QTL in mice. Mamm Genome. 2004;15:865–871. doi: 10.1007/s00335-004-2392-2. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Li H, Zhang X, Yang T, Yue M, Zhang Y, Chen S, Cui N, Yuan C, Li J, Zhu SJ, Liu W. Akkermansia muciniphila protects mice against an emerging tick-borne viral pathogen. Nat Microbiol. 2023;8:91–106. doi: 10.1038/s41564-022-01279-6. [DOI] [PubMed] [Google Scholar]
- 21.Bai M, Guo H, Zheng XY. Inflammatory bowel disease and Clostridium difficile infection: clinical presentation, diagnosis, and management. Therap Adv Gastroenterol. 2023;16:17562848231207280. doi: 10.1177/17562848231207280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, Barnich N, Ng SC, Colombel JF. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–587. doi: 10.1136/gutjnl-2017-314903. [DOI] [PubMed] [Google Scholar]
- 23.Zamani S, Hesam Shariati S, Zali MR, Asadzadeh Aghdaei H, Sarabi Asiabar A, Bokaie S, Nomanpour B, Sechi LA, Feizabadi MM. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017;9:53. doi: 10.1186/s13099-017-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, Lynch T, Allen-Vercoe E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17:1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 25.Chung CY, Alden SL, Funderburg NT, Fu P, Levine AD. Progressive proximal-to-distal reduction in expression of the tight junction complex in colonic epithelium of virally-suppressed HIV+ individuals. PLoS Pathog. 2014;10:e1004198. doi: 10.1371/journal.ppat.1004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 27.Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med. 2010;10:579–595. doi: 10.2174/1566524011009060579. [DOI] [PubMed] [Google Scholar]
- 28.Boss O, Bergenhem N. Adipose targets for obesity drug development. Expert Opin Ther Targets. 2006;10:119–134. doi: 10.1517/14728222.10.1.119. [DOI] [PubMed] [Google Scholar]
- 29.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XH, Feng ST, Cao QH, Coffey JC, Baker ME, Huang L, Fang ZN, Qiu Y, Lu BL, Chen ZH, Li Y, Bettenworth D, Iacucci M, Sun CH, Ghosh S, Rieder F, Chen MH, Li ZP, Mao R. Degree of Creeping Fat Assessed by Computed Tomography Enterography is Associated with Intestinal Fibrotic Stricture in Patients with Crohn's Disease: A Potentially Novel Mesenteric Creeping Fat Index. J Crohns Colitis. 2021;15:1161–1173. doi: 10.1093/ecco-jcc/jjab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakarov S, Blériot C, Ginhoux F. Role of adipose tissue macrophages in obesity-related disorders. J Exp Med. 2022;219 doi: 10.1084/jem.20211948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan S, Luck H, Winer S, Winer DA. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat Commun. 2021;12:2598. doi: 10.1038/s41467-021-22727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JM, Kim J, Lee YJ, Bae SU, Lee HW. Inflammatory bowel disease-associated intestinal fibrosis. J Pathol Transl Med. 2023;57:60–66. doi: 10.4132/jptm.2022.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mak JWY, Ng SC. Epidemiology of fibrostenosing inflammatory bowel disease. J Dig Dis. 2020;21:332–335. doi: 10.1111/1751-2980.12853. [DOI] [PubMed] [Google Scholar]
- 37.Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019;178:1041–1056. doi: 10.1016/j.cell.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, Salem JE, Vaillant JC, Oppert JM, Laugerette F, Michalski MC, Wind P, Rousset M, Brot-Laroche E, Leturque A, Clément K, Thenet S, Poitou C. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol. 2018;246:217–230. doi: 10.1002/path.5134. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q, Zhu W. Dietary Non-digestible Polysaccharides Ameliorate Intestinal Epithelial Barrier Dysfunction in IL-10 Knockout Mice. J Crohns Colitis. 2016;10:1076–1086. doi: 10.1093/ecco-jcc/jjw065. [DOI] [PubMed] [Google Scholar]
- 44.Mariadason JM, Catto-Smith A, Gibson PR. Modulation of distal colonic epithelial barrier function by dietary fibre in normal rats. Gut. 1999;44:394–399. doi: 10.1136/gut.44.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Vinci A, Behnsen J, Cheng C, Jellbauer S, Raffatellu M, Sousa KM, Edwards R, Nguyen NT, Stamos MJ, Pigazzi A. Bariatric surgery attenuates colitis in an obese murine model. Surg Obes Relat Dis. 2017;13:661–668. doi: 10.1016/j.soard.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rieder F, de Bruyn JR, Pham BT, Katsanos K, Annese V, Higgins PD, Magro F, Dotan I. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;8:1166–1178. doi: 10.1016/j.crohns.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Tabuso M, Adya R, Stark R, Gopalakrishnan K, Tsang YW, James S, White A, Fisk A, Dimitri F, Christian M, Arasaradnam RP. Fibrotic Phenotype of Peritumour Mesenteric Adipose Tissue in Human Colon Cancer: A Potential Hallmark of Metastatic Properties. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22052430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desmoulière A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 49.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr. 2022;76:489–501. doi: 10.1038/s41430-021-00991-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Wu D, Wang H, Xie L, Hu F. Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases. Front Endocrinol (Lausanne) 2022;13:908868. doi: 10.3389/fendo.2022.908868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, Hendrick G, Caldera JR, Arias C, Moskowitz JE, Ho Sui SJ, Yang S, Underhill D, Brady MJ, Knott S, Kaihara K, Steinbaugh MJ, Li H, McGovern DPB, Knight R, Fleshner P, Devkota S. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell. 2020;183:666–683.e17. doi: 10.1016/j.cell.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed in this paper have been provided in the main manuscript and in supplementary documents, and it is permissible to contact the corresponding author to try to obtain it if necessary.