Abstract
Gastric polyps (GPs) are increasingly common. On upper endoscopy, they should be examined with white light and occasionally chromoendoscopy, and their morphology classified according to the Paris classification. Most GPs have a typical endoscopic appearance and can be associated with diseases like Helicobacter pylori infection. Histological examination is necessary for an accurate diagnosis. While most polyps are non-neoplastic and do not require treatment, some carry a risk of malignancy or are already malignant. Therefore, understanding the diagnosis, classification, and management of GPs is crucial for patient prognostication. Our new classification categorizes GPs into "good", "bad", and "ugly" based on their likelihood of becoming malignant. We aim to provide descriptions of the endoscopic appearance, pathology, treatment, and follow-up for different GPs, as well as clinical management flowcharts.
Keywords: Gastric polyps, Fundic gland polyps, Hyperplastic polyps, Adenomas, Neuroendocrine tumors, Early gastric cancer
Core Tip: During upper gastrointestinal endoscopy, gastric polyps are frequently encountered, however, most are benign. Despite this, it is crucial that endoscopist have a thorough understanding of diagnostic approaches, management strategies, and screening protocols, particularly for polyps with neoplastic potential. We have developed a new classification system for gastrointestinal polyps based on their likelihood of becoming malignant, categorizing them into "good", "bad", and "ugly" groups. This classification aims to assist clinicians in managing and treating polyps effectively.
INTRODUCTION
Gastric polyps (GPs) are luminal lesions that arise above the mucosal surface[1]. This simple definition encompasses a wide range of lesions with varying histology and neoplastic potential. The detection of GPs is becoming increasingly common in clinical practice, with an estimated current incidence of 6% of upper gastrointestinal endoscopic procedures in the United States[2]. This trend likely underestimates the actual occurrence, as the majority of GPs are asymptomatic, reflecting the widespread access to esophago-gastroduodenoscopy (OGD) in recent years.
During an OGD, it is important to thoroughly examine the gastric mucosa and any polyps encountered using white light and narrow band imaging (NBI) to classify their morphology according to the Paris classification[3,4]. Chromoendoscopy may be used in some occasions. Most GPs have a typical endoscopic appearance in the stomach and can be associated with diseases such as Helicobacter pylori (H. pylori) infection, autoimmune gastritis, or inherited polyposis syndrome[5]. However, it is essential to conduct histological examination of GPs and the surrounding mucosa for an accurate assessment and diagnosis[6]. While most polyps are non-neoplastic and do not necessitate treatment, some GPs pose a risk of malignancy or are already malignant at the time of endoscopic examination[7]. Therefore, a deep understanding of the diagnosis, classification, and management of GPs is crucial for patient prognostication.
It can be a complex task for clinicians to classify and manage GPs due to uncertainties about lesion characterization, sampling, treatment necessity, therapy type, and long-term monitoring. It is crucial not to underestimate lesions with malignant potential and to treat them appropriately. As such, our new classification of gastrointestinal polyps is based on categorizing them into three groups according to their likelihood of becoming malignant: "Good" [polyps that generally do not progress to cancer, such as fundic GPs (FGPs), inflammatory fibroid polyps (IFPs), and ectopic pancreas (EP)], "bad" [polyps that pose a risk of malignancy, such as large hyperplastic polyps, adenomas, type 1 and 2 neuroendocrine tumors, and hamartomatous polyps (HaPs)], and "ugly" [the most aggressive and invasive polyps, such as type 3 neuroendocrine tumors and early gastric cancer (EGC)]. We aim to provide descriptions of the endoscopic appearance, pathology, treatment, and follow-up for different gastrointestinal polyps, as well as a clinical management flowchart.
A table summarizing the main characteristics of each type of GPs as well as clinical flow charts, are provided in Table 1 and Supplementary Figures 1-8.
Table 1.
Types of gastric polyps
|
|
|
Frequency
|
Risk factors
|
Associated conditions
|
Age/sex
|
Location
|
Size
|
Symptoms
|
Endoscopic features
|
Neoplastic potential
|
| Good | ||||||||||
| Fundic gland | Sporadic | 80% | Proton-pump inhibitors | None | Middle age/women | Body/fundus | < 8 mm | Asymptomatic | Sessile, smooth surface | Very rare |
| Syndromic | Hereditary | Familial adenomatous polyposis, MUTHY | Early age/no sex difference | Body, multiple (> 90%) | < 6 mm | Asymptomatic | Rare | |||
| Inflammatory fibroid | Rare (< 0.1%) | Not known |
May be familial, Devon polyposis syndrome-associated with PDGFRA mutation | Older (6th-7th decade) (adults/slight predominace in women) | Antrum-pylorus | Mean size 1-5 cm, up to 9 cm | Early satiety or good, sometimes bleeding | Sessile, pedunculated, +/- ulcerated | Very rare | |
| Ectopic pancreas | 0.5%-13% | None | None | Not reported | Antrum-prepyloric region | Variable | Incidental | Firm round or oval subepithelial lesion with a central depression | Benign, no follow-up needed | |
| Hamartomatous polyp | Sporadic | 1% | Not known | - | Not reported | Anywhere | Variable | Incidental findings | Sessile, differential dx H. pylori | Benign |
| Bad | ||||||||||
| Hamartomatous | Syndromic | PJS, Juvenile polyposis syndrome and phosphatase and tensin homolog hamartoma syndrome | STK11 (PJS) | - | - | The 0.1-3 cm (PJS) | - | Lifetime malignancy risk (up to 29%) | ||
| Hamartomatous | Solitary | Gastric inverted Hamartomatous Polyps | Common (up to 20%) | |||||||
| Hyperplastic | 15% | H. pylori, chronic atrophic gastritis | Not reported | Middle age/no sex difference | Antrum (60%), any site, solitary, more common multiple | Usually < 2 cm, but up to 12 cm | Asymptomatic, incidental findings | Smooth or lobulated, sessile or pedunculated | Dysplasia 15%, cancer risk < 1% | |
| Adenoma | 6%-10% | Atrophy, intestinal Metaplasia | None | Middle age/men (intestinal type) | More common, antrum (intestinal type), any site (other types) | Variable (few mm to cm) | Asymptomatic, anemia, bleeding, rarely obstruction | Sessile, pedunculated | Depends on size; histology (high-grade dysplasia 30% at 5 years); likely gene-disrupting 3% at 5 years | |
| Gastric neuroendocrine tumors | < 2% gastric neoplasm | |||||||||
| GNET type 1 | 80% all G-NET | Autoimmune gastritis | None | Middle age/old | Body, fundus | Small, multiple | Asymptomatic anemia | Reddish | Low (< 1%), locoregional MTS depends on size, grading and mm propria invasion: 5 years survival 100% | |
| GNET type 2 | 5% | Gastrinoma (multiple endocrine neoplasia type 1) | Young | Body, fundus | Small, multiple | Diarrhea, abdominal pain bleeding from peptic ulcer |
Small, yellow, often multiple ulceration | Lymph node MTS 30% with good prognosis | ||
| Ugly | ||||||||||
| GNET type 3 | The 10%-20% all GNET | No predisposing factors | None | Adults/no sex difference | Anywhere, preference antrum | Large, solitary | Asymptomatic | Single lesion | The 50% risk MTS. Survival 70% at 5 years | |
| Early gastric cancer | Sporadic hereditary (1%-3%) |
Equal to gastric adenomaE-cadherin gene | None | Adult/old/male | Antrum (50%) corpus (35%), cardia (15%) | Epigastric pain glycemic index bleeding, anemia, vomiting/nausea | Polyp, ulcer | The 5 years survival 75%, the 80% lifetime cancer risk |
GNET: Gastro-intestinal neuroectodermal tumor; PJS: Peutz-Jeghers syndrome; H. pylori: Helicobacter pylori; MTS: Metastasis.
THE GOOD
Inclusion criteria are polyps with generally no progression to cancer such as: FGPs, IFPs, and ectopic pancreatic tissue.
FGPs
FGPs are the most commonly encountered type of GPs, constituting about 80% of all GPs[2]. While FGPs are typically sporadic, they can also occur in conjunction with polyposis syndromes like familial adenomatous polyposis (FAP) or MUTYH-associated polyposis (MAP)[8,9]. Given the rarity of FAP and MAP patients, in addition to the low likelihood of developing gastric cancer, both types of FGPs have been categorized as "good" polyps. However, there is a slight variation in the management of syndromic and non-syndromic patients, particularly regarding the common occurrence of dysplasia in FGPs associated with FAP[10].
Sporadic FGPs are closely related to the use of proton-pump inhibitors (PPIs). PPIs increase serum gastrin levels which leads to polyps consisting of large fundic gland cysts with parietal, chief, and some mucous cells[11]. PPIs administration increases the size and number of FGPs while the withdrawal of PPIs leads to regression of FGPs, emphasizing the link between PPIs consumption and FGPs development[12,13]. It has been noted that the incidence of H. pylori infection is very low in patients with FGPs. In contrast, it has been linked to FGPs regression, suggesting a protective role of H. pylori in reducing FGPs occurrence[14,15].
Sporadic FGPs are more common in middle-aged woman, possibly due to hormonal imbalances during menopause[16,17]. They grow in the gastric body or fundus, are often multiple, less than 8 mm in diameter, isochromatic, and a sessile shape with a smooth surface (Figure 1). On NBI, dot-shaped crypt regular openings with dense regular vessels can be visible. Sporadic FGPs are generally regarded as benign lesions, but rarely (1%-6% of cases) they are associated with dysplasia of the overlying foveolar epithelium with very slow progression to cancer[18,19]. The surrounding mucosa is generally normal appearing or shows signs of PPIs[11].
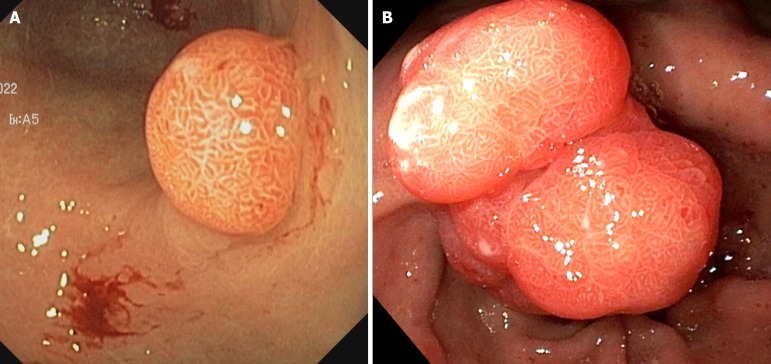
Figure 1.
Multiple fundic gland polyps in a patient on proton pump inhibitors.
The suggested management of sporadic FGPs on OGD is to take biopsies of one or more representative FGPs, while carefully inspecting the remaining polyps. When a polyp displays an atypical morphology or is larger than 1 cm, ulcerated, or located in the antrum, the best course of action is to remove it to confirm the diagnosis and eliminate the possibility of dysplasia. Although bleeding from FGPs is rare, it can be treated with polyp resection and PPIs therapy discontinuation[20]. Periodic surveillance for sporadic FGPs is not typically recommended.
Syndromic FGPs often develop at an earlier age, sometimes even in those less than 20 years old, and this occurs without distinction between genders[16]. In cases where FGPs are associated with FAP/MAP, it has been reported that dysplasia occurs in up to 54% of cases[8,19]. Nevertheless, the risk of developing malignancy in these cases remains low[21]. In over 90% of cases, syndromic FGPs manifest as multiple growths (more than 20) and primarily affect the gastric body. Similar to sporadic FGPs, they are generally sessile and have an average size of less than 6 mm[10]. The endoscopic appearance of syndromic FGPs without carcinoma is indistinguishable from that of sporadic FGPs. However, certain characteristics such as redness, irregular surface, depressed areas, erosions, and irregular vessels under NBI have been associated with FGPs containing carcinoma. It is recommended to conduct endoscopic surveillance for syndromic FGPs[22], although the optimal timing for this surveillance has not yet been standardized[23].
To summarize, when FGPs are detected on OGD, inspect them with white light and if needed, chromoendoscopy. If polyps are found in the antrum, size larger than 1 cm, red appearing, irregular surface, depressed areas, erosions and/or irregular vessels are present, remove them or take biopsies. Moreover, in patients with more than 20 FGPs, FGPs in the antrum, and onset of FGPs prior to 40 years, it is necessary to perform colonoscopy to rule out the possibility of polyposis syndrome. Reduction, discontinuation of PPIs therapy, or switching to a different PPI can be considered in patients with multiple FGPs and hypergastrinemia, or in cases of anemia caused by FGPs[20,24].
IFPs
IFPs are an extremely rare entity, representing less than 0.1% of all GPs[25]. They generally arise within the submucosa of the gastrointestinal tract and penetrate through the lamina propria leading to bulging of the mucosal layer. They may originate from dendritic cells[26].
IFPs are more commonly small (< 15 mm) and located in the antrum of older adults. In the majority of cases, IFPs are asymptomatic, however, large IFPs can cause early satiety, gastric outlet obstruction, or ulcerate the overlying mucosa causing bleeding and anemia. On OGD they appear as solitary, sessile or pedunculated, sometimes ulcerated polyps and typically have a solid pale tan cut surface. On endoscopic ultrasonography (EUS) they present as a homogeneous hypoechoic mass originating from the second or third layer without involvement of the fourth layer and without a capsule[27]. On histology, IFPs display a proliferation of spindle cells with an eosinophilic-rich inflammatory infiltrate and mutations in the platelet-derived growth factor receptor alpha gene that may lead to a misdiagnosis of gastrointestinal stromal tumour (GIST)[28,29]. The stroma in most lesions is positive for CD34 but negative for CD117.
Diagnosis of IFPs is typically confirmed through histopathological analysis, as they do not have a pathognomonic endoscopic appearance, and only 10% are diagnosed in the preoperative setting with endoscopic biopsy or EUS.
Endoscopic resection of IFPs is generally not associated with a risk of recurrence. Furthermore, since IFPs are considered benign, surveillance is not recommended. However, in sporadic cases, IFPs have been reported to be malignant which may necessitate scheduled follow-ups[30].
EP
EP is a rare congenital anomaly characterized by aberrant pancreatic tissue that lacks direct vascular or neural connections with the true pancreas[31]. This anomalous tissue can be found in various locations throughout the body, including the gastrointestinal tract, biliary system, liver, lung, mediastinum, and brain. In the gastrointestinal tract, it is most commonly located in the gastric antrum and the prepyloric region on the greater curvature or posterior wall, followed by the duodenum and jejunum[32]. EP tissue can originate from the mucosa, muscularis mucosae propria, submucosa, or muscularis propria.
The majority of gastric EPs are typically asymptomatic and are usually discovered incidentally during OGD. They generally do not require any treatment or ongoing monitoring. However, in some instances, they may manifest with symptoms such as bleeding or pain, and there have been reported cases of gastric outlet obstruction, resembling hypertrophic pyloric stenosis, in both adults and children[33]. Malignant transformation of EP is rare[34]. During OGD, EP typically appears as a submucosal nodule covered by normal mucosa with an umbilicated shape[35] (Figure 2). However, when lacking central dimpling, EP can be indistinguishable from other submucosal lesions such as GIST, gastrointestinal autonomic nerve tumors and carcinoid tumors. In these cases, EUS with endoscopic-guided fine needle aspiration plays a pivotal role in making an accurate diagnosis[36].
Figure 2.
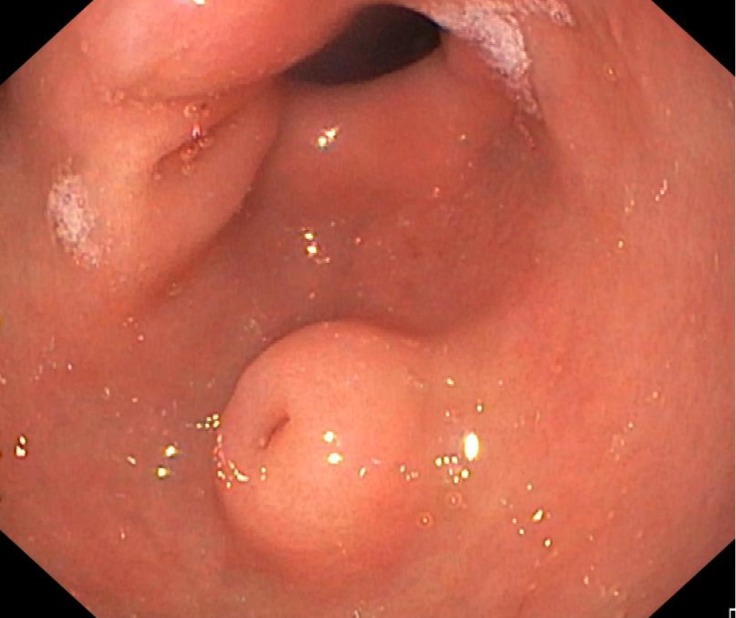
Antral ectopic pancreas with typical umbilicated shape.
Histologically, EPs are classified into four distinct categories according to Fuentes’s classification. The most common tissue type of EP is similar to that found in the normal pancreas, containing all cellular components such as acini, ducts, and pancreatic islet cells. The second histological type consists only of ducts, the third of acini (exocrine cells), and the fourth of pancreatic islet cells (endocrine cells)[37]. Asymptomatic gastric EPs do not require any treatment or follow-up.
THE BAD
In this class we identify polyps with a low risk of malignancy such as hyperplastic polyps, adenomas, type 1 and 2 neuroendocrine tumors, and HaPs.
Hyperplastic polyps
Gastric hyperplastic polyps (GHPs) are common epithelial polyps found in the stomach, with an incidence ranging from 15% in areas where H. pylori infection is less common to 75% in populations with widespread H. pylori infection[38]. GHPs are a reactive response to chronic inflammatory stimuli, primarily caused by H. pylori infection, as well as chronic atrophic gastritis, pernicious anemia, stomas, and sites of ulcers and erosions[39].
GHPs shows no predilection towards males or females; however, they most commonly affect individuals in the middle to late stages of life. On OGD, GHPs can appear in various forms, ranging from smooth to lobulated, and can be either sessile or pedunculated, with sizes generally less than 2 cm, although larger dimensions of up to 12 cm have been reported[40]. They typically have a reddish surface and often show erosions, particularly as they grow in size (Figure 3). The morphological characteristics of GHPs can make it challenging to distinguish them from polypoid foveolar hyperplasia or gastric adenomatous polyps, underscoring the need for biopsy sampling[41]. The antrum is the most commonly affected site in the stomach (60%), although GHPs can occur in any area of the stomach, including the cardia. They may appear as solitary growths or, more commonly, as multiple growths[38]. Over time, their size can remain stable or increase, especially in the presence of the inflammatory agent. Conversely, the eradication of H. pylori infection significantly increases the rate of GHPs elimination by more than 20 times, as shown in a meta-analysis[42].
Figure 3.
Antral hyperplastic polyps. A: Small sessile; B: Giant polyp.
GHPs are usually asymptomatic and are found incidentally on OGD. However, they can cause bleeding when eroded, or intermittent gastric outlet obstruction when pedunculated and prolapsing from the antrum into the bulb[43]. Microscopically, GHPs are characterized by elongated, dilated, branching, distorted, cystic foveolar glands with mucinous cytoplasm and an inflamed stroma[11].
GHPs have long been considered to be benign growths, however, their development within a background of chronic inflammation may increase the risk of neoplastic transformation, following the adenoma-carcinoma sequence[44]. On histological examination, 15% of GHPs exhibit intestinal metaplasia, while nearly 5% show signs of dysplasia, and cancer is present in less than 1%[38]. Notably, GHPs larger than 1 cm, displaying a pedunculated morphology, arising in individuals who have undergone gastrectomy, or coexisting with other neoplastic lesions, are all factors that predispose GHPs to neoplastic transformation[45]. When examining GHPs, it is essential to extensively sample the surrounding mucosa to rule out H. pylori infection, atrophy, metaplasia, or dysplasia, especially when they show characteristics of underlying chronic inflammatory conditions[11].
All polyps greater than 5 mm in size should be removed endoscopically and all patients with H. pylori infection should be adequately treated to eradicate the infection[46]. Surveillance after removal of GHPs should be based on polyp histology (i.e., dysplasia) and cancer risk due to concurrent H. pylori infection, autoimmune gastritis, family history of gastric cancer, migrants from areas with high gastric cancer incidence and operative link of gastritis assessment stage[47]. Furthermore, it is advisable to conduct a follow-up endoscopy three to six months after polyp resection to ensure that there are no residual GHPs and that H. pylori has been successfully eradicated.
Gastric adenomas
Gastric adenomas (GAs) account for 6% to 10% of all GPs in Western countries, with higher rates in countries like Japan and China[48]. Atrophic gastritis and intestinal metaplasia often underlie these polyps, although they can also occur in patients without gastric inflammatory conditions. There is no confirmed association with H. pylori infection, and no evidence suggests that PPIs treatment increases the risk of developing GAs.
Like the other types of polyps discussed, they usually do not cause any symptoms, but when they become symptomatic, can present with anemia, bleeding or rarely obstruction[48]. On OGD, they are typically single lesions that can be flat, sessile, or pedunculated in shape, with a size ranging from a few millimeters to centimeters (Figure 4). Unlike colonic adenomas, the pit-pattern of GAs observed through chromoendoscopy and magnification is not correlated with the histopathology of the lesion. Microscopically, there are four types of GAs: Intestinal type, foveolar type, pyloric gland type, and oxyntic gland type[11].
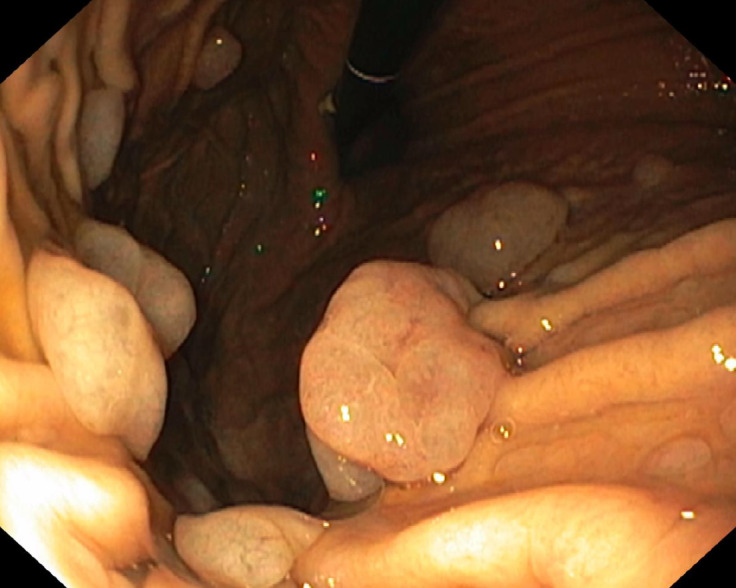
Figure 4.
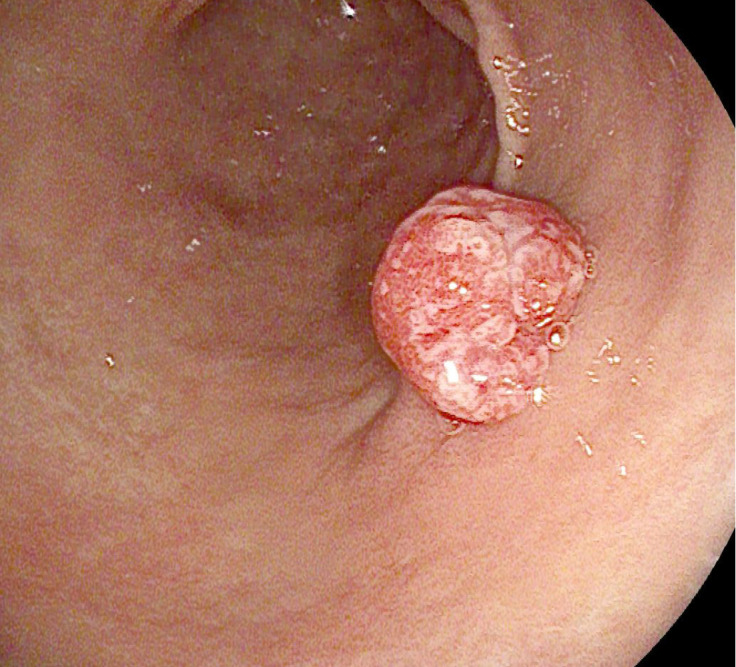
Antral gastric adenoma.
Intestinal-type adenomas are the most frequent type of GAs. Middle-late age men are the most affected. These polyps mainly arise in an antropyloric region of the stomach affected by intestinal metaplasia, with H. pylori infection and autoimmune gastritis as predisposing factors. Histopathological analysis shows dysplastic columnar epithelium cells, goblet cells, endocrine cells, and Paneth cells[11]. Like colonic adenomas, they can be distinguished as tubular, villous, or tubulovillous. Forty percent of intestinal-type adenomas show high-grade dysplasia and may progress to cancer[49].
Foveolar-type adenomas possess a dysplastic foveolar epithelium. They are rare, equally distributed between genders, and diagnosed at a mean age of 44 years[49]. They can be sporadic or associated with polyposis syndromes like FAP. In contrast to intestinal-type adenomas which predominate in the gastric antrum, foveolar-type adenomas occur mostly in the gastric body and fundus with normal surrounding mucosa[21,49]. Histologically, they are composed of dysplastic foveolar-type epithelium rich in mucin and rarely present high grade dysplasia or carcinoma[11].
Pyloric gland-type is another rare form of GAs consisting of densely packed pyloric glands. They are mostly found in the fundus of patients with autoimmune atrophic gastritis and pyloric metaplasia, but can arise in FAP patients with non-atrophic gastritis. Dysplasia can occur in up 50% of cases while submucosal invasion is less than 10%[11].
Oxyntic gland-type GAs is the least common form of GAs. They predominantly affect the mucosa of the proximal stomach. Histologically, they appear as packed oxyntic glands. Often, there is submucosal invasion, although lymphovascular invasion and malignant potential are very low[11].
Adenomas in the stomach, especially those of the intestinal type, have the potential to develop into cancer. The likelihood of malignancy rises with the size of the adenoma and is particularly elevated in flat adenomas[50]. GAs with high-grade dysplasia have a risk of progressing to cancer within five years that is more than 10 times greater than that of low-grade GAs (30% vs 3%, respectively)[51].
GAs should be completely excised en-bloc to identify the presence of any malignant foci. Moreover, as the presence of GAs is strongly associated with separate foci of intestinal metaplasia, dysplasia and synchronous or metachronous gastric adenocarcinoma[52], the background mucosa should be thoroughly inspected and biopsied. Surveillance after removal of GAs should be based on polyp histology and individual cancer risk. An OGD at one year following complete resection is suggested[45].
Gastric neuroendocrine tumors
Gastric neuroendocrine tumors (G-NETs) encompass well-differentiated neuroendocrine tumors found in the stomach. Along with a small percentage of poorly differentiated neuroendocrine carcinomas, they make up gastric neuroendocrine neoplasms (G-NENs). Additionally, they are part of the larger group of gastroenteropancreatic neuroendocrine tumors, which include well-differentiated NETs from the entire gastrointestinal tract. Originating from the enterochromaffin-like (ECL) cells of the stomach, G-NETs are a relatively uncommon type of gastrointestinal neoplasms, accounting for less than 2% of all gastric neoplasms[53].
The World Health Organization divided G-NETs into three histological grades (G1, G2, G3) depending on the mitotic rate and ki-67 index[54]. In addition, well differentiated G-NETs are distinguished into three clinical types (type 1, type 2 and type 3) according to etiology, behavior, and prognosis[53]. To assess G-NETs, it is essential to biopsy the polyp, gastric antrum, and body/fundus, as this may provide information regarding the etiology and prognosis of the tumor[55]. Biopsies of the polyp evaluate the grade of the tumor, while it should be remembered that NETs are usually located deep in the mucosa.
Type 1 neuroendocrine tumors
Type 1 G-NETs develop in the presence of ECL cell hyperplasia due to elevated gastrin levels in autoimmune atrophic gastritis. These tumors make up to 80% of all G-NENs. During an OGD, type 1 G-NETs manifest as small, red, and multiple polyps, primarily located in the fundus and the body of the stomach (Figure 5). The surrounding mucosa, which should be sampled, exhibits mucosal cell atrophy, neuroendocrine cell hyperplasia, and parietal cell depletion[53]. Gastric juice sampling for pondus hydrogenii (pH) measurement shows high pH levels. Blood tests reveal increased serum gastrin level, vitamin B12 deficiency with or without macrocytic anemia, and 80% positivity for antiparietal cell antibodies[56]. Chromogranin A (CgA) levels are elevated but not useful as a tumor marker.
Figure 5.
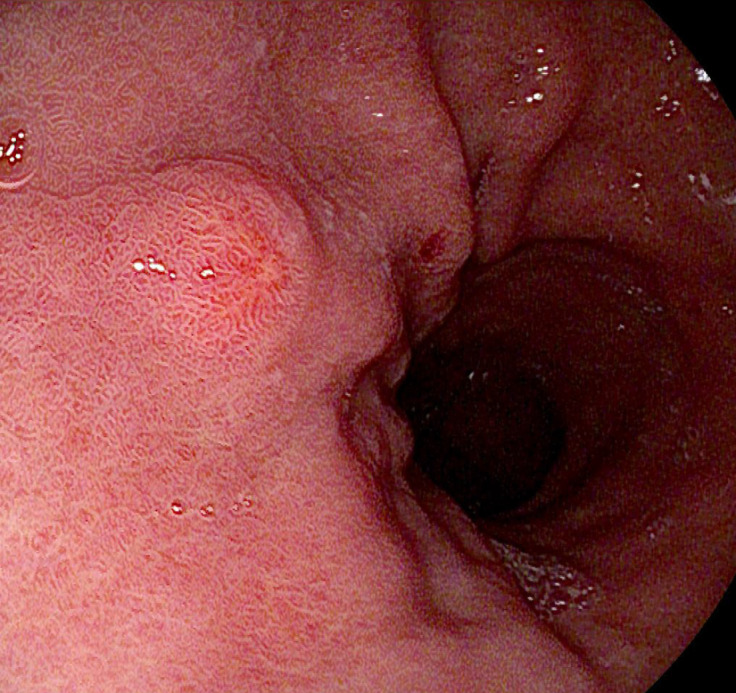
Small type 1 gastric neuroendocrine tumor.
In cases of type 1 G-NETs, the prognosis is generally positive, with a less than 1% risk of metastasis in tumors smaller than 10 mm. Surgical removal is recommended for lesions that are 10 mm or larger[55]. For type 1 G-NETs smaller than 10 mm, regular endoscopic follow-up at 6-month intervals and subsequently every 12 months is suggested. Rebiopsy is not necessary if the polyp's characteristics remain unchanged[55].
EUS is recommended for tumors > 1 cm and those with G2 grade regardless of lesion size, in order to define the depth of invasion and lymph node involvement. Cross-sectional imaging with magnetic resonance imaging (MRI) and contrast-enhanced thoracoabdominal computed tomography (CT), and functional imaging with somatostatin receptor positron emission tomography (PET)/CT (68Ga-SSA-PET-CT), are required only in cases of metastases or high-risk features (G2 on biopsies, vessels or lymphatic invasion, suspected T2 on EUS) or R1 margins after endoscopic resection[57].
Overall, the rate of locoregional lymph node metastasis of type 1 G-NETs is 3.3%. However, this rate is higher in lesions ≥ 10 mm compared with lesions < 10 mm (15.3% vs 0.8%), G2 vs G1 (10% vs 6.7%) and T2 (muscularis propria invasion) vs T1 (29.4% vs 3.1%). Nevertheless, the reported 5-year disease-specific survival rate is 100% in patients with and without locoregional lymph node metastasis[58].
For type 1 G-NETs the treatment of choice is endoscopic resection including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and full-thickness resection (FTR). Although ESD and FTR result in higher rates of R0 resection, the choice of technique is not standardized and depends on the location, characteristics of the lesion and local expertise[59]. According to the European Neuroendocrine Tumor Society’s recently published guidance statements, in cases of R1 resection a step-up approach (EMR > ESD > FTR > surgery) is suggested[55]. After R0 resection of type 1 G-NETs, follow-up should be scheduled with OGD at 1 year.
Although rare, tumors > 20 mm in size or suspicious of muscularis propria invasion, should undergo surgery with lymph node sampling. Somatostatin analogues can be used continuously in patients with metastatic disease or in patients who are not fit for surgical resection.
Type 2 neuroendocrine tumors
Type 2 G-NETs constitute a smaller, less common subset of G-NETs (approximately 5%)[55]. Similar to type 1 G-NETs, the excessive production of gastrin leads to hypertrophy and hyperplasia of ECL cells. However, in the case of type 2 G-NETs, gastrin is secreted by a gastrinoma associated with Zollinger-Ellison syndrome, typically occurring in patients with multiple endocrine neoplasia type 1 (MEN-1) syndrome.
On upper endoscopy, type 2 G-NETs appear as small, multiple, and yellowish polyps located mostly in the gastric body and fundus, often surrounded by peptic ulceration. Histologically, the surrounding mucosae are normal, hypertrophic or mildly inflamed but not atrophic. Furthermore, pH measurement of the gastric juice reveals low pH due to hyperchlorhydria. Patients may suffer from diarrhea, steatorrhea, abdominal pain, and present with bleeding due to peptic ulceration. When type 2 G-NET is suspected, blood examinations with parathormone, calcium, gastrin, and insulin levels, should be tested to rule out the possibility of MEN.
Compared to type 1 G-NETs, type 2 G-NETs tend to impact younger patients and carry a higher risk of lymph node metastases (about 30%), although the overall prognosis is favorable[60]. The optimal treatment approach for type 2 G-NETs has not been definitively established and is contingent upon the management of MEN-1 syndrome. For G-NETs larger than 10 mm, resection should be considered following prior EUS evaluation, while lesions smaller than 10 mm may be monitored through OGD surveillance. Treatment options include endoscopic resection or surgery involving partial or total gastrectomy. Additionally, consideration should be given to removal of the gastrinoma. In cases where resection of the gastrinoma is not indicated or feasible, reducing basal acid output using PPIs or histamine H2 receptor antagonists is essential. The use of somatostatin analogues has not been uniformly standardized[61].
HaPs
Gastric HaPs are a rare category of GPs, accounting for approximately 1% of all GPs[62]. HaPs are often associated with syndromes such as Peutz-Jeghers syndrome (PJS), juvenile polyposis syndrome (JPS), phosphatase and tensin homolog, and hamartoma syndrome[63]. JPS is the most common hereditary gastrointestinal polyposis syndrome, with a prevalence of 1/100000-160000[64]. It is an autosomal dominant disorder caused by a mutation in SMAD4, BMPRA1, or ENG genes[65]. PJS is another autosomal dominant inherited disorder characterized by a mutation in STK11, with a reported prevalence of 1/200000[66]. Typical features of PJS include mucocutaneous melanosis, gastrointestinal polyps, and intestinal and extraintestinal tumors (pancreas, breast, ovary, and testis).
On OGD, HaPs appear mostly as sessile, solitary, or multiple polyps, morphologically resembling hyperplastic polyps. Sometimes a submucosal bulge covered by normal gastric mucosa with a red or irregular depression at the top is seen. Histologically, they result from disordered growth of tissues indigenous to the site, deriving from any of the 3 embryonic layers[52] and formed of cystically dilated oxyntic glands and irregularly deformed oxyntic glands.
Sporadic HaPs are also known as gastric inverted HaPs (GIHP). These are submucosal lesions characterized by inverted growth of gastric glands on histology[63]. While the risk of malignancy is low in sporadic HaPs, certain polyps such as GIHP and those associated with PJS and JPS carry a higher risk of malignant transformation. About one in every five GIHP cases has been linked to adenocarcinoma[62], and PJS patients face a 29% risk of developing gastric cancer at a median age of 30-40 years[67].
When it comes to solitary polyps, distinguishing between hyperplastic polyps and HaPs based on endoscopic appearance can be challenging. Therefore, histological examination is essential for assessing the risk of malignancy. In cases of multiple gastric HaPs and mucocutaneous melanosis, genetic testing should be conducted to rule out the possibility of a hereditary polyp syndrome[63].
THE UGLY
In this class, we identify polyps with a high risk of malignancy such as type 3 neuroendocrine tumors and EGC.
Type 3 neuroendocrine tumors
Type 3 G-NETs represent 10%-20% of all G-NETs. They are sporadic and usually not associated with predisposing clinical conditions. Gastrin levels are normal as well as the pH of the gastric juice and the surrounding mucosae. The clinical presentation of G-NETs is not specific, and they are usually diagnosed during OGDs for symptoms such as abdominal pain, dyspepsia, nausea, bleeding or anemia. Bleeding has been reported more commonly in type 3 G-NETs.
On endoscopic examination, type 3 G-NETs are usually found as solitary lesions, primarily located in the antrum. Unlike type 1 and type 2 G-NETs, type 3 G-NETs are more aggressive, with a 50% risk of metastasis and a 70% 5-year survival rate[55]. Therefore, after endoscopic diagnosis, further investigations are necessary to rule out lymph node involvement and metastatic disease. This typically involves cross-sectional imaging (such as CT or MRI) and, often, functional imaging (such as 68Ga-SSA-PET-CT). Once metastatic disease has been ruled out and if type 3 G-NETs are small (< 20 mm) and low grade (G1-G2), EUS should be performed to assess regional lymph node involvement and evaluate the integrity of the muscle layer. For patients with localized type 3 G1 G-NETs ≤ 10 mm in size (not invading the muscle layer and without lymph node involvement) and for those with localized type 3-low G2 (Ki-67 3%-10%) G-NETs < 15 mm, when the risk of surgery is high, endoscopic treatment may be considered[55,68]. Both EMR and ESD can be used as resection techniques.
Type 3 G-NETs > 10 mm, G3, invading the muscle layer or with lymph node involvement, require surgical resection. Partial gastrectomy with nodal sampling should be proposed to patients with type 3 G1-2 G-NETs 10-20 mm, without lymph node involvement (whether invading or not invading the muscle layer on EUS). Moreover, it can be considered in patients with positive margins after endoscopic resection.
Conversely, total or subtotal gastrectomy with lymphadenectomy represents the best choice for patients with type 3 G3 (Ki 67 > 20%) G-NETs > 20 mm or with lymph node involvement. Moreover, it should be considered in patients with nodal localization, higher tumor grade compared with initial staging, lymphovascular invasion, or R1 resection after initial wedge resection[55].
After the resection of type 3 G-NETS, it is important to include both endoscopic examination and cross-sectional imaging in the follow-up process, although the exact protocol for this is not yet well-defined. Patients who have undergone endoscopic treatment or partial gastrectomy with lymph node sampling should undergo OGD after 3 months to evaluate the resection site, followed by cross-sectional imaging and endoscopy. For patients who have had total gastrectomy with lymphadenectomy, the follow-up schedule is similar to that for gastric adenocarcinoma. Additionally, monitoring CgA levels is recommended as it serves as a useful circulating tumor marker for tracking disease progression, treatment response, and early detection of recurrence after treatment[55].
Patients with metastatic disease may be treated palliatively with different techniques such as resection of metastases, regional therapies, somatostatin analogues, target specific-molecular drugs or chemotherapy[53].
EGC
EGC is defined as gastric cancer confined to the mucosa or submucosa, regardless of lymph node metastasis[69]. In most cases, it has a negligible risk of lymph node metastasis and can be treated effectively with endoscopic resection. Endoscopically benign-appearing polyps and apparently normal mucosa sometimes may mask the presence of adenocarcinoma. It is extremely important to be familiar with such findings, as it helps guide diagnosis and offers the best therapy for improving survival.
EGCs are usually asymptomatic and detected as incidental findings during endoscopy. Definitive diagnosis is based on histopathological examination. However, the choice of therapeutic strategy relies on very close endoscopic examination of the lesion. Therefore, endoscopic technologies are continuously moving forward to enhance the detection of these lesions and allow optimal delineation of tumor margin and precise estimation of the lesion’s depth[70].
Advanced gastric adenocarcinoma appears more frequently as a friable, ulcerated mass, a big gastric ulcer with thickened margins or linitis plastica, whereas endoscopic treatment is not a possible curative option. On the other hand, the endoscopic appearance of EGC may vary but it is often flat and indistinct (Figure 6). EGC usually appears as an abnormal irregular pale or reddish area, superficially elevated or depressed. Other endoscopic features are mucosal friability, ulceration, and an irregular vascular and structural pattern.
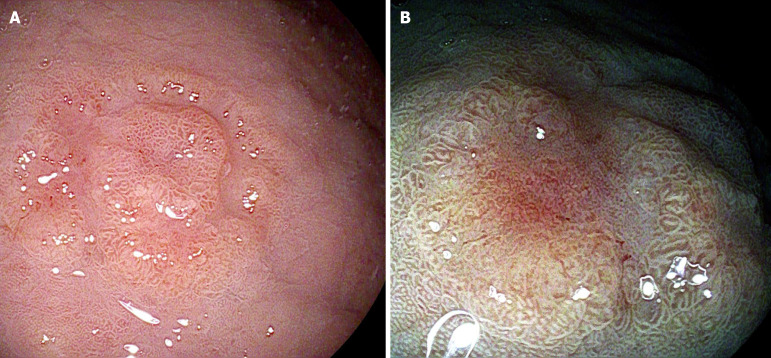
Figure 6.
Early gastric cancer (i-SCAN II, Pentax Medical). A: White light endoscopy; B: Chromoendoscopy.
Careful examination of the lesion characteristics can provide useful information about the depth of tumor invasion. Smooth surface protrusion or depression, slight marginal elevation, and smooth tapering of converging folds usually underline tumors limited to the gastric mucosa, while an irregular surface, markedly elevated margins, and clubbing, abrupt cutting, or fusion of converging folds may mask the presence of a submucosal lesion.
The morphology of polyps should be described in accordance with the Paris classification, as it is linked to the invasiveness of cancer[71]. Specifically, protruding and superficially elevated lesions (0-I and 0-IIa, respectively) are more likely to be differentiated cancer, whereas superficially depressed and ulcer-like lesions (0-IIc and 0-III, respectively) often conceal an undifferentiated adenocarcinoma[72]. The combination of virtual or dye-based chromoendoscopy with magnifying endoscopy, focusing on the disappearance of fine mucosal structure, microvascular dilation, and heterogeneous vessel shape, is extremely helpful in determining the horizontal margins of the lesion and potentially predicting the differentiation and depth of invasion of EGC[73,74]. Various classifications, such as vascular surface, magnifying endoscopy simple diagnostic algorithm for early gastric cancer, and the more recent unified magnifying endoscopic classification and endocytoscopy, have been described in this field[70]. However, unlike colonic lesions, a validated endoscopic classification for EGC is not yet available.
To determine whether endoscopic treatment is feasible for a lesion, it is necessary to have information about histologic type, size, invasion depth, and the presence or not of ulcers[73]. The endoscopic techniques for EGC resection are based on EMR and ESD. Compared with EMR, ESD is associated with higher rates of en-bloc and complete resection and a lower recurrence rate, but it is associated with higher risk of perforation and longer procedural time; post-procedural bleeding is similar between the two procedures[71]. According to ESGE guidelines, indications for ESD are differentiated intramucosal adenocarcinoma of any size in the absence of ulceration, < 3 cm when ulceration is present. ESD can also be extended to undifferentiated carcinoma < 2 cm in size and without ulcers. EMR can be considered for Paris 0-IIa < 10 mm lesions with low likelihood of malignancy, providing an en-bloc resection[71].
It is not recommended to routinely conduct pre-treatment EUS, CT, or PET for EGC that are considered endoscopically resectable due to the low risk of distant metastasis and the high rate of under-staging and over-staging. Additionally, ESD is regarded as the best method for determining T-staging[71]. Complete staging is advised after endoscopic resection in cases where noncurative resection or the risk of lymph node metastasis is present, such as positive vertical margins, lymphovascular invasion, submucosal invasion exceeding 500 µm from the muscularis mucosae, ulceration, or size larger than 2 cm in poorly differentiated lesions[71]. In these instances, surgery is recommended for patients.
ESGE guidelines suggests OGD at 3-6 months and then annually for at least 5 years after curative resection[71]. Although the risk of lymph node metastasis is very low, there is still the risk of metachronous lesions during follow-up. Patients showing endoscopic findings of atrophy, intestinal metaplasia, nodularity, enlarged folds and gastric xanthomas, have an increased risk[73] and should be followed up accordingly[47].
CONCLUSION
In this article we have made an overview of the most frequently encountered classes of GPs, presenting a new classification based on potential malignant evolution that can guide clinicians in polyp management. We provide an overview of clinical presentation, diagnosis, treatment, and follow up. Additionally, we offer a practical approach with flowcharts.
One challenge of this classification is the complexity of categorizing GPs into distinct malignancy classes, as seemingly benign polyps could contain precancerous areas that have the potential to develop into cancer. To address this issue, we have chosen to categorize polyps with a low likelihood of malignancy as "good", those with a moderate likelihood as "bad", and those with a high likelihood as "ugly".
The challenge lies in accurately identifying polyps based on their morphology and endoscopic appearance, often requiring histological examination for diagnosis. Additionally, consensus on assessing submucosal invasion during endoscopy remains difficult, particularly in Western countries. This challenge may lead to both overtreatment and undertreatment when planning resections. Furthermore, treatment should be tailored to each individual patient, considering not only polyp-related factors but also the patient's characteristics and clinical history. Lastly, clinical guidelines do not always clearly define surveillance protocols.
Given that GPs are frequently encountered in clinical practice, it is essential for endoscopists to be knowledgeable in the diagnosis and classification of polyps. This information not only guides treatment but also reflects prognosis. Additionally, evaluating the surrounding mucosa to characterize the environment in which the polyp has developed is crucial for accurate diagnosis. Therefore, endoscopists should perform biopsies not only on the polyps themselves but also on the surrounding tissue. We believe that a thorough and conscious examination during OGD can enhance the stratification of GPs based on their risk of malignancy, thereby ensuring appropriate therapy when required and avoiding unnecessary surveillance. Ultimately, this approach can reduce mortality and morbidity rates while optimizing resource utilization.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Liu JY S-Editor: Luo ML L-Editor: Webster JR P-Editor: Zheng XM
Contributor Information
Deborah Costa, Department of Digestive Endoscopy and Gastroenterology, AULSS2, Conegliano Hospital, Conegliano 31015, Italy.
Daryl Ramai, Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women's Hospital, Boston, MA 02115, United States.
Alberto Tringali, Department of Digestive Endoscopy and Gastroenterology, AULSS2, Conegliano Hospital, Conegliano 31015, Italy. albtri10@gmail.com.
References
- 1.Park DY, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008;132:633–640. doi: 10.5858/2008-132-633-GPCAM. [DOI] [PubMed] [Google Scholar]
- 2.Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524–1532. doi: 10.1038/ajg.2009.139. [DOI] [PubMed] [Google Scholar]
- 3.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 4.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 5.Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659–665. doi: 10.1055/s-2007-1009061. [DOI] [PubMed] [Google Scholar]
- 6.Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaib YH, Rugge M, Graham DY, Genta RM. Management of gastric polyps: an endoscopy-based approach. Clin Gastroenterol Hepatol. 2013;11:1374–1384. doi: 10.1016/j.cgh.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998;22:293–298. doi: 10.1097/00000478-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Vogt S, Jones N, Christian D, Engel C, Nielsen M, Kaufmann A, Steinke V, Vasen HF, Propping P, Sampson JR, Hes FJ, Aretz S. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976–1985. doi: 10.1053/j.gastro.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Sano W, Inoue F, Hirata D, Iwatate M, Hattori S, Fujita M, Sano Y. Sporadic fundic gland polyps with dysplasia or carcinoma: Clinical and endoscopic characteristics. World J Gastrointest Oncol. 2021;13:662–672. doi: 10.4251/wjgo.v13.i7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos S, van der Post RS, Brosens LAA. Gastric Epithelial Polyps: When to Ponder, When to Panic. Surg Pathol Clin. 2020;13:431–452. doi: 10.1016/j.path.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Martin FC, Chenevix-Trench G, Yeomans ND. Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther. 2016;44:915–925. doi: 10.1111/apt.13800. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Chae HS, Kim HK, Cho YS, Park YW, Son HS, Han SW, Choi KY. [Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with omeprazole] Korean J Gastroenterol. 2008;51:305–308. [PubMed] [Google Scholar]
- 14.Watanabe N, Seno H, Nakajima T, Yazumi S, Miyamoto S, Matsumoto S, Itoh T, Kawanami C, Okazaki K, Chiba T. Regression of fundic gland polyps following acquisition of Helicobacter pylori. Gut. 2002;51:742–745. doi: 10.1136/gut.51.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Notsu T, Adachi K, Mishiro T, Ishimura N, Ishihara S. Fundic gland polyp prevalence according to Helicobacter pylori infection status. J Gastroenterol Hepatol. 2020;35:1158–1162. doi: 10.1111/jgh.14934. [DOI] [PubMed] [Google Scholar]
- 16.Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol. 1996;27:896–903. doi: 10.1016/s0046-8177(96)90215-4. [DOI] [PubMed] [Google Scholar]
- 17.Sipponen P, Laxén F, Seppälä K. Cystic 'hamartomatous' gastric polyps: a disorder of oxyntic glands. Histopathology. 1983;7:729–737. doi: 10.1111/j.1365-2559.1983.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd IE, Kohlmann WK, Gligorich K, Hall A, Lyon E, Downs-Kelly E, Samowitz WS, Bronner MP. A Clinicopathologic Evaluation of Incidental Fundic Gland Polyps With Dysplasia: Implications for Clinical Management. Am J Gastroenterol. 2017;112:1094–1102. doi: 10.1038/ajg.2017.125. [DOI] [PubMed] [Google Scholar]
- 19.Jalving M, Koornstra JJ, Boersma-van Ek W, de Jong S, Karrenbeld A, Hollema H, de Vries EG, Kleibeuker JH. Dysplasia in fundic gland polyps is associated with nuclear beta-catenin expression and relatively high cell turnover rates. Scand J Gastroenterol. 2003;38:916–922. doi: 10.1080/00365520310005433. [DOI] [PubMed] [Google Scholar]
- 20.Takeda T, Asaoka D, Tajima Y, Matsumoto K, Takeda N, Hiromoto T, Okubo S, Saito H, Aoyama T, Shibuya T, Sakamoto N, Hojo M, Osada T, Nagahara A, Yao T, Watanabe S. Hemorrhagic polyps formed like fundic gland polyps during long-term proton pump inhibitor administration. Clin J Gastroenterol. 2017;10:478–484. doi: 10.1007/s12328-017-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brosens LA, Wood LD, Offerhaus GJ, Arnold CA, Lam-Himlin D, Giardiello FM, Montgomery EA. Pathology and Genetics of Syndromic Gastric Polyps. Int J Surg Pathol. 2016;24:185–199. doi: 10.1177/1066896915620013. [DOI] [PubMed] [Google Scholar]
- 22.Bertoni G, Sassatelli R, Nigrisoli E, Pennazio M, Tansini P, Arrigoni A, Rossini FP, Ponz de Leon M, Bedogni G. Dysplastic changes in gastric fundic gland polyps of patients with familial adenomatous polyposis. Ital J Gastroenterol Hepatol. 1999;31:192–197. [PubMed] [Google Scholar]
- 23.Yang J, Gurudu SR, Koptiuch C, Agrawal D, Buxbaum JL, Abbas Fehmi SM, Fishman DS, Khashab MA, Jamil LH, Jue TL, Law JK, Lee JK, Naveed M, Qumseya BJ, Sawhney MS, Thosani N, Wani SB, Samadder NJ. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020;91:963–982. doi: 10.1016/j.gie.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Graham DY, Tansel A. Interchangeable Use of Proton Pump Inhibitors Based on Relative Potency. Clin Gastroenterol Hepatol. 2018;16:800–808. doi: 10.1016/j.cgh.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klingbeil KD, Balaban A, Fertig RM, Gamret AC, Gong Y, Torres C, Satahoo SS. Inflammatory fibroid polyp of the gastric antrum presenting as hypovolemic shock: Case report and literature review. Intractable Rare Dis Res. 2017;6:304–309. doi: 10.5582/irdr.2017.01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allibone RO, Nanson JK, Anthony PP. Multiple and recurrent inflammatory fibroid polyps in a Devon family ('Devon polyposis syndrome'): an update. Gut. 1992;33:1004–1005. doi: 10.1136/gut.33.7.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997;46:53–57. doi: 10.1016/s0016-5107(97)70210-4. [DOI] [PubMed] [Google Scholar]
- 28.Bjerkehagen B, Aaberg K, Steigen SE. Do Not Be Fooled by Fancy Mutations: Inflammatory Fibroid Polyps Can Harbor Mutations Similar to Those Found in GIST. Case Rep Med. 2013;2013:845801. doi: 10.1155/2013/845801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokouchi Y, Imaoka M, Sayama A, Jindo T, Sanbuissho A. Inflammatory fibroid polyp in the duodenum of a common marmoset (Callithrix jacchus) Toxicol Pathol. 2013;41:80–85. doi: 10.1177/0192623312452491. [DOI] [PubMed] [Google Scholar]
- 30.Harima H, Kimura T, Hamabe K, Hisano F, Matsuzaki Y, Sanuki K, Itoh T, Tada K, Sakaida I. Invasive inflammatory fibroid polyp of the stomach: a case report and literature review. BMC Gastroenterol. 2018;18:74. doi: 10.1186/s12876-018-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fam S, O'Briain DS, Borger JA. Ectopic pancreas with acute inflammation. J Pediatr Surg. 1982;17:86–87. doi: 10.1016/s0022-3468(82)80338-2. [DOI] [PubMed] [Google Scholar]
- 32.Allison JW, Johnson JF 3rd, Barr LL, Warner BW, Stevenson RJ. Induction of gastroduodenal prolapse by antral heterotopic pancreas. Pediatr Radiol. 1995;25:50–51. doi: 10.1007/BF02020846. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SB, Gupta V. Ectopic pancreas as a cause of gastric outlet obstruction in an infant. Indian J Gastroenterol. 2004;23:219. [PubMed] [Google Scholar]
- 34.Cazacu IM, Luzuriaga Chavez AA, Nogueras Gonzalez GM, Saftoiu A, Bhutani MS. Malignant Transformation of Ectopic Pancreas. Dig Dis Sci. 2019;64:655–668. doi: 10.1007/s10620-018-5366-z. [DOI] [PubMed] [Google Scholar]
- 35.Sakorafas GH, Sarr MG. Ectopic gastric submucosal pancreatic tissue. JOP. 2003;4:214–215. [PubMed] [Google Scholar]
- 36.Riyaz A, Cohen H. Ectopic pancreas presenting as a submucosal gastric antral tumor that was cystic on EUS. Gastrointest Endosc. 2001;53:675–677. doi: 10.1067/mge.2001.113270. [DOI] [PubMed] [Google Scholar]
- 37.Li CF, Li QR, Bai M, Lv YS, Jiao Y. Overview of ectopic pancreas. World J Gastrointest Surg. 2024;16:284–288. doi: 10.4240/wjgs.v16.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch. 2006;448:80–84. doi: 10.1007/s00428-005-0068-2. [DOI] [PubMed] [Google Scholar]
- 40.Ghazi A, Ferstenberg H, Shinya H. Endoscopic gastroduodenal polypectomy. Ann Surg. 1984;200:175–180. doi: 10.1097/00000658-198408000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markowski AR, Markowska A, Guzinska-Ustymowicz K. Pathophysiological and clinical aspects of gastric hyperplastic polyps. World J Gastroenterol. 2016;22:8883–8891. doi: 10.3748/wjg.v22.i40.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang Y, Zhang W, Huang Y, Wang Y, Shao Q, Wu X, Lu N, Xie C. Effect of Helicobacter pylori eradication on hyperplastic gastric polyps: A systematic review and meta-analysis. Helicobacter. 2021;26:e12838. doi: 10.1111/hel.12838. [DOI] [PubMed] [Google Scholar]
- 43.Aydin I, Ozer E, Rakici H, Sehitoglu I, Yucel AF, Pergel A, Sahin DA. Antral hyperplastic polyp: A rare cause of gastric outlet obstruction. Int J Surg Case Rep. 2014;5:287–289. doi: 10.1016/j.ijscr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijkhuizen SM, Entius MM, Clement MJ, Polak MM, Van den Berg FM, Craanen ME, Slebos RJ, Offerhaus GJ. Multiple hyperplastic polyps in the stomach: evidence for clonality and neoplastic potential. Gastroenterology. 1997;112:561–566. doi: 10.1053/gast.1997.v112.pm9024310. [DOI] [PubMed] [Google Scholar]
- 45.Imura J, Hayashi S, Ichikawa K, Miwa S, Nakajima T, Nomoto K, Tsuneyama K, Nogami T, Saitoh H, Fujimori T. Malignant transformation of hyperplastic gastric polyps: An immunohistochemical and pathological study of the changes of neoplastic phenotype. Oncol Lett. 2014;7:1459–1463. doi: 10.3892/ol.2014.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginsberg GG, Al-Kawas FH, Fleischer DE, Reilly HF, Benjamin SB. Gastric polyps: relationship of size and histology to cancer risk. Am J Gastroenterol. 1996;91:714–717. [PubMed] [Google Scholar]
- 47.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 48.Chandrasekhara V, Ginsberg GG. Endoscopic management of gastrointestinal stromal tumors. Curr Gastroenterol Rep. 2011;13:532–539. doi: 10.1007/s11894-011-0224-6. [DOI] [PubMed] [Google Scholar]
- 49.Abraham SC, Montgomery EA, Singh VK, Yardley JH, Wu TT. Gastric adenomas: intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol. 2002;26:1276–1285. doi: 10.1097/00000478-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Sakaguchi H, Enjoji M. Depressed adenoma of the stomach. Cancer. 1988;62:2197–2202. doi: 10.1002/1097-0142(19881115)62:10<2197::aid-cncr2820621021>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 51.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 52.Islam RS, Patel NC, Lam-Himlin D, Nguyen CC. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (N Y) 2013;9:640–651. [PMC free article] [PubMed] [Google Scholar]
- 53.Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850–856. doi: 10.4251/wjgo.v12.i8.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115–154. doi: 10.1007/s12022-022-09708-2. [DOI] [PubMed] [Google Scholar]
- 55.Panzuto F, Ramage J, Pritchard DM, van Velthuysen MF, Schrader J, Begum N, Sundin A, Falconi M, O'Toole D. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1-G3. J Neuroendocrinol. 2023;35:e13306. doi: 10.1111/jne.13306. [DOI] [PubMed] [Google Scholar]
- 56.Hung OY, Maithel SK, Willingham FF, Farris AB 3rd, Kauh JS. Hypergastrinemia, type 1 gastric carcinoid tumors: diagnosis and management. J Clin Oncol. 2011;29:e713–e715. doi: 10.1200/JCO.2011.35.3235. [DOI] [PubMed] [Google Scholar]
- 57.Rinzivillo M, Panzuto F, Esposito G, Lahner E, Signore A, Annibale B. Usefulness of 68-Gallium PET in Type I Gastric Neuroendocrine Neoplasia: A Case Series. J Clin Med. 2022;11:1641. doi: 10.3390/jcm11061641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsolakis AV, Ragkousi A, Vujasinovic M, Kaltsas G, Daskalakis K. Gastric neuroendocrine neoplasms type 1: A systematic review and meta-analysis. World J Gastroenterol. 2019;25:5376–5387. doi: 10.3748/wjg.v25.i35.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panzuto F, Magi L, Esposito G, Rinzivillo M, Annibale B. Comparison of Endoscopic Techniques in the Management of Type I Gastric Neuroendocrine Neoplasia: A Systematic Review. Gastroenterol Res Pract. 2021;2021:6679397. doi: 10.1155/2021/6679397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 61.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 62.Odashima M, Otaka M, Nanjo H, Jin M, Horikawa Y, Matsuhashi T, Ohba R, Koizumi S, Kinoshita N, Takahashi T, Shima H, Watanabe S. Hamartomatous inverted polyp successfully treated by endoscopic submucosal dissection. Intern Med. 2008;47:259–262. doi: 10.2169/internalmedicine.47.0360. [DOI] [PubMed] [Google Scholar]
- 63.Vyas M, Yang X, Zhang X. Gastric Hamartomatous Polyps-Review and Update. Clin Med Insights Gastroenterol. 2016;9:3–10. doi: 10.4137/CGast.S38452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latchford AR, Neale K, Phillips RK, Clark SK. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum. 2012;55:1038–1043. doi: 10.1097/DCR.0b013e31826278b3. [DOI] [PubMed] [Google Scholar]
- 65.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adolph VR, Bernabe K. Polyps in children. Clin Colon Rectal Surg. 2008;21:280–285. doi: 10.1055/s-0028-1089943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 68.Exarchou K, Kamieniarz L, Tsoli M, Victor A, Oleinikov K, Khan MS, Srirajaskanthan R, Mandair D, Grozinsky-Glasberg S, Kaltsas G, Howes N, Pritchard DM, Toumpanakis C. Is local excision sufficient in selected grade 1 or 2 type III gastric neuroendocrine neoplasms? Endocrine. 2021;74:421–429. doi: 10.1007/s12020-021-02775-1. [DOI] [PubMed] [Google Scholar]
- 69.Ngamruengphong S, Abe S, Oda I. Endoscopic Management of Early Gastric Adenocarcinoma and Preinvasive Gastric Lesions. Surg Clin North Am. 2017;97:371–385. doi: 10.1016/j.suc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Fujiyoshi MRA, Inoue H, Fujiyoshi Y, Nishikawa Y, Toshimori A, Shimamura Y, Tanabe M, Ikeda H, Onimaru M. Endoscopic Classifications of Early Gastric Cancer: A Literature Review. Cancers (Basel) 2021;14:100. doi: 10.3390/cancers14010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591–622. doi: 10.1055/a-1811-7025. [DOI] [PubMed] [Google Scholar]
- 72.Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 73.Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663–698. doi: 10.1111/den.13684. [DOI] [PubMed] [Google Scholar]
- 74.Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T, Goda K, Imazu H, Arakawa H, Mochizuki K, Tajiri H. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310–315. doi: 10.1055/s-0028-1119639. [DOI] [PubMed] [Google Scholar]