Abstract
Context:
Many clinicians, trainers, and athletes do not have a true understanding of the effects of commonly used performance-enhancing drugs (PEDs) on performance and health.
Objective:
To provide an evidence-based review of 7 commonly used pharmacological interventions for performance enhancement in athletes.
Data Sources:
PubMed and Scopus databases were searched on April 8, 2022.
Study Selection:
Systematic reviews (SRs) and meta-analyses (MAs) assessing the performance-enhancing effects of the following interventions were included: androgenic anabolic steroids (AAS), growth hormone (GH), selective androgen receptor modulators (SARMs), creatine, angiotensin-converting enzyme (ACE)-inhibitors, recombinant human erythropoietin (rHuEPO), and cannabis.
Study Design:
Umbrella review of SRs and MAs.
Level of Evidence:
Level 4.
Data Extraction:
Primary outcomes collected were (1) body mass, (2) muscle strength, (3) performance, and (4) recovery. Adverse effects were also noted.
Results:
A total of 27 papers evaluating 5 pharmacological interventions met inclusion criteria. No studies evaluating SARMs or ACE-inhibitors were included. AAS lead to a 5% to 52% increase in strength and a 0.62 standard mean difference in lean body mass with subsequent lipid derangements. GH alters body composition, without providing a strength or performance benefit, but potential risks include soft tissue edema, fatigue, arthralgias, and carpel tunnel syndrome. Creatine use during resistance training can safely increase total and lean body mass, strength, and performance in high-intensity, short-duration, repetitive tasks. Limited evidence supports rHuEPO benefit on performance despite increases in both VO2max and maximal power output, and severe cardiovascular risks are documented. Cannabis provides no performance benefit and may even impair athletic performance.
Conclusion:
In young healthy persons and athletes, creatine can safely provide a performance-enhancing benefit when taken in controlled doses. AAS, GH, and rHuEPO are associated with severe adverse events and do not support a performance benefit, despite showing the ability to change bodily composition, strength, and/or physiologic measures. Cannabis may have an ergolytic, instead of ergogenic, effect.
Keywords: athletes, doping, performance-enhancing drugs, sport supplements
Performance-enhancing drugs (PEDs) are substances taken for their desired ergogenic benefit. Perceived benefits include enhancing training adaptations, improving exercise efficiency or performance, mitigating recovery times, or aiding in injury prevention. 46 Major classes of PEDs include anabolic agents, hematologic agents, stimulants, beta-blockers, beta-2-agonists, diuretic agents, and anxiolytics and analgesics; more recently, athletes have explored gene doping.39,92 Athletes may take various PEDs based on their sport, with agents such as anabolic steroids and growth hormone (GH) often taken for their potential for increasing muscle mass and thus, force generation. Meanwhile, hematologic agents, such as recombinant human erythropoietin (rHuEPO), may be beneficial for endurance athletes in sports such as cycling or running.54,85
Doping in sports is not a new phenomenon, as the use of substances to enhance performance dates back to the ancient times of Greek and Roman gladiators.9,16,91 Still today, athletes are continuously utilizing a variety of resources in an attempt to optimize performance.9,16,27 In some cases, the pressures and desires to succeed can push athletes to use dietary, and even pharmacological, interventions to gain edges over competition.84,92 Even the smallest improvements in performance can mean the difference between winning or losing at the highest level of sport.
The prevalence of PEDs in amateur and college athletes ranges from 1.1% to 18.3%.12,18,30,53,58,64,77,82 While the true prevalence of PED use in athletes is difficult to determine, it is generally thought that the number of people using these substances is underestimated due to response errors in self-reported surveys and suboptimal drug detection methods.65,88 In addition, the prevalence varies widely based on what substance, sporting event, and athletic level is being evaluated.1,30,42 While historically it was thought that most persons using PEDs were high level athletes, amateur and recreational athletes as well as regular gym-goers are among those trying and consistently using PEDs.21,34,72 This increase in overall use is concerning due to the risk of serious adverse effects associated with taking PEDs, especially when they are not properly dosed, monitored, or administered.9,54,90
The list of PEDs is long, diverse, and continuously evolving, of which some of the most common are as follows: androgenic anabolic steroids (AAS), GH, selective androgen receptor modulators (SARMs), creatine, angiotensin-converting enzyme (ACE)-inhibitors, rHuEPO, and cannabis. While several studies have reviewed the efficacy and safety of the aforementioned substances, there is still no consensus on whether their ergogenic effects impact sports performance, due to limited substantial high-quality evidence. Thus, the purpose of this study was to compile data reported in high-quality systematic reviews (SRs) and meta-analyses (MAs) on common PEDs used by athletes to provide an evidence-based overview of the reported changes in biological and sport-dependent parameters that affect athletic performance.
Methods
Study Identification and Selection
Two independent reviewers performed a literature search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. 71 The search results were reviewed with the senior author in the event of disagreement. All search results underwent title and abstract review and potentially eligible studies advanced to a full-text review. In addition, the reference lists of all eligible studies were screened for additional articles that met the inclusion criteria.
Search Strategy and Eligibility Criteria
The PubMed and Scopus databases were searched on April 8, 2022, using the search terms noted in Appendix 1 (available in the online version of this article). SRs and MAs were identified. To be included, the studies had to be pertinent to one of the following preselected PEDs: (1) AAS, (2) GH (or growth hormone-releasing hormone, GHRH), (3) SARMs, (4) creatine, (5) ACE-inhibitors, (6) rHuEPO, or (7) cannabis. In addition, the studies had to be pertinent to athletes/sports performance, such as muscle mass, strength, cardiac parameters, or recovery. Exclusion criteria included papers not written in the English language or any study design or report that was not an SR or MA.
Outcome Measures and Data Extraction
Study characteristics including author name, year, study design, number of studies included in review, total number of participants, and athletic level of participants were extracted for included studies. In addition, drug intervention, dose, duration, and timing of administration were collected when reported. Primary outcomes collected were categorized into 4 main categories: (1) body mass, (2) muscle strength, (3) performance, and (4) recovery. Complications and adverse effects of each intervention were also noted. For papers that mentioned multiple substances or various testing populations, only the information of the aforementioned substances and healthy populations of interest for this umbrella review were extracted.
Data Analysis
Included papers and extracted data were synthesized into Appendix 2 (available online). Heterogeneity among the included studies precluded formal MA. Table 1 summarizes combined data for each intervention.
Table 1.
Primary outcomes by intervention
| Intervention | Body Mass | Muscle Strength | Sports Performance | Recovery |
|---|---|---|---|---|
| Anabolic Steroids | Evidence favors increase in lean body mass | Evidence favors benefit for trained athletes; conflicting results in untrained | Insufficient evidence | Insufficient evidence |
| Growth Hormone | Evidence shows increase in body weight and lean body mass and decrease in fat mass | Evidence shows no benefit | Potential benefit for anaerobic exercise capacity but not sports performance | Insufficient evidence |
| Creatine | Evidence supports increased body mass and lean body mass and no effect on fat mass | Evidence suggests a positive effect on upper and lower body strength, at least with the short-term use | Evidence shows potential benefit for short-burst anaerobic performance, but not aerobic performance. Conflicting results on change in peak power during exercise | Inconclusive evidence, but possible decrease on muscle damage markers |
| Erythropoietin | Insufficient evidence | Insufficient evidence | Evidence shows possible endurance benefit, V02max increase, and increase in max power output. Insufficient evidence to show that this difference translates to improvements in running, cycling, or swimming | Evidence suggests no benefit |
| Cannabinoids | Insufficient evidence | Evidence suggests no benefit, and possible increased weakness | Evidence shows no benefit of cannabis use on athletic or exercise performance; possible negative influence on it | Insufficient evidence |
Methodological Quality Assessment
Risk of bias analysis was performed by 2 authors utilizing the Assessment of Multiple Systematic Reviews (AMSTAR) on the 27 studies that met the inclusion/exclusion criteria. 80 (Appendix 3, available online). AMSTAR is composed of an 11-question tool used for evaluating the methodological quality of systematic reviews. 80 Each question receives an answer of “Yes,” “No,” “Can’t answer,” or “Not applicable,” and the results were then converted to a numerical score to provide an assessment of overall bias: low quality (score 0-3), moderate quality (score 4-7), or high quality (score 8-11). This tool assesses bias by evaluating topics including the literature search, inclusion/exclusion transparency, quality of included papers, conflicts of interest, etc. The methodology quality of each individual study within the reviews was not evaluated. This was because some reviews did not assess the methodological quality of each individual study within their review and, for those that did, there was a large amount of heterogeneity in the bias assessment tool used.
Results
Study Selection
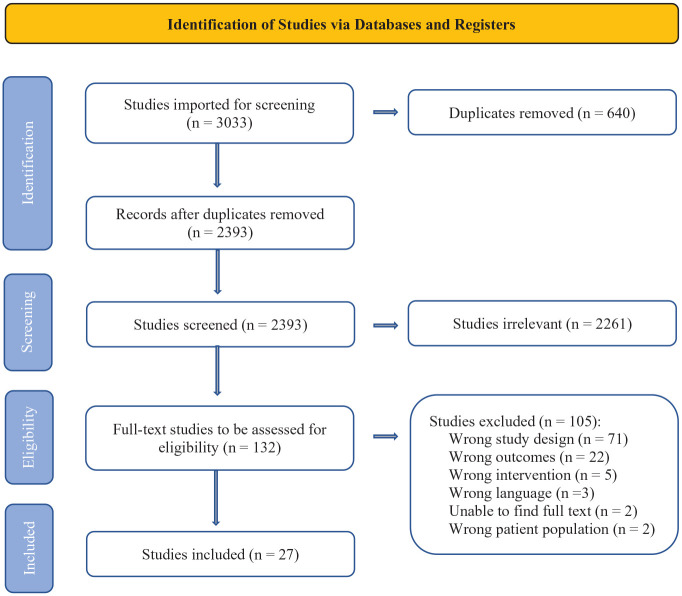
There were 3033 studies imported for screening. Ultimately, data were extracted from 27 studies meeting the inclusion/exclusion criteria after full-text review (Figure 1). While 7 substances were included in our literature search, only 5 substances were included in studies that met our screening criteria. These included AAS, GH, creatine, rHuEPO, and cannabis. No studies evaluating SARMs and ACE-inhibitors met inclusion/exclusion criteria.
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Study Characteristics and Demographics
The mean number of reviews per substance was 5.4 (range, 2-14), with a mean number of studies per review of 18.5 (range, 3-96), and a mean number of participants per substance of 1736.8 (range, 453-5912).
Risk of bias analyses revealed 3 reviews scored as low quality of evidence,24,41,45 12 reviews as moderate quality, and 12 reviews as high quality (Appendix 3, available online).
Primary Outcomes
The primary outcomes for each substance, including body mass, muscle strength, performance, and recovery, are seen below and in Table 1.
Body Mass
For measures regarding body mass, there was evidence that AAS favor an increase in lean body mass (standard mean difference, 0.62). 2 In addition, GH favors an increase in body weight and lean body mass, and a decrease in fat mass (mean difference, 0.96 kg, 2.56 kg, and -0.93kg, respectively).35,56,78 Finally, creatine can increase body mass and lean body mass (mean difference, 1.2 kg and 2.2 kg, respectively) with no effect on fat mass.10,69 There was insufficient evidence to make any assessment of the effect of rHuEPO and cannabinoids on body mass measures.
Muscle Strength
AAS were found to improve strength in trained athletes (5% to 52% increase),2,24 while there is conflicting evidence of that effect in untrained athletes. GH showed no strength benefit in the present literature. Creatine had a positive effect on upper and lower body strength, with short-term use (upper body increase, 6.85 kg; range, 5.24-8.47; lower body increase, 9.76 kg; range, 3.37-16.15). 17 Cannabis was found to have no ergogenic benefit, but there was evidence linking it with strength impairments.45,48,86 There was insufficient evidence to make any assessment on the effect of rHuEPO on measures of muscle strength.
Performance
For measures of performance, there was minimal evidence that GH provides a potential benefit for anaerobic exercise capacity (mean difference in Wingate value, 0.6 kJ), 35 while creatine may benefit short-burst anaerobic performance, but not aerobic performance. For creatine, there were conflicting results on changes in peak power during exercise. rHuEPO provides a potential increase in aerobic capacity or endurance, increased time to exhaustion (TTE), a VO2max increase (7% to 9.7%), and an increase in max power output (25-35 W).38,79 However, there is insufficient evidence to correlate this difference to improvements in running, cycling, or swimming. Lastly, the evidence for cannabis showed no benefit on athletic or exercise performance but rather a potential negative influence. There was insufficient evidence to make assessments on the effects of AAS on performance.
Recovery
When evaluating recovery metrics, there is no evidence to support that creatine has a recovery benefit. However, there were 2 studies showing that creatine may decrease muscle damage markers such as creatine kinase and lactate dehydrogenase.20,44 For rHuEPO, results showed that it provided no recovery benefit. Finally, there was insufficient evidence to make assessments on the effects of AAS, GH, or cannabis on measures of recovery.
Adverse Events
There are several adverse effects associated with many of the performance-altering substances that were investigated. For example, AAS use was correlated with a decrease in high-density lipoproteins (HDLs), increase in low-density lipoproteins (LDLs), increased irritability or mood changes, and an elevation in liver enzymes. 2 Other reported side effects of AAS use included injection-site reactions, alopecia, acne, increased hematocrit, and decreased testicular size. 2 For GH, side effects were related to abuse and may include soft tissue edema, fatigue, arthralgias, and carpal tunnel syndrome. 56 When evaluating the findings of 14 reviews for creatine, the results showed that it was generally safe when used in the short term and using dosing recommendations within the respective studies. Most studies on creatine use noted a small risk of gastrointestinal irritation, rash, or headaches with use. In addition, the long-term effects of creatine use are less known due to lack of evidence. Adverse effects associated with rHuEPO use were related primarily to the increased viscosity of blood, leading to increased risk of thrombotic events such as myocardial infarction (MI), pulmonary embolism (PE), and stroke. 38 Lastly, cannabis was found to be relatively safe; however, there are reports of associated tachycardia, decreased resting blood pressure, and decreased balance. 45
A summary statement for each PED, including their adverse events, are noted in Table 2.
Table 2.
Summary of primary findings and adverse events by intervention
| Intervention (No. of Reviews) | No. of Studies in Reviews per Intervention (Total No. of Participants Receiving Intervention) | Adverse Events | Summary Statement |
|---|---|---|---|
| Anabolic Steroids (2) | 42 (984) | Decrease in HDL, increase in LDL, irritability and mood changes, elevations in liver enzymes such as aspartate aminotransferase may all be present. Other reported side effects include injection site reactions, alopecia, acne, increased hematocrit, and decreased testicular size | AAS use in healthy trained adults is associated with small increases in strength and lean body mass. There are risks associated with use |
| GH (3) | 49 (559) | Soft tissue edema, fatigue, arthralgias, and carpal tunnel syndrome | GH administration may lead to changes in body composition (lean body mass, body weight, extracellular water content, fat mass), but it does not seem to increase either muscle strength or improve physical performance in healthy, young subjects. There are adverse effects associated with use |
| Creatine (14) | 351 (5912) | Generally safe when properly used short-term (small chance of GI upset, rash, or headache have been reported), long-term effects are largely unknown due to lack of evidence | There is sufficient evidence to conclude that total and lean body mass, strength, and performance in high-intensity, short-duration, repetitive tasks are improved when resistance training is augmented with properly dosed and monitored creatine use |
| Erythropoietin (3) | 34 (453) | Cardiovascular effects (risk of thrombotic events such as MI, PE, and stroke), encephalopathy | There is weak scientific evidence supporting EPO on athletic performance, despite evidence for an increase in both VO2max and maximal cycling power output |
| Cannabinoids (5) | 32 (776) | Tachycardia, decreased resting blood pressure, and decreased balance | Based on the low quality of evidence available, THC does not enhance aerobic exercise or strength and may impair athletic performance |
AAS, androgenic anabolic steroids; EPO, erythropoietin; GH, growth hormone; GI, gastrointestinal; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; PE, pulmonary embolism; THC, tetrahydrocannabinol.
Discussion
Anabolic Steroids
Ergogenic Potential
There is a lack of current evidence on the association between AAS use and sports performance, despite its ability to increase lean body mass and strength.2,24 The noted increase in muscle strength associated with AAS use is supported by many studies demonstrating a dose-dependent increase in muscle mass resulting from AAS administration. In fact, 1 study concluded that the effect of AAS plus exercise on muscle strength was approximately 52% greater than the increase in strength attributable to exercise alone. In addition, when coupled with consistent weekly exercise (3-4 times per week), lean body mass increases 0.62 standard deviations above what would be gained from exercise alone, were reported. 2
Safety
The most common adverse events recorded were changes in lipids, mood, and liver enzymes. Specifically, AAS were associated with a statistically significant decrease in HDL levels in 3 of 5 studies,11,29,47 but an increase in LDL levels was only seen in 1 of 5 studies. 29 However, it should be noted that these changes may return to normal after cessation of anabolic steroid use. 33 Of the 5 studies that monitored mood, 2 found that subjects in the anabolic steroid group were irritable and had more significant mood changes compared with a placebo.28,43 Lastly, while the clinical significance could be uncertain, 1 of the 4 studies noted increased AST levels when compared with placebo. 29 Other reported adverse effects include injection-site reactions, alopecia, acne, increased hematocrit, and decreased testicular size. 2 A review examining the adverse effects of doping with AAS in competitive athletics, recreational sports, and bodybuilding further found that the increase in hematocrit levels may lead to venous thromboembolism, MI, and stroke. 90 Long-term users were also found to have a higher incidence of arrythmia, atherosclerosis, and ventricular myocardial hypertrophy. In women who abuse AAS, anovulation leading to infertility, dysmenorrhea, and hirsutism has been reported. 68 Lastly, 17-alpha-alkylated AAS are hepatotoxic and may lead to cholestasis, peliosis, hepatic adenomas, and hepatic carcinomas. As a secondary affect, hyperbilirubinemia can cause cholemic nephrosis and lead to renal failure.
Regulation and Testing
According to the World Anti-Doping Agency (WADA), anabolic steroids are the most abused PEDs, accounting for approximately 50% of all violations in human sports since the International Olympic Committee (IOC) banned anabolic steroids for the first time for the 1976 Olympic games in Montreal, Canada. 5 Initially, the American College of Sports Medicine issued a statement on how the administration of anabolic steroids to healthy humans below the age of 50 in medically approved therapeutic doses does not bring about any significant performance improvements. This was later revised in 1984 given new data that reported greater gains in strength in persons taking AAS combined with high-intensity exercise regimens when compared with placebo and, since then, AAS have been on the banned substances list. Furthermore, AAS are tested for and prohibited by many American sporting associations, including the National Collegiate Athletic Association (NCAA), Major League Baseball (MLB), National Football League (NFL), National Hockey League (NHL), and the National Basketball Association (NBA).
Per WADA guidelines, the key principle for the detection of AAS use is the establishment of an athlete’s biological passport. For example, an athlete’s urinary and serum testosterone, dihydrotestosterone, or epitestosterone are measured at baseline in the blood or urine followed by repeat measurements of the athlete’s steroid and steroid metabolite concentrations longitudinally. This allows for monitoring of significant deviations from baseline over time.
Distinguishing between an athlete with naturally high testosterone concentrations and an athlete using testosterone or testosterone precursor supplements is difficult. There have been technological advancements that have dramatically improved the measurement of AAS, but there remain challenges, particularly as the development of novel, designer AAS advances rapidly.
Growth Hormone
Ergogenic Potential
The main findings in our current study are that, although GH may not improve muscle strength or physical performance in young athletes, it does have the capacity to change body composition.35,56,78
All 3 studies examined found an increase in lean body mass (range, 2.1-2.86 kg), while 2 of the studies found a significant decrease in fat mass (range, 0.67-1.22 kg).35,56,78 Mixed results were found when measuring significant changes in body weight.35,56 No studies showed improvements in muscle strength, when measured by 1-repetition maximum voluntary strength testing of biceps brachii or quadriceps femoris strength, and 2 studies also found no improvement when measuring isometric deadlifts or maximal explosive jump strength.35,78 When evaluating measures of performance, GH did not provide any benefit in bicycling speed, exercising energy expenditure, power output, or VO2max.35,56 However, 1 review found that there was some evidence suggesting GH may increase anaerobic work capacity when assessed using the Wingate test (mean difference in Wingate value, 0.60 kJ; 95% CI, 0.23 to 0.97 kJ; P < 0.01). 62 Lastly, 1 paper looked at recovery metrics and found that, in 2 out of 3 studies, blood lactate levels were significantly higher in the GH-treated group, showing that GH may decrease exercise stamina.7,56
Safety
Only 1 review in our study evaluated the potential adverse effects of GH-treated participants. 56 They found that participants in the GH group had more soft tissue edema and fatigue (44% vs 1% and 35% vs 0%, respectively) when compared with those in control groups. In addition, this review found that the development of arthralgias and carpal tunnel syndrome were more common in the GH-treated group.
Aside from edema, fatigue, arthralgias, and carpel tunnel syndrome, which has also been found in previous reviews, other significant multisystem adverse effects include cardiovascular insults and neuropsychiatric complaints with high-dose regimens.40,81 More research is needed to better understand the long-term adverse effects of GH supplementation in healthy athletes.
Regulation and Testing
The WADA 2022 Prohibited Drug List shows that peptide hormones and their releasing factors, which includes GH and GH releasing factors, as well as their analogues, are always prohibited. 93 This list of drugs is adopted by the IOC, thus prohibiting Olympic athletes from using these substances. In addition, the use of this class of drugs is prohibited by several American sporting associations, including NCAA, MLB, NFL, NHL, and NBA.
Detecting recombinant human GH is challenging, as it is both structurally identical to the body’s native GH and secreted in a pulsatile fashion. 40 Thus, application of the athlete biological passport may provide improved testing accuracy. 25 The 2 most common tests used in identifying exogenous GH use are the isoforms differential immunoassay and the human GH biomarkers tests.25,81
Creatine
Ergogenic Potential
Creatine monohydrate has numerous ergogenic benefits reported in the literature. Several studies have demonstrated that creatine may increase higher power and heavier weight/lower repetition strength feats.10,17,51,52,63,69,95 These benefits of creatine supplementation likely lie primarily in its additive properties to the phosphagen (adenosine trisphosphate-creatine phosphate [ATP-CrP]) energy system. The ATP-CrP system is an anaerobic energy system that provides energy for approximately 30 seconds of activities, such as in weightlifting or sprinting. 6
It is therefore logical that, in several studies, the benefits of creatine are noted at short timeframes and involve anaerobic efforts, such as in the Wingate test.51,52,63 Strength gains and muscular hypertrophy achieved with creatine use may be attributed to greater time under tension (ie, more repetitions or more work performed in total) that creatine permits. 50 By increasing total work capacity, creatine supplementation may contribute to greater training tolerance.
Creatine appears to have benefits in both upper and lower body strength tasks.51,52 Those utilizing creatine may expect improvement in lean body mass.10,69,95 In addition, creatine supplementation may allow for enhanced recovery between working sets and contributes its benefits in repetitious bouts of exercise.10,41 Particular sports that may benefit from creatine use include those in which athletes sprint intermittently and recover, such as in American football, soccer, basketball, and tennis.50,63 Oral creatine supplementation may also benefit biomarkers of muscle damage and improve carbohydrate storage capacity, although data on these outcomes are mixed.20,44,67,70
Safety
Overall, the side effects from creatine are minimal and many studies do not report adverse effects. Several studies have examined the side effects of creatine in high-level athletes, and most demonstrate no increases in cramping or any changes in health markers.31,49 These findings persist even to those using creatine long term.75,76 In fact, some studies suggest that creatine use may reduce injury rates and augment rehabilitation from injury by limiting disuse atrophy.31,36 There are no consistent data supporting the idea that creatine may lead to kidney damage. 3 In addition, creatine appears safe for use regardless of ambient temperature. 57
There is no specific population in which creatine use appears unsafe, with most data suggesting its safety in both adolescents and older adults, untrained and competitive athletes, and male and female athletes.3,15
Regulation and Testing
Creatine supplementation has increased rapidly not just in the professional sports world but also in young athletes. 61 The most popular form of oral creatine supplementation in the United States (US) is creatine monohydrate, which is readily available for purchase. The US Food and Drug Administration recently recognized creatine monohydrate as a safe substance, and therefore exempted it from premarket approval. 83 Additional forms of creatine exist, such as creatine hydrochloride, creatine citrate, creatine nitrate, creatine pyruvate, and other creatine salts. However, current evidence does not support their use over creatine monohydrate.3,26 There are inconsistencies in the literature on the optimal dosing of creatine, and whether a loading dose is required. 3 Furthermore, it is important to note that creatine may be obtained from animal-based foods, though not as at high of a concentration as with supplementation. 94 Creatine is currently not banned by any major sports organizations or the IOC. However, the NCAA does have a policy that prohibits member institutions from providing creatine to student-athletes. 66
Erythropoitin
Ergogenic Potential
There is weak evidence supporting rHuEPO use for improved athletic performance, despite evidence showing an increase in physiologic parameters.38,79,86
The hematological effects of rHuEPO include increasing hemoglobin and hematocrit concentrations, which may lead to increased oxygen carrying capacity of blood.38,79,86 In addition, all 3 studies found that rHuEPO increased VO2max and maximal power output.38,79,86 Further, when TTE was measured, there was an improvement in medium-dose and high-dose participants when evaluated in the short term, but no effect was found with low-dose rHuEPO. 86 Interestingly, this increased TTE may be smaller in trained athletes when compared with untrained athletes. 38
However, what was unclear is whether these changes in physiological parameters translate into an improvement in performance or recovery. VO2max and TTE measures generally lack reproducibility due to the high variability in study protocols. Two studies evaluating race performance times have found minimal, if any, benefits of rHuEPO on performance.8,22 This may be due to the fact these studies were performed with doses lower than those used by athletes who abuse these substances. Nevertheless, despite the benefit of rHuEPO on physiologic measures, there is a lack of sufficient evidence to support its ability to enhance performance outcomes in aerobic sporting events, such as running or cycling.
Safety
rHuEPO use is associated with several cardiovascular effects, including increased blood pressure and viscosity, leading to greater risk of thrombotic events such as stroke, MI, and PE. 38 Other associated side effects may include hypertensive posterior encephalopathy and enhanced tumor growth. 38 Additional papers on the topic have found similar adverse events, with cardiovascular consequences such as hypertension and an increased risk of severe thrombotic events being the most commonly reported.23,37,73
Regulation and Testing
rHuEPO and other erythropoietin-stimulating agents are always prohibited, both in and out of competition, by the WADA 2022 Prohibited Drug List. As mentioned previously, this list is used by the IOC and other international cycling agencies such as the Union Cycliste Internationale to help control doping. 93 In addition, rHuEPO use is prohibited by all major American sports organizations.
Due to strategic doping regimens and short windows of detection, detecting rHuEPO use in athletes is challenging. 79 However, utilizing the athletic biological passport and methods such as sodium-dodecyl-sulfate-polyacrylamide gel electrophoresis, iso-electric focusing, and biotinylated anti-EPO antibodies have improved detection.14,59,60
Cannabis
Ergogenic Potential
The performance-enhancing potential of cannabis use is a controversial topic, with limited high-quality data. Cannabis consumption is reported to have analgesic properties, which polled athletes indicate as a primary reason they consume cannabis during athletic activities. 55 The feelings of euphoria and increased sociability may alleviate stress associated with athletic activities and may improve overall performance. 74 The cardiovascular and pulmonary effects of cannabis use during athletic performance are reported heterogeneously and lack consensus. In vivo and in vitro studies have previously shown effects relating to tachycardia, vasodilation, and bronchodilation. 32 Recently, an SR analyzing these cannabinoid effects on athletic performance concluded that cannabis consumption rather imposes ergolytic effects that potentially impair performance. 13 For example, 2 studies showed that cannabis use may decreased exercise duration,45,87 and 3 studies demonstrated that it may decrease work capacity.13,19,45 However, due to limited data investigating cannabis use and exercise potential, the ergogenic effects of cannabis consumption in athletes remains unclear and without consensus.
Safety
The adverse effects of cannabis use during athletic performance are not reported widely in the literature. Studies on the effects of cannabis consumption and driving ability have shown a strong association with driving and cognitive impairment. 89 In addition, cardiovascular events—including MI and stroke—have also been associated with cannabis consumption outside of athletic performance. 45 Cannabis use has also been shown to lead to panic disorders, paranoia, and psychosis. 74 The current lack of data regarding cannabis use during athletic performance, in conjunction with the known adverse effects, highlights a gap in the research that requires further investigation.
Regulation and Testing
Despite these inconclusive findings regarding ergogenic potential and gaps in the literature regarding adverse effects, cannabis use remains extremely popular with athletes. Recently, a study performed by Docter et al 19 indicated that approximately 25% of elite and university athletes consume cannabis. The cannabis plant includes at least 60 cannabinoid compounds, with tetrahydrocannabinol (THC) and cannabidiol being the most widely known. 13 The pharmacodynamic effects of cannabis consumption are caused primarily by the compound THC. The metabolized product of THC is 11-nor-9-carboxy-THC (THCCOOH), which is monitored in the urine of competing athletes by WADA.32,45 In addition to WADA, the NFL, NBA, and NCAA actively test for cannabis using varying thresholds. 19 Although highly restricted and tested, public attitudes towards cannabis use have become increasingly positive in the past decade, as many states have legalized its use. 4
Limitations
This study has significant limitations. The first is that only 2 databases, Scopus and PubMed, were searched, making it possible that some relevant papers not found in these 2 databases were not included in our review. In addition, there was a limited number of current SRs/MAs evaluating many of the examined agents, including AAS, GH, and rHuEPO; each of these substances had ≤3 reviews on the topic, preventing the formation of any strong conclusions. Another limitation of this umbrella review is that, despite the systematic nature of this study, there was a significant heterogeneity within each review, limiting the ability to compare outcomes for each agent. For example, the populations evaluated, dosing regimens, and exercises performed, while similar, varied across reviews. Last, as is the case with any SR, our search strategy and eligibility criteria may have unintentionally omitted relevant data.
It should be noted that this current review covers only the aforementioned interventions, which were chosen based on previous literature on the subject.65,85,92 Due to the wide variety of substances used for performance-enhancing effects in a range of settings, we appreciate that this review is not comprehensive, nor does it elaborate on the nuances of each substance. The focus of this umbrella review is to provide an evidence-based overview of the possible pharmacological interventions for performance enhancement in healthy athletes with a focus on interventions that have already been studied in SRs or MAs. While there is brief mention of the regulation surrounding these substances as well as the safety profile of the interventions, these are not the primary outcomes of our paper and further discussion would be outside of the scope of the current review. Using the findings presented in the current review, there is the potential, unlike in narrative reviews, for improved synthesis and relay of higher quality evidence to providers, athletic trainers, and athletes on the effects and risks of using these agents to augment athletic performance.
Conclusion
In studies evaluating young healthy persons and athletes, creatine can safely provide a performance-enhancing benefit when taken in controlled doses. AAS, GH, and rHuEPO are associated with severe adverse events and do not support an ergogenic effect, despite showing the ability to change bodily composition, strength, and/or physiologic measures. Cannabis may have an ergolytic, instead of ergogenic, effect. Limited available data on SARMs and ACE-inhibitors prevented inclusion in this review. Further high-quality studies should be performed to evaluate the performance-enhancing effect of these substances.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health
Supplemental material, sj-docx-2-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health
Supplemental material, sj-docx-3-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health
Footnotes
The following author declared potential conflicts of interest: J.C. has received consulting fees from Smith & Nephew, Ossur, and Miach, honoraria from Smith & Nephew, and holds stock in Springloaded and Overture. J.M.T. has received royalties, honoraria, a grant, and a patent from Arthrex, and consulting fees from DePuy Mitek.
ORCID iD: Sachin Allahabadi  https://orcid.org/0000-0002-1185-3039
https://orcid.org/0000-0002-1185-3039
Contributor Information
Alec A. Warrier, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Eric N. Azua, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Luke B. Kasson, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Sachin Allahabadi, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Zeeshan A. Khan, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Enzo S. Mameri, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
Hasani W. Swindell, Department of Orthopedic Surgery, Columbia University Irving Medical Center, New York, New York.
John M. Tokish, Mayo Clinic Arizona, Phoenix, Arizona.
Jorge Chahla, Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, Illinois.
References
- 1. Aguilar-Navarro M, Muñoz-Guerra J, del Mar Plara M, Del Coso J. Analysis of doping control test results in individual and team sports from 2003 to 2015. J Sport Health Sci. 2020;9(2):160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews MA, Magee CD, Combest TM, Allard RJ, Douglas KM. Physical effects of anabolic-androgenic steroids in healthy exercising adults: a systematic review and meta-analysis. Curr Sports Med Rep. 2018;17(7):232-241. [DOI] [PubMed] [Google Scholar]
- 3. Antonio J, Candow DG, Forbes SC, et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J Int Soc Sports Nutr. 2021;18(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators - national survey on drug use and health, United States, 2002-2014. MMWR Surveill Summ. 2016;65(11):1-28. [DOI] [PubMed] [Google Scholar]
- 5. Beckett AH, Cowan DA. Misuse of drugs in sport. Br J Sports Med. 1978;12(4):185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berg JM. Biochemistry. New York: Freeman and Company, 2011. [Google Scholar]
- 7. Billat VL, SirvenT P, Py G, Koralsztein JP, Mercier J. The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med. 2003;33(6):407-426. [DOI] [PubMed] [Google Scholar]
- 8. Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32(7):1238-1243. [DOI] [PubMed] [Google Scholar]
- 9. Botrè F, Pavan A. Enhancement drugs and the athlete. Neurol Clin. 2008;26(1):149-167. [DOI] [PubMed] [Google Scholar]
- 10. Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13(2):198-226. [DOI] [PubMed] [Google Scholar]
- 11. Broeder CE, Quindry J, Brittingham K, et al. The Andro Project: physiological and hormonal influences of androstenedione supplementation in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000;160(20):3093-3104. [DOI] [PubMed] [Google Scholar]
- 12. Campian MD, Flis AE, Teramoto M, Cushman DM. Self-reported use and attitudes toward performance-enhancing drugs in ultramarathon running. Wilderness Environ Med. 2018;29(3):330-337. [DOI] [PubMed] [Google Scholar]
- 13. Charron J, Carey V, Marcotte L'heureux V, Roy P, Comtois AS, Ferland PM. Acute effects of cannabis consumption on exercise performance: a systematic and umbrella review. J Sports Med Phys Fitness. 2021;61(4):551-561. [DOI] [PubMed] [Google Scholar]
- 14. Clark B, Woolford SM, Eastwood A, Sharpe K, Barnes PG, Gore CJ. Temporal changes in physiology and haematology in response to high- and micro-doses of recombinant human erythropoietin. Drug Test Analysis. 2017;9(10):1561-1571. [DOI] [PubMed] [Google Scholar]
- 15. De Guingand DL, Palmer KR, Snow RJ, Davies-Tuck ML, Ellery SJ. Risk of adverse outcomes in females taking oral creatine monohydrate: a systematic review and meta-analysis. Nutrients. 2020;12(6):1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Rose, Eduardo H. Doping in athletes - an update. Clin Sports Med. 2008;27(1):107-130. [DOI] [PubMed] [Google Scholar]
- 17. Dempsey RL, Mazzone MF, Meurer LN. Does oral creatine supplementation improve strength? A meta-analysis. J Family Prac. 2002;51(11):945-951. [PubMed] [Google Scholar]
- 18. Dietz P, Ulrich R, Dalaker R, et al. Associations between physical and cognitive doping - a cross-sectional study in 2,997 triathletes. PLoS ONE. 2013;8(11):e78702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Docter S, Khan M, Gohal C, et al. Cannabis use and sport: a systematic review. Sports Health. 2020;12(2):189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doma K, Ramachandran AK, Boullosa D, Connor J. The paradoxical effect of creatine monohydrate on muscle damage markers: a systematic review and meta-analysis. Sports Med. 2022;52(7):1623-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunn M, White V. The epidemiology of anabolic-androgenic steroid use among Australian secondary school students. J Sci Med Sport. 2011;14(1):10-14. [DOI] [PubMed] [Google Scholar]
- 22. Durussel J, Daskalaki E, Anderson M, et al. Haemoglobin mass and running time trial performance after recombinant human erythropoietin administration in trained men. PLoS ONE. 2013;8(2):e56151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekblom B. Blood doping and erythropoietin: the effects of variation in hemoglobin concentration and other related factors on physical performance. Am J Sports Med. 1996;24(6):S40-S42. [PubMed] [Google Scholar]
- 24. Elashoff JD, Jacknow AD, Shain SG, Braunstein GD. Effects of anabolic-androgenic steroids on muscular strength. Ann Int Med. 1991;115(5):387-393. [DOI] [PubMed] [Google Scholar]
- 25. Equey T, Pastor A, de la Torre Fornell R, et al. Application of the athlete biological passport approach to the detection of growth hormone doping. J Clin Endocrinol Metab. 2022;107(3):649-659. [DOI] [PubMed] [Google Scholar]
- 26. Fazio C, Elder CL, Harris MM. Efficacy of alternative forms of creatine supplementation on improving performance and body composition in healthy subjects. J Strength Condit Res. 2022;36(9):2663-2670. [DOI] [PubMed] [Google Scholar]
- 27. Garthe I, Maughan RJ. Athletes and supplements: prevalence and perspectives. Int J Sport Nutr Exerc Metab. 2018;28(2):1-138. [DOI] [PubMed] [Google Scholar]
- 28. Giorgi A, Weatherby RP, Murphy PW. Muscular strength, body composition and health responses to the use of testosterone enanthate: a double-blind study. J Sci Med Sport. 1999;2(4):341-355. [DOI] [PubMed] [Google Scholar]
- 29. Granados J, Gillum TL, Christmas KM, Kuennen MR. Prohormone supplement 3β-hydroxy-5α-androst-1-en-17-one enhances resistance training gains but impairs user health. J Appl Physiol. 2014;116(5):560-569. [DOI] [PubMed] [Google Scholar]
- 30. Green G, Uryasz F, Petr T, Bray CD. NCAA study of substance use and abuse habits of college student-athletes. Clin J Sport Med. 2001;11(1):51-56. [DOI] [PubMed] [Google Scholar]
- 31. Greenwood M, Kreider RB, Melton C, et al. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Mol Cell Biochem. 2003;244(1-2):83-88. [PubMed] [Google Scholar]
- 32. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327-360. [DOI] [PubMed] [Google Scholar]
- 33. Hartgens K, Wijnen JA, Keizer HA. Body composition, cardiovascular risk factors and liver function in long term androgenic-anabolic steroids using bodybuilders three months after drug withdrawal. Int J Sports Med. 1996;17(6):429-433. [DOI] [PubMed] [Google Scholar]
- 34. Henning AD, Dimeo P. The new front in the war on doping: amateur athletes. Int J Drug Policy. 2017;51:128-136. [DOI] [PubMed] [Google Scholar]
- 35. Hermansen K, Bengtsen M, Kjær M, Vestergaard P, Jørgensen JOL. Impact of GH administration on athletic performance in healthy young adults: a systematic review and meta-analysis of placebo-controlled trials. Growth Horm IGF Res. 2017;34:38-44. [DOI] [PubMed] [Google Scholar]
- 36. Hespel P, Op’t Eijnde B, Leemputte MV, et al. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Phys. 2001;536(2):625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heuberger JAAC, Posthuma JJ, Ziagkos D, et al. Additive effect of erythropoietin use on exercise-induced endothelial activation and hypercoagulability in athletes. Eur J Appl Physiol. 2020;120(8):1893-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heuberger JAAC, Cohen Tervaert JM, Schepers FML, et al. Erythropoietin doping in cycling: lack of evidence for efficacy and a negative risk-benefit. Br J Clin Pharmacol. 2013;75(6):1406-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heuberger JAAC, Cohen AF. Review of WADA prohibited substances: limited evidence for performance-enhancing effects. Sports Med. 2018;49(4):525-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holt RI, Ho KK. The use and abuse of growth hormone in sports. Endocrine Rev. 2019;40(4):1163-1185. [DOI] [PubMed] [Google Scholar]
- 41. Hopwood MJ, Graham K, Rooney KB. Creatine supplementation and swim performance: a brief review. J Sports Sci Med. 2006;5:10-24. [PMC free article] [PubMed] [Google Scholar]
- 42. Horn S, Gregory P, Guskiewicz KM. Self-reported anabolic-androgenic steroids use and musculoskeletal injuries: findings from the center for the study of retired athletes health survey of retired NFL players. Am J Phys Med Rehabil. 2009;88(3):192-200. [DOI] [PubMed] [Google Scholar]
- 43. Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Sreekumaran Nair K. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93(2):534-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiaming Y, Rahimi MH. Creatine supplementation effect on recovery following exercise-induced muscle damage: a systematic review and meta-analysis of randomized controlled trials. J Food Biochem. 2021;45(10):e13916-n/a. [DOI] [PubMed] [Google Scholar]
- 45. Kennedy MC. Cannabis: exercise performance and sport. A systematic review. J Sci Med Sport. 2017;20(9):825-829. [DOI] [PubMed] [Google Scholar]
- 46. Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. King DS, Sharp RL, Vukovich MD, et al. Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men: a randomized controlled trial. JAMA. 1999;281(21):2020-2028. [DOI] [PubMed] [Google Scholar]
- 48. Kramer A, Sinclair J, Sharpe L, Sarris J. Chronic cannabis consumption and physical exercise performance in healthy adults: a systematic review. J Cannabis Res. 2020;2(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kreider RB, Melton C, Rasmussen CJ, et al. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem. 2003;244(1-2):95-104. [PubMed] [Google Scholar]
- 50. Kreider RB, Kalman DS, Antonio J, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage FX, Dutheil F. Creatine supplementation and upper limb strength performance: a systematic review and meta-analysis. Sports Med. 2016;47(1):163-173. [DOI] [PubMed] [Google Scholar]
- 52. Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage FX, Dutheil F. Creatine supplementation and lower limb strength performance: a systematic review and meta-analyses. Sports Med. 2015;45(9):1285-1294. [DOI] [PubMed] [Google Scholar]
- 53. Lazuras L, Barkoukis V, Loukovitis A, et al. I want it all, and I want it now: lifetime prevalence and reasons for using and abstaining from controlled performance and appearance enhancing substances (PAES) among young exercisers and amateur athletes in five European countries. Front psychol. 2017;8:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liddle DG, MD, Connor DJ, MD. Nutritional supplements and ergogenic aids. Primary Care. 2013;40(2):487-505. [DOI] [PubMed] [Google Scholar]
- 55. Lisano J, Phillips K, Smith J, Barnes MJ, Stewart LK. Patterns and perceptions of cannabis use with physical activity. Cannabis. 2019;2(2):151-164. [Google Scholar]
- 56. Liu H, Bravata DM, Hoffman AR, et al. Systematic review: the effects of growth hormone on athletic performance. Ann Int Med. 2008;148(10):747-758. [DOI] [PubMed] [Google Scholar]
- 57. Lopez RM, Casa DJ, McDermott BP, Ganio MS, Armstrong LE, Maresh CM. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J Athl Train. 2009;44(2):215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Madden PM, McCarty EC, Young CC, McCarty EC. Netter’s Sports Medicine. Philadelphia, PA: Saunders, 2022:186-199. [Google Scholar]
- 59. Martin L, Kafi R, Zhou X, Zhang L, Ericsson M, Marchand A. Detection of recombinant erythropoietin biosimilar Jimaixin™ after administration in healthy subjects. Drug Test Anal. 2022;14(1):72-79. [DOI] [PubMed] [Google Scholar]
- 60. Martin L, Martin J, Collot D, et al. Improved detection methods significantly increase the detection window for EPO microdoses. Drug Test Anal. 2021;13(1):101-112. [DOI] [PubMed] [Google Scholar]
- 61. McGuine T, Sullivan J, Bernhardt D. Creatine supplementation in high school football players. Clin J Sport Med. 2001;11(4):247-253. [DOI] [PubMed] [Google Scholar]
- 62. Meinhardt U, Nelson AE, Hansen JL, et al. The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Int Med. 2010;152(9):568-577. [DOI] [PubMed] [Google Scholar]
- 63. Mielgo-Ayuso J, Calleja-Gonzalez J, Marqués-Jiménez D, Caballero-García A, Córdova A, Fernández-Lázaro D. Effects of creatine supplementation on athletic performance in soccer players: a systematic review and meta-analysis. Nutrients. 2019;11(4):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miskulin I, Grbic DS, Miskulin M. Doping attitudes, beliefs, and practices among young, amateur Croatian athletes. Sports (Basel). 2021;9(2):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Momaya A, Fawal M, Estes R. Performance-enhancing substances in sports: a review of the literature. Sports Med. 2015;45(4):517-531. [DOI] [PubMed] [Google Scholar]
- 66. NCAA. The NCAA’s advertising and promotional guidelines effective September 2022. https://www.ncaa.com/_flysystem/public-s3/images/2022/09/16/Advertising_Promotional_Standards_2022.pdf. Accessed September 23, 2022.
- 67. Nelson AG, Arnall DA, Kokkonen J, Day R, Evans J. Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med Sci Sports Exerc. 2001;33(7):1096-1100. [DOI] [PubMed] [Google Scholar]
- 68. Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): adverse effects on non-reproductive organs and functions. Rev Endoc Metabol Dis. 2015;16(3):199-211. [DOI] [PubMed] [Google Scholar]
- 69. Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. Scand J Med Sci Sports. 2003;13(4):272. [DOI] [PubMed] [Google Scholar]
- 70. Northeast B, Clifford T. The effect of creatine supplementation on markers of exercise-induced muscle damage: a systematic review and meta-analysis of human intervention trials. Int J Sport Nutr Exerc Metabol. 2021;31(3):276-291. [DOI] [PubMed] [Google Scholar]
- 71. Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Piacentino D, Sani G, Kotzalidis GD, et al. Anabolic androgenic steroids used as performance and image enhancing drugs in professional and amateur athletes: toxicological and psychopathological findings. Hum Psychopharmacol. 2021;37(1):e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Salamin O, Kuuranne T, Saugy M, Leuenberger N. Erythropoietin as a performance-enhancing drug: its mechanistic basis, detection, and potential adverse effects. Mol Cell Endocrinol. 2018;464:75-87. [DOI] [PubMed] [Google Scholar]
- 74. Saugy M, Avois L, Saudan C, et al. Cannabis and sport. Br J Sports Med. 2006;40(Supplement 1):i13-i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schilling BK, Stone MH, Keith R, et al. Creatine supplementation and health variables: a retrospective study. Med Sci Sports Exerc. 2001;33(2):183-188. [DOI] [PubMed] [Google Scholar]
- 76. Schröder H, Terrados N, Tramullas A. Risk assessment of the potential side effects of long-term creatine supplementation in team sport athletes. Eur J Nutr. 2005;44(4):255-261. [DOI] [PubMed] [Google Scholar]
- 77. Seifarth S, Dietz P, Disch AC, Engelhardt M, Zwingenberger S. The prevalence of legal performance-enhancing substance use and potential cognitive and or physical doping in German recreational triathletes, assessed via the randomised response technique. Sports. 2019;7(12):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sergeeva KV, Miroshnikov AB, Smolensky AV. Effect of growth hormone administration on the mass and strength of muscles in healthy young adults: a systematic review and meta-analysis. Hum Physiol. 2019;45(4):452-460. [Google Scholar]
- 79. Sgrò P, Sansone M, Sansone A, Romanelli F, Di Luigi L. Effects of erythropoietin abuse on exercise performance. Phys Sportsmed. 2018;46(1):105-115. [DOI] [PubMed] [Google Scholar]
- 80. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Siebert DM, Rao AL. The use and abuse of human growth hormone in sports. Sports Health. 2018;10(5):419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Simon P, Striegel H, Aust F, Dietz K, Ulrich R. Doping in fitness sports: estimated number of unreported cases and individual probability of doping. Addiction. 2006;101(11):1640-1644. [DOI] [PubMed] [Google Scholar]
- 83. Smedley KO. Creatine Monohydrate. 2020;2022(September 8). Food and Drug Administration. https://www.fda.gov/media/143525/download [Google Scholar]
- 84. Smith ACT, Stavros C. Exploring the progressive use of performance enhancing substances by high-performance athletes. Subst Use Misuse. 2020;55(6):914-927. [DOI] [PubMed] [Google Scholar]
- 85. Tokish JM, Kocher MS, Hawkins RJ. Ergogenic aids a review of basic science, performance, side effects, and status in sports. Am J Sports Med. 2004;32(6):1543-1553. [DOI] [PubMed] [Google Scholar]
- 86. Trinh KV, Diep D, Chen KJQ, Huang L, Gulenko O. Effect of erythropoietin on athletic performance: a systematic review and meta-analysis. BMJ Open Sport Exer Med. 2020;6(1):e000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Trinh K, Diep D, Robson H. Marijuana and its effects on athletic performance: a systematic review. Clin J Sports Med 2018;28(4):350-357. [DOI] [PubMed] [Google Scholar]
- 88. Ulrich R, Pope HG, Jr, Cléret L, et al. Doping in two elite athletics competitions assessed by randomized-response surveys. Sports Med. 2017;48(1):211-219. [DOI] [PubMed] [Google Scholar]
- 89. Volkow ND. Adverse health effects of marijuana use. New Engl J Med. 2014;370(23):2219-2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vorona E, Nieschlag E. Adverse effects of doping with anabolic androgenic steroids in competitive athletics, recreational sports and bodybuilding. Minerva Endocrinol. 2018;43(4):476-488. [DOI] [PubMed] [Google Scholar]
- 91. Wadler GI, Hainline B. Drugs and the athlete. Philadelphia, PA: Davis Co; 1989. [Google Scholar]
- 92. Watson CJ, Stone GL, Overbeek DL, Chiba T, Burns MM. Performance-enhancing drugs and the Olympics. J Intern Med. 2022;291(2):181-196. [DOI] [PubMed] [Google Scholar]
- 93. World Anti-Doping Agency. World anti-doping code international standard prohibited list 2022. https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf. Accessed September 18, 2022.
- 94. Wu G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids. 2020;52(3):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu S, Chen K, Hsu C, et al. Creatine supplementation for muscle growth: a scoping review of randomized clinical trials from 2012 to 2021. Nutrients. 2022;14(6):1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health
Supplemental material, sj-docx-2-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health
Supplemental material, sj-docx-3-sph-10.1177_19417381231197389 for Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses by Alec A. Warrier, Eric N. Azua, Luke B. Kasson, Sachin Allahabadi, Zeeshan A. Khan, Enzo S. Mameri, Hasani W. Swindell, John M. Tokish and Jorge Chahla in Sports Health