Abstract
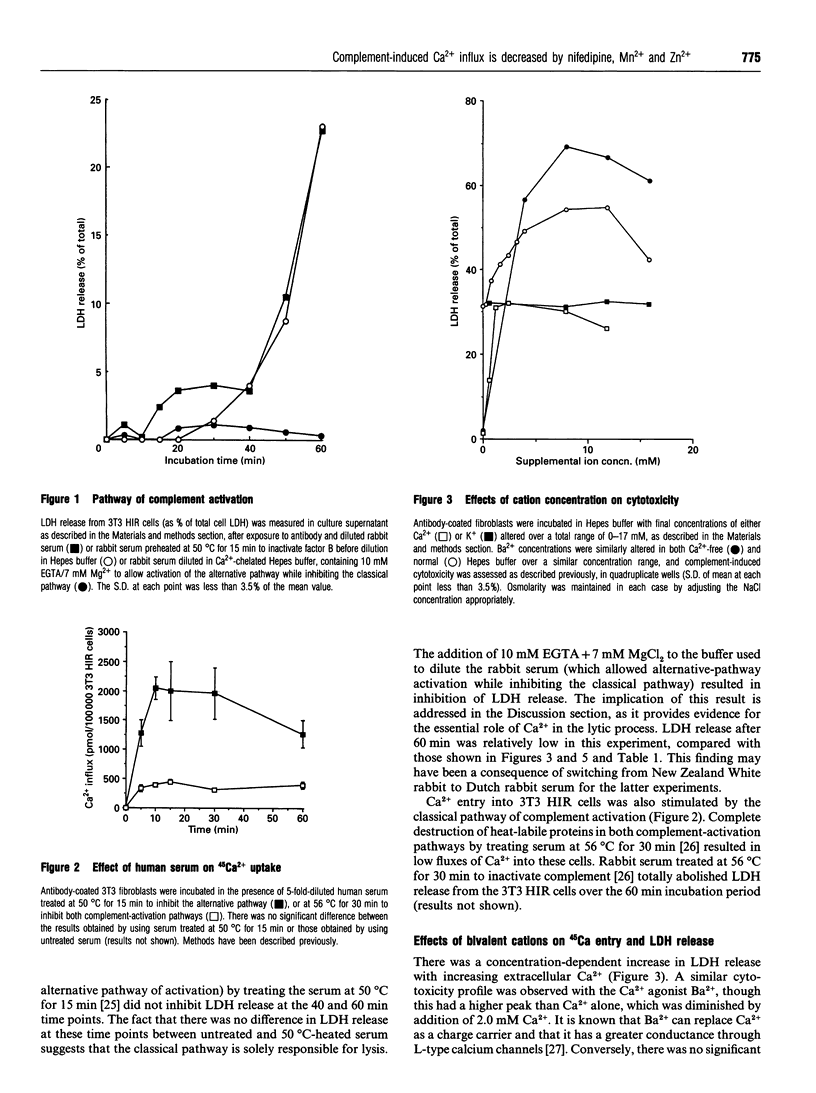
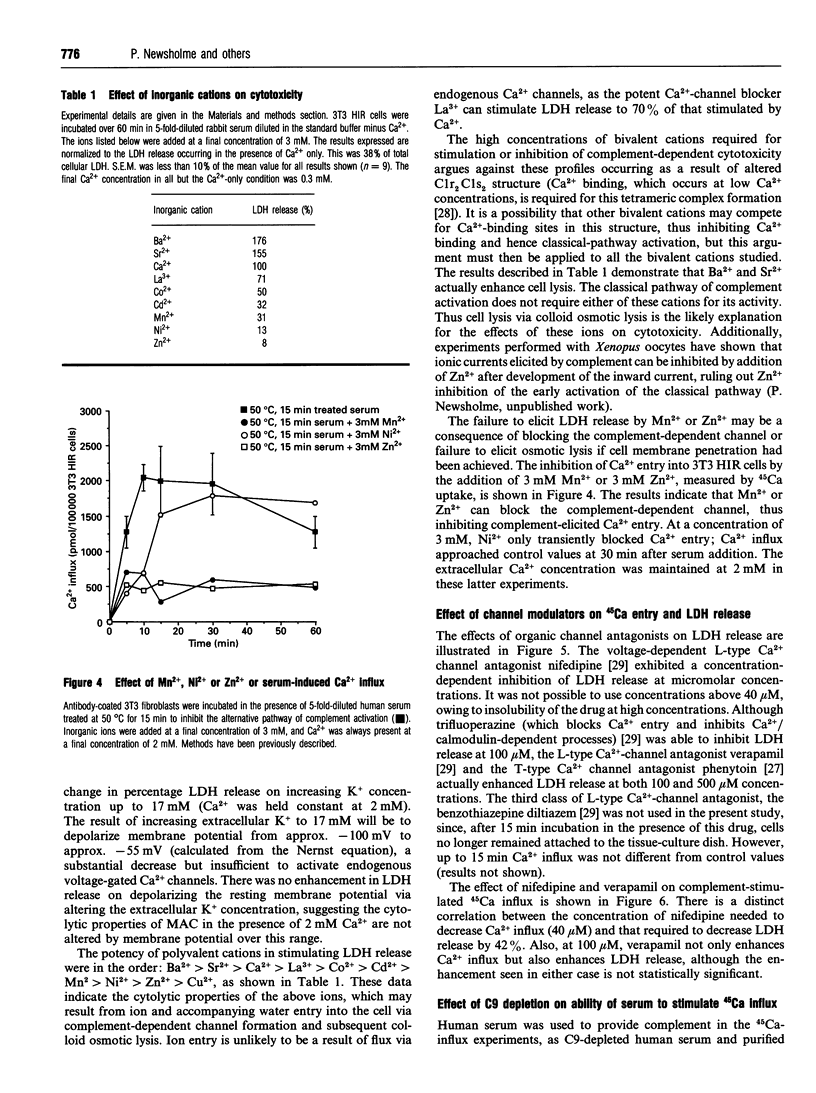
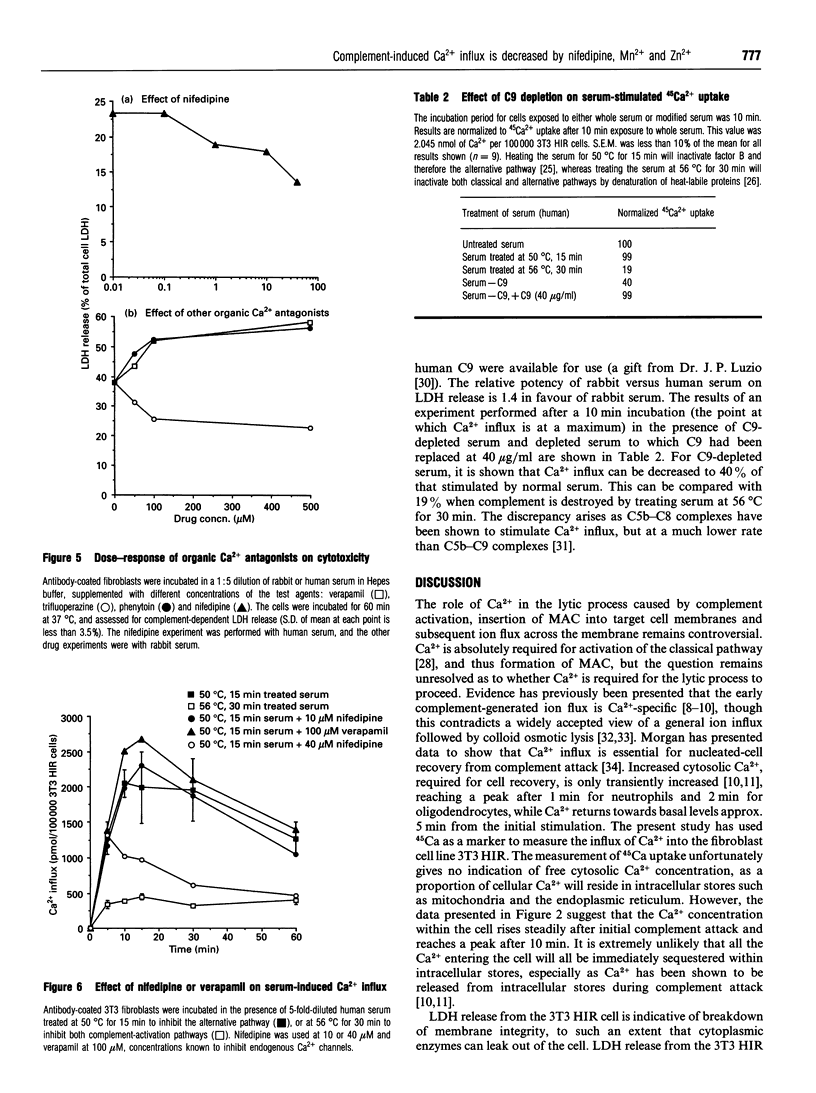
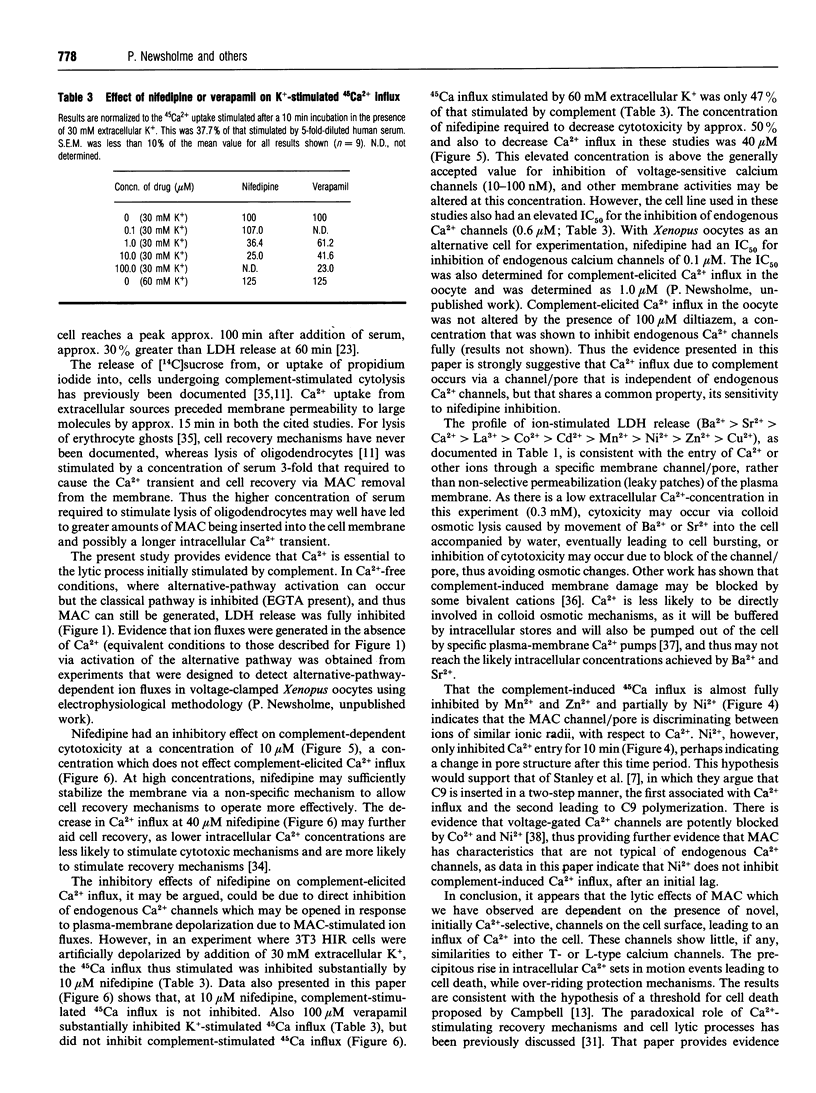
The effects of different extracellular cations or organic Ca(2+)-channel modulators on complement-induced changes in intracellular Ca2+ and cell death have been investigated in the transfected NIH-3T3 HIR 3.5 cell line, which overexpresses the human insulin receptor. Cells were incubated with mouse anti-(human insulin receptor) monoclonal antibodies before exposure to rabbit or human serum (sources of heterologous complement). Changes in intracellular Ca2+ were complement-dependent (measured by influx of 45Ca), as was cytotoxicity (monitored by leakage of lactate dehydrogenase into the culture supernatant). Addition of a dihydropyridine Ca(2+)-channel antagonist (nifedipine) or some bivalent inorganic cations caused inhibition of 45Ca entry via a novel channel distinct from endogenous voltage-gated Ca2+ channels. Nifedipine decreased, but conversely the addition of a phenylalkylamine Ca(2+)-channel antagonist (verapamil) or the inorganic Ca2+ agonists Ba2+ and Sr+ increased, complement-induced cytotoxicity. These agents had no effect on cell viability at the studied concentrations, in the absence of complement. It is concluded that complement-induced cytotoxicity is mediated by Ca2+ influx through novel specific transmembrane channels which are sensitive to the Ca(2+)-channel antagonist nifedipine, but otherwise show little resemblance to L- or T-type voltage-gated Ca2+ channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Benz R., Schmid A., Wiedmer T., Sims P. J. Single-channel analysis of the conductance fluctuations induced in lipid bilayer membranes by complement proteins C5b-9. J Membr Biol. 1986;94(1):37–45. doi: 10.1007/BF01901011. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Complement lysis: a hole is a hole. Immunol Today. 1991 Sep;12(9):318–321. doi: 10.1016/0167-5699(91)90007-G. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Studies on the terminal stages of antibody-complement-mediated killing of a tumor cell. I. Evidence for the existence of an intermediate, T. J Immunol. 1976 May;116(5):1272–1275. [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Hallett M. B., Luzio J. P. Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J. 1981 Feb 15;194(2):551–560. doi: 10.1042/bj1940551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Luzio J. P. Intracellular free calcium as a pathogen in cell damage initiated by the immune system. Experientia. 1981 Oct 15;37(10):1110–1112. doi: 10.1007/BF02085041. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Esser A. F. Big MAC attack: complement proteins cause leaky patches. Immunol Today. 1991 Sep;12(9):316–321. doi: 10.1016/0167-5699(91)90006-F. [DOI] [PubMed] [Google Scholar]
- FRANK M. M., RAPP H. J., BORSOS T. STUDIES ON THE TERMINAL STEPS OF IMMUNE HEMOLYSIS. I. INHIBITION BY TRISODIUM ETHYLENEDIAMINETETRAACETATE (EDTA). J Immunol. 1964 Sep;93:409–413. [PubMed] [Google Scholar]
- Farber J. L. The role of calcium in cell death. Life Sci. 1981 Sep 28;29(13):1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Linington C., Morgan B. P., Scolding N. J., Wilkins P., Piddlesden S., Compston D. A. The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain. 1989 Aug;112(Pt 4):895–911. doi: 10.1093/brain/112.4.895. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Presidential address to the American Association of Immunologists, delivered in Chicago, Illinois, April 6, 1977. Mechanism of cytolysis by lymphocytes: A comparison with complement. J Immunol. 1977 Oct;119(4):1195–1203. [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Luzio J. P., Campbell A. K. Inhibition of complement-induced [14C]sucrose release by intracellular and extracellular monoclonal antibodies to C9: evidence that C9 is a transmembrane protein. Biochem Biophys Res Commun. 1984 Jan 30;118(2):616–622. doi: 10.1016/0006-291x(84)91347-0. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Luzio J. P., Campbell A. K. Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium. 1986 Dec;7(5-6):399–411. doi: 10.1016/0143-4160(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Porzig H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev Physiol Biochem Pharmacol. 1990;114:209–262. doi: 10.1007/BFb0031020. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Activation and control of the complement system. Essays Biochem. 1986;22:27–68. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Scolding N. J., Houston W. A., Morgan B. P., Campbell A. K., Compston D. A. Reversible injury of cultured rat oligodendrocytes by complement. Immunology. 1989 Aug;67(4):441–446. [PMC free article] [PubMed] [Google Scholar]
- Shiver J. W., Dankert J. R., Esser A. F. Formation of ion-conducting channels by the membrane attack complex proteins of complement. Biophys J. 1991 Oct;60(4):761–769. doi: 10.1016/S0006-3495(91)82110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. Repolarization of the membrane potential of blood platelets after complement damage: evidence for a Ca++ -dependent exocytotic elimination of C5b-9 pores. Blood. 1986 Aug;68(2):556–561. [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Page M., Campbell A. K., Luzio J. P. A mechanism for the insertion of complement component C9 into target membranes. Mol Immunol. 1986 May;23(5):451–458. doi: 10.1016/0161-5890(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Tepikin A. V., Voronina S. G., Gallacher D. V., Petersen O. H. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem. 1992 Jul 15;267(20):14073–14076. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegar B. D., Kelly R., Lansman J. B. Block of current through single calcium channels by Fe, Co, and Ni. Location of the transition metal binding site in the pore. J Gen Physiol. 1991 Feb;97(2):351–367. doi: 10.1085/jgp.97.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Young T. M. Channel fluctuations induced by membrane attack complex C5B-9. Mol Immunol. 1990 Oct;27(10):1001–1007. doi: 10.1016/0161-5890(90)90123-h. [DOI] [PubMed] [Google Scholar]
- Zoeteweij J. P., van de Water B., de Bont H. J., Mulder G. J., Nagelkerke J. F. Involvement of intracellular Ca2+ and K+ in dissipation of the mitochondrial membrane potential and cell death induced by extracellular ATP in hepatocytes. Biochem J. 1992 Nov 15;288(Pt 1):207–213. doi: 10.1042/bj2880207. [DOI] [PMC free article] [PubMed] [Google Scholar]


