Abstract
Background
Adenocarcinomas and pancreatic neuroendocrine tumors (pNETs) display some similarities and differences. The aim of this study was to compare preoperative data and morphological parameters, and to assess postoperative complications after resection.
Material/Methods
Data of 162 patients who underwent distal pancreatic resection for neuroendocrine or adenocarcinoma tumor were retrospectively analyzed. After applying inclusion and exclusion criteria, 131 patients were included in the study. The preoperative data analyzed included age, sex, and ASA-PS (American Society of Anesthesiologists Physical Status) grade. The diameter of the pancreatic duct and the texture of the pancreas were analyzed. Postoperative data included grading (G1–G3), the presence of PanIN (pancreatic intraepithelial neoplasia), infiltration of structures, and postoperative complications.
Results
Patients with adenocarcinoma were statistically older and had a higher ASA-PS class than patients with NET (P<0.001). Statistically significantly more patients with adenocarcinoma had a histopathological diagnosis of G3 (p<0.001). In patients with adenocarcinomas infiltration of structures occurred more frequently. Pancreatic duct diameter ≥3 mm was more common in patients with adenocarcinoma (P=0.045). Clinically significant pancreatic fistulas were more frequent in patients with neuroendocrine tumors (P=0.044).
Conclusions
Adenocarcinomas in the pancreatic body and tail are more aggressive, they cause more frequent infiltration of structures, and more often metastasize to lymph nodes compared to NETs. NETs tend to have softer pancreatic texture and higher incidence of clinically significant pancreatic fistulas, but postoperative complications of Clavien-Dindo grade ≥III occur at a similar rate in both groups.
Keywords: Adenocarcinoma, Neuroendocrine Tumors, Pancreatic Fistula, Postoperative Complications
Introduction
The incidence of pancreatic adenocarcinomas is 4–5/100 000 population [1] and it is one of the most common pancreatic tumors [1]. Pancreatic neuroendocrine tumors (pNETs) are much less common than adenomas and account for only 1–2% of pancreatic tumors [2]. Their incidence is 1–2/100 000 people and has been increasing in recent years [3]. Adenocarcinomas are more than twice as common in the head of the pancreas than in the body and tail [1], and neuroendocrine tumors occur twice as often in distal locations (the body and tail) compared to the head of the pancreas [4].
Adenocarcinomas and neuroendocrine tumors have different prognoses [5–7]. Adenocarcinomas are associated with a much shorter 5-year survival rate compared to pancreatic NETs (around 5.0% for adenocarcinomas) [5], while the 5-year survival rate of patients with pancreatic neuroendocrine tumors is 51.3% [5]. In addition, the differences between the above-mentioned histological types of pancreatic tumors also concern morphological parameters such as the frequency of metastases to lymph nodes and distant metastases, as well as the presence of intraepithelial neoplasia (PanIN) [6,8].
In the case of tumor localization in the body or tail of pancreas, distal resection with or without splenectomy is a standard procedure [9]. The decision to perform splenectomy depends, among other things, on the local advancement of the tumor [10]. Pancreatic fistulas are one of the most common postoperative complications after distal resections [9]. Risk factors for this type of complication include pancreatic texture and main pancreatic duct diameter [9,11]. Soft pancreas and pancreatic duct diameter <3 mm are risk factors for postoperative pancreatic fistula [9,11]. The texture of the pancreas is related to the histopathological type of the tumor [12]. Soft pancreas is more often found in neuroendocrine tumors compared to adenocarcinomas [13]. In addition, adenocarcinomas are more likely to have dilation of the Wirsung duct >3 mm [14]. The Fistula Risk Score (FRS), used to assess the risk of postoperative pancreatic fistula, considers histopathological diagnosis “other than adenocarcinoma or inflammatory tumor” as a risk factor for this type of complication [11].
Few studies have compared pancreatic adenocarcinomas and pancreatic neuroendocrine tumors located in the body or tail; therefore, the present study focused on the comparative analysis of these 2 histopathological types of pancreatic neoplasms. The aim of this study was a comparative analysis of preoperative data, morphological parameters of adenocarcinomas and neuroendocrine tumors of the body and tail of the pancreas, as well as the assessment of postoperative complications after resection.
Material and Methods
We retrospectively analyzed data of 162 patients who underwent distal pancreatic resection for neuroendocrine or adenocarcinoma tumors at the Department of Gastrointestinal Surgery of the Medical University of Silesia in Katowice in 2015–2021. Inclusion criteria were:
Age ≥18 years.
Location of the tumor in the body or tail of the pancreas.
Diagnosis of a neuroendocrine tumor or adenocarcinoma in histopathological examination of the surgical specimen.
Performing the procedure by a surgeon with extensive experience in pancreatic surgery (more than 20 procedures per year) [15].
Performing distal pancreatectomy with or without splenectomy using the same method (open method via laparotomy. Pancreatic duct being closed with 2–3 single sutures. The cross-section of the pancreas was closed with single sutures in 2 layers.)
Availability of documentation containing complete data.
The exclusion criteria were:
The presence of other pathologies in the head, uncinate process, isthmus of the pancreas, or within the papilla of Vater.
Diagnosis of mixed neoplasm (pancreatic neuroendocrine tumor with adenocarcinoma) (to ensure homogeneity of the study group).
Performing multi-organ resection or tumor enucleation.
Treatment of another cancer within the 12 months before distal pancreatectomy.
Treatment with neoadjuvant chemotherapy before surgery.
After applying the above inclusion and exclusion criteria, 131 patients were included in the analysis.
Patients were treated according to the latest ESMO guidelines [16] for ductal adenocarcinomas and according to the ENETS Center of Excellence guidelines for neuroendocrine tumors [17]. Multidisciplinary treatment involved the cooperation of a surgeon, gastroenterologist, radiologist, and oncologist. The basic imaging test performed to assess the pancreas and the stage of cancer advancement is computed tomography (CT) of the abdominal cavity with contrast [18], which allows to determination of tumor size and resectability [18]. In each case in the study group, qualification for surgery was based on this imaging test. Computed tomography of the abdominal cavity was performed using the pancreatic protocol involving triphasic (arterial, late arterial, and venous phases) cross-sectional imaging with thin slices using multidetector CT (Figure 1). Moreover, in the study group, endoscopic ultrasonography (EUS) with biopsy was performed to obtain preoperative histopathological results.
Figure 1.
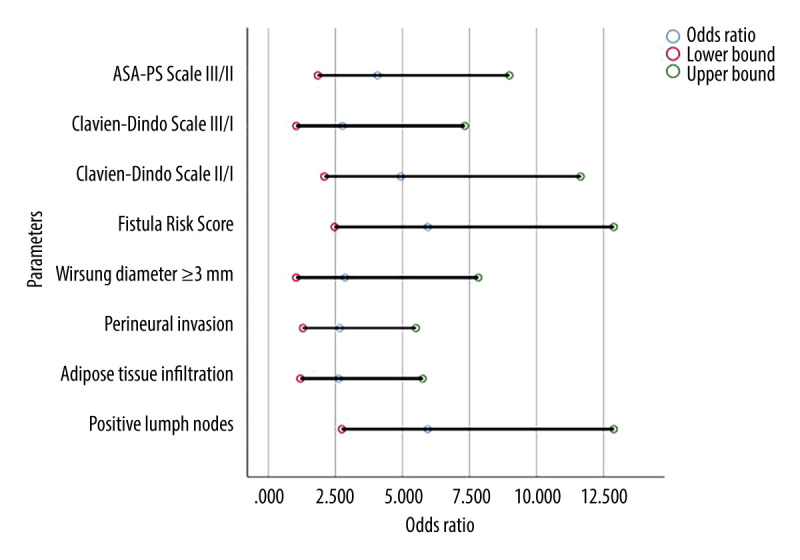
Odds ratios with upper and lower confidence intervals of significant results comparing adenocarcinomas to neuroendocrine tumors. ASA-PS – American Society of Anesthesiologists physical status classification system.
Patients were divided into 2 groups depending on the histological type of the tumor: patients in group 1 had pancreatic adenocarcinoma and patients in group 2 had pancreatic neuroendocrine tumor. An analysis of the significance of differences between the 2 groups in terms of preoperative data, morphological parameters, and postoperative complications was performed.
We analyzed preoperative data on age, sex, and ASA-PS grade. The ASA-PS scale was used to assess the clinical condition of patients before the procedure. The texture of the pancreas and the diameter of the pancreatic duct were assessed intraoperatively by the operating surgeon. The texture of the pancreas was defined as normal or soft. Patients were divided into 2 groups according to the diameter of the pancreatic duct: <3 mm and ≥3 mm [19,20]. Postoperative data included grading (G1–G3) and the presence of PanIN neoplasia. Tumors have been classified according to the 2019 WHO classification [21]. The classification of pancreatic intraepithelial neoplasia (PanIN) was presented in accordance with the publication Takaori et al on the presence of PanIN [22]. In addition, the following histopathological parameters were analyzed in the 2 groups: presence of lymph nodes and distant metastases, infiltration of adipose tissue and nerve trunks, and presence of vascular occlusions. The presence of postoperative pancreatic fistulas and the severity of complications were also analyzed from the postoperative data. The occurrence and classification of pancreatic fistulas were assessed according to the classification of the International Study Group of Pancreatic Fistula (ISGPF) [23]. As defined by ISGPF, pancreatic fistula is an abnormal communication between the pancreatic ductal “system” and another epithelial surface [23]. For diagnosis, the necessary threshold is any measurable volume of drained fluid on or after postoperative day 3, with amylase level >3 times the upper limit of normal amylase for each specific institution [23]. Moreover, in the diagnosis of pancreatic fistulas, CT of the abdominal cavity with contrast was first used. In case of contraindications to this examination, MRI of the abdominal cavity with contrast was performed. The risk of postoperative pancreatic fistula was assessed according to the Fistula Risk Score (FRS) [11] and the severity of complications was assessed according to the Clavien-Dindo scale, which is used to assess the severity of surgical complications after surgery.
Ethics Statement
The was a retrospective study of patients’ medical records. All data were fully anonymized. Written informed consent was obtained from all participants. All procedures were performed in accordance with the Helsinki Declaration on Human Medical Research and its subsequent amendments or comparable ethical standards. Our retrospective analysis of patients’ medical records fell into a category exempt from Institutional Review Board (IRB) approval according to local law.
Statistical Analysis
A descriptive analysis was carried out. A 95% confidence interval was used. The distribution of quantitative variables was analyzed. Normally distributed variables are expressed as means and their standard deviations, while non-normally distributed variables are given as medians and their interquartile ranges. An analysis of differences between the groups of patients divided according to the histological type of cancer (NET or pancreatic adenocarcinoma) was performed. The following factors were taken into account in the analysis: age, sex, ASA-PS grade, histological grade (G1–G3), PanIN, presence of lymph node and distant metastases, adipose tissue and nerve trunk infiltration, presence of vascular occlusions, diameter of the pancreatic duct, texture of the pancreas, occurrence of pancreatic fistulas, risk group according to the FRS scale, and the severity of postoperative complications according to the Clavien-Dindo scale. The level of statistical significance was set at P<0.05. Homogeneity of variance was tested using Levene’s test. Quantitative variables were analyzed using the Mann-Whitney U test. Analysis of nominal variables was performed using the chi-square test or Fischer’s exact test when necessary. The correlation strength was calculated using the Phi coefficient for 2×2 matrices or Cramer’s V for larger matrices. A regression analysis showing the odds ratio along with the lower and upper confidence intervals was performed. All calculations and statistical analyzes were performed in IBM SPSS Statistics 26 (Armonk, New York, USA).
Results
The study group consisted of 61 men (46.6%) and 70 women (53.4%) with a median age of 63 years (IQR 51–69). Seventy-eight patients (59.5%) were ≥60 years old; while 30 patients (22.9%) were ≥70 years old. Seventy-seven patients (58.8%) had a neuroendocrine tumor, and 54 (41.2%) had an adenocarcinoma. Most patients were ASA-PS grade II (84 patients – 64.1%). The characteristics of the patients in the study population are shown in Table 1.
Table 1.
Significance of difference in clinical parameters and complications between NET and adenocarcinoma.
| NET (n=77) | PDAC (n=54) | Significance (p-value) | Strength of correlation* | ||
|---|---|---|---|---|---|
| Age (median (IQR)) | 58 (45.5–65.0) | 68.5 (61.8–74.0) | <0.001 | 0.418 | |
| Male sex | 37 (48.1%) | 24 (44.4%) | 0.724 | 0.036 | |
| ASA-PS Scale | I | 5 (6.5%) | 0 (0.0%) | <0.001 | 0.357 |
| II | 57 (74.0%) | 27 (50.0%) | |||
| III | 15 (19.5%) | 27 (50.0%) | |||
| Histopathological diagnosis | G1 | 32 (41.6%) | 16 (29.6%) | <0.001 | 0.346 |
| G2 | 44 (57.1%) | 26 (48.1%) | |||
| G3 | 1 (1.3%) | 12 (22.2%) | |||
| PanIN | None | 26 (33.8%) | 8 (14.8%) | <0.001 | 0.608 |
| PanIN 1 | 46 (59.7%) | 13 (24.1%) | |||
| PanIN 1–2 | 2 (2.6%) | 2 (3.7%) | |||
| PanIN 1–3 | 3 (3.9%) | 31 (57.4%) | |||
| Adipose tissue infiltration | 44 (57.1%) | 42 (77.8%) | 0.016 | 0.214 | |
| Vascular invasion | 45 (58.4%) | 33 (61.1%) | 0.857 | 0.027 | |
| Perineural invasion | 33 (42.9%) | 36 (66.7%) | 0.008 | 0.235 | |
| Lymph nodes metastasis | 25 (32.5%) | 40 (74.1%) | <0.001 | 0.410 | |
| Distant metastases | 13 (16.9%) | 6 (11.1%) | 0.453 | −0.081 | |
| Wirsung diameter ≥3 mm | 7 (9.1%) | 12 (22.2%) | 0.045 | 0.184 | |
| Soft pancreas | 23 (29.9%) | 12 (22.2%) | 0.423 | −0.085 | |
| Pancreatic fistula | A or none | 62 (80.5%) | 51 (94.4%) | 0.044 | −0.214 |
| B | 10 (13.0%) | 3 (5.6%) | |||
| C | 5 (6.5%) | 0 (0.0%) | |||
| Fistula Risk Score | Low | 2 (2.6%) | 7 (13.0%) | <0.001 | −0.355 |
| Moderate | 53 (68.8%) | 43 (79.6%) | |||
| High | 22 (28.6%) | 2 (3.7%) | |||
| Clavien-Dindo | I | 47 (61.0%) | 17 (31.5%) | <0.001 | 0.376 |
| II | 14 (18.2%) | 25 (46.3%) | |||
| III | 12 (15.6%) | 12 (22.2%) | |||
| IV | 4 (5.2%) | 0 (0.0%) | |||
NET – neuroendocrine tumors; PDAC – pancreatic ductal adenocarcinoma; IQR – interquartile range; ASA-PS – American Society of Anesthesiologists physical status classification system; PanIN – pancreatic intraepithelial neoplasia;
Negative correlation means that clinical parameters occur more frequently or have higher values in NET tumors; Positive correlation means that they occur more frequently or have high values in adenocarcinomas.
Morphological parameters are shown in Table 1. Tumors were classified according to the WHO classification into 3 groups: G1, G2, and G3 [21]. The G1 group included 48 (36.6%) patients, G2–70 (53.4%), and G3–13 (9.9%) patients. Among patients, PanIN 1 was the most common (59 patients – 45%), followed by 34 patients (30%) in both the PanIN 1–3 group and those without pancreatic intraepithelial neoplasia. PanIN 1–2 was the least common (4 patients – 3%). Nineteen (14.5%) patients had pancreatic duct diameter ≥3 mm. Soft consistency of the pancreas was found in 35 (26.7%) cases. Tumor metastases to local nodes were present in 65 (49.6%) patients. Distant metastases, mainly to the liver, were found in 19 patients (14.5%). Other parameters, such as perineural invasion, adipose tissue, and vascular occlusion, are also shown in Table 1.
In the analyzed population, the most common finding was no fistula or type A pancreatic fistula, in 113 (86.3%) patients. Type B fistulas affected 13 patients (10%), including 10 patients in the group with NET and 3 in the group of patients with adenocarcinoma. Type C fistula was found only in the NET group of patients, with 5 cases (3.8%). Most patients belonged to the moderate risk group according to the FRS scale (96 patients – 73.3%). Postoperative complications were presented using the Clavien-Dindo classification [24]. The most common severity of complications in patients with a diagnosis of neuroendocrine tumor was grade I in 64 (48.9%) patients. In contrast, among the group of patients with a diagnosis of adenocarcinoma, the most common was grade II in 25/54 (46.3%). The above data are presented in Table 1.
Patients with adenocarcinoma were significantly older than patients with NET (median age 68.5 and 58 years, respectively, P<0.001; OR=1.085). Patients with adenocarcinoma were more likely to have a higher ASA-PS class than patients with NET (P<0.001; OR (ASA-PS III/II)=4.071). More patients with adenocarcinoma had a histopathological diagnosis of G3 (12 vs 1 NET patient; P<0.001; OR (G3/G1)=24.0). Lymph node metastasis (P<0.001; OR=5.943), infiltration of adipose tissue (P=0.016; OR=2.625), and nerve trunks (P=0.045; OR=2.667) were more frequent in the group with adenocarcinoma as well as the pancreatic duct diameter ≥3 mm (P=0.045; OR=2.857). Clinically significant pancreatic fistulas were more common in patients with neuroendocrine tumors (P=0.044), and this group of patients also had a higher risk of complications in the form of postoperative pancreatic fistula as assessed by the FRS score (P<0.001; OR=5.943). Correlations between the study groups are shown in Table 1. The odds ratio of statistically significant results along with their lower and upper 95% confidence intervals are shown in Table 2. There was a strong statistical trend concerning the histopathological diagnosis G3 compared to G1 in adenocarcinomas (odds ratio of 24.0) (Table 2). A similar significant result was found between ASA-PS Scale III in comparison to ASA-PS Scale II. ASA-PS Scale III are more than 4 times likely to occur in adenocarcinomas than in NETs in comparison to ASA-PS Scale II (odds ratio 4.071). Lymph node metastasis was almost 6 times more common in adenocarcinomas than in NETs (odds ratio 5.943). These results are shown in Figures 1 and 2.
Table 2.
Odds Ratios with upper and lower confidence intervals of significant results.
| Odds ratio | Lower bound of CI | Upper bound of CI | |
|---|---|---|---|
| Age | 1.085 | 1.045 | 1.126 |
| ASA-PS Scale III/II | 4.071 | 1.845 | 8.984 |
| Histopathological diagnosis: G3/G1 | 24.0 | 2.862 | 201.238 |
| PanIN 1–3/none | 33.583 | 8.072 | 139.719 |
| Lymph nodes metastasis | 5.943 | 2.743 | 12.877 |
| Adipose tissue infiltration | 2.625 | 1.198 | 5.752 |
| Perineural invasion | 2.667 | 1.293 | 5.499 |
| Wirsung diameter ≥3 mm | 2.857 | 1.043 | 7.826 |
| Fistula Risk Score* High/Low | 38.5 | 4.545 | 326.102 |
| Clavien-Dindo II/I | 4.937 | 2.094 | 11.641 |
| Clavien-Dindo III/I | 2.765 | 1.044 | 7.320 |
CI – 95% confidence interval; ASA-PS – American Society of Anesthesiologists physical status classification system; G3 – grade 3; G1 – grade 1; PanIN – pancreatic intraepithelial neoplasia;
Odds ratio for NET (for the rest of the variables it is for adenocarcinoma).
Figure 2.
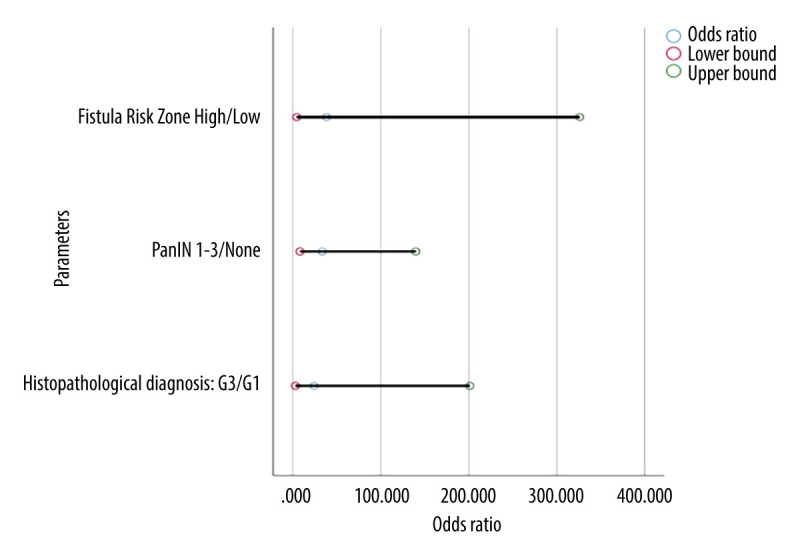
Odds ratios with upper and lower confidence intervals of significant results comparing neuroendocrine tumors to adenocarcinomas. PanIN – pancreatic intraepithelial neoplasia; G3 – grade 3; G1 – grade 1.
Discussion
Pancreatic neuroendocrine tumors and adenocarcinomas differ in biology and prognosis [4], but these 2 histological types of tumors can coexist [25,26]. Multiple primary malignancies (MPMs), defined as 2 or more malignant primary tumors arising in the same patient, are uncommon but well-recognized [27,28]. This type of tumor is challenging to diagnose and requires accurate imaging [29]. Multiple primary malignancies were not found in the population analyzed in the present study.
Age
Chen et al reported that 38.4% of pancreatic neuroendocrine tumors occur in patients over age 60 years [30]. This is consistent with the results of our analysis. In our study, 33/77 (42.9%) of NET patients were over 60 years old, while only 10/77 (13%) were over 70 years old. patient age is an important issue affecting the course of postoperative complications and overall survival after NET resection, as demonstrated by Liu et al, who found that younger patients had a better long-term prognosis compared to older patients [31]. Similar report concerns patients with adenocarcinoma [32], although Cai et al found no relationship between long survival and patient age after resection of adenocarcinoma [33] because adenocarcinomas have a more aggressive course than pancreatic neuroendocrine tumors [33]. In the analyzed group, patients with adenocarcinoma were older (P<0.001). In this group 45/54 (83.3%) of patients were over 60 years old and 20/54 (37%) were over 70 years old. In our study, the median age of patients with adenocarcinoma was 68.5 years, which is comparable to that reported in other studies [32,33]. Partelli et al found a significantly older average age of patients with adenocarcinoma compared to patients with NET (64 vs 56 years, P<0.0001) [34].
Sex
In the analyzed groups, there was no significant difference in the occurrence of NETs and PDAC according to sex. Males accounted for 48.1% of neuroendocrine tumors and 44.4% of adenocarcinomas. Some authors reported a higher percentage of men among patients with pancreatic NETs, amounting to approximately 55% [35]. According to the literature, the incidence of adenocarcinoma by sex is similar to that found in our analysis [18]. In both analyzed types of pancreatic tumors, long-term survival is lower in men compared to women [18,36].
ASA-PS
Nell et al showed that most patients with NETs had a low ASA-PS grade (89% in ASA-PS I or II) [37]. Adenocarcinomas occur a lower percentage (approximately 75%) of patients with ASA-PS I and II compared to neuroendocrine tumors [36], probably due to age differences. There are differences in the frequency of ASA-PS grades between different studies, but most indicate a higher percentage of patients with ASA-PS III or higher in the group with adenocarcinoma compared to NET, which is consistent with the results of our study. In our analysis, most NET patients were ASA-PS I or II (62/77, 80.5%), while half of the adenocarcinoma patients were ASA-PS III (27/54, 50%); which shows a significant difference in the ASA-PS scale between patients in both groups (P<0.001; OR=4.071).
Grading
According to the literature, pancreatic neuroendocrine tumors G1 account for 40–50% of surgically treated NETs [38,39]. In our analysis, G1 tumors accounted for 41.6% of all surgically treated NETs. According to the literature, in patients with adenocarcinomas, G2 and G3 tumors are more common than G1 tumors [5]. In our study, G2 and G3 adenocarcinomas accounted for 48.1% and 22.2% of cases, respectively. On the other hand, G1 tumors were found in 29.6% of cases. The above results agree with the analysis of Yadav et al, which showed that neuroendocrine tumors have a lower grade of histological malignancy compared to adenocarcinomas [5].
PanIN
Pancreatic intraepithelial neoplasia (PanIN) is one of the precursor lesions in pancreatic adenocarcinomas [40]. It is most common in this histopathological type of pancreatic tumors, but it is also found in neuroendocrine tumors and chronic pancreatitis [8,40]. In neuroendocrine tumors, PanIN 1 predominates, with an incidence of 60–75% [8,40]. However, PanIN 1–3 is more common in adenocarcinomas, occurring in about 75% of cases [41]. In the analyzed group, PanIN 1 predominated in the group of patients with neuroendocrine tumors (59.7%). Among patients diagnosed with adenocarcinoma, PanIN 1–3 was found more often than in patients with neuroendocrine tumors (57.4%).
According to the literature, PanIN 1–3 is twice as common in patients over 60 years old [40]. The more frequent occurrence of PanIN 1–3 in our study group may be related to the fact that among patients diagnosed with adenocarcinoma, the median age was higher compared to patients with neuroendocrine tumors. Hassid et al showed that as the grade of intraepithelial neoplasia increases, the percentage of tumors that have metastasized to the lymph nodes increases [42]. In our analysis, PanIN 1–3 was more common in patients with adenocarcinoma, which was more likely to metastasize to lymph nodes.
Infiltration of Structures
The presence of adipose tissue and perineural infiltration and vascular occlusions is associated with a greater aggressiveness of the tumor and worsens the long-term prognosis [6]. In the case of perineural infiltrations, they are more common in patients with adenocarcinomas than neuroendocrine tumors [6]. In the analyzed population, a statistically significant difference was found between the study groups in terms of adipose tissue and perineural infiltration. In the group of patients with adenocarcinoma, perineural and adipose tissue infiltration were 1.5 times more frequent than in patients with neuroendocrine tumors. Takahashi et al showed that vascular occlusions occur twice as often in the case of adenocarcinomas compared to neuroendocrine tumors [6]. We found a higher percentage of patients with NETs (58.4%) with vascular occlusions compared to reports in the literature showing that they occur in about 40% of patients [6]. This may be the reason for not observing statistically significant differences between the groups in the presence of vascular occlusions.
Lymph Nodes and Sistant Metastases
In patients with pancreatic neuroendocrine tumors, lymph node metastases occur 9–38% of cases [6,43]. In the analyzed group, nodal metastases were found in 32.5% of cases. Compared to pancreatic neuroendocrine tumors, ductal adenocarcinomas metastasize more often, metastasizing to lymph nodes in up to 75% of cases [6]. In our study, nodal metastases occurred in 74.1% of patients with adenocarcinomas, which is consistent with the literature [6].
According to Dasari et al, distant metastases occur in about 15% of patients with neuroendocrine tumors [44]. The present study found that distant metastases occurred in 16.9% of patients with NETs. According to the available literature, pancreatic adenocarcinomas have distant metastases at the time of diagnosis in about 15% of cases [45]. In our study, distant metastases occurred in 11.1% of patients with in adenocarcinomas. We found more distant metastases in patients with neuroendocrine tumors compared to adenocarcinomas, but the difference was not statistically significant. In patients with pancreatic adenocarcinomas, most distant metastases occur metachronously [45], but in patients with neuroendocrine tumors, most distant metastases occur synchronously [46]. Our analysis included only synchronous distant metastases due to the retrospective nature of the analysis. Therefore, the number of metachronous metastases in the analyzed group is underestimated.
Wirsung Diameter
In distal pancreatic resections, complications often arise from the difficulty of visualizing the pancreatic duct and closing it safely [47]. In our study, pancreatic duct diameter ≥3 mm was present in 7 (9.1%) patients with NET and in 12 (22.2%) patients with adenocarcinoma (P=0.045, OR=2.857). Also, authors of other papers comparing the incidence of dilated pancreatic duct in patients with NET and in those with adenocarcinoma reported that they were significantly more common (2–3 times) in patients with adenocarcinomas [14,48]. Jin et al reported a 22.6% incidence of pancreatic duct ≥3 mm in patients with neuroendocrine tumors [49], but it was a study discussing the dilatation of the diameter of the pancreatic duct based on preoperative magnetic resonance imaging. The diameter of the pancreatic duct may differ in preoperative and intraoperative studies because it is often measured at different locations. We found that a dilated pancreatic duct was significantly more common in patients with adenocarcinoma than in patients with NET (P<0.01). According to Baxi et al, pancreatic duct dilatation occurs up to 8 times more often in patients with adenocarcinomas compared to those with neuroendocrine tumors, regardless of the location of the lesion in the pancreas [14]. This is due to the greater “hardness” of the adenocarcinoma tumor [14], and this is another factor contributing to the higher incidence of pancreatic fistulas in patients with neuroendocrine tumors [50].
Pancreatic Texture
Assessment of the texture of the pancreas is a subjective evaluation, made by the operating surgeon [12], but for an experienced surgeon, it is a method that is recognized in research [12]. In the group we analyzed, soft pancreas was present in 23 (29.9%) patients with NET and in 12 (22.2%) patients with adenocarcinomas, but the difference was not statistically significant (P=0.423). In neuroendocrine tumors but not in adenocarcinomas, there is no desmoplastic reaction in the pancreas. This results in less frequent pancreatic fibrosis in neuroendocrine tumors compared to other neoplastic lesions and chronic inflammatory tumors of the pancreas [11]. As a result, the soft consistency of the pancreas is more often found in patients with neuroendocrine tumors compared to adenocarcinomas [13]. According to Partelli et al, soft pancreas is 2 times more common in patients with NET compared to adenocarcinomas [34].
Pancreatic Fistulas and Fistula Risk Score
The overall incidence of pancreatic fistulas after pancreatic resection surgery was 5–30% without histological grade differentiation [50,51]. Clinically significant pancreatic fistulas are more common in distal resections of neuroendocrine tumors compared to adenocarcinomas [52–54]. According to the literature, pancreatic fistulas are 1.5 times more common in pancreatic patients with NETs [53,55]. The analysis showed that type B and C pancreatic fistulas were found in 13.5% and 6.0% of neuroendocrine tumor cases, respectively. Type B pancreatic fistulas occurred in only 6.0% of cases. No case of type C pancreatic fistula was found in patients with adenocarcinoma.
The histopathological diagnosis of a tumor “other than adenocarcinoma” is also a risk factor for postoperative pancreatic fistula on the FRS scale [11,19]. The results calculated according to the FRS scale were similar in the 2 groups. Twenty-two (28.6%) patients with a neuroendocrine tumor were classified as being at high risk for postoperative pancreatic fistula. In contrast, among patients with adenocarcinoma, only 3.7% were found to be at high risk of pancreatic fistula.
Clavien-Dindo Complications
In our study, clinically significant (≥III) complications on the Clavien-Dindo scale were 16/77 (21%) for NET and 12/54 (22%) for adenocarcinoma. Heidsma et al presented the “textbook outcome” (TO) of patients operated on for neuroendocrine tumors [52]. TO was defined as no significant postoperative complications (Clavien-Dindo ≥III), no 90-day postoperative mortality, no prolonged hospital stay, no rehospitalization within 90 days, and no R0 resection [52]. For distal TO pancreatectomy, it was 56.7% for neuroendocrine tumors [52]. According to the literature, clinically significant complications occur in 20–30% of patients operated on for NET [37,39]. Beek et al points to the extent of resection as a key risk factor for major complications on the Clavien-Dindo scale after resections of neuroendocrine tumors [56]. Researchers indicate a significantly higher risk of clinically significant complications when pancreatoduodenectomy is performed as compared to distal resections (OR: 3.13; CI 95%: 1.32–7.41) [56]. On the other hand, for adenocarcinomas, postoperative complications of grade ≥III on the Clavien-Dindo scale affect 19–26% of patients [20,57]. The literature contains many reports of the higher incidence of significant postoperative complications after resections of adenocarcinomas compared to neuroendocrine tumors. In the population we analyzed concerning distal resections, clinically significant postoperative complications occurred with similar frequency in both groups, although the overall incidence of postoperative complications was higher for adenocarcinomas than neuroendocrine tumors. Patients with NETs tended to be younger than those with adenocarcinoma, which may influence the frequency of postoperative complications. However, there are also other factors such as soft pancreas and non-dilated pancreatic duct in NETs, which may have influenced our results.
Surgical Technique
Modifications of surgical technique aimed at reducing the rate of complications after pancreatic resection procedures are subject to ongoing discussion [58–60]. The use of minimally invasive surgery in distal pancreatic resections improves some short-term results [58,59]. According to Karunakaran et al, the use of minimally invasive methods clearly shortened the length of hospital stay [61]. However, there are reports showing the rate of clinically significant complications after minimally invasive procedures does not differ from open surgery [55,61], but Adams et al found a higher incidence of pancreatic fistulas after laparoscopic distal pancreatic resections [58]. This may be due to the use of a mechanical suture closing the cross-section of the remaining pancreatic parenchyma [58]. However, Tieftrunk et al showed the advantage of mechanical suture over manual suture in the prevention of postoperative pancreatic fistula after distal pancreatic resections [62].
The impact of the use of sealants, such as fibrin glue or fibrin patch, on the occurrence of postoperative complications, including pancreatic fistula, remains unclear [60,63]. According to Serra et al, the use of sealants in pancreatic surgery requires further validation [63]. Each modification of the surgical technique during pancreatic resection procedures should be individualized and adapted to the specific clinical case [61]. We did not analyze the surgical technique in the context of postoperative complications because there were no differences in the technique in our patients – all patients in the study population were operated on using the open method via laparotomy. The pancreatic duct was closed with 2–3 single sutures and the cross-section of the pancreas was closed with single sutures in 2 layers.
The undoubted limitation of the study is the lack of follow-up, which results, among other things, in an underestimation of the number of metachronous distant metastases in both groups, which prevents a complete characterization of the studied tumor types. Another limitation is the retrospective nature of the analysis, but, taking into account the small number of publications comparing adenocarcinomas and neuroendocrine tumors of the pancreas with distal localization, the present study has high didactic utility.
Conclusions
Patients undergoing distal resections tend to be older and have a higher ASA-PS score for adenocarcinomas compared to those with neuroendocrine tumors. Adenocarcinomas in the pancreatic body and tail are more aggressive than NETs and tend to have a higher grade of histological malignancy. They cause more frequent infiltration of adipose tissue and perineural invasion, and more often metastasize to lymph nodes. Synchronous distant metastases occurred at a similar rate in both groups.
A pancreatic duct ≥3 mm in diameter is more commonly found in pancreatic adenocarcinomas. In contrast, the softer texture of the pancreas and a less dilated pancreatic duct were more common in patients with neuroendocrine tumors. This may contribute to the higher incidence of clinically significant pancreatic fistulas in patients with NETs, although postoperative complications of Clavien-Dindo scale grade III and above occurred to a similar degree in both groups.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; A SEER-based study. BMC Cancer. 2018;18:688. doi: 10.1186/s12885-018-4610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallet J, Law CHL, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–97. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 3.Kos-Kudła B, Rosiek V, Borowska M, et al. Pancreatic neuroendocrine neoplasms – management guidelines (recommended by the Polish Network of Neuroendocrine Tumours) Endokrynol Pol. 2017;68:169–97. doi: 10.5603/EP.2017.2016. [DOI] [PubMed] [Google Scholar]
- 4.Beane JD, Borrebach JD, Billderback A, et al. Small pancreatic neuroendocrine tumors: Resect or enucleate? Am J Surg. 2021;222:29–34. doi: 10.1016/j.amjsurg.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Yadav S, Sharma P, Zakalik D. Comparison of demographics, tumor characteristics, and survival between pancreatic adenocarcinomas and pancreatic neuroendocrine tumors: A population-based study. Am J Clin Oncol. 2018;41:485–91. doi: 10.1097/COC.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi D, Kojima M, Morisue R, et al. Comparison of morphological features in lymph node metastasis between pancreatic neuroendocrine neoplasms and pancreatic ductal adenocarcinomas. Pancreatology. 2020;20:936–43. doi: 10.1016/j.pan.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Heng X, Chen B, Zhao K, et al. Comparison of nomogram for Primary Nonfunctional Pancreatic Neuroendocrine Tumors based on the 7th vs 8th edition of the AJCC cancer staging manual. PLoS One. 2023;18:e0284930. doi: 10.1371/journal.pone.0284930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszka Ł, Pająk J, Mrowiec S, et al. Precursor lesions of early onset pancreatic cancer. Virchows Archiv. 2011;458:439–51. doi: 10.1007/s00428-011-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiwani A, Chawla T. Risk factors of pancreatic fistula in distal pancreatectomy patients. Surg Res Pract. 2019;2019:4940508. doi: 10.1155/2019/4940508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L, Ning D, Chen X. Prevention and treatment of pancreatic fistula after pancreatic body and tail resection: Current status and future directions. Front Med. 2020;14:251–61. doi: 10.1007/s11684-019-0727-3. [DOI] [PubMed] [Google Scholar]
- 11.Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Zhu S, Luo G, Huang G. Soft pancreas should be assessed histopathologically for fibrosis to predict postoperative pancreatic fistula after pancreaticoduodenectomy. Asian J Surg. 2021;44:421–22. doi: 10.1016/j.asjsur.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Bartolini I, Bencini L, Risaliti M, et al. Current management of pancreatic neuroendocrine tumors: From demolitive surgery to observation. Gastroenterol Res Pract. 2018;2018:9647247. doi: 10.1155/2018/9647247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxi AC, Jiang Q, Hao J, et al. The effect of solid pancreatic mass lesions on pancreatic duct diameter at endoscopic ultrasound. Endosc Ultrasound. 2017;6:103. doi: 10.4103/2303-9027.204812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casciani F, Trudeau MT, Asbun HJ, et al. Surgeon experience contributes to improved outcomes in pancreatoduodenectomies at high risk for fistula development. Surgery. 2021;169:708–20. doi: 10.1016/j.surg.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 17.Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 19.Miller BC, Christein JD, Behrman SW, et al. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172–80. doi: 10.1007/s11605-013-2337-8. [DOI] [PubMed] [Google Scholar]
- 20.Serenari M, Ercolani G, Cucchetti A, et al. The impact of extent of pancreatic and venous resection on survival for patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2019;18:389–94. doi: 10.1016/j.hbpd.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–88. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas. 2004;28:257–62. doi: 10.1097/00006676-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Clavien PA, Barkun J, De Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 25.Nießen A, Schimmack S, Weber TF, et al. Presentation and outcome of mixed neuroendocrine non-neuroendocrine neoplasms of the pancreas. Pancreatology. 2021;21:224–35. doi: 10.1016/j.pan.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Dhali A, Ray S, Ghosh R, et al. Mixed neuroendocrine-non-neuroendocrine tumour of pancreas mimicking groove pancreatitis: Case report. Int J Surg Case Rep. 2021;88:106524. doi: 10.1016/j.ijscr.2021.106524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corvino A, Setola SV, Sandomenico F, et al. Synchronous tumours detected during cancer patient staging: Prevalence and patterns of occurrence in multidetector computed tomography. Pol J Radiol. 2020;85:e261. doi: 10.5114/pjr.2020.95781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corvino A, Corvino F, Radice L, Catalano O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: Report of a case and review of literature. Clin Imaging. 2015;39:538–42. doi: 10.1016/j.clinimag.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Maurea S, Corvino A, Imbriaco M, et al. Simultaneous non-functioning neuroendocrine carcinoma of the pancreas and extra-hepatic cholangiocarcinoma. A case of early diagnosis and favorable post-surgical outcome. JOP. 2011;12:255–58. [PubMed] [Google Scholar]
- 30.Chen J, Yang Y, Liu Y, Kan H. Prognosis analysis of patients with pancreatic neuroendocrine tumors after surgical resection and the application of enucleation. World J Surg Oncol. 2021;19:1–11. doi: 10.1186/s12957-020-02115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Sun X, Zhang Z, et al. The clinical characteristics and survival associations of pancreatic neuroendocrine tumors: Does age matter? Gland Surg. 2021;10:574. doi: 10.21037/gs-20-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramai D, Lanke G, Lai J, et al. Early- and late-onset pancreatic adenocarcinoma: A population-based comparative study. Pancreatology. 2021;21:124–29. doi: 10.1016/j.pan.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Wang Y, Cai Y, et al. The effect of age on short- and long-term outcomes in patients with pancreatic ductal adenocarcinoma undergoing laparoscopic pancreaticoduodenectomy. Pancreas. 2020;49:1063–68. doi: 10.1097/MPA.0000000000001620. [DOI] [PubMed] [Google Scholar]
- 34.Partelli S, Tamburrino D, Cherif R, et al. Risk and predictors of postoperative morbidity and mortality after pancreaticoduodenectomy for pancreatic neuroendocrine neoplasms: A comparative study with pancreatic ductal adenocarcinoma. Pancreas. 2019;48:504–9. doi: 10.1097/MPA.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg JA, Ivanov NA, Egan CE, et al. Sex-based clinicopathologic and survival differences among patients with pancreatic neuroendocrine tumors. J Gastrointest Surg. 2022;26:2321–29. doi: 10.1007/s11605-022-05345-6. [DOI] [PubMed] [Google Scholar]
- 36.Gavazzi F, Capretti G, Giordano L, et al. Pancreatic ductal adenocarcinoma and invasive intraductal papillary mucinous tumor: Different prognostic factors for different overall survival. Dig Liver Dis. 2022;54:826–33. doi: 10.1016/j.dld.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Nell S, Borel Rinkes IHM, Verkooijen HM, et al. Early and late complications after surgery for MEN1-related nonfunctioning pancreatic neuroendocrine tumors. Ann Surg. 2018;267:352–56. doi: 10.1097/SLA.0000000000002050. [DOI] [PubMed] [Google Scholar]
- 38.Albers MB, Almquist M, Bergenfelz A, Nordenström E. Complications of surgery for gastro-entero-pancreatic neuroendocrine neoplasias. Langenbecks Arch Surg. 2020;405:137. doi: 10.1007/s00423-020-01869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindner K, Binte D, Hoeppner J, et al. Resection of non-functional pancreatic neuroendocrine neoplasms – a single-center retrospective outcome analysis. Curr Oncol. 2021;28:3071–80. doi: 10.3390/curroncol28040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zińczuk J. Assessment of the presence of pancreatic intraepithelial neoplasia 43. Progress in Health Sciences. 2017;7:2017–60. [Google Scholar]
- 41.Recavarren C, Labow DM, Liang J, et al. Histologic characteristics of pancreatic intraepithelial neoplasia associated with different pancreatic lesions. Hum Pathol. 2011;42:18–24. doi: 10.1016/j.humpath.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Hassid B, Lucas AL, Salomao M, et al. Absence of pancreatic intraepithelial neoplasia (PanIN) predicts poor survival following resection of pancreatic cancer. Pancreas. 2014;43:1073–77. doi: 10.1097/MPA.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren S-J, Tan Q-Q, Cao D, et al. Prognostic role and predictors of lymph node involvement in pancreatic neuroendocrine tumors. Eur J Radiol. 2023;162:110772. doi: 10.1016/j.ejrad.2023.110772. [DOI] [PubMed] [Google Scholar]
- 44.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lou X, Li J, Wei YQ, et al. Comparable prevalence of distant metastasis and survival of different primary site for LN + pancreatic tumor. J Transl Med. 2020;18:266. doi: 10.1186/s12967-020-02438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mou Y, Wang ZY, Tan CL, et al. The role of primary tumor resection in patients with pancreatic neuroendocrine tumors with liver metastases. Front Oncol. 2022;12:767. doi: 10.3389/fonc.2022.838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin AN, Narayanan S, Turrentine FE, et al. Pancreatic duct size and gland texture are associated with pancreatic fistula after pancreaticoduodenectomy but not after distal pancreatectomy. PLoS One. 2018;13:e0203841. doi: 10.1371/journal.pone.0203841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta N, Kankotia R, Sahakian A, et al. Endoscopic ultrasound assessment of pancreatic duct diameter predicts neuroendocrine tumors and other pancreas masses. Pancreas. 2019;48:66–69. doi: 10.1097/MPA.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 49.Jin F, Wang K, Qin T, et al. Pancreatic neuroendocrine neoplasms: Correlation between MR features and pathological tumor grades. J Huazhong Univ Sci Technol Med Sci. 2017;37(4):587–95. doi: 10.1007/s11596-017-1777-x. [DOI] [PubMed] [Google Scholar]
- 50.Atema JJ, Jilesen APJ, Busch ORC, et al. Pancreatic fistulae after pancreatic resections for neuroendocrine tumours compared with resections for other lesions. HPB (Oxford) 2015;17:38–45. doi: 10.1111/hpb.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMillan MT, Christein JD, Callery MP, et al. Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2016;159:1013–22. doi: 10.1016/j.surg.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 52.Heidsma CM, Tsilimigras DI, van Dieren S, et al. Indications and outcomes of enucleation versus formal pancreatectomy for pancreatic neuroendocrine tumors. HPB. 2021;23:413–21. doi: 10.1016/j.hpb.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Ecker BL, McMillan MT, Allegrini V, et al. Risk factors and mitigation strategies for pancreatic fistula after distal pancreatectomy: Analysis of 2026 resections from the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269:143–49. doi: 10.1097/SLA.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 54.Molasy B, Zemła P, Mrowiec S, Kusnierz K. Utility of fistula risk score in assessing the risk of postoperative pancreatic fistula occurrence and other significant complications after different types of pancreatic neuroendocrine tumor resections. Ann Surg Treat Res. 2022;103:340–49. doi: 10.4174/astr.2022.103.6.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang JH, Hossain MS, Stackhouse K, et al. The role of minimally invasive surgery in resectable distal pancreatic adenocarcinoma. HPB (Oxford) 2023;25(10):1213–22. doi: 10.1016/j.hpb.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 56.van Beek D-J, Takkenkamp TJ, Wong-Lun-Hing EM, et al. Risk factors for complications after surgery for pancreatic neuroendocrine tumors. Surgery. 2022;171(1):127–36. doi: 10.1016/j.surg.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Neeman U, Lahat G, Goykhman Y, et al. Prognostic significance of pancreatic fistula and postoperative complications after pancreaticoduodenectomy in patients with pancreatic ductal adenocarcinoma. Surgeon. 2020;18:24–30. doi: 10.1016/j.surge.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Adams AM, Russell DM, Carpenter EL, et al. Minimally invasive versus open distal pancreatectomy: A matched analysis using ACS-NSQIP. Surg Endosc. 2023;37:617–23. doi: 10.1007/s00464-022-09363-y. [DOI] [PubMed] [Google Scholar]
- 59.Chang JH, Hossain MS, Stackhouse K, et al. The role of minimally invasive surgery in resectable distal pancreatic adenocarcinoma. HPB. 2023;25:1213–22. doi: 10.1016/j.hpb.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Lai M, Zhou S, He S, et al. Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database Syst Rev. 2023;6:CD009621. doi: 10.1002/14651858.CD009621.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karunakaran M, Barreto SG. Surgery for pancreatic cancer: Current controversies and challenges. Future Oncol. 2021;17:5135–62. doi: 10.2217/fon-2021-0533. [DOI] [PubMed] [Google Scholar]
- 62.Tieftrunk E, Demir IE, Schorn S, et al. Pancreatic stump closure techniques and pancreatic fistula formation after distal pancreatectomy: Meta-analysis and single-center experience. PLoS One. 2018;13:e0197553. doi: 10.1371/journal.pone.0197553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serra F, Bonaduce I, Rossi EG, et al. The using of sealants in pancreatic surgery: A systematic review. Ann Med Surg (Lond) 2021;64:102244. doi: 10.1016/j.amsu.2021.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]


