Abstract
Introduction
Transitions in temporal niche have occurred many times over the course of mammalian evolution. These are associated with changes in sensory stimuli available to animals, particularly with visual cues, because levels of light are so much higher during the day than at night. This relationship between temporal niche and available sensory stimuli elicits the expectation that evolutionary transitions between diurnal and nocturnal lifestyles will be accompanied by modifications of sensory systems that optimize the ability of animals to receive, process, and react to important stimuli in the environment.
Methods
This study examines the influence of temporal niche on investment in sensory brain tissue of 13 rodent species (five diurnal; eight nocturnal). Animals were euthanized and the brains immediately frozen on dry ice; olfactory bulbs were subsequently dissected and weighed, and the remaining brain was weighed, sectioned, and stained. Stereo Investigator was used to calculate volumes of four sensory regions that function in processing visual (lateral geniculate nucleus, superior colliculus) and auditory (medial geniculate nucleus, inferior colliculus) information. A phylogenetic framework was used to assess the influence of temporal niche on the relative sizes of these brain structures and of olfactory bulb weights.
Results
Compared to nocturnal species, diurnal species had larger visual regions, whereas nocturnal species had larger olfactory bulbs than their diurnal counterparts. Of the two auditory structures examined, one (medial geniculate nucleus) was larger in diurnal species, while the other (inferior colliculus) did not differ significantly with temporal niche.
Conclusion
Our results indicate a possible indirect association between temporal niche and auditory investment and suggest probable trade-offs of investment between olfactory and visual areas of the brain, with diurnal species investing more in processing visual information and nocturnal species investing more in processing olfactory information.
Keywords: Diurnal, Nocturnal, Activity pattern, Mosaic evolution, Rodents
Introduction
Over a 24-h period, there are predictable patterns of change in temperature and light levels. There are also temporal differences in biotic factors, such as the presence of predators or competitors and resource availability. These differences between day and night result in distinct sensory environments, which most animals have evolved to exploit by concentrating their activity to specific times. An animal’s temporal niche, or daily activity pattern, refers to the time in which the animal is most likely to be awake and active. There is great diversity in the types of daily activity patterns seen in vertebrates. Animals may be active during the night (nocturnal), during the day (diurnal), at dusk and dawn (crepuscular), or during both night and day (cathemeral). In addition to these discrete categories, there are varying levels of flexibility in patterns of activity [1–3]. Flexibility exists in multiple forms. Even within strict temporal niche categories, patterns of activity can vary in a variety of ways, e.g., in the number of activity peaks exhibited (unimodal, bimodal, or polymodal). Some species switch their most active period from one time of the day to another in response to stressors, such as predators or competitors [2], and some display predictable seasonal shifts in activity patterns [4, 5]. Furthermore, intraspecific variability in daily activity patterns occurs in many species [6–8].
Despite the diversity of daily activity patterns currently observed in mammals, there is strong evidence that the earliest mammals were nocturnal [9–11]. This was likely a mechanism to avoid the dominant diurnal dinosaurs of that time [12]. Nocturnal behavior was largely conserved in mammals through most, or all, of the Mesozoic, but with the empty niches resulting from the extinction of dinosaurs at the end of that era (circa 66 million years ago), the range of mammalian temporal niches expanded [11]. Daily activity patterns are constrained phylogenetically, with closely related species more likely to occupy similar temporal niches [10, 13]. However, a study including nearly half of the approximately 6,450 extant mammalian species found that several orders include species that occupy different temporal niches [14]. In addition, many families include both diurnal and nocturnal species [15], and even within genera, there can be interspecific variability in daily activity patterns. This indicates that, despite phylogenetic conservatism, evolutionary transitions in temporal niche have occurred many times within Class Mammalia.
How and why these transitions occur is a subject of ongoing research. Ankel-Simons and Rasmussen [16] suggest that some temporal niche transitions simply reflect the opportunistic filling of an empty niche. Temporal niche transitions might also be driven by changes in food availability [17, 18], or the appearance of new competitors [4, 6, 19], or predators [18–20]. It is likely that all of these have played a role in shifting temporal niches in different lineages.
Regardless of the selective forces driving temporal niche transitions, adaptation to a new one requires physiological and sensory system changes. A vital component of an animal’s fitness is the ability to sense and respond to the external environment. Different sensory modalities capitalize on different forms of stimuli and those stimuli vary across a 24-h day. Photic cues, for example, are abundant during the day but limited at night, and it is generally thought that diurnal species rely more heavily on vision for foraging, hunting, and predator avoidance than nocturnal species [21]. If diurnal species have more robust visual systems, it raises the question of whether other sensory systems, e.g., olfactory and auditory, might be better developed in nocturnal species to help guide their activity in the dark. Moreover, if diurnal and nocturnal species rely differently on these senses, what adaptations to the sensory system should be expected?
While sense organs receive sensory stimuli and may do some initial processing, the brain functions to do the more sophisticated and refined processing that enables an animal to extract crucial information from those signals and respond appropriately to external cues. The quantity and quality of different types of sensory information available should therefore impact how an animal invests not only in the collection of sensory information through sensory organs but also in the processing of that information by structures within the brain. Since neural tissue is among the most energetically expensive there is [22], selection would be expected to optimize the relative investment in the various components of the sensory brain (i.e., areas of the brain that receive, integrate, and process sensory information) with the caveat that these regions may not be developmentally independent.
The influence of developmental constraints on brain evolution has received considerable attention, much of it is focused on the relative importance of concerted processes (i.e., change in one structure is accompanied by proportional changes in other structures) versus mosaic ones (i.e., one structure evolves independently from other structures). Many studies have shown that brain size and certain brain divisions exhibit distinct allometry and scale in a phylogenetically conserved manner in vertebrates [23–26], supporting the concerted evolution hypothesis. Other studies have provided evidence for mosaic patterns of evolutionary changes in the brain [27–30]. It seems likely that concerted and mosaic evolution have taken place concurrently and been shaped by the unique history and demands of the structures under selective pressure. Indeed, Moore and DeVoogd [31] found clear evidence that both concerted and mosaic processes have shaped the evolution of song circuits in the brains of passerine birds.
Several studies have examined the relationship between daily activity pattern and sensory investment in vertebrates. Differences in olfactory investment between nocturnal and diurnal species have been found in birds [32], insectivores, and primates [33], with nocturnal species possessing larger olfactory bulbs (OB). Iglesias et al. [34] found that shifts to more nocturnal activity correspond with a decrease in the size of the optic tectum in teleosts. Studies of primates have shown that diurnal species have a larger visual cortex relative to hindbrain volume [35] than nocturnal species. Campi and Krubitzer [36] described differences in visual, auditory, and somatosensory/motor regions of the cortex in two species of diurnal squirrels relative to the nocturnal brown rat (Rattus norvegicus). The diurnal squirrels had significantly larger visual regions and smaller somatosensory and auditory regions in the cortex than the brown rat. Campi et al. [37] observed a similar pattern when comparing visual and somatosensory cortices of the diurnal African grass rat (Arvicanthis niloticus) and nocturnal brown rat in that primary visual cortex was larger, and primary somatosensory cortex was smaller in the diurnal than the nocturnal species. However, in contrast to the findings of Campi and Krubitzer [36], this study found that the two regions of the auditory cortex were larger in the diurnal (African grass rat) than in the nocturnal species (brown rat). In a subsequent comparison of the African grass rat and brown rat, Shuboni-Mulligan et al. [38] found that two visual brain structures, the lateral geniculate nucleus (LGN) and superior colliculus (SC), were larger in the diurnal compared to the nocturnal species. This contrasts with the finding of Finlay et al. [26] that the volume of the LGN is not correlated with daily activity pattern in mammals. Additionally, two comparative studies examining the relative sizes of ventral and dorsal subregions of the LGN found substantial species differences, though they did not investigate whether these were correlated with temporal niche [39, 40]. Many components of the visual system are highly variable, even between individuals of the same species. Ankel-Simons and Rasmussen [16] have suggested that this variability makes the visual system highly susceptible to evolutionary change. It may be that this enables temporal niche transitions to occur more readily than would otherwise be possible.
Taken together, these studies suggest that an animal’s daily activity pattern may influence how it invests in brain tissues that process olfactory and visual information. However, the diversity of species examined thus far is limited, and there is ambiguity with respect to the relationship between daily activity pattern and auditory investment. To address these issues, additional species need to be examined within a phylogenetic framework, ideally with a focus on investment in multiple sensory modalities. The latter permits identification of possible energetic trade-offs and ensures standardization of methods. It thus has the potential to clarify contradictions in the literature that may reflect differences in experimental design, including studies that combine data from multiple sources.
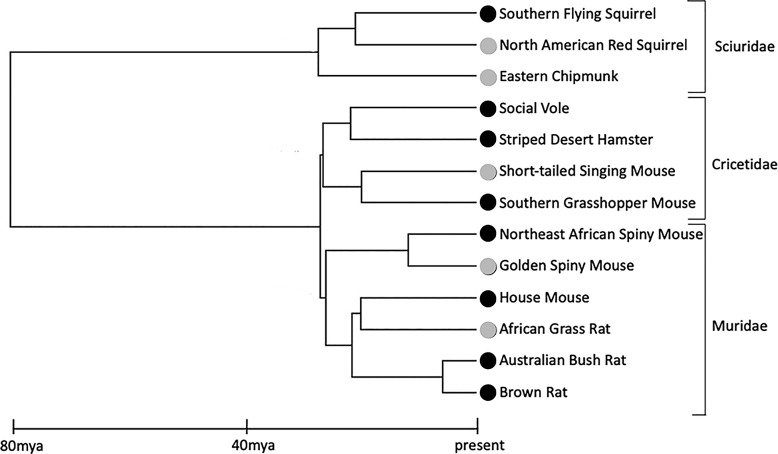
In this study, we investigate the influence of temporal niche on investment in subcortical sensory brain regions supporting olfaction, vision, and audition across 13 rodent species (eight nocturnal, five diurnal), representing a minimum of five independent transitions in temporal niche (Fig. 1). We test the hypothesis that evolutionary transitions from nocturnality to diurnality, or vice versa, are accompanied by changes in regions of the brain that process sensory information. We predict trade-offs between vision and olfaction and vision and audition, with diurnal species devoting proportionally more neural tissue to processing visual information and nocturnal species investing more to processing olfactory and auditory information, as the cues these systems process are equally available across a 24-h period. Additionally, we investigate if, and to what extent, brain size and the size of these sensory regions are phylogenetically constrained. To accomplish this, we estimate phylogenetic signal in each measure and model different modes of evolution for each area of interest. Lastly, we discuss the extent to which these sensory areas have evolved in a mosaic or concerted fashion.
Fig. 1.
Phylogeny and temporal niche of 13 rodent species (gray: diurnal, black: nocturnal). Phylogenetic relationships and divergence times were established from Fabre et al. [41]. Mya, million years ago.
Methods
Specimens
We collected data from 13 rodent species, representing three extant families: Sciuridae, Cricetidae, and Muridae (Table 1; Fig. 1). The sample includes eight nocturnal species (southern flying squirrel [Glaucomys volans], social vole [Microtus socialis], striped desert hamster [Phodopus sungorus], southern grasshopper mouse [Onychomys torridus], Northeast African spiny mouse [Acomys cahirinus], house mouse [Mus musculus], Australian bush rat [Rattus fuscipes], and brown rat [Rattus norvegicus]) and five diurnal species (North American red squirrel [Tamiasciurus hudsonicus], eastern chipmunk [Tamias striatus], short-tailed singing mouse [Scotinomys teguina], golden spiny mouse [Acomys russatus], and African grass rat [Arvicanthis niloticus]). The families Cricetidae and Muridae likely had nocturnal ancestors [13], but within both clades, diurnality has evolved several times independently, including in 3 lineages that are examined here (Fig. 1). The earliest sciurids, in contrast, were probably diurnal, as are most extant members of this family [13]. A single transition back to nocturnality appears to have occurred approximately 18 million years ago at the origin of tribe Pteromyini, today represented by 58 species of flying squirrels [58, 59].
Table 1.
Common name, genus and species, source, sample size (N) with numbers of males (m) and females (f) used, activity pattern, and references for activity pattern designation
| Common Name | Genus and Species | Source | N (m, f) | Activity pattern | References |
|---|---|---|---|---|---|
| Southern flying squirrel | Glaucomys volans | Live-trapped, East Lansing, MI | 3 (0, 3) | Nocturnal | [42, 43] |
| North American red squirrel | Tamiasciurus hudsonicus | Live-trapped, East Lansing, MI | 3 (1, 2) | Diurnal | [44] |
| Eastern chipmunk | Tamias striatus | Live-trapped, East Lansing, MI | 4 (2, 2) | Diurnal | [45] |
| Social vole | Microtus socialis | Kronfeld-Schor Lab, Tel Aviv University | 3 (2, 1) | Nocturnal | [46] |
| Striped desert hamster | Phodopus sungorus | Nelson Lab, Ohio State University | 6 (3, 3) | Nocturnal | [47] |
| Short-tailed singing mouse | Scotinomys teguina | Phelps Lab, University of Texas, Austin | 6 (3, 3) | Diurnal | [48] |
| Southern grasshopper mouse | Onychomys torridus | Rowe Lab, Michigan State University | 3 (1, 2) | Nocturnal | [49, 50] |
| Northeast African spiny mouse | Acomys cahirinus | Kronfeld-Schor Lab, Tel Aviv University | 5 (4, 1) | Nocturnal | [51] |
| Golden spiny mouse | Acomys russatus | Kronfeld-Schor Lab, Tel Aviv University | 4 (2, 2) | Diurnal | [6, 52, 53] |
| House mouse | Mus musculus | Live-trapped, Lansing, MI | 6 (3, 3) | Nocturnal | [54] |
| African grass rat | Arvicanthis niloticus | Smale Lab, Michigan State University | 6 (3, 3) | Diurnal | [55] |
| Australian bush rat | Rattus fuscipes | Live-trapped, NSW Australia | 5 (2, 3) | Nocturnal | [4, 56] |
| Brown rat | Rattus norvegicus | Lab, Charles River | 5 (3, 2) | Nocturnal | [57] |
MI, Michigan; NSW, New South Wales.
Southern flying squirrels, North American red squirrels, eastern chipmunks, and house mice were live-trapped in and around East Lansing, Michigan, between October 2015 and December 2017 (Table 1). Australian bush rats were live-trapped in NSW, Australia, in August 2015. Brown rats were purchased from Charles River Laboratories. Southern grasshopper mice and African grass rats were obtained from Michigan State University laboratory colonies. Striped desert hamsters were obtained from a laboratory colony at Ohio State University. Intact whole brains from social voles, Northeast African spiny mice, and golden spiny mice were obtained from Tel Aviv University and those from short-tailed singing mice were obtained from a University of Texas at Austin laboratory.
The number of individuals of each species ranged from 3 to 6 (Table 1). We used only adult individuals and tried to sample both males and females. However, the southern flying squirrels were all female in this study. All animals were handled according to protocols approved by the following institutional and regional authorities: American Society of Mammalogists [60], Michigan State University Institutional Animal Care and Use Committee (protocol # 07/16-116-00), Office of Environment and Heritage (License #SL100634), and New South Wales Department of Industry and Investment Animal Research Authority (ORA 14/17/009).
Data Collection and Measurements
Regions of Interest
The structures chosen to estimate investment in olfaction, vision, and audition, respectively, included the OB; the LGN and SC; and the medial geniculate nucleus (MGN) and inferior colliculus (IC).
Olfaction
The main OB receives input directly from the olfactory neurons of the olfactory epithelium, along with the Grueneberg ganglion and septal organ, all of which are in the nasal cavity [61, 62]. The accessory OB in rodents receives input from the vomeronasal organ and lie dorsocaudally on the main OB [63]. Estimation of investment in olfaction was accomplished by measuring the combined mass of the main and accessory OB.
Vision
The LGN is a visual brain structure located in the thalamus. The LGN receives direct afferents from the retina, sends and receives projections from the SC [64], and sends projections out to the primary visual cortex [65]. It is comprised of three distinct subdivisions, i.e., dorsal LGN (dLGN), intergeniculate leaflet (IGL), and ventral LGN (vLGN), the latter two of which are also known to function in the patterning of activity across the day [66]. All three subdivisions of the LGN were combined in our initial measurement. To determine how different subdivisions contribute to the overall size of the LGN, we also separately measured the dLGN and the combined volume of the IGL and vLGN. The latter were combined due to obscure boundaries between them and their similar functions in circadian rhythms. Hereafter, we treat the IGL and vLGN as a single structure, referred to as the vLGN.
The SC is a visual sensorimotor structure in the midbrain that can be divided into seven functionally distinct layers in rodents [67]. The SC projects to the LGN, and in the case of the vLGN, those connections are reciprocal. The SC also has connections with the pulvinar complex, another thalamic visual structure [64]. Reciprocal connections between the SC and pulvinar complex have been identified in some rodents but appear to be uncommon in other mammalian taxa [68]. For the purposes of this study, we focus on the three superficial layers of the SC (i.e., the zonal layer, superficial gray layer, and optic nerve layer), as they receive most of the retinal input to this structure and function almost exclusively in processing visual information. The deeper layers of the SC function in multiple forms of sensory processing, including auditory and somatosensory [69, 70].
Audition
The MGN of the thalamus plays an important role in auditory processing and it conveys information between the IC and the auditory cortex [71]. While the MGN is the main target of projections from the IC and serves as the major input to the auditory cortex, it also receives and integrates information from the auditory nuclei in the brain stem and has connections with the amygdala and frontal cortex [72]. In mammals, the MGN is subdivided into three functionally distinct parts: dorsal MGN, medial MGN, and ventral MGN [72, 73]. All three subdivisions of the MGN were included in our measurement.
The IC has the most diverse connections of the regions measured here and is an important site of convergence within the auditory pathway [74]. In addition to its connections with the MGN, it functions to integrate auditory information from the brain stem and the auditory cortex [72]. The IC is subdivided into three distinct parts: the central nucleus, dorsal cortex, and lateral cortex. All three subdivisions of the IC were included in our measurement.
Brain Collection and Histology
Fresh, unfixed tissue was used to avoid issues of uneven shrinkage of brain tissues. Each animal was euthanized with a lethal dose of sodium pentobarbital, administered intraperitoneally. Immediately after death, the animal was weighed to the nearest gram, and its brain was extracted, placed in powdered dry ice for 2–5 min, and transferred to a −80° freezer until further processing. After removal from the freezer, each brain was trimmed immediately caudal to the medulla oblongata and weighed to the nearest milligram. OBs were then separated from the brain just anterior to the olfactory peduncles and weighed to the nearest milligram. The portion of the brain extending from the anterior thalamus to just caudal to the auditory tectum was coronally sectioned at 40 µm thickness on a cryostat, except for the house mice brains which, because of their small size, were sectioned at 20 µm thickness. Three alternate series of brain tissue sections were mounted onto superfrost slides, and one was stained for acetylcholinesterase as follows: slides were incubated for 5 h in a solution of 0.0072% ethopropazine HCl, 0.075% glycine, 0.05% cupric sulfate, 0.12% acetylthiocholine iodide, and 0.68% sodium acetate (pH 5.0); rinsed 2 times (3 min each) with distilled H2O; and developed in a 0.77% sodium sulfide solution (pH 7.8) for 45 min. Slides were then rinsed with 2 changes of distilled H2O (3 min each), then run through a series of ascending ethanol concentrations (70%, 95%, 100%, and 100%) for 1 min each (to dehydrate the adhering tissue), cleared through 2 changes of xylenes for 5 min each, and cover slipped using DPX mounting medium. The two remaining slide series were set aside for future work.
Measurements
Estimation of investment in olfaction was accomplished by measuring the combined mass of the main and accessory OB. For the other regions of interest (LGN, dLGN, vLGN, SC, MGN, and IC), photomicrographs of AChE-stained sections (Fig. 2, 3) were taken with a digital camera (MBF Bioscience CX9000) attached to a Zeiss light microscope (Carl Zeiss, Gottingen, Germany, ×5 objective), using the 2D slide scanning module on Stereo Investigator 2017 (MBF Bioscience). The Cavalieri method was used (100 × 100 μm grid, every third section) to calculate volumetric measurements in Stereo Investigator. Boundaries of each brain structure were determined according to the rat brain atlas [75]. For each structure, only one side (left or right) was measured, and that value was doubled to obtain total volume.
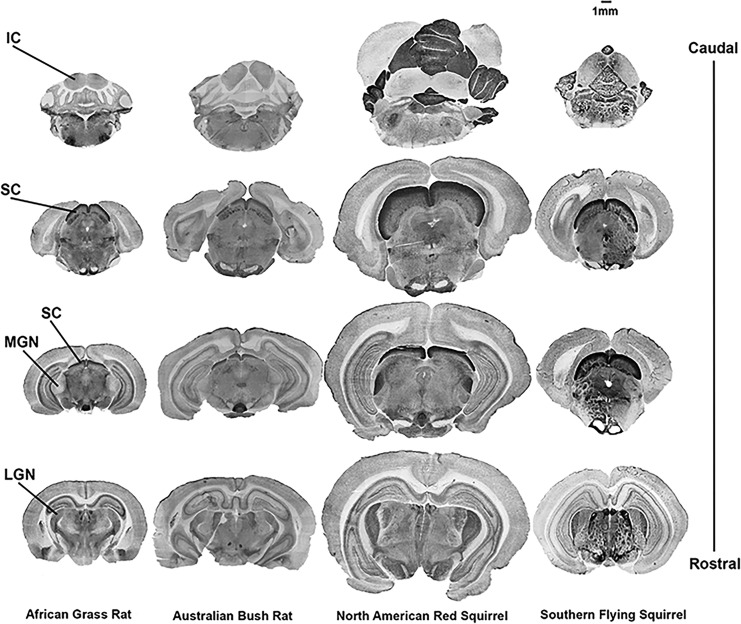
Fig. 2.
Photomicrographs of AChE-stained brain sections indicating regions measured in this study: lateral geniculate nucleus (LGN), superior colliculus (SC), medial geniculate nucleus (MGN), and inferior colliculus (IC). Labels are restricted to African grass rat (diurnal). Other species shown are Australian bush rat (nocturnal), North American red squirrel (diurnal), and southern flying squirrel (nocturnal). Scale bar in upper right.
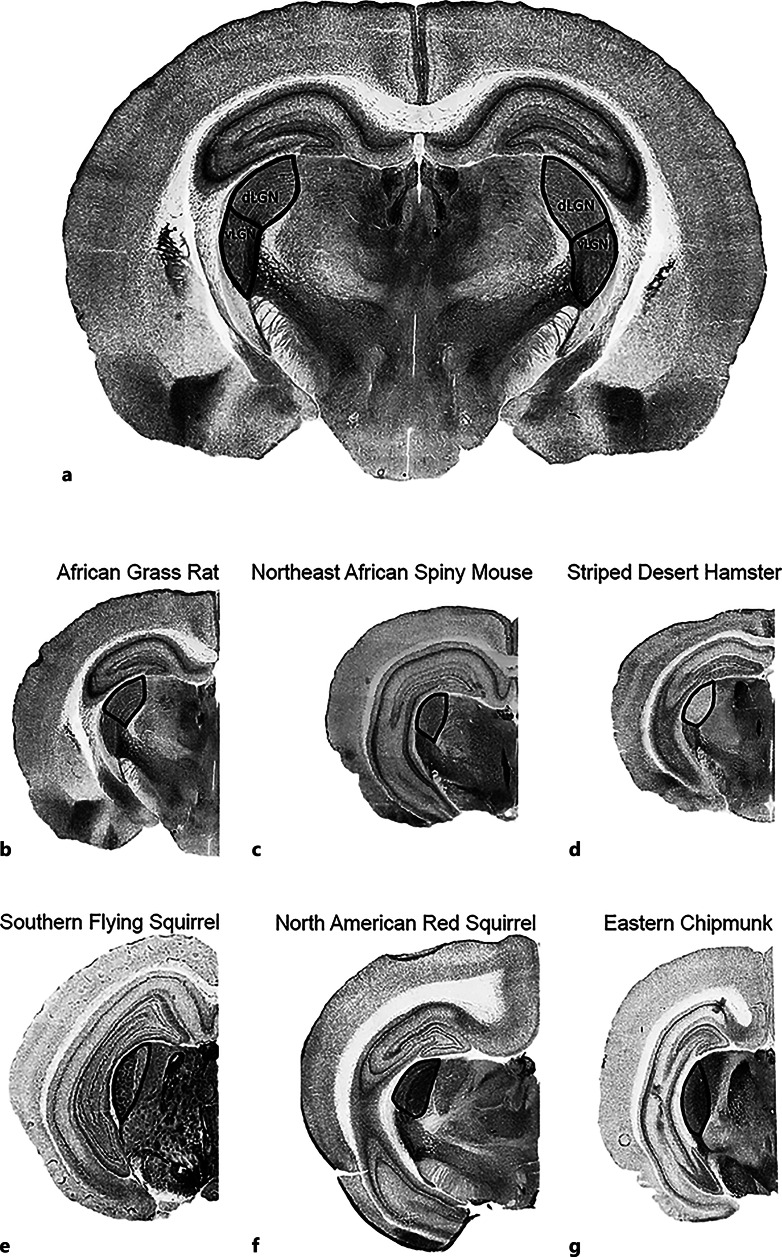
Fig. 3.
Photomicrographs of AChE-stained brain sections illustrating boundaries of dorsal lateral geniculate nucleus (dLGN) and ventral lateral geniculate nucleus (vLGN); the latter includes the intergeniculate leaflet (IGL). a African grass rat with dLGN and vLGN boundaries highlighted; African grass rat (b), Northeast African spiny mouse (c), striped desert hamster (d), southern flying squirrel (e), North American red squirrel (f), and eastern chipmunk (g) with dLGN boundary highlighted. Brains are not to scale. Boundaries identified using Paxinos and Watson [75].
While neuronal density, including neuron/glial proportions, would provide a more accurate indicator of investment in brain tissue, it is more difficult to measure, and thus many studies, including this one, have used size (i.e., mass or volume) as an alternative proxy for investment. Neuron density scales closely with volume in sensory brain structures of rodents [40, 73, 76].
Data Analysis
Variables and Transformations
Continuous variables used in the analyses include body, brain, and OB mass, as well as LGN, dLGN, vLGN, SC, MGN, and IC volume. The vLGN/dLGN ratio was arcsine transformed prior to analysis, and all other analyses were carried out using log-transformed data. All data transformations and analyses were carried out in RStudio [77]. All species were assigned to one of two categorical states, diurnal or nocturnal, based on descriptions of daily activity patterns gleaned from field studies reported in the literature (Table 1). Laboratory studies were not considered in determining activity patterns.
Phylogenetic Signal Estimations
To estimate the influence of phylogeny on each variable, we calculated Blomberg’s K (based on 1,000 randomizations for p value) and Pagel’s λ (based on likelihood ratio tests) using the phylosig package in R [78]. Estimations were carried out using the residuals from linear regressions. Brain size was regressed on body size, and the size of each sensory region (OB, LGN, dLGN, vLGN, SC, MGN, and IC) was regressed on brain size. The vLGN/dLGN ratio was the only measure not regressed against another structure prior to calculating Blomberg’s K and Pagel’s λ.
Modes of Evolution and ANCOVA
For each brain structure of interest, different modes of evolution were modeled using phylogenetic general least squares in the package phylolm in RStudio [79]. Most models incorporated one of three different branch length transformations: lambda (λ), delta (δ), or kappa (κ). For a λ transformation, the internal branch lengths are multiplied by a constant, but the tip branches are left unaffected. A λ value of 0 is equivalent to no phylogenetic effect, whereas a λ value of 1 is equivalent to a fixed Brownian motion model [80]. For a δ transformation, all the values of the phylogenetic covariance matrix are raised to the power of δ. This transforms the sum of the length of shared branches between two tips [80]. For a κ transformation, all branch lengths are raised to the power of κ. In this case, the elements of the phylogenetic covariance matrix are the sum of the individually transformed branch lengths [80].
We compared the following models for brain size and each brain region measurement: λ set to 0 (equivalent to no phylogenetic effect), λ set to 1 (equivalent to a fixed Brownian model), λ set to maximum likelihood (ML), δ set to ML, and κ set to ML. We also modeled early burst evolution and the Ornstein-Uhlenbeck (OU) evolutionary model. We then compared the seven models using sample size corrected Akaike’s information criterion (AICc). An ANCOVA was performed using the best linear model for each brain region as a function of total brain size and activity pattern (nocturnal vs. diurnal). The ANCOVAs that were performed using a phylogenetic regression were carried out in the CAPER package in RStudio [81].
ANOVA
To investigate the relationship between vLGN/dLGN ratios and activity pattern, we carried out non-phylogenetic and phylogenetic ANOVAs, the latter using the phytools package 0.7–70 [78]. The ANOVA that performed best was used to compare vLGN/dLGN ratios between diurnal and nocturnal species.
Results
Phylogenetic Signal
Blomberg’s K was marginally significant for brain size, and significant for OB size, SC size, and vLGN/dLGN ratio, indicating a modest phylogenetic signal in brain size and vLGN/dLGN ratio, and a strong signal in OB and SC size (Table 2). Pagel’s λ, in contrast, detected a significant phylogenetic signal only in relative brain size. These differences in detected phylogenetic signal may reflect the small sample size used for this study. Blomberg’s K is typically more reliable than Pagel’s λ when working with smaller sample sizes [82]. The results of the Blomberg’s K estimations were consistent with the models of evolution selected (based on AICc) for the ANCOVAs and ANOVA.
Table 2.
Phylogenetic signal estimates (Blomberg’s κ and Pagel’s λ) for brain mass, olfactory bulb mass (OB), vLGN/dLGN ratio, and volumes of lateral geniculate nucleus (LGN), dorsal lateral geniculate nucleus (dLGN), ventral lateral geniculate nucleus (vLGN), superior colliculus (SC), medial geniculate nucleus (MGN), and inferior colliculus (IC)
| Measure | κ | p value | λ | p value |
|---|---|---|---|---|
| Brain | 0.603 | 0.065 | 0.594 | 0.031 |
| OB | 0.850 | 0.009 | 1 | 0.110 |
| LGN | 0.534 | 0.175 | <0.001 | 1 |
| dLGN | 0.556 | 0.134 | <0.001 | 1 |
| vLGN | 0.415 | 0.438 | <0.001 | 1 |
| vLGN/dLGN | 0.739 | 0.019 | 0.596 | 0.190 |
| SC | 0.861 | 0.011 | 1 | 0.118 |
| MGN | 0.489 | 0.235 | <0.001 | 1 |
| IC | 0.448 | 0.339 | <0.001 | 1 |
Each measure, except vLGN/dLGN ratio, is size-independent, i.e., based on the residuals from a linear regression.
Brain Size
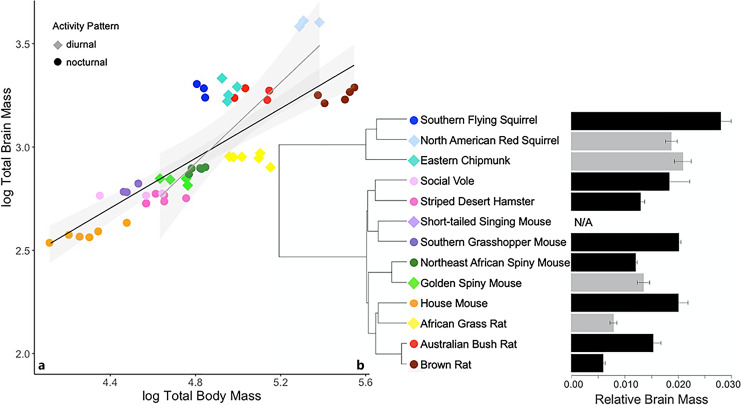
Brain mass was not recorded for the short-tailed singing mouse. Among the other 12 species, brain mass ranged from 0.61% of body mass in the brown rat to 2.81% in the southern flying squirrel (Fig. 4). The optimal linear model for brain size was Brownian motion, which explained 51.5% of the variation (F3,8 = 4.893, p = 0.032). Body size is a significant predictor of brain size (Table 3). Brain size increases with body size in both diurnal and nocturnal species. Activity pattern does not have a significant influence on relative brain size.
Fig. 4.
a Log brain mass regressed against log body mass of 12 rodent species. Shading represents 95% confidence intervals. b Mean brain mass relative to body mass; error bars are SEM (gray: diurnal, black: nocturnal).
Table 3.
ANCOVAs examining effects of temporal niche on total brain mass as a proportion of body mass; olfactory bulb (OB) mass as a proportion of total brain mass; and volumes of the lateral geniculate nucleus (LGN), dorsal lateral geniculate nucleus (dLGN), ventral lateral geniculate nucleus (vLGN), superior colliculus (SC), medial geniculate nucleus (MGN), and inferior colliculus (IC) as proportions of total brain mass
| Regions | Factors | DF | Mean Sq | F value | Pr(>F) | |
|---|---|---|---|---|---|---|
| Brain | Temporal niche | 1 | 0.0001 | 0.0125 | 0.914 | |
| Body size | 1 | 0.0118 | 14.261 | 0.005** | ||
| Temporal niche*body | 1 | 0.0003 | 0.4040 | 0.543 | ||
| Residuals | 8 | 0.0008 | ||||
| Olfactory structure | OB | Temporal niche | 1 | 0.0006 | 5.1631 | 0.049* |
| Brain size | 1 | 0.0191 | 154.66 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0005 | 4.1801 | 0.071 | ||
| Residuals | 9 | 0.0001 | ||||
| Visual structures | LGN | Temporal niche | 1 | 0.2294 | 40.269 | <0.001*** |
| Brain size | 1 | 4.6037 | 808.31 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0281 | 4.925 | 0.031* | ||
| Residuals | 55 | 0.0057 | ||||
| dLGN | Temporal niche | 1 | 0.2398 | 23.248 | <0.001*** | |
| Brain size | 1 | 4.5876 | 444.67 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0159 | 1.545 | 0.2193 | ||
| Residuals | 53 | 0.0103 | ||||
| vLGN | Temporal niche | 1 | 0.1610 | 38.332 | <0.001*** | |
| Brain size | 1 | 2.8730 | 684.07 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0528 | 12.566 | <0.001*** | ||
| Residuals | 53 | 0.0042 | ||||
| SC | Temporal niche | 1 | 0.0043 | 6.8836 | 0.028* | |
| Brain size | 1 | 0.0227 | 36.153 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0017 | 2.6839 | 0.136 | ||
| Residuals | 9 | 0.0006 | ||||
| Auditory structures | MGN | Temporal niche | 1 | 0.1824 | 10.391 | 0.002** |
| Brain size | 1 | 5.9126 | 336.91 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.0007 | 0.0397 | 0.843 | ||
| Residuals | 54 | 0.0175 | ||||
| IC | Temporal niche | 1 | 0.0284 | 2.782 | 0.102 | |
| Brain size | 1 | 3.6597 | 358.49 | <0.001*** | ||
| Temporal niche*brain | 1 | 0.1573 | 15.407 | <0.001*** | ||
| Residuals | 50 | 0.0102 |
*p < 0.05.
**p < 0.01.
***p < 0.001.
Olfactory System
OB
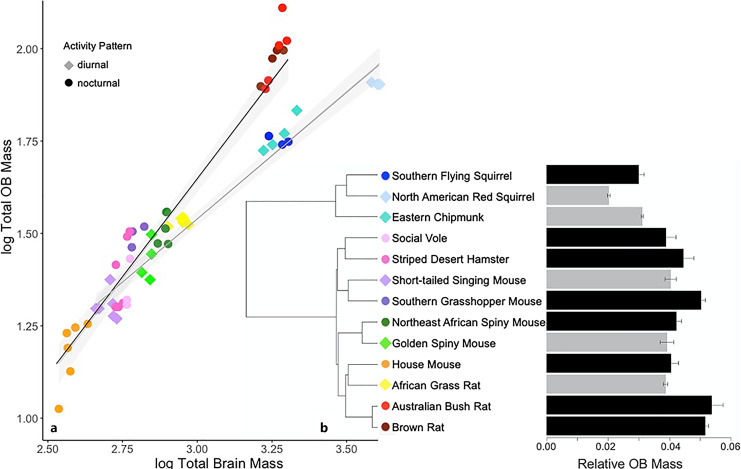
OB size ranged from 2.02% of brain mass in the North American red squirrel to 5.35% of brain mass in the Australian bush rat (Fig. 5). The three squirrel species exhibited the smallest relative OB size compared to all other species. The Brownian motion model of evolution performed best for OB data, explaining 93.1% of the variation in OB size (F3,9 = 54.6, p < 0.001). The phylogenetic ANCOVA shows that brain size and activity pattern are both significant predictors of relative OB size (Table 3), with nocturnal species possessing larger OBs than diurnal species.
Fig. 5.
a Log olfactory bulb (OB) mass regressed against log brain mass of 13 rodent species. Shading represents 95% confidence intervals. b Mean OB mass relative to brain mass; error bars are SEM (gray: diurnal, black: nocturnal).
Visual System
LGN
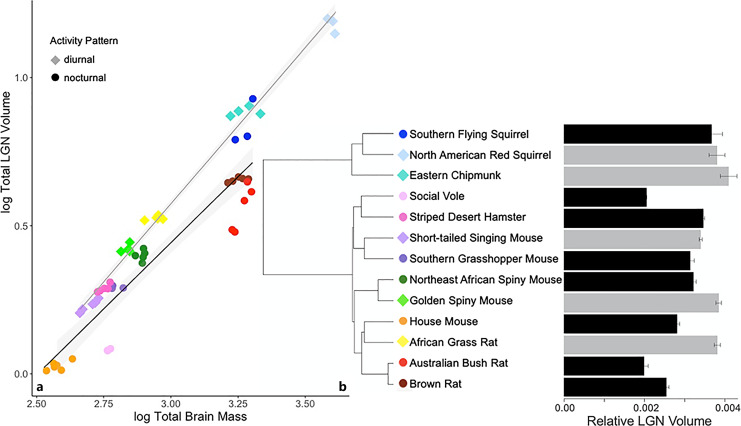
The volume of the LGN ranged from 0.2% of brain mass, in the Australian bush rat, to 0.41% of brain mass in eastern chipmunk (Fig. 6). The LGN showed phylogenetic independence when comparing the different regression models of evolution, with the non-phylogenetic linear model explaining 93.6% of the variation in LGN size (F3,55 = 284.5, p < 0.001). The ANCOVA found that brain size and activity pattern are both significant predictors of LGN volume, and there is also a significant interaction between the two (Table 3). Diurnal species have a larger LGN than nocturnal species.
Fig. 6.
a Log lateral geniculate nucleus (LGN) volume regressed against log brain mass of 13 rodent species. Shading represents 95% confidence intervals. b Mean LGN volume relative to brain mass; error bars are SEM (gray: diurnal, black: nocturnal).
LGN Subregions
Volume of the dLGN ranged from 0.12% of total brain mass in social vole to 0.32% in eastern chipmunk, and volume of the vLGN ranged from 0.07% in Australian bush rat to 0.15% in African grass rat. Both dLGN and vLGN volumes showed phylogenetic independence when comparing the different regression models of evolution, with the non-phylogenetic linear model explaining 89.3% of the variation in dLGN size (F3,53 = 156.5, p < 0.001) and 92.9% of the variation in vLGN size (F3,53 = 245, p < 0.001). The ANCOVA found that brain size and activity pattern are significant predictors of both dLGN and vLGN volumes (Table 3). Diurnal species exhibited significantly larger dLGN and vLGN volumes (relative to brain size) than nocturnal species, the same pattern as seen for the overall size of the LGN.
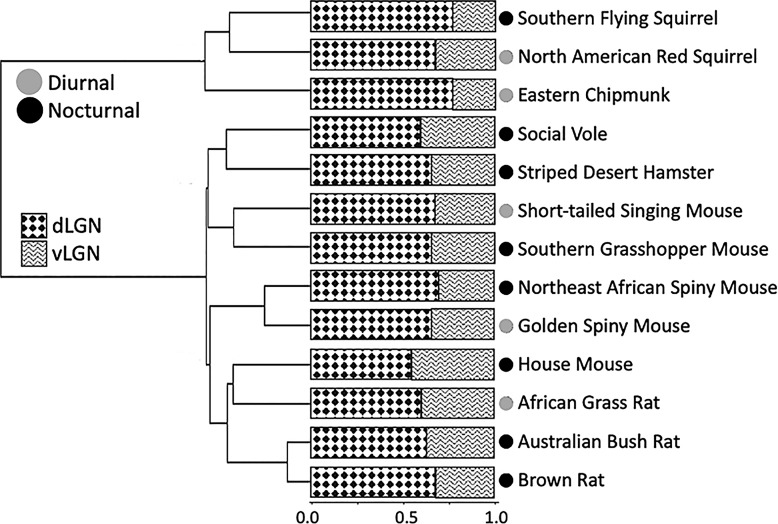
In contrast to the findings for the LGN and its individual subregions, the ratio of vLGN to dLGN did have a significant phylogenetic signal (Table 2) and was not significantly influenced by activity pattern. Both the non-phylogenetic ANOVA (F = 1.20, p = 0.278) and phylogenetic ANOVA (F = 0.44, p = 0.597) found no difference between diurnal and nocturnal species. However, the vLGN/dLGN ratio varied markedly among species (F = 27.64, p < 0.001), ranging from 0.300 in the eastern chipmunk to 0.831 in the house mouse (Fig. 7).
Fig. 7.
Proportion of LGN volume occupied by dLGN and vLGN in 13 rodent species. Phylogenetic relationships and divergence times were established from Fabre et al. [41].
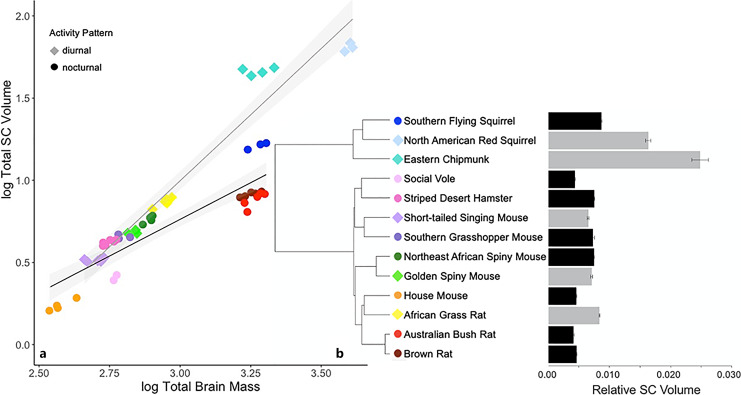
SC
Compared to the LGN, the SC exhibited a greater range of variation in relative size, with two sciurids, the North American red squirrel and eastern chipmunk, exhibiting much larger values for SC than the other species (Fig. 8). The SC showed a strong phylogenetic component, and the Brownian motion model performed best, explaining 78.1% of the variation in SC size (F3,9 = 15.24, p < 0.001). The phylogenetic ANCOVA of the SC showed that brain size and activity pattern are both significant predictors of SC size (Table 3), with diurnal species possessing a larger SC than nocturnal species.
Fig. 8.
a Log superior colliculus (SC) volume regressed against log brain mass of 13 rodent species. Shading represents 95% confidence intervals. b Mean SC volume relative to brain mass; error bars are SEM (gray: diurnal, black: nocturnal).
Auditory System
MGN
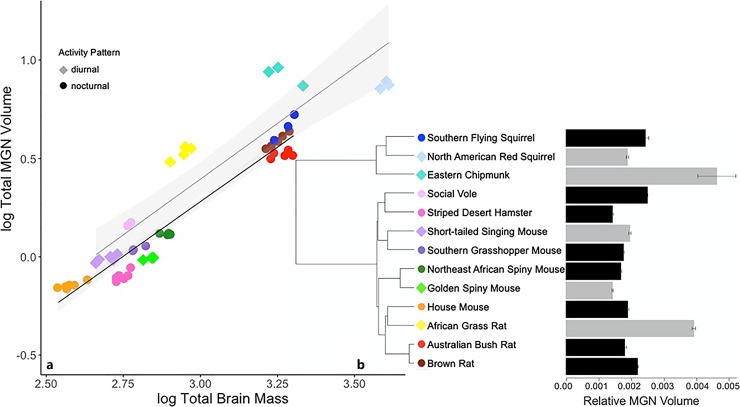
Volume of the MGN ranged from 0.14% of brain mass in the striped desert hamster, to 0.46% of brain mass in the eastern chipmunk (Fig. 9). The non-phylogenetic regression performed best for the MGN data, explaining 85.9% of the variation in MGN size (F3,54 = 115.8, p < 0.001). The ANCOVA results showed that brain size and activity pattern are both statistically significant predictors of MGN size (Table 3), with diurnal species exhibiting a larger MGN than nocturnal species.
Fig. 9.
a Log medial geniculate nucleus (MGN) volume regressed against log brain mass of 13 rodent species. Shading represents 95% confidence intervals. b Mean MGN volume relative to brain mass; error bars are SEM (gray: diurnal, black: nocturnal).
IC
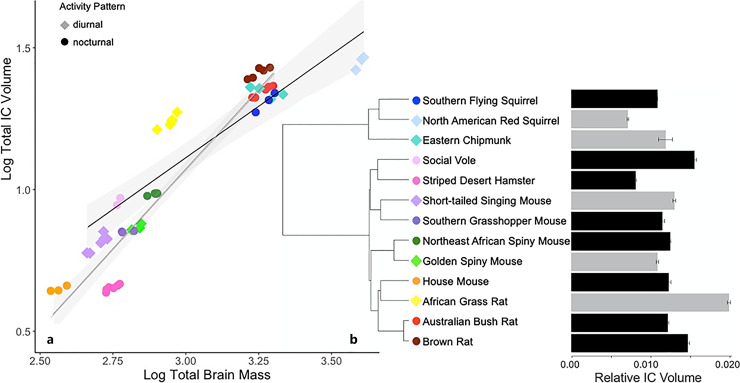
Volume of the IC ranged from 0.71% of brain mass in the North American red squirrel to 1.97% of brain mass in the African grass rat (Fig. 10). The model that best fit the IC size data was a non-phylogenetic regression. That model explained 87.6% of the variation in IC size (F3,50 = 125.6, p < 0.001). The ANCOVA of the IC showed that brain size is a significant predictor of IC size (Table 3). While activity pattern alone is not a significant factor influencing IC size, there is a highly significant interaction between brain size and activity pattern, reflected in the different slopes of the linear regressions.
Fig. 10.
a Log inferior colliculus (IC) volume regressed against log brain mass of 13 rodent species. Shading represents 95% confidence intervals. b Mean IC volume relative to brain mass; error bars are SEM (gray: diurnal, black: nocturnal).
Discussion
The overall size of the brain had the largest impact on the sizes of the sensory regions within it, as expected, however, the sizes of sensory regions were also influenced by temporal niche (Table 3), and there appear to be trade-offs between investment in visual and olfactory regions of the brain. Specifically, nocturnal species had significantly larger OBs than their diurnal counterparts, while diurnal species had larger LGNs and SCs, the two visual areas examined. These findings suggest a mosaic pattern of change that may be influenced by trade-offs in investment in some tissues associated with temporal niche. There was also a significant difference between diurnal and nocturnal species in the size of the MGN, but in a direction that was the reverse of what we predicted. Specifically, the MGN, like the LGN, was larger in diurnal than nocturnal species. This may reflect a tendency for these two thalamic structures to evolve in a concerted manner. Below we discuss some of the issues raised by these data.
Brain Size
A transition to nocturnality is thought to have occurred as mammals evolved from their diurnal synapsid ancestors [12, 83] and with it came a major expansion in brain size [84]. It has been suggested that this temporal niche transition contributed to overall enlargement of the brain in early mammals, as they developed sensory systems that went well beyond those of their ancestors to guide their behavior in the darkness of the night [85]. This raises the question of whether subsequent transitions back to diurnality might have been accompanied by changes in brain size. Our data, collected from animals representing at least four of these transitions, did not find evidence for this in Rodentia, i.e., nocturnal and diurnal species did not differ significantly in brain size relative to body size (Table 3; Fig. 4). However, although unrelated to the size of the brain overall, temporal niche was associated with sizes of different sensory structures within it.
Olfactory Bulbs
OBs, which exhibited a strong phylogenetic signal, were significantly larger in nocturnal than in the diurnal species (Fig. 5). These results are consistent with the Barton et al. [33] findings for insectivores and primates. OBs receive chemical stimuli via olfactory epithelium, which may contain information about the presence of food, predators, and competitors, as well as reproductive condition of a male or female conspecific. Olfactory stimuli can be detected from the three-dimensional world around an animal, and the OB play a role in mapping odorants in space [86]. Unlike visual or auditory cues, chemical ones can last for several days and thus provide information about the recent past as well as the present, and they may do this at all phases of the day-night cycle. The relatively large size of OBs in nocturnal species could reflect a history of more intense selection for animals with abilities of these sorts.
The smallest OBs relative to body size were in the three sciurids. Diurnality was likely present in the first sciurids, which appeared in the fossil record approximately 36 million years ago [58], thus the members of this lineage have had a long time to adapt to a day-active way of life in which the increase in visual information may have made olfactory processing less crucial. The transition back to nocturnality of the flying squirrel lineage approximately 18 mya [58] might be expected to result in selection for increasing OB size. Indeed, the OB of flying squirrels was 47.9% larger than that of the other tree squirrel (i.e., the North American red squirrel), raising the possibility that processing of olfactory information became more important as these animals branched off from other tree squirrels and returned to their ancestral, nocturnal, condition. However, the OBs of the ground-dwelling diurnal sciurid, the eastern chipmunk, were similar to those of the flying squirrel (Fig. 5). The relatively small OBs of the two tree squirrels raises the possibility that the terrestrial (vs. arboreal) lifestyle is a driver of OB evolution. Regardless of how the differences evolved, they suggest that nocturnal species may be able to use olfactory cues more effectively than their diurnal relatives.
Visual System
Lateral Geniculate Nucleus
The relative sizes of the LGN and its constituent subregions were significantly larger in diurnal than nocturnal species. As a part of the visual pathway, the LGN receives direct input from the retina, receives projections from the SC, and projects to the primary visual cortex. This enables animals to extract different kinds of information about the surrounding world from light, such as information about form, distance, location, movement, and reflection of different wavelengths, e.g., [87]. Our data suggest that selection for diurnality in rodents may have been accompanied by increases, to varying degrees, in the ability to use light to obtain such information.
The relative size of the LGN was notably high in the two diurnal squirrels, which is consistent with behavioral evidence that the visual systems of diurnal sciurids are especially well developed, e.g., [88, 89]. The only nocturnal sciurid examined here, the southern flying squirrel, presents an interesting case in that its LGN, though smaller (relative to brain weight) than in diurnal sciurids, is substantially larger than in the other nocturnal species examined here (Fig. 6). This might reflect the history of this lineage, with its relatively recent emergence from diurnal sciurid ancestors approximately 18 mya [58]. Also, visual information may be of greater value to animals that glide than to those that use other forms of locomotion at night. Wavelength information is limited in flying squirrels as they have mutations that have rendered short wavelength sensitive photopigments nonfunctional [90]. The reduction of functional cones may manifest as a diminution of tissue within the LGN where cells involved in wavelength discrimination have been described in primates [91]. Examination of the LGN and sensory behavior in other rodents, both diurnal and nocturnal, are needed to better understand both the differences between flying squirrels and the other nocturnal species examined and between them and other sciurids.
Interestingly, when Finlay et al. [26] analyzed the size of the LGN in 31 species of mammals (5 primates and 26 non-primate species), they saw no overall effect of temporal niche. The differences between those results and ours likely reflect the species examined: their analysis included only five rodents, and only one of those was diurnal. Their results and ours together suggest the possibility of a link between temporal niche and LGN volume that exists in Rodentia but is absent in primates and perhaps other mammals. Additionally, Finlay et al. [26] estimated Pagel’s λ for size of the LGN in 82 mammalian species and found a strong phylogenetic component, whereas no phylogenetic signal was detected in our sample. This may reflect the much broader taxonomic sampling and larger sample size in the Finlay et al. [26] study.
LGN Subregions
We also investigated the contributions of dLGN and vLGN to the overall LGN size difference between diurnal and nocturnal species. Both subregions exhibited patterns of size change similar to that of the total LGN. The significant relationship between the dLGN and diurnality was not unexpected as this region is devoted almost entirely to processing visual information. In addition to interspecific differences in dLGN size (relative to brain size), we observed variation in its organization. Notably, there are visible vertical components in the dLGN of the squirrels that are not apparent in our other species (Fig. 3). Kaas et al. [92] and Van Hooser et al. [93] described a five-layered laminar organization in the gray squirrel (Sciurus carolinensis), different from that of the three-layered dLGN of the albino rat [94]. We cannot compare our results directly to those due to differences in methodology. However, our findings provide additional evidence of a difference between sciuromorph and myomorph rodents in organization of the dLGN.
Like the dLGN, the vLGN (here including the IGL) was larger in diurnal species. The vLGN plays a key role in modulating some effects of photic cues on daily rhythms that differ between nocturnal and diurnal species [66, 95]. Perhaps the vLGN size difference we see contributes to those functional differences. However, it may also be related to visuosensory and visuomotor functions carried out by vLGN regions beyond those of the IGL [96, 97]. Gaillard et al. [98] have described a bilaminar organization in the magnocellular division of the vLGN in the African grass rat, which has also been identified in the hamster (Mesocricetus auratus) [99] and two ground squirrels (Citellus tridecemlineatus; Spermophilus beecheyi) [100, 101], but not yet in other rats or mice. Our histological methods do not allow us to investigate this, but future work examining the fine structure of the vLGN across a larger sampling of rodents might be of value in elucidating factors that impact the overall organization of this structure.
Interestingly, while the relative volumes of the dLGN and vLGN were associated with activity pattern, their ratio was not. Furthermore, there was a moderate phylogenetic signal and significant interspecific variation in this ratio. The reasons for these patterns are unclear. Brauer et al. [39] found that mammals with high levels of neocorticalization have a smaller vLGN/dLGN ratio, which might explain the phylogenetic signal observed here. Further studies of this issue and of other possible factors (e.g., developmental timing, habitat, locomotion, diet), with increased sampling within and between taxonomic groups, are needed to better understand the variation in vLGN to dLGN ratio observed in this study.
Superior Colliculus
The SC, which exhibited a notable phylogenetic effect (Table 2), was significantly larger in our diurnal species compared to the nocturnal ones (Fig. 8). The SC was strikingly large in the diurnal squirrels, which could reflect the long diurnal history of this lineage. The SC receives direct input from the retina, and it provides information about light to the cortex through parallel, though interconnected, output pathways [67]. One of these is via its projection to the LGN; the other is via its projection to the pulvinar complex, which then projects to multiple extra-striate regions of the cortex [102]. The photic information appears to be processed in different ways along these pathways and to serve somewhat different functions. The latter pathway is particularly interesting, considering that reciprocity between the SC and pulvinar is found in some rodents but is not common in other mammals [68, 103]. Fredes et al. [103] suggest that the projections between the SC and pulvinar complex may differ between nocturnal and diurnal species, and this difference may be reflected in the relative size of the superficial layers of the SC.
The fact that both the LGN and SC were larger (relative to brain size) in diurnal species, compared to nocturnal species, could mean these two visual regions are developmentally linked and selection on one leads to changes in the other. If that were the case, we would expect to see species rank similarly (i.e., largest to smallest) in the size of the LGN and SC. While species are similarly ranked, the magnitudes of species’ differences in the sizes of these regions are not consistent. The fact that the SC functions in two parallel visual pathways, whereas the LGN functions in only one, could explain this imbalance. Comparing the size of the pulvinar complex, which is involved in the extrageniculate pathway, could provide clarification. It is also possible that each structure was independently expanded by somewhat different selective pressures that may have arisen because diurnal species live in a world in which light is more important.
Auditory System
Medial Geniculate Nucleus
Diurnal species had a significantly larger MGN than nocturnal species (Fig. 9). The MGN acts to process and relay auditory information between the IC and the auditory cortex; it also receives projections from several auditory nuclei in the brainstem [71]. More specifically, it functions in processing frequency, intensity, and location of sounds [104]. It also acts as a selection filter, as it is the last opportunity for auditory information to be processed before reaching the auditory cortex [105].
Our findings for MGN are of particular interest in that two diurnal species, the eastern chipmunk and African grass rat, exhibited values at least twice those of any other species (Fig. 9). It is unclear why this should be the case, but one possibility is that it reflects the importance of vocal communication in these species. Although the eastern chipmunk is solitary, it is known to be extremely vocal, particularly in the context of territorial communication and alarm calls [106, 107]. The African grass rat is a social species and begins to develop vocal communication very early after birth; African grass rats are known to be highly vocal as adults [108]. While our data do not allow us to evaluate this hypothesis, future studies could test for such an association by comparing components of the auditory system between species with varying levels of communicative complexity.
Inferior Colliculus
The size of the IC was not significantly different between diurnal and nocturnal species, but it was affected by a strong interaction between temporal niche and brain size (Table 3). In species with smaller brains, diurnal species had a larger IC, whereas in species with larger brains, nocturnal species had a larger IC (Fig. 10). The IC organizes inputs from auditory nuclei in the brainstem and projects to the MGN, which in turn projects to the auditory cortex [72]. It is a convergence point in which sensory, motor, and cognitive information are integrated to carry out higherorder auditory functions, such as localizing sounds, distinguishing between important and insignificant sounds, and perceiving and generating vocal communication [109].
The African grass rat had a very large MGN and the largest IC relative to brain size. The IC of the chipmunk, although not as extreme as in the African grass rat, was also notably large (Fig. 9, 10). Both species are highly vocal, but while African grass rats are social and live in colonial burrows [110], eastern chipmunks are solitary, with only one adult individual per burrow [111]. It could be that sociability requires more integration of other forms of information to carry out complex social behaviors.
Variation in the MGN and IC
Another interesting pattern seen in our auditory data is the degree of interspecific variation. Diurnal species showed very high levels of interspecific variation in both the MGN and IC, whereas nocturnal species exhibited much less interspecific variation in both structures (Fig. 9, 10). Could this suggest that there are a greater variety of selection factors acting on auditory systems of animals that are active during the day?
Shelley and Blumstein [112] investigated the relationship between sociality, vocal alarm calls, and diurnality in rodents. They established an association between sociality and diurnality and sociality and alarm calls, but there was a stronger, directional relationship between diurnality and alarm calls. They demonstrated that the evolution of diurnality preceded the evolution of vocal alarm communication in rodents. It has also been established that prey typically alarm call only when there is enough light to detect and track predators [113]. If diurnality is indeed predominantly responsible for the evolution of alarm calls, this suggests a coupling of visual cues with auditory communication. Such coupling could have created selection acting on the auditory system of diurnal animals that is not present, or present to a lesser degree, in night-active animals.
Conclusions
The species differences observed in the sizes of olfactory and visual brain areas support the hypothesis of a trade-off related to temporal niche, specifically that with the evolution of diurnality, and concomitant increase in available visual cues, overall investment in visual processing increases, and reliance on olfactory cues decreases. The brain components of these two sensory modalities appear to have evolved in a segregated manner, separate from other brain structures, which represents a mosaic pattern of evolutionary change. These results support earlier work by Finlay and Darlington [114] and Finlay et al. [115] which found that OBs do not change in concert with other brain structures. It is possible that OBs may have fewer constraints compared to other brain regions.
Our data also suggest that there may be some level of coevolution between the visual and auditory systems. Specifically, diurnality may have encouraged the coevolution of certain types of communicative behaviors that involve both visual and auditory components. While our results support mosaic evolution of specific brain regions, overall brain size did not differ with activity pattern, suggesting that brain size is conserved to some degree. Finlay et al. [115] have shown that the size of larger regions of the brain, such as the neocortex, diencephalon, cerebellum, and medulla, are highly conserved and change in concert with one another. It appears that while larger regions of the brain are constrained, smaller regions within those may show dissociative changes, as we found for some components of the sensory brain examined here. These data add support to the growing body of evidence that mosaic and concerted processes both play a role in brain evolution and that these processes may vary depending on the structures under pressure and the taxa investigated.
The species included in this study ordinarily exhibit either diurnal or nocturnal rhythms. However, interspecific variation in mammals extends well beyond this simple dichotomy. For example, in some species, activity is more evenly distributed across the day and night, while in others, peaks occur at both dawn and dusk. In addition, many species exhibit plasticity in activity pattern in nature or when conditions are experimentally altered in the laboratory, reviewed in [21]. The sensory worlds in which these species operate are highly variable, reflecting a range of rhythm patterns and varying levels of plasticity. An important question for future investigation is how sensory brain evolution is impacted by these factors.
Acknowledgments
We thank Noga Kronfeld-Schor, Stephen Phelps, Ashley Rowe, and Randy Nelson for animals and brains used in this study, and Catherine Lindell and Heather Eisthen for their many helpful comments on an early version of the manuscript. We are also indebted to Michigan State University Museum for loaning traps and trapping gear.
Statement of Ethics
All animals were handled according to protocols approved by the following institutional and regional authorities: American Society of Mammalogists [60], Michigan State University Institutional Animal Care and Use Committee (protocol # 07/16-116-00), Office of Environment and Heritage (License #SL100634), and New South Wales Department of Industry and Investment Animal Research Authority (ORA 14/17/009).
Conflict of Interest Statement
The authors declare no competing interests.
Funding Sources
This research was supported by NSF award DBI-0939454.
Author Contributions
Conceived of and designed study: A.M. and L.S.; animal trapping: A.M., L.S., P.M., and B.L.; brain sectioning, tissue staining, photomicrographs, measurements, and analyzed data: A.M.; and contributed to writing of the manuscript: A.M., B.L., and L.S.
Funding Statement
This research was supported by NSF award DBI-0939454.
Data Availability Statement
The data supporting the findings of this study are available in the online supplementary files (for all online suppl. material, see https://doi.org/10.1159/000538090). Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Refinetti R. The diversity of temporal niches in mammals. Bio Rhythm Res. 2008;39(3):173–92. [Google Scholar]
- 2. Castillo-Ruiz A, Paul MJ, Schwartz WJ. In search of a temporal niche: social interactions. Prog Brain Res. 2012;199:267–80. [DOI] [PubMed] [Google Scholar]
- 3. Helm B, Visser ME, Schwartz W, Kronfeld-Schor N, Gerkema M, Piersma T, et al. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Philos T R Soc B. 2017;372(1734):20160246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meek PD, Zewe F, Falzon G. Temporal activity patterns of the swamp rat (Rattus lutreolus) and other rodents in north-eastern New South Wales, Australia. Aust Mammal. 2012;34(2):223–33. [Google Scholar]
- 5. Ikeda T, Uchida K, Matsuura Y, Takahashi H, Yoshida T, Kaji K, et al. Seasonal and diel activity patterns of eight sympatric mammals in Northern Japan revealed by an intensive camera-trap survey. PLoS One. 2016;11(10):e0163602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutman R, Dayan T. Temporal partitioning: an experiment with two species of spiny mice. Ecology. 2005;86(1):164–73. [Google Scholar]
- 7. Refinetti R. Variability of diurnality in laboratory rodents. J Comp Physiol. 2006;192(7):701–14. [DOI] [PubMed] [Google Scholar]
- 8. Hertel AG, Swenson JE, Bischof R. A case for considering individual variation in diel activity patterns. Behav Ecol. 2017;28(6):1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall MI, Kamilar JM, Kirk EC. Eye shape and the nocturnal bottleneck of mammals. Proc Biol Sci. 2012;279(1749):4962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson SR, Wiens JJ. Out of the dark: 350 million years of conservatism and evolution in diel activity patterns in vertebrates. Evolution. 2017;71(8):1944–59. [DOI] [PubMed] [Google Scholar]
- 11. Maor R, Dayan T, Ferguson-Gow H, Jones KE. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat Ecol Evol. 2017;1(12):1889–95. [DOI] [PubMed] [Google Scholar]
- 12. Gerkema MP, Davies WIL, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc Biol Sci. 2013;280(1765):20130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roll U, Dayan T, Kronfeld-Schor N. On the role of phylogeny in determining activity patterns of rodents. Evol Ecol. 2006;20(5):479–90. [Google Scholar]
- 14. Bennie JJ, Duffy JP, Inger R, Gaston KJ. Biogeography of time partitioning in mammals. Proc Natl Acad Sci USA. 2014;111(38):13727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis DJ, Rasmussen MA. The evolution of cathemerality in primates and other mammals: a comparative and chronoecological approach. Folia Primatol. 2006;77(1–2):178–93. [DOI] [PubMed] [Google Scholar]
- 16. Ankel-Simons F, Rasmussen DT. Diurnality, nocturnality, and the evolution of primate visual systems. Am J Phys Anthropol. 2008;137(S47):100–17. [DOI] [PubMed] [Google Scholar]
- 17. van der Vinne V, Tachinardi P, Riede SJ, Akkerman J, Scheepe J, Daan S, et al. Maximising survival by shifting the daily timing of activity. Ecol Lett. 2019;22(12):2097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Y, Wang H. Convergent evolution of bird-mammal shared characteristics for adapting to nocturnality. Proc Biol Sci. 2019;286(1897):20182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gliwicz J, Dabrowski MJ. Ecological factors affecting the diel activity of voles in a multi-species community. Ann Zool Fenn. 2008;45(4):242–7. [Google Scholar]
- 20. Wu Y, Wang H, Wang H, Feng J. Arms race of temporal partitioning between carnivorous and herbivorous mammals. Sci Rep. 2018;8(1):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: environmental factors. Prog Brain Res. 2012;199:281–304. [DOI] [PubMed] [Google Scholar]
- 22. Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211(Pt 11):1792–804. [DOI] [PubMed] [Google Scholar]
- 23. Chalfin BP, Cheung DT, Muniz JAPC, De Lima Silveira LC, Finlay BL. Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates, and mammals. J Comp Neurol. 2007;504(3):265–74. [DOI] [PubMed] [Google Scholar]
- 24. Yopak KE, Lisney TJ, Darlington RB, Collin SP, Montgomery JC, Finlay BL. A conserved pattern of brain scaling from sharks to primates. Proc Natl Acad Sci USA. 2010;107(29):12946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finlay BL, Hinz F, Darlington RB. Mapping behavioural evolution onto brain evolution: the strategic roles of conserved organization in individuals and species. Philos T R Soc B. 2011;366(1574):2111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finlay BL, Charvet CJ, Bastille I, Cheung DT, Muniz JAPC, de Lima Silveira LC. Scaling the primate lateral geniculate nucleus: niche and neurodevelopment in the regulation of magnocellular and parvocellular cell number and nucleus volume. J Comp Neurol. 2014;522(8):1839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405(6790):1055–8. [DOI] [PubMed] [Google Scholar]
- 28. Safi K, Dechmann DKN. Adaptation of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera). Proc Biol Sci. 2005;272(1559):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corfield JR, Wild JM, Parsons S, Kubke MF. Morphometric analysis of telencephalic structure in a variety of neognath and paleognath bird species reveals regional differences associated with specific behavioral traits. Brain Behav Evol. 2012;80(3):181–95. [DOI] [PubMed] [Google Scholar]
- 30. Montgomery SH, Mundy NI, Barton RA. Brain evolution and development: adaptation, allometry, and constraint. Proc Biol Sci. 2016;283(1838):20160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore JM, DeVoogd TJ. Concerted and mosaic evolution of functional modules in songbird brains. Proc Biol Sci. 2017;284(1854):20170469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Healy S, Guilford T. Olfactory-bulb size and nocturnality in birds. Evolution. 1990;44(2):339–46. [DOI] [PubMed] [Google Scholar]
- 33. Barton RA, Purvis A, Harvey PH. Evolutionary radiation of visual and olfactory brain systems in primates, bats, and insectivores. Philos Trans R Soc Lond B. 1995;348(1326):381–92. [DOI] [PubMed] [Google Scholar]
- 34. Iglesias TL, Dornburg A, Warren D, Wainwright PC, Schmitz L, Economo EP. Eyes wide shut: the impact of dim-light vision on neural investment in marine teleosts. J Evol Biol. 2018;31(8):1082–92. [DOI] [PubMed] [Google Scholar]
- 35. Barton RA. Evolutionary specialization in mammalian cortical structure. J Evol Biol. 2007;20(4):1504–11. [DOI] [PubMed] [Google Scholar]
- 36. Campi KL, Krubitzer L. Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J Comp Neurol. 2010;518(22):4491–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campi KL, Collins CE, Todd WD, Kaas J, Krubitzer L. Comparison of area 17 cellular composition in laboratory and wild-caught rats including diurnal and nocturnal species. Brain Behav Evol. 2011;77(2):116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shuboni-Mulligan DD, Cavanaugh BL, Tonson A, Shapiro EM, Gall AJ. Functional and anatomical variations in retinorecipient brain areas in Arvicanthis niloticus and Rattus norvegicus: implications for the circadian and masking systems. Chronobiol Int. 2019;36(11):1464–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brauer K, Winkelmann E, Nawka S, Strnad W. Comparative volumetric investigations on the lateral geniculate body of mammals. Z Mikrosk-Anat Forsch. 1982;96(3):400–6. [PubMed] [Google Scholar]
- 40. Najdzion J, Wasilewska B, Bogus-Nowakowska K, Rowniak M, Szteyn S, Robak A. A morphometric comparative study of the lateral geniculate body in selected placental mammals: the common shrew, the bank vole, the rabbit, and the fox. Folia Morphol. 2009;68(2):70–8. [PubMed] [Google Scholar]
- 41. Fabre PH, Hautier L, Dimitrov D, Douzery EJP. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol. 2012;12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aschoff J. Circadian activity pattern with two peaks. Ecology. 1966;47(4):657–62. [Google Scholar]
- 43. Muul I. Behavioral and physiological influences on the distribution of the flying squirrel, Glaucomys volans. Miscellaneous Publications, Mus Zoolog Univ Mich. 1968;134(3). [Google Scholar]
- 44. Pauls R. Behavioral strategies relevant to the energy economy of the red squirrel (Tamiasciurus hudsonicus). Can J Zool. 1978;56(7):1519–25. [Google Scholar]
- 45. Elliott L. Social behavior and foraging ecology of the eastern chipmunk (Tamias striatus) in the Adirondack Mountains. Smithson Contrib Zool. 1978;265:1–107. [Google Scholar]
- 46. Haim A, Shanas U, Brandes O, Gilboa A. Suggesting the use of integrated methods for vole population management in alfalfa fields. Integr Zool. 2007;2(3):184–90. [DOI] [PubMed] [Google Scholar]
- 47. Wynne-Edwards KE, Surov AV, Telitzina AY. Differences in endogenous activity within the genus Phodopus. J Mammal. 1999;80(3):855–65. [Google Scholar]
- 48. Hooper ET, Carleton MD. Reproduction, growth and development in two contiguously allopatric rodent species, genus Scotinomys. Univ. of Mich. Miscellaneous Publications; 1975. [Google Scholar]
- 49. O’Farrell MJ. Seasonal activity patterns of rodents in a sagebrush community. J Mammal. 1974;55(4):809–23. [Google Scholar]
- 50. Upham NS, Hafner JC. Do nocturnal rodents in the Great Basin Desert avoid moonlight? J Mammal. 2013;94(1):59–72. [Google Scholar]
- 51. Weber ET, Hohn VM. Circadian activity rhythms in the spiny mouse, Acomys cahirinus. Physiol Behav. 2005;86(4):427–33. [DOI] [PubMed] [Google Scholar]
- 52. Shargal E, Kronfeld-Schor N, Dayan T. Population biology and spatial relationships of coexisting spiny mice (Acomys) in Israel. J Mammal. 2000;81(4):1046–52. [Google Scholar]
- 53. Levy O, Dayan T, Kronfeld-Schor N, Porter WP. Biophysical modeling of the temporal niche: from first principles to the evolution of activity patterns. Am Nat. 2012;179(6):794–804. [DOI] [PubMed] [Google Scholar]
- 54. Robbers Y, Koster EAS, Krijbolder DI, Ruijs A, van Berloo S, Meijer JH. Temporal behaviour profiles of Mus musculus in nature are affected by population activity. Physiol Behav. 2015;139:351–60. [DOI] [PubMed] [Google Scholar]
- 55. Blanchong JA, Smale L. Temporal patterns of activity of the unstriped Nile rat, Arvicanthis niloticus. J Mammal. 2000;81(2):595–9. [Google Scholar]
- 56. Wood DH. The ecology of Rattus fuscipes and Melomys cervinipes (Rodentia: Muridae) in south-east Queensland rain forest. Aust J Zool. 1971;19(4):371–92. [Google Scholar]
- 57. Taylor KD. Range of movement and activity of common rats (Rattus norvegicus) on agricultural land. J Appl Ecol. 1978;15(3):663–77. [Google Scholar]
- 58. Mercer JM, Roth VL. The effects of Cenozoic global change on squirrel phylogeny. Science. 2003;299(5612):1568–72. [DOI] [PubMed] [Google Scholar]
- 59. Burgin CJ, Wilson DE, Mittermeier RA, Rylands AB, Lacher ET Jr, Sechrest W. Illustrated checklist of the mammals of the world. Barcelona: Lynx Edicons; 2020. [Google Scholar]
- 60. Sikes RS; Animal Care and Use Committee of the American Society of Mammalogists . 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal. 2016;97(3):663–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gruneberg H. A ganglion probably belonging to the N. terminalis system in the nasal mucosa of the mouse. Z Anat Entwickl Gesch. 1973;140(1):39–52. [PubMed] [Google Scholar]
- 62. Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24(38):8383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70(3):245–318. [DOI] [PubMed] [Google Scholar]
- 64. Baldwin MK, Wong P, Reed JL, Kaas JH. Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): evidence for four subdivisions within the pulvinar complex. J Comp Neurol. 2011;519(6):1071–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Horng S, Kreiman G, Ellsworth C, Page D, Blank M, Millen K, et al. Differential gene expression in the developing lateral geniculate nucleus and medial geniculate nucleus reveals novel roles for Zic4 and Foxp2 in visual and auditory pathway development. J Neurosci. 2009;29(43):13672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav R. 1997;21(5):705–27. [DOI] [PubMed] [Google Scholar]
- 67. May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–78. [DOI] [PubMed] [Google Scholar]
- 68. Deichler A, Carrasco D, Gonzalez-Cabrera C, Letelier JC, Marin G, Mpodozis J. The nucleus pretectalis principalis: a pretectal structure hidden in the mammalian thalamus. J Comp Neurol. 2019;527(2):372–91. [DOI] [PubMed] [Google Scholar]
- 69. Gaese BH, Johnen A. Coding for auditory space in the superior colliculus of the rat. Eur J Neurosci. 2000;12(5):1739–52. [DOI] [PubMed] [Google Scholar]
- 70. McHaffie JG, Kao C, Stein BE. Nociceptive neurons in rat superior colliculus: response properties, topography, and functional implications. J Neurophysiol. 1989;62(2):510–25. [DOI] [PubMed] [Google Scholar]
- 71. Hu B, Senatorov V, Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. J Physiol. 1994;479(Pt 2):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Winer JA, Schreiner CE. The inferior colliculus. York, NY: Springer+Business Media, Inc. New; 2005. [Google Scholar]
- 73. Najdzion J, Wasilewska B, Rowniak M, Bogus-Nowakowska K, Szteyn S, Robak A. A morphometric comparative study of the medial geniculate body of the rabbit and the fox. Anat Histol Embryol. 2011;40(5):326–34. [DOI] [PubMed] [Google Scholar]
- 74. Kulesza RJ, Vinuela A, Saldana E, Berrebi AS. Unbiased stereological estimates of neuron number in subcortical auditory nuclei of the rat. Hear Res. 2002;168(1–2):12–24. [DOI] [PubMed] [Google Scholar]
- 75. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. San Diego, CA: Academic Press; 2014. [Google Scholar]
- 76. Herculano-Houzel S, Ribeiro P, Campos L, Valotta da Silva A, Torres LB, Catania KC, et al. Updated neuronal scaling rules for the brains of glires (Rodents/Lagomorphs). Brain Behav Evol. 2011;78(4):302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. RStudio Team . RStudio. Boston, MA: Integrated Development for R. RStudio, PBC; 2020. Available from: http://www.rstudio.com/. [Google Scholar]
- 78. Revell LJ. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 2012;3(2):217–23. [Google Scholar]
- 79. Ho LST, Ane C. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst Biol. 2014;63(3):397–408. [DOI] [PubMed] [Google Scholar]
- 80. Harmon LJ. Phylogenetic comparative methods. 2019. [Google Scholar]
- 81. Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, et al. Caper: comparative analyses of phylogenetics and evolution in R; 2018. [Google Scholar]
- 82. Munkemuller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, et al. How to measure and test phylogenetic signal. Methods Ecol Evol. 2012;3(4):743–56. [Google Scholar]
- 83. Davis DD, Walls GL. The vertebrate eye (and its adaptive radiation). J Mammal. 1942;23(4):453. [Google Scholar]
- 84. Jerison HJ. Basic selection pressures for enlarged brains. In: Jerison HJ, editor. Evolution of the brain and intelligence. Academic Press; 1973. p. 256–81. [Google Scholar]
- 85. Jerison HJ. On theory in comparative psychology. In: Sternberg RJ, Kaufman JC, editors. The evolution of intelligence. Lawrence Erlbaum Associates Publishers; 2002. p. 251–88. [Google Scholar]
- 86. Jacobs LF. From chemotaxis to the cognitive map: the function of olfaction. P Natl Acad Sci. 2012;109(Suppl 1):10693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Glickfeld LL, Reid RC, Andermann ML. A mouse model of higher visual cortical function. Curr Opin Neurobiol. 2014;24(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jacobs GH, Birch DG, Blakeslee B. Visual acuity and spatial contrast sensitivity in tree squirrels. Behav Process. 1982;7(4):367–75. [DOI] [PubMed] [Google Scholar]
- 89. Van Hooser SD, Nelson SB. The squirrel as a rodent model of the human visual system. Vis Neurosci. 2006;23(5):765–78. [DOI] [PubMed] [Google Scholar]
- 90. Carvalho LS, Cowing JA, Wilkie SE, Bowmaker JK, Hunt DM. Shortwave visual sensitivity in tree and flying squirrels reflects changes in lifestyle. Curr Biol. 2006;16(3):R81–R83. [DOI] [PubMed] [Google Scholar]
- 91. De Valois RL, Abramov I. Color Vision. Annu Rev Neurosci. 1966;17:337–62. [DOI] [PubMed] [Google Scholar]
- 92. Kaas JH, Hall WC, Diamond IT. Visual cortex of the grey squirrel (Sciurus carolinensis): architectonic subdivisions and connections from the visual thalamus. J Comp Neurol. 1972;145(3):273–305. [DOI] [PubMed] [Google Scholar]
- 93. Van Hooser SD, Heimel JAF, Nelson SB. Receptive field properties and laminar organization of the lateral geniculate nucleus in the Gray Squirrel (Sciurus carolinensis. J Neurophysiol. 2003;90(5):3398–418. [DOI] [PubMed] [Google Scholar]
- 94. Montero VM, Brugge JF, Beitel RE. Relation of the visual field to the lateral geniculate body of the albino rat. J Neurophys. 1968;31(2):221–36. [DOI] [PubMed] [Google Scholar]
- 95. Brock O, Gelegen C, Sully P, Salgarella I, Jager P, Menage L, et al. A role for thalamic projection GABAergic neurons in circadian responses to light. J Neurosci. 2022;42(49):9158–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Conley M, Friederich-Ecsy B. Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): II. Connections with the cortex, thalamus, and brainstem. J Comp Neurol. 1993;328(1):21–42. [DOI] [PubMed] [Google Scholar]
- 97. Livingston CA, Fedder SR. Visual-ocular motor activity in the macaque pregeniculate complex. J Neurophysiol. 2003;90(1):226–44. [DOI] [PubMed] [Google Scholar]
- 98. Gaillard F, Karten HJ, Sauve Y. Retinorecipient areas in the diurnal Murine rodent Arvicanthis niloticus: a disproportionally large superior colliculus. J Comp Neurol. 2013;521(8):Spc1–1726. [DOI] [PubMed] [Google Scholar]
- 99. Frost DO, So KF, Schneider GE. Postnatal development of retinal projections in Syrian hamsters: a study using autoradiographic and anterograde degeneration techniques. Neuroscience. 1979;4(11):1649–77. [DOI] [PubMed] [Google Scholar]
- 100. Agarwala S, Petry HM, May JG III. Retinal projections in the ground squirrel (Citellus tridecimlineatus). Vis Neurosci. 1989;3(6):537–49. [DOI] [PubMed] [Google Scholar]
- 101. Major DE, Rodman HR, Libedinsky C, Karten HJ. Pattern of retinal projections in the California ground squirrel (Spermophilus beecheyi): anterograde tracing study using cholera toxin. J Comp Neurol. 2003;463(3):317–40. [DOI] [PubMed] [Google Scholar]
- 102. Baldwin MKL, Balaram P, Kaas JH. The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol. 2017;525(15):3207–26. [DOI] [PubMed] [Google Scholar]
- 103. Fredes F, Vega-Zuniga T, Karten H, Mpodozis J. Bilateral and ipsilateral ascending tectopulvinar pathways in mammals: a study in the squirrel (Spermophilus beecheyi). J Comp Neurol. 2012;520(8):1800–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Winer JA, Morest DK. The neuronal architecture of the dorsal division of the medial geniculate body of the cat. A study with the rapid Golgi method. J Comp Neurol. 1983;221:1–30. [DOI] [PubMed] [Google Scholar]
- 105. Blundon JA, Zakharenko SS. Presynaptic gating of postsynaptic synaptic plasticity: a plasticity filter in the adult auditory cortex. Neuroscientist. 2013;19(5):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Burke da Silva K, Mahan C, Da Silva J. The trill of the chase: eastern chipmunks call to warn kin. J Mammal. 2002;83(2):546–52. [Google Scholar]
- 107. Baack J, Switzer P. Alarm calls affect foraging behavior in Eastern Chipmunks (Tamias striatus). Ethology. 2000;106(12):1057–66. [Google Scholar]
- 108. Delany M, Monro R. Growth and development of wild and captive Nile rats, Arvicanthis niloticus (Rodentia: Muridae). Afr J Ecol. 1985;23(2):121–31. [Google Scholar]
- 109. Gruters KG, Groh JM. Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus. Front Neural Circuit. 2012;6:96–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Senzota RBM. Activity patterns and social behaviors of the grass rats (Arvicanthis niloticus (desmarest)) in the serengeti national park, Tanzania. Trop Ecol. 1990;31(2):35–40. [Google Scholar]
- 111. Snyder DP. Tamias striatus. Mammalian species. 1982. 168:1–8. [Google Scholar]
- 112. Shelley EL, Blumstein DT. The evolution of vocal alarm communication in rodents. Behav Ecol. 2005;16(1):169–77. [Google Scholar]
- 113. Blumstein DT, Armitage KB. Alarm calling in yellow-bellied marmots: I. The meaning of situationally variable alarm calls. Anim Behav. 1997;53(1):143–71. [Google Scholar]
- 114. Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268(5217):1578–84. [DOI] [PubMed] [Google Scholar]
- 115. Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;24(2):263–78. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the online supplementary files (for all online suppl. material, see https://doi.org/10.1159/000538090). Further inquiries can be directed to the corresponding author.