Abstract
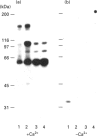
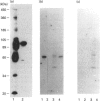
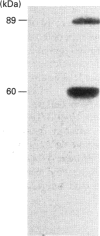
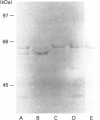
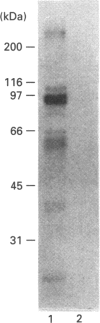
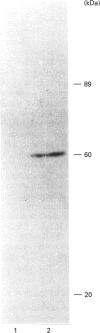
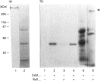
Activation of a calmodulin (CaM)-dependent protein kinase associated with rabbit skeletal-muscle sarcoplasmic reticulum (SR) results in the phosphorylation of polypeptides of 450, 360, 165, 105, 89, 60, 34 and 20 kDa. Radioligand-binding studies indicated that a membrane-bound 60 kDa polypeptide contained both CaM- and ATP-binding domains. Under renaturing conditions on nitrocellulose blots, the 60 kDa polypeptide of the membrane exhibited CaM-dependent autophosphorylation activity, suggesting that it was the CaM-dependent protein kinase of SR. Ca2+/CaM-independent autophosphorylation of polypeptides of 62 and 45 kDa was found to occur in the light SR, whereas the Ca2+/CaM-dependent autophosphorylation activity was enriched in the heavy SR. Both these kinase activities were absent from transverse tubules, although these membranes were enriched in CaM-binding polypeptides of 160, 100 and 80 kDa. In the absence of Ca2+, CaM bound to a 33 kDa polypeptide of the membrane. The purified ryanodine receptor was not phosphorylated by the purified CaM kinase, although it was a substrate for protein kinase C. Affinity-purified antibodies to brain CaM kinase II cross-reacted with the 60 kDa polypeptide in Western blots and immunoprecipitated the 60 kDa polypeptide, along with the 360, 105, 89, 34 and 20 kDa phosphoproteins, from Nonidet-P-40-solubilized SR membranes. Antibodies raised against the 60 kDa kinase polypeptide did not cross-react with the other phosphoproteins, suggesting that these polypeptides were distinct and unrelated. Subcellular distribution of the 60 kDa kinase indicated the specific association of the polypeptide with the junctional-face membrane of SR. The CaM-dependent incorporation of 32P into various membrane proteins was inhibited by the CaM kinase II fragment (290-309), with an IC50 value of 2 nM for the inhibition of incorporation into the 60 kDa kinase polypeptide. Recent studies [Wang and Best (1992) Nature (London) 359, 739-741] have shown that a CaM kinase activity intrinsic to the membrane can inactivate the Ca(2+)-release channel of skeletal muscle SR. Since our results demonstrate that the 60 kDa polypeptide of SR is a CaM-dependent protein kinase, we suggest that this kinase, through its associations, may be responsible for gating the Ca(2+)-release channel.
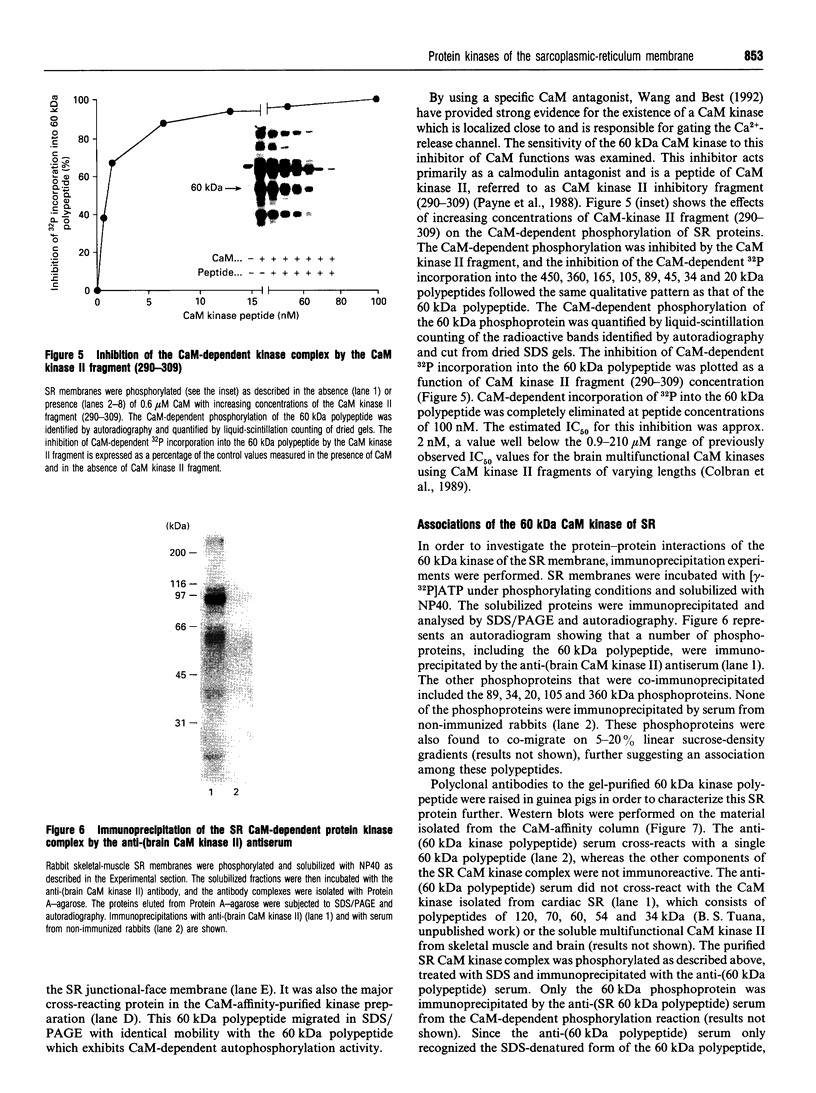
Full text
PDF





Images in this article
Selected References
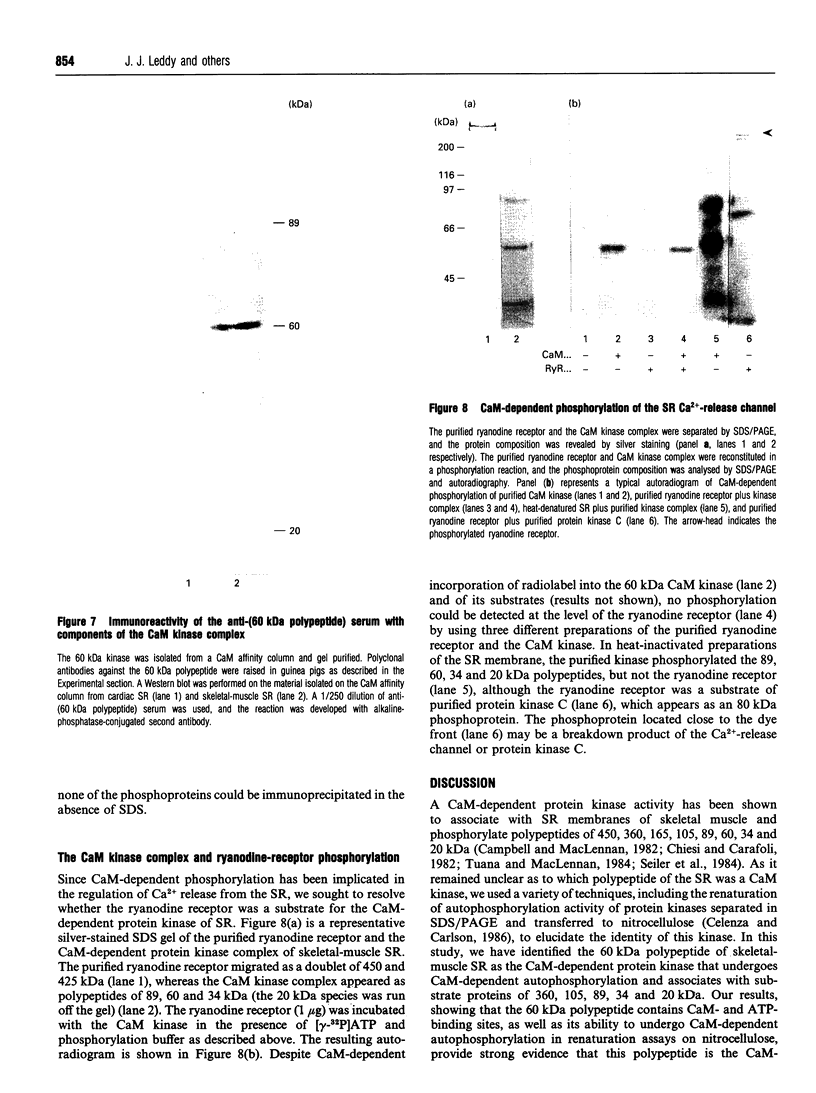
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Phosphorylation of a 60,000-dalton protein. J Biol Chem. 1982 Feb 10;257(3):1238–1246. [PubMed] [Google Scholar]
- Campbell K. P., Shamoo A. E. Phosphorylation of heavy sarcoplasmic reticulum vesicles: identification and characterization of three phosphorylated proteins. J Membr Biol. 1980 Oct 31;56(3):241–248. doi: 10.1007/BF01869479. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991 Mar 8;64(5):871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Carafoli E. Role of calmodulin in skeletal muscle sarcoplasmic reticulum. Biochemistry. 1983 Feb 15;22(4):985–993. doi: 10.1021/bi00273a043. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Carafoli E. The regulation of Ca2+ transport by fast skeletal muscle sarcoplasmic reticulum. Role of calmodulin and of the 53,000-dalton glycoprotein. J Biol Chem. 1982 Jan 25;257(2):984–991. [PubMed] [Google Scholar]
- Chu A., Saito A., Fleischer S. Preparation and characterization of longitudinal tubules of sarcoplasmic reticulum from fast skeletal muscle. Arch Biochem Biophys. 1987 Oct;258(1):13–23. doi: 10.1016/0003-9861(87)90317-1. [DOI] [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Inesi G., Jay S. D., Campbell K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990 Jun 26;29(25):5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Schworer C. M., Hashimoto Y., Fong Y. L., Rich D. P., Smith M. K., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Biochem J. 1989 Mar 1;258(2):313–325. doi: 10.1042/bj2580313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello B., Chadwick C., Fleischer S. Isolation of the junctional face membrane of sarcoplasmic reticulum. Methods Enzymol. 1988;157:46–50. doi: 10.1016/0076-6879(88)57067-2. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C. Regulation of calcium channel activity by GTP binding proteins and second messengers. Biochim Biophys Acta. 1991 Jan 10;1091(1):68–80. doi: 10.1016/0167-4889(91)90224-l. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Murphy B. J., Tuana B. S. Modification of a discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide gel system for minigel electrophoresis. Anal Biochem. 1990 Nov 1;190(2):209–211. doi: 10.1016/0003-2697(90)90182-9. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Tuana B. S. Identification of low molecular weight GTP-binding proteins and their sites of interaction in subcellular fractions from skeletal muscle. J Biol Chem. 1991 Sep 15;266(26):17613–17620. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983 Apr;4(2):233–252. doi: 10.1007/BF00712033. [DOI] [PubMed] [Google Scholar]
- Fujii J., Maruyama K., Tada M., MacLennan D. H. Expression and site-specific mutagenesis of phospholamban. Studies of residues involved in phosphorylation and pentamer formation. J Biol Chem. 1989 Aug 5;264(22):12950–12955. [PubMed] [Google Scholar]
- Gechtman Z., Orr I., Shoshan-Barmatz V. Involvement of protein phosphorylation in activation of Ca2+ efflux from sarcoplasmic reticulum. Biochem J. 1991 May 15;276(Pt 1):97–102. doi: 10.1042/bj2760097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard M. J., Dent P., Smythe C., Cohen P. Targetting of protein phosphatase 1 to the sarcoplasmic reticulum of rabbit skeletal muscle by a protein that is very similar or identical to the G subunit that directs the enzyme to glycogen. Eur J Biochem. 1990 Apr 30;189(2):243–249. doi: 10.1111/j.1432-1033.1990.tb15483.x. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörl W. H., Heilmeyer L. M., Jr Evidence for the participation of a Ca2+-dependent protein kinase and protein phosphatase in the regulation of the Ca2+ transport ATPase of the sarcoplasmic reticulum. 2. Effect of phosphorylase kinase and phosphorylase phosphatase. Biochemistry. 1978 Mar 7;17(5):766–772. doi: 10.1021/bi00598a002. [DOI] [PubMed] [Google Scholar]
- James P., Inui M., Tada M., Chiesi M., Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989 Nov 2;342(6245):90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Kanaseki T., Ikeuchi Y., Sugiura H., Yamauchi T. Structural features of Ca2+/calmodulin-dependent protein kinase II revealed by electron microscopy. J Cell Biol. 1991 Nov;115(4):1049–1060. doi: 10.1083/jcb.115.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Ikemoto N. Involvement of 60-kilodalton phosphoprotein in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1986 Sep 5;261(25):11674–11679. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels in neurons and other cells. Annu Rev Neurosci. 1988;11:119–136. doi: 10.1146/annurev.ne.11.030188.001003. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol Sci. 1990 Mar;11(3):107–111. doi: 10.1016/0165-6147(90)90195-e. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Campbell K. P., Takisawa H., Tuana B. S. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:393–401. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Jan 14;25(1):244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation of sarcoplasmic reticulum from skeletal muscle. Methods Enzymol. 1974;31:238–246. doi: 10.1016/0076-6879(74)31025-7. [DOI] [PubMed] [Google Scholar]
- Meyer T., Hanson P. I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992 May 22;256(5060):1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Mitchell R. D., Palade P., Fleischer S. Purification of morphologically intact triad structures from skeletal muscle. J Cell Biol. 1983 Apr;96(4):1008–1016. doi: 10.1083/jcb.96.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii H., Takisawa H., Yamamoto T. A possible role of protein phosphorylation in the inactivation of a Ca2+-induced Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biochem. 1987 Aug;102(2):263–271. doi: 10.1093/oxfordjournals.jbchem.a122050. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Nishimoto I., Wagner J. A., Schulman H., Gardner P. Regulation of Cl- channels by multifunctional CaM kinase. Neuron. 1991 Apr;6(4):547–555. doi: 10.1016/0896-6273(91)90057-7. [DOI] [PubMed] [Google Scholar]
- Orr I., Gechtman Z., Shoshan-Barmatz V. Characterization of Ca(2+)-dependent endogenous phosphorylation of 160,000- and 150,000-Dalton proteins of sarcoplasmic reticulum. Biochem J. 1991 May 15;276(Pt 1):89–96. doi: 10.1042/bj2760089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Schulman H., Lou L. L. Multifunctional Ca2+/calmodulin-dependent protein kinase: domain structure and regulation. Trends Biochem Sci. 1989 Feb;14(2):62–66. doi: 10.1016/0968-0004(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Seiler S., Wegener A. D., Whang D. D., Hathaway D. R., Jones L. R. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease. J Biol Chem. 1984 Jul 10;259(13):8550–8557. [PubMed] [Google Scholar]
- Shoshan-Barmatz V. Activation of Ca2+ release in isolated sarcoplasmic reticulum. J Membr Biol. 1988 Jul;103(1):67–77. doi: 10.1007/BF01871933. [DOI] [PubMed] [Google Scholar]
- Tada M., Kadoma M., Inui M., Fujii J. Regulation of Ca2+-pump from cardiac sarcoplasmic reticulum. Methods Enzymol. 1988;157:107–154. doi: 10.1016/0076-6879(88)57073-8. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Furukawa K., Ogurusu T., Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991 Jan;109(1):163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuana B. S., MacLennan D. H. Calmidazolium and compound 48/80 inhibit calmodulin-dependent protein phosphorylation and ATP-dependent Ca2+ uptake but not Ca2+-ATPase activity in skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1984 Jun 10;259(11):6979–6983. [PubMed] [Google Scholar]
- Tuana B. S., MacLennan D. H. Isolation of the calmodulin-dependent protein kinase system from rabbit skeletal muscle sarcoplasmic reticulum. FEBS Lett. 1988 Aug 1;235(1-2):219–223. doi: 10.1016/0014-5793(88)81266-3. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Jones L. R. Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J Biol Chem. 1984 Feb 10;259(3):1834–1841. [PubMed] [Google Scholar]
- Williams A. J. Ion conduction and discrimination in the sarcoplasmic reticulum ryanodine receptor/calcium-release channel. J Muscle Res Cell Motil. 1992 Feb;13(1):7–26. doi: 10.1007/BF01738423. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]
- Witcher D. R., Strifler B. A., Jones L. R. Cardiac-specific phosphorylation site for multifunctional Ca2+/calmodulin-dependent protein kinase is conserved in the brain ryanodine receptor. J Biol Chem. 1992 Mar 5;267(7):4963–4967. [PubMed] [Google Scholar]
- Woodgett J. R., Davison M. T., Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983 Nov 15;136(3):481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]