Abstract
Objective:
To compare positron emission tomography (PET)/magnetic resonance imaging (MRI) to the standard of care imaging (SCI) for the diagnosis of peritoneal carcinomatosis (PC) in primary abdominopelvic malignancies.
Summary Background Data:
Identifying PC impacts prognosis and management of multiple cancer types.
Methods:
Adult subjects were prospectively and consecutively enrolled from April 2019 to January 2021. Inclusion criteria were: 1) acquisition of whole-body contrast-enhanced (CE) 18F-fluorodeoxyglucose PET/MRI, 2) pathologically confirmed primary abdominopelvic malignancies. Exclusion criteria were: 1) greater than 4 weeks interval between SCI and PET/MRI, 2) unavailable follow-up. SCI consisted of whole-body CE PET/computed tomography (CT) with diagnostic quality CT, and/or CE-CT of the abdomen and pelvis, and/or CE-MRI of the abdomen±pelvis. If available, pathology or surgical findings served as the reference standard, otherwise, imaging followup was used. When SCI and PET/MRI results disagreed, medical records were checked for management changes. Follow-up data were collected until August 2021.
Results:
One hundred sixty-four subjects were included, 85 (52%) were female, and the median age was 60 years (interquartile range 50–69). At a subject level, PET/MRI had higher sensitivity (0.97, 95% CI 0.86–1.00) than SCI (0.54, 95% CI 0.37–0.71), P < 0.001, without a difference in specificity, of 0.95 (95% CI 0.90–0.98) for PET/MRI and 0.98 (95% CI 0.93–1.00) for SCI, P ¼ 0.250. PET/MRI and SCI results disagreed in 19 cases. In 5/19 (26%) of the discordant cases, PET/MRI findings consistent with PC missed on SCI led to management changes.
Conclusion:
PET/MRI improves detection of PC compared with SCI which frequently changes management.
Keywords: contrast-enhanced, FDG, peritoneal metastases, peritoneal carcinomatosis, PET/MRI, whole-body
Peritoneal carcinomatosis (PC) is the malignant involvement of the peritoneum from a primary cancer, which can occur in many abdominopelvic tumors and is associated with a decreased overall survival.1,2 The identification of peritoneal metastases may change management in several manners, such as reconsidering regional therapy for hepatic metastases, introducing systemic chemotherapy in the face of recurrent disease, changing the operative strategy, considering a palliative/noncurative pathway, or opting for cytoreduc-tive surgery and/or peritoneal chemotherapy.3–5 Imaging plays a key role in the preoperative detection of peritoneal metastases. The imaging patterns suggestive of PC vary, ranging from subtle nodular stranding to overt masses. Differential diagnoses for such findings include inflammation, infection, trauma, and developmental disorders.6
To date, there is no established imaging reference standard, and direct peritoneal visualization at laparotomy or laparoscopy remains the most reliable method for the diagnosis of PC.1 The diagnostic performance of contrast-enhanced (CE) computed tomography (CT), 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/ CT, and magnetic resonance imaging (MRI) for PC has been studied predominantly in subjects with primary gastrointestinal and gynecologic malignancies.7–15 A recent meta-analysis found a similar performance of PET/CT and MRI, noting improved sensitivity of both modalities compared with CE-CT.16
There are limited data regarding the use of FDG-PET/MRI in the evaluation of peritoneal metastases, with preliminary studies finding improved delineation of the extent of peritoneal carcinoma-tosis compared with both FDG-PET/CT and MRI.17,18 This study aimed to compare the diagnostic performance of FDG-PET/MRI to the standard of care imaging (SCI) encompassing CE-CT, CE-FDG-PET/CT, or CE-MRI in the detection of peritoneal metastases.
METHODS
Subject Selection
This study was approved by the institutional review board (protocols 2019P000410/2020P001367), and written informed consent was obtained. Adult subjects with pathologically proven primaryabdominopelvic cancers and prior SCI were enrolled as they were referred for whole-body CE-FDG-PET/MRI by their treating providers from April 1, 2019 to January 31, 2021. Exclusion criteria were: 1) the interval between the preceding SCI and PET/MRI exceeded 4 weeks, 2) pathology, laparoscopy, or follow-up imaging results were not available. Additionally, for subjects who underwent more than 1 PET/MRI during the study period, only the first pair of PET/MRI and SCI were evaluated to assure the independence of observations.
PET/MRI Acquisition
Simultaneous, whole-body CE-FDG-PET/MRI was acquired in a hybrid 3.0T Biograph mMR scanner (Siemens Healthineers, Erlangen, Germany). Subjects fasted for at least 6 hours and had their blood glucose levels checked before the examination to ensure it was less than 140 mg/dL. If glucose levels were higher than this threshold, the scan was rescheduled. Subjects were invited to void before the scan to avoid FDG accumulation in the bladder. The injected FDG activity was 4.5 MBq/Kg, with an incubation time of 1 hour.
According to the patient’s body habitus, 4 to 5 bed positions were acquired to scan the whole body. The PET/MRI protocol included the following precontrast whole-body sequences, acquired from the base of the neck to the mid-thighs: axial T2-weighted single-shot fast spinecho, axial simultaneous multi-slice free-breathing diffusion-weighted images (DWI) with b-values of 50 and 800 s/mm2 and the derived computed apparent diffusion coefficient maps, axial T1-weighted gradient-echo Dixon sequences. Dedicated pre-and postcontrast upper abdominal sequences were also acquired: axial T1-weighted in and out of phase gradient-echo, coronal T2-weighted single-shot fast spinecho, axial T2-weighted fat-suppressed fast spinecho, axial T1-weighted fat-saturated gradient-echo before and after contrast injection in arterial, portal, and delayed phases. A dose of 0.1 mL/kg body weight of gadoxetate disodium (Eovist, Bayer Health-Care Pharmaceuticals Inc, Wayne, NJ), or gadobutrol (Gadavist, Bayer HealthCare Pharmaceuticals Inc, Wayne, NJ) or gadoterate (Dotarem, Guerbet LLC, Princeton, NJ), was injected as contrast media. Dedicated pelvic sequences (axial, sagittal, and coronal T2 weighted highresolution fast spinecho) were also acquired in the presence of a known pelvic primary tumor. Finally, late (10–15 minutes after contrast injection) 3 mm thick CE T1-weighted fat-saturated gradientecho sequences in the axial and coronal planes of the whole-body were acquired (for more technical details regarding the protocol, please refer to supplemental digital Table 1.1, http://links.lww.com/SLA/D689). The costs of the PET/MRI were covered by institutional funds and by an industry sponsor (Bayer Healthcare).
PET/MRI Image Analysis
The PET/MRI images were evaluated in consensus by 2 board-certified radiologists (S.A.E. and O.A.C.), with 4 and 9 years of expertise in PET/MRI, respectively. The readers were blinded to SCI images and/or reports. Additionally, PET/MRI and SCI for the same patient were read at least 4 weeks apart to reduce recall bias. PET/MRI findings of PC were assessed on a subject level, regardless of the number of detected lesions, based on specific findings of peritoneal involvement according to the criteria described bellow.
Positive PET/MRI findings were classified according to MRI features-nodular thickening/enhancement, linear thickening/enhancement, diffuse thickening/enhancement; and/or PET imaging features—focal FDG uptake, diffuse peritoneal FDG uptake, and linear FDG uptake. Peritoneal enhancement was assessed in the late whole-body images acquired 10 to 15 minutes after contrast injection and was considered pathologic when the peritoneum was subjectively enhancing more than the pleura as seen in the left posterior costophrenic sulcus.
Nodular thickening/enhancement was characterized by any peritoneal nodularity with high signal intensity on DWI or late postcontrast T1 sequences that was not perceived as fat necrosis or vascular ectasia. Linear thickening/enhancement corresponded to short segment thickening and/or enhancement of the peritoneum on DWI or late postcontrast T1 whole-body acquisitions. If the same kind of involvement was observed diffusely throughout the upper and/or lower abdomen, it was considered diffuse thickening/enhancement. PET features were described as increased FDG accumulation (ie, more than background liver/mediastinal uptake) focally (focal), smoothly over a small portion of the abdomen or pelvis (linear), or extensively (diffuse).
To ascertain that any difference of performance, if observed, was truly due to the simultaneous PET/MRI acquisition rather than the simple combination of PET and MRI information, PET and MRI images were separately reviewed at least 4 weeks apart. The MRI sequences were also divided into CE-MRI (delayed axial and coronal whole-body T1 weighted volumetric interpolated breath-hold examinations) and non-CE-MRI (whole-body T2-weighted SSFSE and DWI, non-CE upper abdomen and pelvis sequences), to determine the role of contrast administration. At first, one set of unlabeled PET, non-CE MRI, or CE-MRI components was read in a random order for the whole cohort. The process was repeated for the 2 remaining image sets for each subject. The reviewers were blinded to the other images in all instances. Lastly, the reviewers analyzed all the unlabeled fused PET/MRI images, this time with access to all acquired sequences, including the CE ones. The detection of PC in stand-alone PET, standalone non-CE-MRI, stand-alone CE-MRI, and fused images was assessed to determine the differences in sensitivity and/or specificity following contrast administration and/or image fusion.
Management Changes
All the subjects were followed until August 31, 2021 to assess whether any new clinical information yielded by the PET/MRI reading, at the time of acquisition, led to changes in management. This was evaluated through the review of the provider notes in the subject’s electronic medical records. Management changes were defined as a change in the goals of care (ie, curative intent to palliative intent), change in radiation therapy or systemic therapy regimens, cancellation of previously planned surgeries, or the scheduling of new surgeries due to unforeseen lesions. Management changes were attributed to PET/MRI if the assessment/plan developed by the treating team explicitly cited PET/MRI imaging findings not seen on SCI to justify the rationale.
Standard of Care Imaging
The SCI consisted of whole-body CE-PET/CT with diagnostic-quality (ie, with standard radiation dose) CT and/or CE-CT of the abdomen and pelvis, and/or abdominal CE-MRI (or abdominal and pelvic MRI according to primary tumor site). SCI studies were evaluated by the same 2 board-certified radiologists in consensus, who were blinded to PET/MRI images and/or reports. For each patient, SCI and PET/MRI were assessed at least 4weeks apart. When SCI included more than 1 cross-sectional imaging study, they were analyzed together in the same session, and findings were considered positive if PC was detected in any of them, and negative when none reported compatible findings.
For SCI, PC was considered positive or negative on a subject level, according to standard published criteria, regardless of the number of peritoneal metastases detected, as reported in the literature.1,19
Reference Standard
The reference standard for PC diagnosis was determined via pathology report from tissue sampling or surgical specimens, when available. Subjects who underwent laparoscopy without sampling were also considered positive if the operative note described a macroscopic appearance of peritoneal carcinomatosis. When neither pathology nor surgical reports were available, imaging follow-up was considered the reference standard. Lesions that grew over time or regressed on chemotherapy and/or radiation therapy in concordance with the index lesion were considered malignant. Follow-up data were collected until August 31, 2021.
Statistical Analysis
Categorical variables are summarized as counts and proportions, continuous variables are described by their median and interquartile range (IQR). Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for PET/MRI and SCI were estimated as binomial proportions using the confusion matrices (true-positive, true-negative, false-positive, and false-negative results) according to the set reference standard, and exact confidence intervals for these parameters were obtained using Clopper-Pearson method.
Matched performance of PET/MRI and SCI was assessed using the McNemar test. The performance of stand-alone PET, standalone non-CE-MRI, stand-alone CE-MRI, and fused CE-PET/MRI was evaluated via pairwise comparisons with McNemar test, adjusting for multiple comparisons via the false discovery rate (Benja-mini-Hochberg) method.
The predictive value of PET and MRI features and their respective odds ratios and P values were estimated using a binomial logistic regression based on a generalized linear model. A McFadden pseudo-r-squared value was obtained to evaluate the overall model’s goodness-of-fit.
A P value of less than 0.05 was adopted to establish statistical significance. All P values reported are two-tailed. Statistical calculations were performed on RStudio version 1.4.1103 (RStudio Team, Boston, MA).
Data Availability
The data generated in this study are available upon request from the corresponding author.
RESULTS
Subject Selection and Demographics
Two hundred fifty-two subjects were referred for PET/MRI during the study period. Eighty-eight subjects were excluded: 30 had no follow-up, and 58 had an interval of more than 4weeks between SCI and PET/MRI. Ultimately, 164 subjects remained and were included in the analysis. Out of the 164PET/MRI scans, 4 were acquired with gadoxetate, and the remaining 160 with extracellular contrast media.
The median subject age was 60 years (IQR 50–69 years, range 23–87 years) and 85 (52%) were female. Supplemental digital Table 1.2, http://links.lww.com/SLA/D690 details subject demographics, primary tumor sites, treatment status, SCI distribution, and the reference standards used. The median interval between SCI and PET/MRI was 7 days (IQR 0–17 days).
Reference Standard
The reference standard consisted of pathology reports in 29 cases (18%), visual inspection during the operation for 55 patients (34%), and imaging follow-up in the remaining 79 (48%). According to the reference standard, 37 of 164 (23%) subjects were found to have PC, and the remaining 127 (77%) were negative for PC. Among the 37 subjects with PC, 20 (54%) were confirmed via surgical or pathology findings, and 17 (46%), by imaging follow-up.
Peritoneal Carcinomatosis Detection
At a subject level, the sensitivity, specificity, accuracy, PPV and NPV of PET/MRI were 0.97 (95% CI 0.86–1.00), 0.95 (95% CI 0.90–0.98), 0.96 (95% CI 0.91–0.98), 0.86 (95% CI 0.71–0.95), and 0.99 (95% CI 0.96–1.00) respectively. In the same context, the sensitivity, specificity, accuracy, PPV, and NPV of SCI were 0.54 (95% CI 0.37–0.71), 0.98 (95% CI 0.93–1.00), 0.88 (95% CI 0.82–0.92), 0.87 (95% CI 0.66–0.97), and 0.88 (95% CI 0.81–0.93) respectively. PET/MRI has higher sensitivity than SCI (P < 0.001), whereas there was no significant difference between SCI’s and PET/MRI’s specificity (P = 0.250). Furthermore, PET/MRI carries significantly higher NPV when compared with SCI, while there is not enough evidence to detect a difference in PPV, as demonstrated by the 95% CIs, which do not overlap. The null hypothesis that PET/MRI is not superior to SCI for peritoneal carcinomatosis diagnosis is rejected (OR 0.19 for SCI, 95% CI 0.04–0.66, P = 0.004). The detailed diagnostic performance of PET/MRI and SCI is displayed in Supplemental Digital Table 1.3, http://links.lww.com/SLA/D691.
Regarding the cases deemed positive by the reference standard, PET/MRI was able to detect peritoneal metastases that were missed on SCI in 16/37 (43%). Both modalities equally demonstrated PC in 20/37 (54%) of the patients, and in 1/37 (3%) neither modality was able to identify PC. There were no cases of peritoneal disease detected on SCI missed by PET/MRI.
As for the subjects considered negative per the reference standard, in 3 of the 127 (2%) subjects both their respective PET/MRI and SCI were falsely positive, while in 3 of 127 (2%), PET/MRI was false positive and SCI true negative. Both SCI and PET/MRI were true negative in the remaining 121 (95%) cases.
Stand-Alone PET, Non-CE-MRI, CE-MRI, and Fused PET/MRI Comparison
When evaluating the separate components of the PET/MRI study and then the fused PET/MRI images, stand-alone PET, standalone non-CE-MRI, stand-alone CE-MRI, and fused PET/MRI had sensitivities of 0.43, 0.54, 0.78, and 0.97, respectively. Those differences were statistically significant, with fused PET/MRI outperforming stand-alone CE-MRI (P = 0.023), and PET (P < 0.001). Stand-alone CE-MRI was also significantly superior to PET regarding sensitivity (P = 0.005). Among the stand-alone MRIs, CE-MRI was more sensitive than non-CE MRI (P = 0.039). The specificities of PET, non-CE-MRI, CE-MRI, and fused PET/MRI were 0.98, 0.96, 0.95, and 0.95, respectively. There were no significant differences in specificity between stand-alone PET and stand-alone non-CE-MRI, CE-MRI alone, or fused PET/MRI (P = 0.134). There was no difference in specificities between CE-MRI and non-CE-MRI (P = 1.000).
Imaging Feature Analysis
The individual diagnostic performance of the individual imaging patterns is shown in supplemental digital Table 1.4, http://links.lww.com/SLA/D692, the classification of findings into these features is more detailed in the Methods section. Notably, the features that were significantly correlated with the presence of PC were nodular thickening/enhancement on MRI (OR 39.48, 95% CI 8.96–174.05, P = 0.001) and linear thickening/enhancement on MRI (OR 48.41, 95% CI 7.16–327.45, P = 0.001). None of the remaining variables were statistically significantly correlated with the presence of PC. The overall model fit including all the MRI and PET patterns was moderate to excellent (P < 0.001, McFadden’s pseudo-r-squared = 0.589), which means that the array of features described can be considered good predictors of PC.20
Management Changes
PET/MRI and SCI results disagreed in 19 cases (16 true positives on PET/MRI and false negatives on SCI; 3 false positives on PET/MRI and true negatives on SCI). In 5 of 19 (26%) of the discordant cases, PET/MRI findings consistent with PC missed on SCI led to management changes. One subject with recurrent ovarian cancer underwent resection of a solitary serosal metastasis found on PET/MRI (Fig. 1). Another subject had a uterine leiomyosarcoma metastasis to the splenic serosa detected on PET/MRI, which prompted surgical resection. Conversely, 1 subject with rectal cancer had a previously scheduled liver metastasectomy canceled after PET/ MRI showed PC that was not apparent on SCI. Another subject with cholangiocarcinoma was changed to palliative therapy due to the detection of extensive peritoneal disease by PET/MRI only. Finally, in 1 case of pancreatic adenocarcinoma, the presence of peritoneal implants on PET/MRI that were not previously demonstrated on SCI also raised concern for liver lesions that were previously thought to be inflammatory. These lesions were biopsied and then proven to be metastatic, which ultimately led to a referral for liver-directed therapy. Figure 2 displays another instance of positive PET/MRI and negative SCI, although management was not changed at the time. None of the false positives described by PET/MRI led to management changes: 1 case had a change in systemic chemotherapy due to a tumor thrombus unrelated to PC, 1 patient already had metastatic rectal cancer with bilobar hepatic involvement detected by both PET/ MRI and SCI, for which the same systemic chemotherapy regimen was maintained. The remaining subject already had an indication for systemic therapy due to local tumor extent (T4bN+ rectal cancer) demonstrated in both modalities. Illustrative images from the last false positive case described are shown in Figure 3.
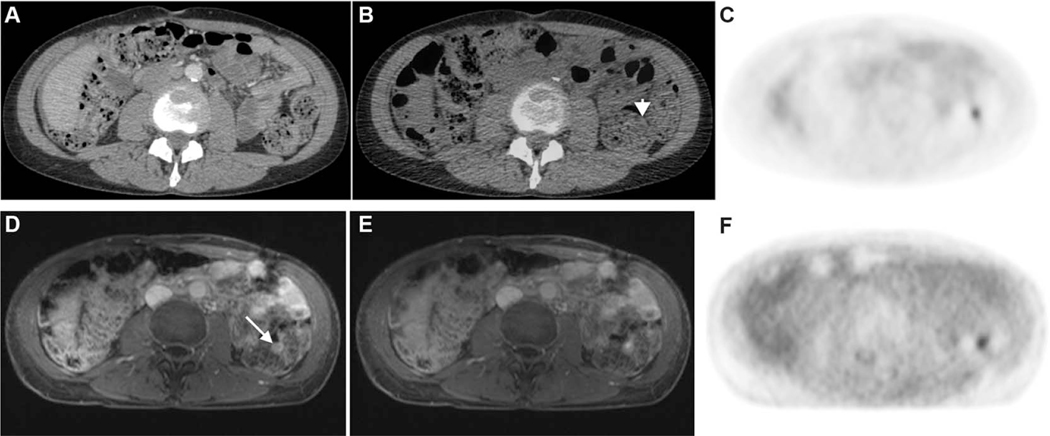
FIGURE 1.
Top panel: CE-CT (A), fused PET/CT (B), and FDG-PET images (C). Bottom panel: CE-MRI (A), fused PET/MRI (B), and FDG-PET images (C). A51-year-old female with a history of high-grade serous ovarian cancer status post complete cytoreduction. A small focus of moderate uptake along the wall of the descending colon (arrowhead in B) does not have an abnormal anatomic correlate on CE-CT to suggest metastasis and was attributed to physiologic bowel uptake. However, on PET/MRI, the focal uptake corresponds to an enhancing nodule on the surface of the colon (arrow in D). This lesion was consistent with a serosal metastasis and was subsequently resected.
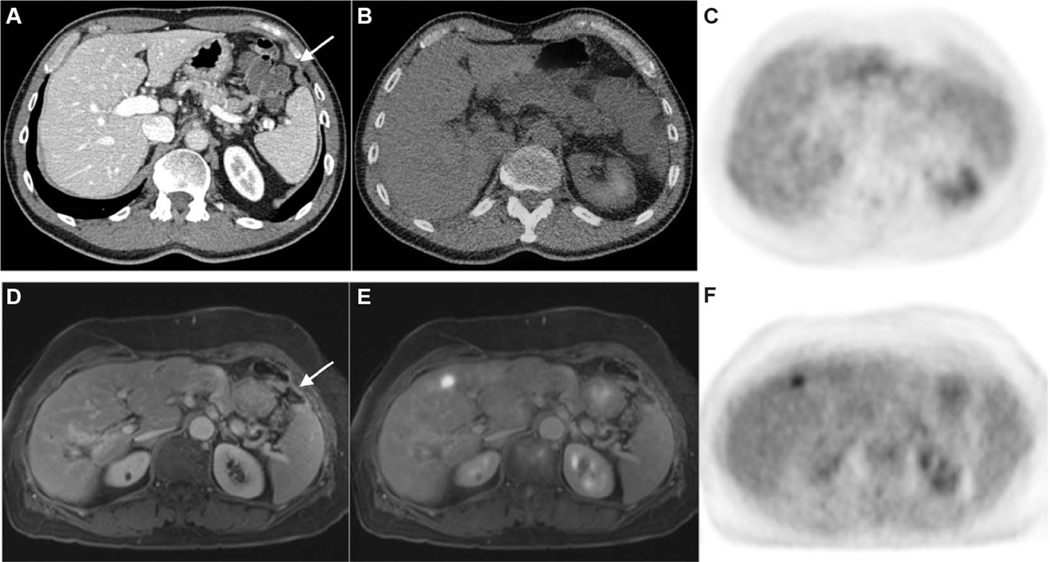
FIGURE 2.
Top panel: CE-CT (A), fused PET/CT (B), and FDG-PET images (C). Bottom panel: CE-MRI (D), fused PET/MRI (E), and FDG-PET images (F). A 65-year-old male with a history of resected adenocarcinoma of the ascending colon. A thin rind of soft tissue (arrow in A) is difficult to discern from the diaphragm on CT, and the degree of radiotracer uptake is similar to hepatic parenchyma on PET and fused PET/CT images. An area of abnormal peritoneal enhancement in the left upper quadrant (arrow in D) is readily perceptible on CE-MRI. This was confirmed to be metastatic adenocarcinoma following surgery. In this case, PET/MRI was able to detect peritoneal metastases approximately 4 months before a positive SCI scan that led to surgery, at which point widespread unresectable disease was found intraoperatively.
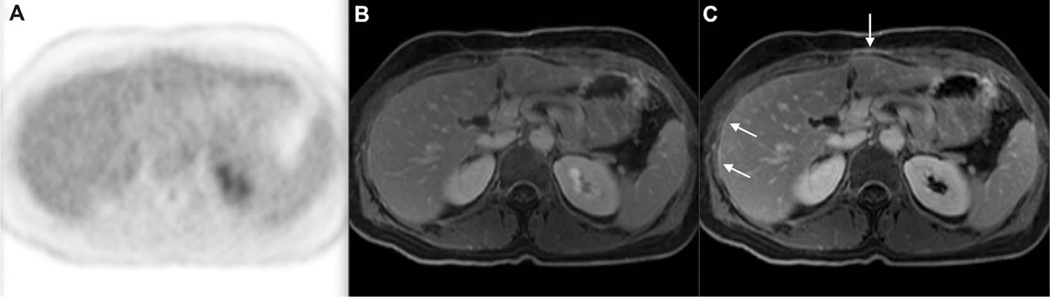
FIGURE 3.
FDG-PET (A), fused PET/MRI (B), and CET1-weighted VIBE MRI (C). Example of a false positive on PET/MRI in a 55-year-old female with metastatic sigmoid colon adenocarcinoma. There is no focus of increased FDG uptake on PET. However, there are some areas of linear thickening and enhancement of the peritoneum on the postcontrast T1-weighted VIBE sequence (arrows in C), which raised suspicion of peritoneal carcinomatosis. These findings regressed on CE-MRI follow-up 5 months later (not shown). VIBE; volumetric interpolated breath-hold examination.
DISCUSSION
The diagnosis of PC is an important prognostic factor and carries management implications in the setting of gastrointestinal and gynecological malignancies.21–27 Therefore, this study sought to compare individual or combinations of SCI against PET/MRI to assess potential diagnostic improvements. Notably, PET/MRI’s sensitivity was superior to SCI’s, while maintaining similarly high specificity. The major benefit of the increased diagnostic yield is most clearly represented by the high NPVof 0.99 (0.96–1.00), which virtually excludes PC. Notwithstanding, SCI’s sensitivity in this cohort was in the lower range of the intervals reported in the literature.16 This could be a result of pooling CT together with the more sensitive MRI and PET/CT. CT detection of PC is primarily limited by the soft tissue contrast resolution in subcentimeter nodules, which tend to blend in with adjacent normal soft tissue structures. This is particularly problematic in miliary small bowel serosal nodules and diffuse mesenteric infiltration as these findings are difficult to observe yet are a common cause of unresectable disease.9,12,16 However, it should be noted that less than one-fourth of the total subjects included in this study had only CT as the comparison against PET/MRI. Most subjects had either CT in combination with PET/CT or MRI, one of those modalities alone, or even a combination of them.
Data on PET/MRI in the setting of PC are still scarce. PET/ MRI was superior to MRI alone when estimating the peritoneal cancer index in subjects with primary gynecologic malignancies.18 However, no direct measurement of sensitivity, specificity, or accuracy at a subject level was reported in that study. Furthermore, CT and PET/CT were not evaluated. Previous studies on stand-alone MRI described an incremental benefit of DWI over anatomic sequences alone for the detection of peritoneal carcinomatosis, with improved sensitivity.28 DWI is particularly advantageous for the detection of serosal and perihepatic metastases.8,12,15
To the best of our knowledge, this study is the first to prospectively compare the diagnostic performance of PET/MRI to standard imaging modalities used in routine clinical practice, namely CT, PET/CT, and MRI, for the detection of peritoneal metastases. Previous studies have highlighted diagnostic challenges in SCI with respect to lesion size, morphology, and location. The combination of anatomic and functional imaging in PET/MRI provides the potential for increased sensitivity and comparable specificity. Figures 1 and 2 are examples of the utility of combining FDG-PET and MRI for the detection of subtle serosal and perihepatic disease, known areas of lower sensitivity and specificity in SCI.
Additionally, specific MRI features, namely nodular thickening/enhancement and linear thickening/enhancement, as assessed in the delayed contrast-enhanced acquisitions, were significantly correlated with the presence of PC and were responsible for the improved sensitivity of CE-MRI over non-CE MRI when evaluated separately. This holds true also regarding the whole PET/MRI acquisition protocol, where the delayed post-contrast T1-weighted sequences (acquired 10–15 minutes after injection) improved the evaluation of PC. Enhancement of the peritoneum was considered pathologic if more intense than that of the pleura at the left posterior costophrenic sulcus (Catalano’s pleuroperitoneal sign). To the best of our knowledge, this criterion had not been previously published. Instead, it relied on previous and unpublished experience of one of the investigators. This criterion might have been, at least in part, responsible for the superior PET/MRI performance in this cohort. Such comparison provides a more objective parameter and may aid in subtle cases. However, even when using state-of-the-art MRI protocols, the inclusion of PET still provides an incremental benefit. The separate component analysis results, where MRI alone and PETalone demonstrated less sensitivity than the fused PET/MRI, reinforces the value of the synergistic nature of PET/MRI.
At this time, it is unclear if the use of dual-timepoint PET could further improve the diagnostic performance of PET/MRI in this setting. However, dual-timepoint PET/CT improves metastasis detection in colorectal cancer.29,30
Importantly, the review of PET/MRI images resulted in clinical management changes in 26% of the discrepant cases (accounting for 16% of the overall positive cases). Management changes included the performance of surgical procedures in oligometastatic cases or the deferral of surgery in cases where unresectable disease was detected. These findings suggest a potentially valuable role for PET/MRI in the management of PC and corroborate the impact already described in colorectal cancer, cholangiocarcinoma, and pancreatic adenocarcinoma.31–34 Nevertheless, additional studies are needed to define the magnitude of this benefit.
PET/MRI was introduced very recently and thus remains largely a research modality. Its availability and usage, despite being still detrimentally affected by high initial acquisition and operational costs, long scan times, and high operational complexity, are slowly increasing worldwide over time.
Limitations
Regarding the evaluation of management changes, because PET/MRI was always performed after SCI, the PET/MRI reader was not blinded to additional clinical information that may have come to light in the interim, although this risk was minimized by excluding cases where the period between PET/MRI and SCI was greater than 4 weeks. A similar limitation resided in how management changes were evaluated; unfortunately, hindsight bias is not avoidable in this design. A more granular analysis, investigating the performance of each of the SCI modalities, was also not possible due to the relatively small sample size for each subgroup. This would make the study underpowered and unable to draw meaningful conclusions, which made us refrain from this approach. Additionally, only 52% of the included subjects had pathology or surgery as the reference standard, and the final determination of peritoneal disease was made using follow-up imaging in 48% of them, most of which were SCI, sharing the same shortcomings. However, it should be noted that this is a common limitation of this kind of studies, in which obtaining tissue sampling without clinical benefit would be unethical given the risk of complications from biopsies. Another possible limitation that might impact the reproducibility of our PET/MRI results in other centers may be related to variable levels of expertise in PET/MRI reading, given that it is not as widespread as the SCI modalities. Finally, study enrollment was at the discretion of the subjects’ treating oncologist or surgeon, and potential biases in the primary malignancy location and extent of disease are not accounted for in this study.
CONCLUSION
PET/MRI increases the sensitivity for peritoneal metastases detection while maintaining high specificity. The resulting negative predictive value enables ruling out PC with high confidence. PET/ MRI might be considered in patients affected by abdominal malignancies whose treatment might change in the case of occurrence of peritoneal carcinomatosis.
Supplementary Material
Acknowledgments
This work was supported by Bayer (grant number IIR-US-2019-6612).
Footnotes
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
REFERENCES
- 1.Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. [DOI] [PubMed] [Google Scholar]
- 3.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. [DOI] [PubMed] [Google Scholar]
- 5.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 6.Bermo MS, Koppula B, Kumar M, et al. The peritoneum: what nuclear radiologists need to know. Semin Nucl Med. 2020;50:405–418. [DOI] [PubMed] [Google Scholar]
- 7.Brendle C, Schwenzer NF, Rempp H, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43:123–132. [DOI] [PubMed] [Google Scholar]
- 8.Soussan M, Des Guetz G, Barrau V, et al. Comparison of FDG-PET/CTand MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol. 2012;22:1479–1487. [DOI] [PubMed] [Google Scholar]
- 9.Dirisamer A, Schima W, Heinisch M, et al. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol. 2009;69:536–541. [DOI] [PubMed] [Google Scholar]
- 10.De Iaco P, Musto A, Orazi L, et al. FDG-PET/CT in advanced ovarian cancer staging: value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur J Radiol. 2011;80:e98–e103. [DOI] [PubMed] [Google Scholar]
- 11.Kim HW, Won KS, Zeon SK, et al. Peritoneal carcinomatosis in patients with ovarian cancer: enhanced CT versus 18F-FDG PET/CT. Clin Nucl Med. 2013;38:93–97. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt S, Meuli RA, Achtari C, et al. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40:371–377. [DOI] [PubMed] [Google Scholar]
- 13.Turlakow A, Yeung HW, Salmon AS, et al. Peritoneal carcinomatosis: role of 18F-FDG PET. J Nucl Med. 2003;44:1407–1412. [PubMed] [Google Scholar]
- 14.Roze JF, Hoogendam JP, van de Wetering FT, et al. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian/fallopian tube/primary peritoneal cancer. Cochrane Database Syst Rev. 2018;10:CD012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dresen RC, De Vuysere S, De Keyzer F, et al. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging. 2019;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van’t Sant I, Engbersen MP, Bhairosing PA, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol. 2020;30:3101–3112. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz MA, Kubik-Huch RA, Hauser N, et al. PET/MRI and PET/CTin advanced gynaecological tumours: initial experience and comparison. Eur Radiol. 2015;25:2222–2230. [DOI] [PubMed] [Google Scholar]
- 18.Jónsdóttir B, Ripoll MA, Bergman A, et al. Validation of 18F-FDGPET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcino-matosis in ovarian and endometrial cancer—a pilot study. Cancer Imaging. 2021;21:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iafrate F, Ciolina M, Sammartino P, et al. Peritoneal carcinomatosis: imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging. Abdom Imaging. 2012;37:616–627. [DOI] [PubMed] [Google Scholar]
- 20.McFadden D. Quantitative methods for analyzing travel behavior of individuals: some recent developments. institute of transportation studies. University of California;. 1977. [Google Scholar]
- 21.Nagata H, Ishihara S, Hata K, et al. Survival and prognostic factors for metachronous peritoneal metastasis in patients with colon cancer. Ann Surg Oncol. 2017;24:1269–1280. [DOI] [PubMed] [Google Scholar]
- 22.Lin C-C, Liang H-P, Lee H-S, et al. Clinical manifestations and survival of hepatocellular carcinoma patients with peritoneal metastasis. J Gastroenterol Hepatol. 2009;24:815–820. [DOI] [PubMed] [Google Scholar]
- 23.Yonemura Y, Prabhu A, Sako S, et al. Long term survival after cytoreductive surgery combined with perioperative chemotherapy in gastric cancer patients with peritoneal metastasis. Cancers (Basel). 2020;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X-J, Yuan P, Li Z-Y, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013;34:463–469. [DOI] [PubMed] [Google Scholar]
- 25.Bhandare M, Patil P, Pai V, et al. Peritoneal carcinomatosis in colorectal cancers—management perspective needs a change. Clin Colorectal Cancer. 2017;16:e1–e6. [DOI] [PubMed] [Google Scholar]
- 26.Seyfried F, von Rahden BH, Miras AD, et al. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin—a longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer. 2015;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komiyama S, Katabuchi H, Mikami M, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol. 2016;21:435–446. [DOI] [PubMed] [Google Scholar]
- 28.Low RN, Sebrechts CP, Barone RM, et al. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings—a feasibility study. AJR Am J Roentgenol. 2009;193:461–470. [DOI] [PubMed] [Google Scholar]
- 29.Dirisamer A, Halpern BS, Schima W, et al. Dual-time-point FDG-PET/CT for the detection of hepatic metastases. Mol Imaging Biol. 2008;10:335–340. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Kim S-K, Lee SM, et al. Detection of hepatic metastases using dualtime-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol. 2011;13:565–572. [DOI] [PubMed] [Google Scholar]
- 31.Ferrone C, Goyal L, Qadan M, et al. Management implications of fluoro-deoxyglucose positron emission tomography/magnetic resonance in untreated intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging. 2020;47:1871–1884. [DOI] [PubMed] [Google Scholar]
- 32.Amorim BJ, Hong TS, Blaszkowsky LS, et al. Clinical impact of PET/MR in treated colorectal cancer patients. Eur J Nucl Med Mol Imaging. 2019;46:2260–2269. [DOI] [PubMed] [Google Scholar]
- 33.Catalano OA, Lee SI, Parente C, et al. Improving staging of rectal cancer in the pelvis: the role of PET/MRI. Eur J Nucl Med Mol Imaging. 2021;48:1235–1245. [DOI] [PubMed] [Google Scholar]
- 34.Furtado FS, Ferrone CR, Lee SI, et al. Impact of PET/MRI in the treatment of pancreatic adenocarcinoma: a retrospective cohort study. Mol Imaging Biol. 2021;23:456–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.