Abstract
Several IAP (inhibitor of apoptosis) proteins regulate cell fate decisions, and the X-linked IAP (XIAP) does so in part by inhibiting caspases, proteases that execute the apoptotic pathway. A tissue-specific homologue of XIAP, known as ILP2 (IAP-like protein 2), has previously been implicated in the control of apoptosis in the testis by direct inhibition of caspase 9. In examining this protein we found that the putative caspase 9 interaction domain is a surprisingly weak inhibitor and is also conformationally unstable. Comparison with the equivalent domain in XIAP demonstrated that the instability is due to the lack of a linker segment N-terminal to the inhibitory BIR (baculovirus IAP repeat) domain. Fusion of a 9-residue linker from XIAP to the N-terminus of ILP2 restored tight caspase 9 inhibition, dramatically increased conformational stability and allowed crystallization of the ILP2 BIR domain in a form strikingly similar to the XIAP third BIR domain. We conclude that ILP2 is an unstable protein, and cannot inhibit caspase 9 in a physiological way on its own. We speculate that ILP2 requires assistance from unidentified cellular factors to be an effective inhibitor of apoptosis in vivo.
Keywords: apoptosis, baculovirus IAP repeat (BIR) domain, caspase, crystallography, inhibitor of apoptosis protein (IAP), spermatogenesis
Abbreviations: Ac-LEHD-AFC, acetyl-Leu-Glu-His-Asp-7-amido-4-fluoromethylcourmarin; BIR, baculovirus IAP repeat; BRUCE, BIR repeat-containing ubiquitin-conjugating enzyme; CARD, caspase recruitment domain; GdmCl, guanidinium chloride; GFP, green fluorescent protein; GST, glutathione S-transferase; (c-)IAP, (cellular) inhibitor of apoptosis; ILP, IAP-like protein; ML-IAP, melanoma IAP; NAIP, neuronal apoptosis inhibitory protein; PDB, Protein Data Bank; RING, really interesting new gene; Smac, second mitochondrial activator of caspases; XIAP, X-linked IAP
INTRODUCTION
The gene family encoding IAP (inhibitor of apoptosis) proteins, originally found in baculoviruses, are present in organisms from viruses to yeast to humans [1]. Their characteristic protein motif is the BIR (baculovirus IAP repeat), an approx. 80-amino-acid zinc-stabilized domain. Up to three tandem copies of the BIR domain can occur within the known IAP family proteins from viruses and animal species (reviewed in [2,3]). There are currently eight human IAP proteins annotated in the Human Genome Nomenclature database: NAIP (neuronal apoptosis inhibitory protein), c-IAP1 (cellular IAP1), c-IAP2, XIAP (X-linked IAP), ML-IAP (melanoma IAP), ILP2 (IAP-like protein 2), survivin and BRUCE (BIR repeat-containing ubiquitin-conjugating enzyme) (reviewed in [2]). As their name suggests, the founding members of the family protect against apoptotic stimuli, in both insect and mammalian cells [1,3]. Further roles for IAPs have been proposed in a variety of cellular processes, including the control of cell division, and a number of signalling cascades, such as transforming growth factor β activation, c-Jun N-terminal kinase regulation and nuclear factor κB activation (reviewed in [3]).
Several IAPs, including XIAP ([4] and multiple references in [3]), c-IAP1 [5], c-IAP2 [5], NAIP [6], ML-IAP [7,8], survivin [9,10], BRUCE [11] and ILP2 [12,13], have been reported to directly interact with and inhibit caspases, cysteine proteases that are the core components of the apoptotic cascade. Ectopic expression of these IAPs has been shown to suppress apoptotic cell death induced by a variety of triggers, such as death receptor activation, growth factor withdrawal and cytotoxic insults (reviewed in [2,14]), suggesting that the caspase-inhibitory property may be central to the function of these proteins as apoptosis inhibitors.
Among the IAPs, XIAP is the most potent modulator of apoptosis, and most well characterized in terms of its caspase-inhibitory activity. It blocks cell death both in vitro and in vivo by inhibiting distinct caspases [4,15–17]. XIAP comprising three tandem BIR domains at its N-terminus and a RING (really interesting new gene) finger domain near its C-terminus. Biochemical dissection and structural analysis of XIAP has shown that the second BIR domain (BIR2) with its N-terminal linker is necessary and sufficient for inhibiting caspases 3 and 7 [15,16,18–20], whereas the third BIR domain (BIR3) is responsible for inhibition of caspase 9 [15,17,21].
ILP2 is the most recently identified member of the human IAP family [12,13]. The coding sequence of ILP2 is very similar to the C-terminal half of XIAP, the region containing the BIR3 and RING domains, with 81% identity at the protein level. Over-expression of ILP2 had no protective effect on death mediated by Fas or tumour necrosis factor, but prevented death induced by Bax or caspase 9 in vitro [12,13], leading to the conclusion that ILP2 blocks the intrinsic apoptotic pathway by directly inhibiting caspase 9. To test this hypothesis we have produced recombinant forms of ILP2 and the equivalent region of XIAP, and determined their stabilities and mode of interaction with caspase 9.
EXPERIMENTAL
Constructs
The bacterial expression plasmid encoding full-length ILP2 (pGEX-ILP2) was a gift from Dr Colin S. Duckett (Department of Pathology, University of Michigan Medical School, Ann Arbor, MI, U.S.A). ΔCARD (lacking caspase recruitment domain) human caspase 9 (lacking the first 138 residues) with alanine substitutions at residues 304 to 306 has been described previously [22]. The cDNAs encoding ILP2 BIR (residues 1–95) and XIAP-BIR (residues 253–356), plus a N-terminal His6-purification tag, were cloned into the expression vector pET15b(+) (Novagen).
All constructs were generated using the following primers (restriction sites are underlined): (i) XIAP-BIR3, residues 253–356, 5′-AAAAAAACATATGTCAACAAATCTTCCAAGAAATCC-3′ and 5′-CGCGGATCCCTATCCCTTCTGTTCTAACAG-3′; (ii) XIAP-BIR3, residues 253–347, 5′-AAAAAAACATATGTCAACAAATCTTCCAAGAAATCC-3′ and 5′-CGCGGATCCCTATGAATGAGTTAAATGAATATTG-3′; (iii) XIAP-BIR3, residues 262–356, 5′-CGCGGATCCCTAAGTAGTTCTTACCAGACA-3′ and 5′-TTTTTTTCATATGGCAGATTATGAAGCACGGATC-3′; (iv) XIAP-BIR3, residues 257–347, 5′-CGCGGATCCCTATCCCTTCTGTTCTAACAG-3′ and 5′-TTTTTTTCATATGCTTCCAAGAAATCCATCCATGGC-3′; (v) XIAP-BIR3, residues 262–347, 5′-CGCGGATCCCTAAGTAGTTCTTACCAGACA-3′ and 5′-TTTTTTTCATATGGCAGATTATGAAGCACGGATC-3′; (vi) XIAP-BIR3, residues 253–336, 5′-AAAAAAACATATGTCAACAAATCTTCCAAGAAATCC-3′ and 5′-CGCGGATCCCTATCCCTTCTGTTCTAACAG-3′; (vii) ILP2 BIR, 5′-TTTTTTTCATATGACGGGTTATGAAGC-3′ and 5′-CGCGGATCCCTAGGTAGTTTGTACCAGAGC-3′; (viii) X-ILP2 BIR (residues 253–261 of XIAP-BIR3 fused on to ICP2 BIR), 5′-CGCGGATCCCTAGGTAGTTTGTACCAGAGC-3′ and 5′-GGAATTCCATATGAGTACTAATCTTCCAAGAAATCCATCTATGACGGGTTATGAAGCC-3′; (ix) X-ILP2 BIR PP/AA (Pro257 and Pro260 mutated to alanine), 5′-GGAATTCCATATGAGTACTAATCTTGCAAGAAATGCATCTATGACGGGTTATGAAGCC-3′ and 5′-CGCGGATCCCTAGGTAGTTTGTACCAGAGC-3′. All constructs were verified by DNA sequencing.
Cell culture and cell death assay
HEK-293T cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 2 mM glutamine. ILP2 BIR and X-ILP2 BIR plasmids were cloned into p3XFLAG vector (Sigma) for mammalian expression. The mammalian expression plasmids encoding Myc-tagged wild-type XIAP (pcDNA3-Myc-XIAP), Bax (pcDNA3-Bax) and Fas (pcDNA3-Fas) have been described previously [15]. For cell death assay, 2×105 HEK-293T cells were co-transfected as described above with GFP (green fluorescent protein). Quantification of cell death was performed by observing the morphology of GFP fluorescent cells with a Leica DM IRB inverted fluorescence microscope, and scoring for overtly apoptotic cells with pycnotic nuclei [4,5]. Immunoblot analysis of transfected proteins was performed with anti-Myc (Santa Cruz) or anti-FLAG M2 (Sigma) antibodies, as described previously [36].
Recombinant proteins
GST (glutathione S-transferase)-tagged full-length ILP2 and ΔCARD caspase 9 were expressed in the Escherichia coli strain BL21(DE3)pLysS and purified as described previously [12,23]. When cells reached a D600 of 0.5, expression of the ILP2 BIR domain and XIAP-BIR constructs was induced with 0.5 mM IPTG (isopropyl β-D-thiogalactoside) for 5 h. Proteins were purified by affinity chromatography using chelating Sepharose (Pharmacia) charged with NiSO4, according to the manufacturer's instructions. Eluted protein was >90% pure as verified by SDS/PAGE. The protein concentration of purified proteins was determined from the A280, based on the molar absorption coefficients calculated from the Edelhoch relationship [24].
SDS/PAGE
PAGE (8–18% linear acrylamide gradient) was performed with a 2-amino-2-methyl-1,3-propanediol/glycine/HCl buffer system with SDS to resolve proteins [25]. Samples for SDS/PAGE were boiled in SDS sample buffer containing 50 mM dithiothreitol for 5 min.
Determination of inhibitory constants
Recombinant ΔCARD caspase 9 was pre-activated in salt-free caspase buffer [20 mM Pipes, 10 mM EDTA, 0.1% CHAPS and 10% (w/v) sucrose, pH 7.2] for 15 min at 37 °C. Following this, a range of inhibitors was pre-incubated with the enzyme for 20 min at 37 °C. The assay was started by the addition of Ac-LEHD-AFC (acetyl-Leu-Glu-His-Asp-7-amido-4-fluoromethylcourmarin) to 100 μM, and measured kinetically for 30 min using an fmax fluorescence plate reader (Molecular Devices) at an excitation wavelength of 405 nm and an emission wavelength of 510 nm. Reaction mixtures were thermostatically controlled at 37 °C. The individual Ki values for the inhibitors [I] were determined from the uninhibited substrate hydrolysis rate (vo) and the inhibited rates (vi), so that a plot of (vo/vi)−1 against [I] gives Ki(app), the equilibrium inhibition constant in the presence of substrate. The inhibition constant Ki for competitive inhibition was calculated by correcting the apparent constant by a factor of 1+S/KM, which accounts for substrate competition (KM=248 μM [23]). All Ki values are averages of at least three independent determinations, with a range of not more than 10%. Individual Figure plots are representative of specific Ki determinations.
Protein unfolding studies
Fluorescence spectra were monitored using a PerkinElmer LS50B luminescence spectrometer coupled with the FL Win Lab software (PerkinElmer, Northwalk, CT, U.S.A.) using a 1-cm pathlength cuvette (Helma). Unfolding studies were carried out by incubating the protein (dialysed in 50 mM Tris/HCl and 100 mM NaCl, pH 8.0), in the presence of incremental increases in guanidinium chloride (GdmCl: range 0–6 M) or urea (0–8 M) for 1 h at room temperature. In the case of GdmCl experiments the intrinsic fluorescence emission was measured at 350 nm following excitation at 295 nm, where a change in fluorescence represented protein unfolding. The fraction of folded protein was simply related to the ratio of initial fluorescence yield to the yield at the various GdmCl concentrations.
In the case of urea unfolding experiments the excitation wavelength was 295 nm and the emission was measured from 300 to 420 nm with a slit width of 10 nm for both excitation and emission. After collecting all the emission spectra, the area under the curve was calculated by summing the product of wavelength increment and emission intensity for each recorded wavelength-intensity pair. To calculate the average wavelength, the product of emission intensity and corresponding wavelength was calculated for each recorded wavelength-intensity pair and divided by values of the area under the curve. The average wavelength was obtained by weighting each wavelength by the fraction of corresponding incremental area (1 nm increment times the intensity) contributing to the total area under the curve [26].
Crystallization
Following purification, the N-terminal His6 tag from X-ILP2 BIR was removed by thrombin digestion and dialysed overnight against 10 mM Hepes (pH 7.3), 100 mM NaCl and 5 mM cysteine. The resulting protein had the sequence GSHMSTNLPRNPSMTGYEARLITFGTWMYSVNKEQLARAGFYAIGQEDKVQCFHCGGGLANWKPKEDPWEQHAKWYPGCKYLLEEKGHEYINNIHLTRSLEGALVQTT. The complex of X-ILP2 and Smac (second mitochondrial activator of caspases) peptide (AVPIAQK) was formed by incubating the two components for 5 min at 37 °C with a 2-fold excess of the peptide. Initial crystallization trials were set up with 8–10 mg/ml X-ILP2 BIR/Smac, and commercially available screening kits from Emerald Biostructures. Crystals were obtained at room temperature by the hanging-drop procedure using 1 μl of both protein and precipitating solution, which consisted of 5% (w/v) poly(ethylene glycol) 3000, 100 mM sodium acetate (pH 4.5) and 100 mM zinc acetate, final pH 5.2. Typically, crystals grew within 2 days as diamond-shaped plates.
Data collection and structure solution
Prior to data collection, crystals were soaked for approx. 1 min in 5% (w/v) poly(ethylene glycol) 3000, 100 mM sodium acetate, pH 5.2, 100 mM zinc acetate and 25% glycerol. Diffraction data was collected at 100 K on an in-house X-ray source (Rigaku Rotating anode, R-axis IV image plate), then processed and scaled using DENZO/SCALEPACK [27]. Crystals from the same drops belonged to two different space groups, namely P3121 and P41212. Based on cell parameters and the molecular mass of X-ILP2 BIR–Smac complex (13 kDa), the most probable number of monomers in the asymmetric unit for both space groups was estimated to be between six and eight (Matthews Probability Calculator, http://www-structure.llnl.gov/mattprob/). Subsequently, a high quality data set from the P41212 crystals was collected at the SSRL (Stanford Synchrotron Radiation Laboratory).
The structure was first solved in the space group P3121 by molecular replacement using the program AMoRe [28]. The search model BIR3 was taken from the PDB (Protein Data Bank) entry 1G73, chain D [29]. The highest correlation factors were obtained when four molecules were placed in the asymmetric unit. Thorough analysis of the molecular replacement solutions revealed that in both crystal forms the molecules pack in ring-like structures. These ring structures could be completed to six-membered rings by placing two additional molecules manually in areas of continuous difference density. In a similar procedure six molecules could be placed in the asymmetric unit of the other crystal form (P41212), forming an almost identical six-membered ring. The two structures were refined in multiple cycles of manual map interpretation in MAIN [30], and crystallographic refinement (simulated annealing, positional and temperature-factor refinement) in CNS [31]. For refinement statistics, please see to Tables 1 and 2.
Table 1. Data processing statistics.
SSRL, Stanford Synchrotron Radiation Laboratory.
| Value | |||
|---|---|---|---|
| Parameter | Space group… | P3121 | P41212 |
| Data collection | Rigaku Rotating anode, R-axis IV | SSRL, Beamline 9-1 | |
| Wavelength (Å) | 1.54178 | 0.984 | |
| Temperature (K) | 95 | 95 | |
| Cell dimensions (Å) | 88.7, 88.7, 191.4 | 86.4, 86.4, 226.8 | |
| Number of monomers in a.u. | 6 | 6 | |
| Solvent content (%) | 56 | 55 | |
| Resolution range (Å) | 50–2.7 | 50–2.2 | |
| No. of observations | 74682 | 260612 | |
| No. of rejected observations | 243 | 908 | |
| No. of unique reflections | 22931 | 44152 | |
| Completeness (%) | 92.9 | 98.2 | |
| Redundancy | 3 | 5.9 | |
| Rsym (%)* | 7.1 | 7.4 | |
| 〈I/σ(I)〉 | 17.5 | 13.1 | |
| Resolution range (Å) | 2.8–2.7 | 2.27–2.20 | |
| Completeness (%) | 96 | 98.5 | |
| Rsym (%) | 37.4 | 38.2 | |
| Reflections with I≥2σ (%) | 67 | 81 | |
* Rsym=Σ_Io−〈I〉_/Σ〈I〉.
Table 2. Refinement statistics.
Numbers in parenthesis refer to the highest resolution shell (2.74–2.70 Å and 2.22–2.20 Å respectively). RMSD, root mean square deviation.
| Value | |||
|---|---|---|---|
| Refinement | Space group… | P3121 | P41212 |
| Data used in refinement | |||
| Resolution range (Å) | 50–2.7 | 50–2.2 | |
| Number of reflections (working/test) | 22904/1111 | 41772/2196 | |
| Completeness (working/test set) | 88.3/4.5 | 93.7/4.9 | |
| Fit to data used in refinement | |||
| Rvalue* (%) | 23.2 (31.7) | 22.3 (23.6) | |
| Rfree† (%) | 27.9 (39.3) | 24.2 (28.7) | |
| Number of non-hydrogen atoms | |||
| Protein atom | 4592 | 4644 | |
| Ligand atom | 202 | 172 | |
| Water | 182 | 313 | |
| Zinc ion | 24 | 24 | |
| RMSD from ideal values | |||
| Bond length (Å) | 0.0064 | 0.0058 | |
| Bond angle (°) | 1.16 | 1.14 | |
| Ramachandran plot | |||
| Core | 84.6 | 84.6 | |
| Allowed | 13.9 | 13.3 | |
| Generous | 1.5 | 1.1 | |
* Rvalue (Rcryst)=Σ_Fo−Fc_/ΣFo.
† Rvalue=Rcryst calculated for 5% of randomly selected reflections that were not included in the refinement.
In most X-ILP2 molecules a number of N- and C-terminal residues are disordered, although continuous density was typically found for residues Leu256 to Leu344. Exceptions are molecules B (P41212 and P3121), E (P3121) and F (P41212), where the complete C-terminus could be traced. In most molecules only four out of seven residues of the Smac peptides could be positioned unambiguously. In addition to the structural zinc ion present in each BIR domain, both structures contain 18 further zinc ions involved in crucial crystal contacts. The zinc ions were placed based on the intensity of the peaks in the different density and their tetrahedral co-ordination. As the exact identity of these solvent molecules is not crucial for the interpretations made in the present study, we made no further attempts to reveal their identity by additional experiments. Yet, we observed that the presence of zinc acetate in the crystallization buffer is required for crystal growth. The coordinates of the X-ILP2 structures have been submitted to the PDB identifiers 1XB0 and 1XB1.
RESULTS
Weak Inhibition of caspase 9 by ILP2
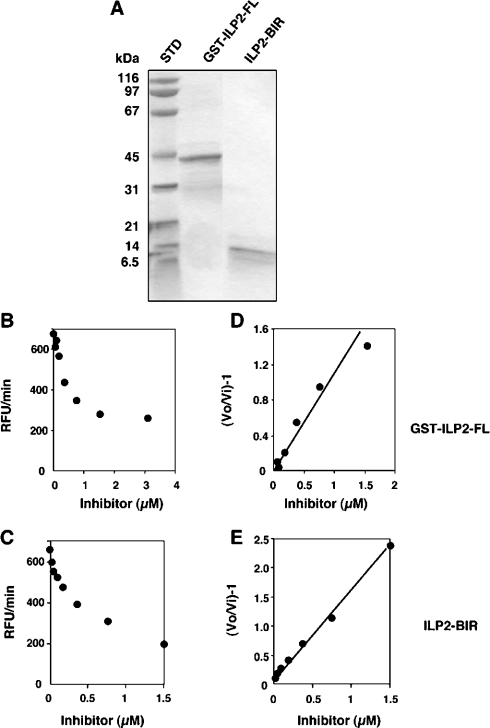
We tested the ability of recombinant GST-tagged full-length ILP2 (GST–ILP2-FL) to repress the activity of caspase 9, using a synthetic substrate reporter of caspase 9 activity (Figure 1). The protein inhibited caspase 9 activity by approx. 50% when used at 1 μM (representing a 5-fold molar excess of inhibitor relative to enzyme), with a Ki of 752 nM, demonstrating only weak suppression of this protease. To examine whether the BIR domain of ILP2 was as efficient in inhibiting caspase 9, ILP2 BIR protein was produced with a His6 tag for purification purposes. ILP2 residues 1–95 correspond to residues 262–356 of XIAP, encompassing the BIR3 domain that had previously been demonstrated to efficiently inhibit caspase 9 [17]. ILP2 BIR protein inhibited caspase 9, about 2-fold more than ILP2 FL, with a Ki of 472 nM. This shows that although the BIR domain of ILP2 alone is sufficient for caspase 9 inhibition, ILP2 is a poor inhibitor of caspase 9 when compared with a similar region of XIAP (Ki in the 20 nM range [17]).
Figure 1. Poor inhibition of caspase-9 by ILP2 derivatives.
ILP2 proteins were expressed and purified, and their degree of purity analysed in SDS/PAGE (A). The proteins were titrated against ΔCARD caspase 9 and residual activity quantified as RFU (relative fluorescence units)/min by release of AFC from the caspase substrate Ac-LEHD-AFC (B and C). Replots according to the relationship described in the Experimental section allows calculation of the apparent Ki from the reciprocal of the slopes of the lines in (D) and (E).
Dissection of XIAP-BIR3: the minimal inhibitory unit
The weak inhibitory activity of ILP2 puzzled us. A sequence alignment (Figure 2) showed that all the amino acid residues essential for inhibition of caspase 9 by XIAP [17,21] are present in ILP2. Therefore the lack of tight inhibition must be due to causes other than those predicted by mutagenesis or protein structures.
Figure 2. Alignment of ILP2 with the equivalent region of XIAP.
ILP2 lacks the regions covering residues 1–261 of XIAP, which contain the BIR1 and BIR2 domains. However, the BIR3 domains (thick underlining) and RING domains (thin underlining) are well conserved, with only 19% differences, most of which are conservative substitutions. Identical residues in both sequences are shaded grey.
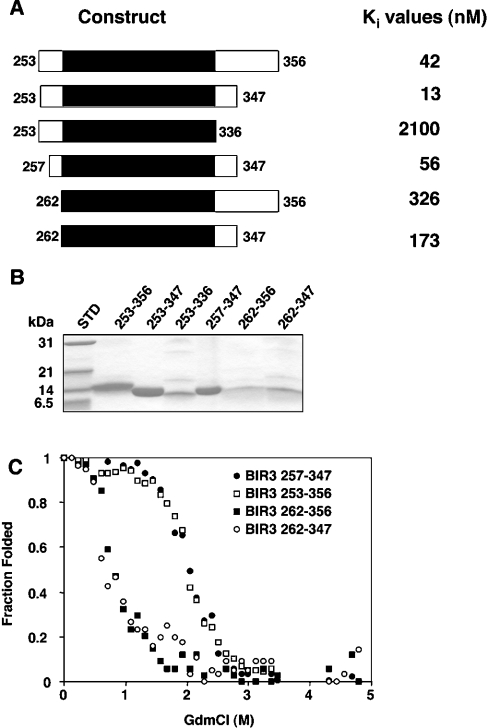
Interestingly, previous dissection of the minimal caspase 9 inhibitory unit of XIAP showed that the region encompassing residues 261–336, which lacks the linker N-terminal to the BIR domain itself, and the last BIR domain α-helix, does not inhibit caspase 9 [17]. To quantify the effect of N- or C-terminal truncations on the ability of XIAP to inhibit caspase 9, we produced several BIR3 domain proteins of XIAP in E. coli (Figure 3).
Figure 3. Conformational stability of the BIR3 domain of XIAP.
The constructs shown in (A) were expressed as His6-tagged proteins in E. coli and purified (B). Expression levels of the most extreme truncations (from 262 at the N-terminus or at 336 in the C-terminus) were much lower than the other constructs, but sufficient quantities could be obtained for unfolding and kinetic analyses, and each mutant was normalized to the same concentration prior to analysis. (C) Proteins were incubated for 1 h at room temperature in the presence of the indicated GdmCl concentrations. Unfolding was measured by fluorescence at 350 nm following excitation at 295 nm. For all experiments the protein concentration was 200 nM.
Each construct was titrated against ΔCARD caspase 9, and the resulting Ki values are shown in Figure 3(A). Deletion of the fifth α-helix (337–347) resulted in a drastic loss of inhibitory activity, which is in agreement with the presence of essential inhibitory interactions in this part of the protein [21]. Interestingly, truncation of the C-terminal linker residues 348–356 led to increased inhibitory potency against caspase 9, but only in the presence of an intact N-terminal linker. All XIAP-BIR3 proteins with a truncation of the N-terminal linker immediately upstream of the BIR domain (beginning at residue 262) were found to have substantially decreased inhibitory activity against caspase 9. In contrast, removal of residues 253–256 had little effect on caspase inhibition, allowing us to conclude that the smallest BIR3 protein (minimal inhibitory unit) that potently inhibits caspase 9 contains residues 257–347. This conclusion is consistent with the known structural requirements for inhibition [21], yet it does not explain why truncation to residue 261 fails to produce efficient inhibition. Indeed, it appears that the region N-terminal to the BIR3 domain of XIAP is essential for inhibition in a physiological range.
Conformational stability of XIAP-BIR3
During the dissection analysis of XIAP-BIR3, we noticed that the expression level of BIR3 protein lacking the N-terminus was dramatically decreased compared with other BIR3 proteins with an extended N-terminus. On the basis of these observations, we hypothesized that the N-terminal residues may be important for the structural integrity of BIR3. To explore this possibility, we analysed the conformation stability of the various proteins.
While deletion of the C-terminal residues (348–356) had no influence on the unfolding behaviour of XIAP-BIR3, truncation up to residue 262 dramatically decreased protein stability (Figure 3C). BIR3 lacking the N-terminal residues started to unfold at very low GdmCl concentrations (≪1 M), with midpoint unfolding occurring at 0.8 M GdmCl and complete unfolding at 3 M GdmCl. These are characteristic of an unstable protein domain [32]. In contrast, BIR3 with intact N-terminal residues started to unfold at 1.5 M and reached midpoint and complete unfolding at 2.2 M and 3 M respectively, in agreement with normal protein stability. All proteins seemed to unfold with a single transition, indicating a simple denaturation. Moreover, unfolding was reversible, since refolding by dilution from 6 M to 0.6 M GdmCl followed by re-examination of the folding transition [33] gave similar curves (results not shown). Taken together, these results show that the N-terminal residues 253–261 are crucial for stability of XIAP-BIR3.
ILP2 BIR domain is unstable: mutation towards a stable domain
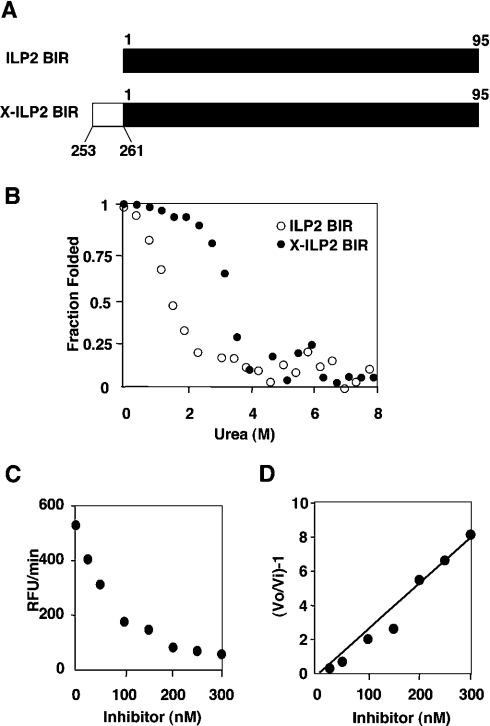
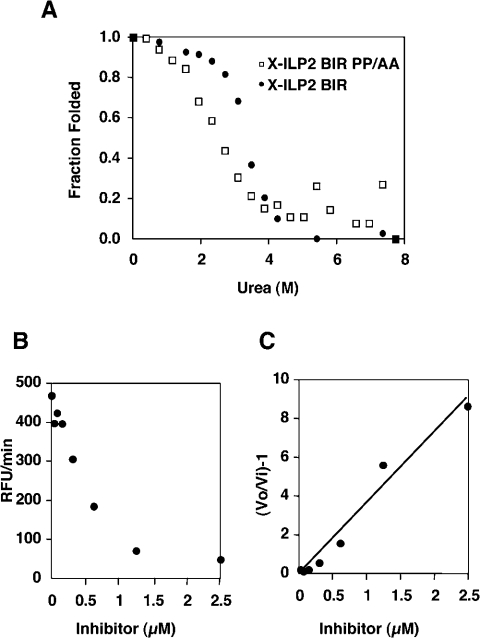
Dissection of XIAP-BIR3 demonstrated that lack of the N-terminal residues results in loss of caspase 9 inhibitory activity and correlates with protein instability. It is therefore most likely that the low caspase 9 inhibitory capacity of ILP2 is because it is an unstable protein. This possibility was tested by urea denaturation (Figure 4), which was used in place of GdmCl because it yields more accurate unfolding transitions at low denaturant. ILP2 BIR unfolded at low urea concentration, reminiscent of the conformational instability of the region of XIAP from 262–356 (Figure 3C). It is therefore an unstable protein at room temperature.
Figure 4. Conversion of ILP2 BIR domain to a stable and functional protein.
ILP2 BIR domain or X-ILP2 BIR domain [shown diagramatically in (A) with the black bar representing ILP2 and the white bar representing the N-terminal addition from XIAP] was incubated for 1 h at room temperature in the presence of the indicated urea concentrations. Unfolding was measured by a change in the peak emission (blue shift) in the range 340–360 nm following excitation at 295 nm. For both experiments the protein concentration was 200 nM (B). X-ILP2 was titrated against ΔCARD caspase 9 and residual activity quantitated as RFU (relative fluorescence units)/min by release of AFC from the caspase substrate Ac-LEHD-AFC (C). A replot according to the relationship described in the Experimental section allows calculation of the apparent Ki from the reciprocal of the slope of the line in (D).
If the conformational stability of ILP2 BIR is due to a naturally truncated N-terminus with respect to XIAP, then addition of the N-terminal residues should create a stable protein. Moreover, we reasoned that this new protein should also be a good caspase 9 inhibitor. To explore this possibility the N-terminal linker residues (253–261, STNLPRNPS) from XIAP-BIR3 were fused on to ILP2 BIR. This fusion protein, which we designate X-ILP2 BIR for convenience, corresponds to residues 253–356 of XIAP-BIR3 (Figure 4A).
Unfolding X-ILP2 BIR was monitored as for ILP2 by changes in intrinsic fluorescence in the presence of urea (Figure 4B). In contrast with ILP2 BIR, X-ILP2 BIR started to unfold around 2 M and complete unfolding occurred at 5 M. Interestingly, the unfolding curve of X-ILP2 BIR resembled that of XIAP-BIR3 253–356 (Figure 3C). We next determined the Ki for inhibition of caspase 9 by X-ILP2 BIR. As predicted, addition of the N-terminal residues resulted in a substantial increase in inhibitory activity, with a Ki value of 35 nM. In contrast, X-ILP2 was unable to inhibit recombinant caspases 3, 6, 7 or 8 (results not shown), and therefore appears to be caspase 9-specific, similar to the BIR3 domain of XIAP [17,37]. These results together strongly indicated that ILP2 BIR is an unstable protein and that addition of the N-terminal linker residues is necessary to form a correctly folded and stable protein.
Cell death protection by X-ILP2
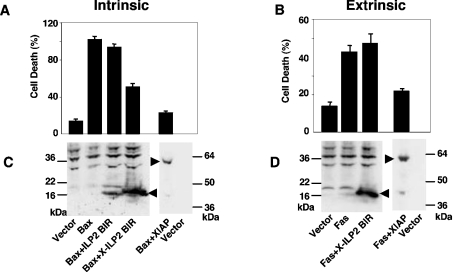
Since we had converted ILP2 into a caspase 9 inhibitor by adding the N-terminal residues from XIAP, we tested whether ectopically expressed X-ILP2 could spare cells from apoptosis. Figure 5 demonstrates that X-ILP2 BIR, but not ILP2 BIR, is able to abrogate cell death triggered by Bax ligation, but not by Fas ligation. This is consistent with Bax requiring the intrinsic, caspase 9, pathway to achieve cell death, and Fas requiring the extrinsic caspase 8 pathway. The result reveals that in a transfected cell culture model X-ILP2 takes on properties similar to the BIR3 domain of XIAP [37]. It is slightly less effective than that of full-length XIAP, presumably because XIAP also contains the BIR2 domain that targets the executioner caspases 3 and 7 [15], which also explains why X-ILP2 fails to rescue cell death triggered by Fas expression. Together these data are consistent with X-ILP2 inhibiting only caspase 9, corroborating the in vitro data (see above). We noted that ILP2 consistently demonstrated lower expression levels than X-ILP2 (Figure 5C), possibly owing to its relative instability and potential removal by the quality control machinery of the cell. Therefore, it is not possible to unequivocally conclude that the lack of cell death protection is due to lack of caspase 9 inhibition. However, given the in vitro data, this remains the most likely explanation, and our data demonstrate that ILP2 is not an efficient apoptosis inhibitor.
Figure 5. Suppression of Bax-mediated cell death by X-ILP2.
HEK-293T cells were transfceted with GFP (0.1 μg) and control, ILP2 BIR, X-ILP2 BIR or XIAP vectors in the presence of Bax [Intrinsic pathway (A)] or Fas [Extrinsic pathway (B)]. After transfection (24 h), viability of GFP-positive cells was evaluated by morphological examination. The results are expressed as the means±S.D. for three independent experiments. (C) and (D) show the relative expression levels of the transfected, FLAG-tagged ILP2 derivatives and Myc-tagged XIAP. The arrowheads point to the epitope-tagged proteins.
Two main properties have been attributed to the BIR3 domain of XIAP: caspase 9 inhibition and Smac binding (reviewed in [3]). Smac primarily operates by binding to a surface groove on BIR3, abrogating caspase 9 binding. Since X-ILP2 BIR shares with XIAP-BIR3 sequence conservation and caspase 9 inhibition, we reasoned that it should also bind Smac, and to this end we determined the structure of the X-ILP2–Smac complex.
Structure of X-ILP2 BIR in complex with Smac heptapeptide
To dissect structure–function relationships of ILP2 we attempted to crystallize ILP2 BIR or X-ILP2 BIR in complex with a heptameric peptide corresponding to the N-terminal region of Smac. No crystals were obtained for ILP2 BIR, but well-diffracting crystals were obtained with X-ILP2 BIR in two different space groups (P3121 and P41212). In both crystals six X-ILP2 BIR molecules form tight hexamaric rings (asymmetric unit), which build up the crystal lattice (Figure 6A). The P3121 ring and the P41212 ring have the same quarternary structure and superimpose almost perfectly. Interestingly, a ring structure is seen in the quaternary structure of the BIR domain of ML-IAP, although in this case the cause is different and due to intermolecule contacts formed by the N-terminus of each domain slotting into the Smac groove of an adjacent molecule [34]. The interactions within the X-ILP2 BIR hexamer are intimate, with an excluded surface of 700–800 Å2 (1 Å=0.1 nm) per molecule interface. For the sake of comparison we refer to the XIAP numbering system to identify residues in X-ILP2 BIR; Figure 2 shows how the residues in the two proteins correspond.
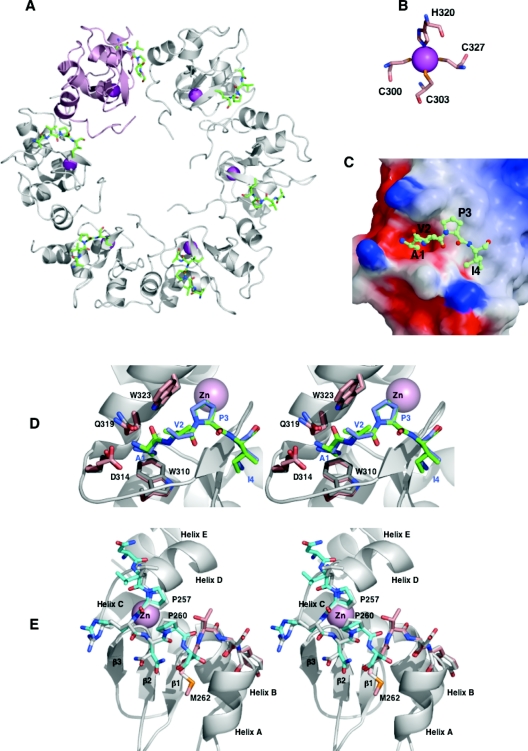
Figure 6. Structural displays of X-ILP2 BIR bound to the Smac peptide.
(A) The asymmetric unit contains six X-ILP2 BIR–Smac units organized as a six-membered ring. One zinc atom per unit (magenta), part of the X-ILP2 BIR molecule, is co-ordinated by three cysteines and one histidine (B). The electrostatic potential surface of X-ILP2 BIR in (C) is given as a point of reference to demonstrate the mode of accommodation of the positively charged α-amine in the negative environment of BIR side chains, typical of Smac pockets in the BIR domains of XIAP [18,29,35,38]. (D) The stereo pair shows a focus on the Smac groove of X-ILP2 BIR (pink side chains) overlaid on the XIAP-BIR3 structure (PDB identifier 1G73, grey). Key residues in Smac binding in each structure are shown, and the corresponding Smac tetrapeptides are green for X-ILP2 BIR and blue for XIAP-BIR3. (E) A close-up on the superimposed XIAP-BIR3 structure (grey) with X-ILP2 BIR (cyan and pink). The cyan color designates the peptide sequence of XIAP added to ILP2 and the pink side chains designate the start of the ILP2 coding sequence (Met262). The two proline residues critical for folding are shown.
Some of the critical interactions (Glu266 with His343) appear to be mediated by zinc ions (results not shown), which is supported by the observation that crystals could only be obtained in the presence of zinc acetate. Even though we have no evidence that this ring structure has a biological significance, it is compelling that the same quaternary structure was observed in two different crystal forms. Despite the high degree of identity between XIAP-BIR3 and ILP2 BIR, the two residues forming critical crystal contact are not conserved (Glu72 is Gln333 and His75 is Gln336).
As expected from the high sequence identity, the structure of X-ILP2 BIR is quite similar to the previously described structure of XIAP-BIR3 [29,35], with an RMSD (root mean square deviation) <0.5 Å for equivalent Cα positions. This structure demonstrates that a correctly folded and stable ILP2 BIR domain contains the elements required for effective Smac binding. The different copies of the X-ILP2 BIR molecule (12 copies total) have a closely similar structure and all bind peptide in a surface groove known as the Smac pocket. Slight backbone differences between XIAP-BIR3 and X-ILP2 BIR are observed as a response to different crystal environments. In most protomers the density becomes unclear after His343 or Leu344, although the complete C-terminus of molecules B, D and E could be traced. In the following we will limit our discussion to the structure of protomer A from spacegroup P41212.
The Smac peptide binds to the Smac pocket previously identified in XIAP-BIR3 [29,35], located between the third β-strand and the third α-helix of X-ILP2 BIR domain (Figure 6D). It adopts an extended conformation with a kink at Pro3 when bound to the BIR protein and lies across the third β-strand of X-ILP2 BIR domain, with the positive charge on its α-amine balanced by the negative electrostatic surface formed by side chains of X-ILP2 BIR residues (Figure 6C). The recognition specificity is achieved through a combination of hydrogen-bond interactions and van der Waals contacts. The amino group of Smac peptide Ala1 donates three hydrogen bonds to Glu314 and Gln319, while its carbonyl group makes two additional contacts to Gln319 and Trp323. The methyl group of Ala1 fits in a hydrophobic pocket formed by the side chains of Leu307, Trp310 and Gln319. Both Smac residues Val2 and Pro3 maintain multiple van der Waals interactions with Trp310. The side chain of Smac residue Ile4 interacts with Gly306 and the aliphatic side chain of Lys297. Although Ala5 of Smac is ordered in the crystals, it does not make important interactions with the BIR3 domain. None of the visible side chains of the Smac peptide make contacts with neighbouring molecules within the ring, suggesting that the binding mode is not influenced by crystal contacts.
Effect of proline mutations on X-ILP2 BIR
Finally, we asked how the N-terminal linker confers conformational stability to the protein. Comparing the N-termini of different BIR3 domains we noticed the conservation of a PXXP motif in XIAP, ML-IAP, Drosophila DIAP1 and DIAP2, but not human cIAP1 and cIAP2. In both XIAP-BIR3 and X-ILP2 BIR, the residues PRNPS of the N-terminal linker pack closely against the loop between β-strands 2 and 3 (Figure 6E). This linker segment contains a short helical turn of residues 260–262, and is followed by helix A starting at Asp264 (XIAP-BIR3), the equivalent position being glycine in X-ILP2 BIR (Figure 6E). Removal of the linker and its short helix would therefore most likely destabilize the A helix, leading to a partial unfolding of the entire protein.
Mutating these two prolines into alanines in X-ILP2 BIR (X-ILP2 BIR PP/AA: STNLARNAS) substantially decreased the stability and the inhibitory capacity of the resulting protein (Figure 7). Whereas X-ILP2 BIR reached midpoint unfolding around 4 M, X-ILP2 BIR mutant reached midpoint at 2.8 M. In addition, the inhibitory capacity of X-ILP2 BIR mutant was substantially decreased, with a Ki of 194 nM (6-fold decrease in Ki compared with X-ILP2 BIR). These results suggest that the N-terminal linker can only fulfil its role as N-terminal cap for helix A when it is made more rigid by the PXXP motif.
Figure 7. Effect of proline mutations on X-ILP2 BIR.
X-ILP2 BIR PP/AA (Pro257 and Pro260 mutated to alanine) was incubated for 1 h at room temperature in the presence of the indicated urea concentration (A). Unfolding was measured as described in Figure 4. X-ILP2 BIR mutant was titrated against ΔCARD caspase 9 and residual activity quantitated as RFU (relative fluorescence units)/min by release of AFC from the caspase substrate Ac-LEHD-AFC (B). A replot according to the relationship described in the Experimental section allows calculation of the apparent Ki from the reciprocal of the slope of the line in (C).
DISCUSSION
ILP2 was identified during genome analysis of XIAP [12,13]. The open reading frame closely resembles the C-terminal half of XIAP, and the most significant difference between ILP2 and XIAP is that ILP2 lacks the first two N-terminal BIR domains present in XIAP. Despite its close similarity to XIAP, ILP2 was found to function in a far more restricted manner, as it is solely expressed in the testis [12,13]. The suggestion was that ILP2 may serve to complement the lack of XIAP expression during spermatocyte meiosis, during which genes on the X chromosome are inactivated, and thus the presence of a compensatory regulator of apoptosis may be essential to maintain sperm cells during their maturation [12,13]. Over-expression of ILP2 failed to inhibit apoptosis induced by death receptors, but potently suppressed death induced by Bax [12]. This implied regulation of the intrinsic apoptosis pathway has led many to believe that ILP2 functions as a caspase 9 inhibitor.
We found that when compared with XIAP, full-length ILP2 or its BIR domain both displayed significantly weaker inhibitory activity against caspase 9, which was unexpected considering the high level of identity (81%) and conservation of predicted caspase 9 interaction residues between XIAP and ILP2. This weak inhibition is due to the limited conformational stability of ILP2 BIR domain, a situation that can be corrected by adding a segment of XIAP to the N-terminus. A potential explanation for the importance of the N-terminal residues in BIR3 stabilization may be that these residues serve as a cap for helix A. Indeed, mutation of Pro257 and Pro260 to alanine results in conformational instability and weakened inhibition, demonstrating the critical role of these residues in the conformation of the short helical segment that constitutes the beginning of the folded BIR domain.
The crystal structure that we present here of X-ILP2 BIR bound to Smac shows that all residues needed for caspase 9 and Smac interactions are conserved between ILP2 and XIAP, and that ILP2 can adopt an authentic BIR fold that contains an intact Smac binding pocket. Additionally, the two auto-ubiquitination sites of XIAP, Lys322 and Lys328 in XIAP [36] equivalent to Lys61 and Lys67 in ILP2, are also maintained in ILP2. Nevertheless, the native protein is minimally stable, and it is unlikely that it can be stabilized in vivo simply by binding to one of its known ligands, Smac or caspase 9, since if this were the case we would expect good caspase 9 inhibition. The instability of the BIR domain may also mean that all potential BIR-dependent functions are lost in ILP2, including some of the signalling functions that have been proposed for other IAPs (reviewed in [3]). We anticipate that the RING E3 ligase of ILP2 is retained, but if the BIR domain represents the targeting function of the ligase, it is unclear whether the activity can be expressed.
An important question that arises in ascertaining the function of ILP2 is whether it is expressed as a protein. Previous studies have detected mRNA expression in testis, but as yet no protein has been identified. Indeed this is not trivial given that ILP2 is so closely related to XIAP, and the size of the protein is very close to the cleavage product of XIAP found in cells [37]. ILP2 is relatively recently descended from XIAP, and is found only in the great apes, and it is significant that all functional residues of the Smac groove and caspase 9 interaction sites are completely conserved between human, gorilla and chimpanzee. On this basis it is likely that ILP2 is a functional protein, and its most likely function is caspase 9 inhibition. Therefore, to be an effective inhibitor in vivo, ILP2 would require a stabilizing protein or other influence to overcome its minimal structural integrity. Since expression of the protein is heavily restricted, we predict that such a stabilizer may be found only in testis.
Acknowledgments
We thank Scott Snipas and Annamarie Price for outstanding technical assistance, Colin Duckett for helpful discussions and for providing the original ILP2 plasmid, Wayne Fairbrother for communicating results before publication, and Robert Liddington and members of his laboratory for assistance with crystallography. This work was supported by the National Institutes of Health grant AG15402.
References
- 1.Uren A. G., Coulson E. J., Vaux D. L. Conservation of baculovirus inhibitor of apoptosis repeat proteins (BIRPs) in viruses, nematodes, vertebrates and yeasts. Trends Biochem. Sci. 1998;23:159–162. doi: 10.1016/s0968-0004(98)01198-0. [DOI] [PubMed] [Google Scholar]
- 2.Deveraux Q. L., Reed J. C. IAP family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen G. S., Duckett C. S. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 4.Deveraux Q., Takahashi R., Salvesen G. S., Reed J. C. X-linked IAP is a direct inhibitor of cell death proteases. Nature (London) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 5.Roy N., Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier J. K., Lahoua Z., Gendron N. H., Fetni R., Johnston A., Davoodi J., Rasper D., Roy S., Slack R. S., Nicholson D. W., MacKenzie A. E. The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J. Neurosci. 2002;22:2035–2043. doi: 10.1523/JNEUROSCI.22-06-02035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vucic D., Stennicke H. R., Pisabarro M. T., Salvesen G. S., Dixit V. M. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 2000;10:1359–1366. doi: 10.1016/s0960-9822(00)00781-8. [DOI] [PubMed] [Google Scholar]
- 8.Kasof G. M., Gomes B. C. Livin, a novel inhibitor-of-apoptosis (IAP) family member. J. Biol. Chem. 2001;276:3238–3246. doi: 10.1074/jbc.M003670200. [DOI] [PubMed] [Google Scholar]
- 9.Conway E. M., Pollefeyt S., Cornelissen J., DeBaere I., Steiner-Mosonyi M., Ong K., Baens M., Collen D., Schuh A. C. Three differentially expressed survivin cDNA variants encode proteins with distinct antiapoptotic functions. Blood. 2000;95:1435–1442. [PubMed] [Google Scholar]
- 10.Shin S., Sung B. J., Cho Y. S., Kim H. J., Ha N. C., Hwang J. I., Chung C. W., Jung Y. K., Oh B. H. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 11.Bartke T., Pohl C., Pyrowolakis G., Jentsch S. Dual Role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell. 2004;14:801–811. doi: 10.1016/j.molcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Richter B. W., Mir S. S., Eiben L. J., Lewis J., Reffey S. B., Frattini A., Tian L., Frank S., Youle R. J., Nelson D. L., et al. Molecular cloning of ILP-2, a novel member of the inhibitor of apoptosis protein family. Mol. Cell. Biol. 2001;21:4292–4301. doi: 10.1128/MCB.21.13.4292-4301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagace M., Xuan J. Y., Young S. S., McRoberts C., Maier J., Rajcan-Separovic E., Korneluk R. G. Genomic organization of the X-linked inhibitor of apoptosis and identification of a novel testis-specific transcript. Genomics. 2001;77:181–188. doi: 10.1006/geno.2001.6635. [DOI] [PubMed] [Google Scholar]
- 14.Verhagen A. M., Coulson E. J., Vaux D. L. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:REVIEWS3009. doi: 10.1186/gb-2001-2-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi R., Deveraux Q., Tamm I., Welsh K., Assa-Munt N., Salvesen G. S., Reed J. C. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 16.Sun C., Cai M., Gunasekera A. H., Meadows R. P., Wang H., Chen J., Zhang H., Wu W., Xu N., Ng S. C., Fesik S. W. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature (London) 1999;401:818–822. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 17.Sun C., Cai M., Meadows R. P., Xu N., Gunasekera A. H., Herrmann J., Wu J. C., Fesik S. W. NMR structure and mutagenesis of the third BIR domain of the inhibitor of apoptosis protein XIAP. J. Biol. Chem. 2000;275:33777–33781. doi: 10.1074/jbc.M006226200. [DOI] [PubMed] [Google Scholar]
- 18.Riedl S. J., Renatus M., Schwarzenbacher R., Zhou Q., Sun S., Fesik S. W., Liddington R. C., Salvesen G. S. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 19.Chai J., Shiozaki E., Srinivasula S. M., Wu Q., Dataa P., Alnemri E. S., Yigong Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y., Park Y. C., Rich R. L., Segal D., Myszka D. G., Wu H. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 21.Shiozaki E. N., Chai J., Rigotti D. J., Riedl S. J., Li P., Srinivasula S. M., Alnemri E. S., Fairman R., Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 22.Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I., Ricci J.-E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. A unified model for apical caspase activation. Mol. Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 23.Renatus M., Stennicke H. R., Scott F. L., Liddington R. C., Salvesen G. S. Dimer formation drives the activation of the cell death protease caspase 9. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967;6:1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 25.Bury A. Analysis of protein and peptide mixtures: evaluation of three sodium dodecyl sulphate-polyacrylamide gel electrophoresis buffer systems. J. Chromatog. 1981;213:491–500. [Google Scholar]
- 26.Shirley B. A. Totowa, New Jersey: Humana Press; 1995. Protein Stability and Folding: Theory and Practice. [Google Scholar]
- 27.Otwinowski Z., Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 28.Navazza J. AMoRe: an automated package for molecular replacement. Acta Cryst. 1994;A50:157–163. [Google Scholar]
- 29.Wu G., Chai J., Suber T. L., Wu J. W., Du C., Wang X., Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature (London) 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 30.Turk D. Munich: Technische Universitaet; 1992. Weiterentwicklung eines Programms fuer Molekuelgraphik und Elektrondichte-Manipulation und seine Anwendung auf verschiedene Protein-Strukturaufklaerungen. PhD Thesis. [Google Scholar]
- 31.Brunger A. T., Adams P. D., Clore G. M., Delano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J.-S., Kuszewski J., Nilges N., Pannu N. S., et al. Crystallography and NMR system (CNS): a new software suite for macromolecular structure determination. Acta Cryst. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg D. P. Analysis of protein conformation by gel electrophoresis. In: Creighton T. E., editor. Protein Structure: A Practical Approach. New York: IRL Press; 1989. pp. 225–250. [Google Scholar]
- 33.Bose K., Clark A. C. Dimeric procaspase-3 unfolds via a four-state equilibrium process. Biochemistry. 2001;40:14236–14242. doi: 10.1021/bi0110387. [DOI] [PubMed] [Google Scholar]
- 34.Franklin M. C., Kadkhodayan S., Ackerly H., Alexandru D., Distefano M. D., Elliott L. O., Flygare J. A., Mausisa G., Okawa D. C., Ong D., et al. Structure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP) Biochemistry. 2003;42:8223–8231. doi: 10.1021/bi034227t. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z., Sun C., Olejniczak E. T., Meadows R. P., Betz S. F., Oost T., Herrmann J., Wu J. C., Fesik S. W. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature (London) 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 36.Shin H., Okada K., Wilkinson J. C., Solomon K. M., Duckett C. S., Reed J. C., Salvesen G. S. Identification of ubiquitination sites on the X-linked inhibitor of apoptosis protein. Biochem. J. 2003;373:965–971. doi: 10.1042/BJ20030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deveraux Q. L., Leo E., Stennicke H. R., Welsh K., Salvesen G. S., Reed J. C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai J., Du C., Wu J. W., Kyin S., Wang X., Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature (London) 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]