Abstract
Ppp5 (protein phosphatase 5) is a serine/threonine protein phosphatase that has been conserved throughout eukaryotic evolution. In mammalian cells, FLAG-tagged Ppp5 and endogenous Ppp5 are found to interact with endogenous Hsp (heat-shock protein) 70, as well as Hsp90. Incubation of cells with arachidonic acid or the microtubule-depolymerizing agent, nocodazole, causes loss of interaction of Hsp70 and Hsp90 with FLAG-tagged Ppp5 and increase of Ppp5 activity. In response to the same treatments, endogenous Ppp5 undergoes proteolytic cleavage of the N- and C-termini, with the subsequent appearance of high-molecular-mass species. The results indicate that Ppp5 is activated by proteolysis on dissociation from Hsps, and is destroyed via the proteasome after ubiquitination. Cleavage at the C-terminus removes a nuclear localization sequence, allowing these active cleaved forms of Ppp5 to translocate to the cytoplasm. The response of Ppp5 to arachidonic acid and nocodazole suggests that Ppp5 may be required for stress-related processes that can sometimes cause cell-cycle arrest, and leads to the first description for in vivo regulation of Ppp5 activity.
Keywords: cellular stress, heat-shock protein 70 (Hsp70), heat-shock protein 90 (Hsp90), nocodazole, protein serine/threonine phosphatase 5 (Ppp5/PP5)
Abbreviations: APC, anaphase-promoting complex; ASK1, apoptosis signal-regulating kinase 1; Cdc, cell-division control; DMEM, Dulbecco's modified Eagle's medium; HA, haemagglutinin; Hop, heat-shock-protein-organizing protein; Hsp, heat-shock protein; Ppp5, protein phosphatase 5; Ppt1, protein phosphatase with tetratricopeptide repeat domain; TPR, tetratricopeptide repeat
INTRODUCTION
Most cellular signalling pathways are mediated by reversible protein phosphorylation, and several kinase cascades have been delineated, but the roles of protein phosphatases are much less clear. Ppp5 (protein phosphatase 5) is a serine/threonine protein phosphatase, which comprises an N-terminal TPR (tetratricopeptide repeat) domain fused to a C-terminal phosphatase catalytic domain (reviewed in [1–3]). Like some other PPP family members, Ppp5 is potently inhibited by the tumour promoters, such as okadaic acid and microcystin [4,5]. From the interaction of its TPR domain with other proteins, Ppp5 has been proposed to participate in several cellular signalling pathways in higher eukaryotes, including those initiated by atrial natriuretic peptide [6], glucocorticoids [7,8], oxidative stress [9] and pathways operating through G-proteins [10]. Ppp5 was found to associate with Hsp (heat-shock protein) 90 and glucocorticoid receptor complexes containing Hsp90 [7,11]. Overexpression of the TPR domain of Ppp5 inhibited glucocorticoid-receptor-mediated transcriptional activation, but it is unclear whether the TPR domain was acting as a dominant-negative mutant or competing with other TPR-containing proteins that bind to Hsp90. In contrast, suppression of Ppp5 expression with antisense oligonucleotides enhanced glucocorticoid-receptor-mediated transcriptional activity and also induced the expression of p21WAF1/Cip1 in a p53-containing cell line, but not in a p53-deficient cell line [8,12]. From these studies, Zuo et al. [8,12] proposed that Ppp5 promotes cellular proliferation by negatively regulating both glucocorticoid and p53-mediated signalling pathways that lead to p21WAF1/Cip1-mediated G1 growth arrest in some cell lines. Suppression of Ppp5 expression was also associated with hyperphosphorylation of p53 at Ser-15 [13]. ASK1 (apoptosis signal-regulating kinase 1), which activates the JNK (Janus kinase) and p38 mitogen-activated protein kinase cascades, was found to interact with Ppp5, using the yeast two-hybrid system [9]. Overexpression of Ppp5 inhibited the H2O2-induced sustained activation of ASK1, as well as ASK1-dependent apoptosis. The Gα12/Gα13 subunits of heterotrimeric G-proteins were found to interact with Ppp5 and stimulate its phosphatase activity, suggesting that Ppp5 is a downstream effector of Gα12/Gα13 signalling pathways [10]. A variety of other proteins have also been reported to interact with Ppp5, using the yeast two-hybrid system or in vitro studies. These include the TPR containing Cdc (cell-division control) 16 and Cdc27 subunits of the APC (anaphase-promoting complex) [14], the human homologue of Arabidopsis thaliana blue-light photoreceptor, cryptochrome 2 [15], the A subunit of PP2A (protein phosphatase 2A) [16] and the Hsp90-dependent haem-regulated eIF2α (eukaryotic inhibition factor 2α) kinase [17], DNA-dependent protein kinase [18] and hRAD17 (human RAD17 homologue) in the DNA-damage-induced pathway [19].
Limited proteolysis and mutagenesis of bacterially expressed Ppp5 has indicated that the low basal phosphatase activity is a consequence of autoinhibition by the TPR domain and a short region at the C-terminus of the phosphatase domain [20–22]. Polyunsaturated fatty acids, such as arachidonic acid, stimulate the phosphatase activity of purified native and bacterially expressed Ppp5, and activation occurs by binding of the lipid to the TPR domain [20,23]. More recently, physiological concentrations of both saturated and unsaturated fatty acyl-CoA esters have been shown to activate Ppp5, suggesting a possible link with fatty acid metabolism [24], but as yet no in vivo physiological role for the lipid activation of Ppp5 has been demonstrated. The crystal structure of the TPR domain of Ppp5 reveals three TPR motifs, each of which comprises a pair of antiparallel α-helices of similar lengths [25]. This is followed by a fourth section with weak TPR similarity that forms an extended α-helix. The three tandem TPR motifs are organized with adjacent α-helices antiparallel like a concertina, but rotated with respect to each other to form a super-helical structure with an amphipathic groove that provides a binding site for interacting proteins. The TPR acceptor site of Hsp90 is located in a 12 kDa fragment at its C-terminus [26], and acidic residues within this fragment were found to interact with basic residues within the groove of the Ppp5 TPR domain [27].
Ppp5 is found in the cytoplasm and nucleus, with prominent localization in the nucleoplasm of some cell lines [4,28]. The region responsible for the nuclear localization of Ppp5 resides in the C-terminal 80 amino acids of Ppp5 [5]. Other studies show a predominantly cytoplasmic location of endogenous Ppp5 [27], while ectopically expressed Ppp5 localized to the mitotic spindle in mitosis [14], to the cytoplasm during interphase [5] and to the cell periphery when co-expressed with a constitutively active form of Gα12 [10].
Putative orthologues of Ppp5 are present in lower eukaryotes, including Trypanosoma brucei [29], Plasmodium falciparum [30], Saccharomyces cerevisiae [4,31,32] and Neurospora crassa [33], where it is termed Ppt1 (protein phosphatase with TPR domain). Ppp5 is found in a wide variety of higher eukaryotes, including plants [34], fruit flies [28], rodents [6,35] and humans [4], and is present in all tissues examined in mammals. Its ubiquity suggests that it may function in one or more cellular process conserved throughout eukaryotic evolution. We show in the present paper that human Ppp5 is bound to two Hsps and that, in response to arachidonic acid or the microtubule-depolymerizing agent nocodazole, Ppp5 undergoes dissociation from these Hsps and proteolytic activation, suggesting that Ppp5 may play a role in stress responses that may sometimes lead to cell-cycle arrest.
MATERIALS AND METHODS
Construction of mammalian expression vectors for FLAG-tagged Ppp5 and the FLAG-tagged TPR domain
For construction of the pCMV5-FLAG-TPR domain expression vector, DNA encoding Ppp5 was amplified by PCR using human Ppp5 template DNA [4,28] and the following oligonucleotide primers: forward primer, 5′-GGGAATTCGCCACCATGGACTACAAGGACGACGATGACAAGGCGATGGCGGAGGGCGAG-3′ incorporating an EcoRI site (underlined) and encoding the FLAG peptide DYKDDDDK (italics), and reverse primer 5′-GAGACAGAGAAGATTACAGTATGTTGATCGAT-3′ incorporating a ClaI site (underlined). The EcoR1/ClaI fragment was ligated into pCR2.1 TOPO, and was then subcloned into the same sites of pCMV5 to produce plasmid pCMV5-FLAG-TPR (encoding amino acids 2–240 of Ppp5). For construction of the pCMV5-FLAG-PPP5 expression vector, a pBluescript subclone of human Ppp5 was digested with HindIII to cleave out the Ppp5 central and C-terminal coding region, which was ligated with the HindIII-digested pCMV5-FLAG-TPR to produce pCMV5-FLAG-PPP5 mammalian expression vector encoding amino acids 2–499. The orientation was checked by restriction enzyme digests, and all constructs were verified by sequencing on an Applied Biosystems 373A automated DNA sequencer using Taq dye terminator cycle sequencing or on an Applied Biosystems model 3730 automated capillary DNA sequencer using Big-Dye version 3.1 chemistry (University of Dundee DNA sequencing service managed by Dr Nick Helps, http://www.dnaseq.co.uk). Ppp5 constructs with HA (haemagglutinin) epitope tags are described in [5].
Cell culture
HEK-293 (human embryonic kidney) cells and HeLa cells were cultured at 37 °C in an atmosphere with 5% CO2 in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) foetal calf serum, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Adherent cells were collected by scraping them from the Petri dishes into the DMEM medium, centrifugation at 500 g for 3 min, rinsing with PBS. A 500 μl volume of ice-cold lysis buffer [50 mM Tris/HCl, pH 7.5, 0.15 M NaCl, 0.03% (v/v) Brij-35, 2 mM EDTA, 0.1 mM EGTA, 5% (v/v) glycerol and Complete™ protease inhibitor cocktail (Roche Diagnostics, Lewes, U.K.)] was added to the cells from each 10-cm-diameter plate. Cells were lysed by ten passages through a 0.8-mm-gauge needle, and the lysates were collected after centrifugation at 13000 g for 10 min. Detached cells were collected from the incubation medium by centrifugation and were manipulated in the same manner. Protein concentrations were determined, and adjusted to similar values before analysis by immunoblotting.
Purification of FLAG-Ppp5-interacting proteins
HEK-293 cells in 50 10-cm-diameter dishes were transfected using the modified calcium phosphate method with pCMV5-FLAG-PPP5 (10 μg/plate) and incubated at 37 °C. Cell lysates were prepared 24 h post-transfection in lysis buffer. The lysate was mixed with 1 ml of anti-FLAG–agarose (Sigma, Poole, U.K.) in a tube by end-over-end rotation for 1 h at 4 °C. The agarose beads were separated by centrifugation and washed four times in lysis buffer. Bound proteins were eluted from a column of anti-FLAG–agarose with 1 ml of 200 μg/ml FLAG peptide in lysis buffer and examined by SDS/PAGE in Tris/glycine, pH 8.3. Proteins that co-eluted with FLAG–Ppp5 were excised from the gel, digested in situ with trypsin, and the tryptic peptide masses were analysed by MS as described in [36].
For elution of FLAG–Ppp5 before immunoblotting, immunopellets from 10–15 dishes of HEK-293 cells were incubated in an ice bath with 50–100 μl of 200 μg/ml FLAG peptide for 20 min with occasional shaking. After centrifugation, the supernatant was analysed by SDS/PAGE.
Analysis of proteins interacting with endogenous Ppp5
Lysates from HEK-293 cells were pre-cleared by mixing with sheep IgG bound non-covalently to Protein-G–Sepharose for 30 min at 4 °C with continuous rotation. Following centrifugation for 5 min at 800 g, the supernatant was collected and incubated with anti-TPR antibodies covalently coupled to Protein-G–Sepharose for 1 h at 4 °C. The pellets were washed four times with lysis buffer, and the bound proteins were eluted with 50–100 μl of 0.1 M glycine (pH 3.5). Eluates were neutralized with 0.1 vol. of Tris/HCl, pH 8.0, and analysed by SDS/PAGE.
Antibodies and immunological techniques
Immunoblotting was performed following fractionation of proteins by SDS/PAGE, using NuPAGE 4–12% BisTris gels (Invitrogen, Groningen, Netherlands). A 0.25 vol. of sample loading buffer containing 50 mM dithiothreitol was added to each sample, and the proteins were denatured by heating at 70 °C for 15 min. Electrophoresis was in NuPAGE Mops, pH 7.7, running buffer containing 10% SDS and NuPAGE antioxidant. Alternatively, where stated, the running buffer was Mes, pH 7.3. Proteins were transferred on to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany), and the blots were probed with the affinity-purified antibodies. Antibody binding was detected using anti-sheep, -mouse and -rabbit IgG antibodies or Protein-G–Sepharose conjugated to horseradish peroxidase, followed by ECL® (enhanced chemiluminescence; Amersham Biosciences, Little Chalfont, Bucks., U.K.).
For immunocytological analyses, HeLa and MCF7 (breast adenocarcinoma) cells were grown, fixed in 2% (w/v) paraformaldehyde and permeabilized using 1% Nonidet P40 as described in [37]. After incubation with primary antibodies, FITC- or Texas-Red-conjugated anti-sheep and anti-mouse secondary antibodies were used to detect the cross-reaction. Indirect immunofluorescence staining was visualized with an Openlab digital imaging system (Improvision, Coventry, U.K.) in conjunction with a Leica DM IRB inverted microscope.
Antibodies against the TPR (amino acids Arg16–Leu181) (S412) and the catalytic domain (Arg181–Met499) (S411) of Ppp5 were raised in sheep by the Scottish Antibody Production Unit (Carluke, Penicuik, Midlothian, U.K.) and were affinity-purified against their respective antigens [20]. Anti-Ppp5 peptide antibodies were produced against peptides AEPPRDEPPADGALKR (S731) and AVPHPNVKPMAYAN (S353), corresponding to human Ppp5 amino acids 12–27 and amino acids 478–491 respectively, and were affinity-purified at the Division of Signal Transduction Therapy, Dundee University, co-ordinated by Dr Hilary McLauchlan and Dr James Hastie. Anti-FLAG antibodies were purchased from Sigma-Aldrich (Poole, U.K.) and anti-GFP (green fluorescent protein) was from Invitrogen (De Schelp, The Netherlands). Mouse anti-Hsp70 and anti-Hsp90 antibodies were from Sigma-Aldrich (St. Louis, MO, U.S.A.), and mouse anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was from Research Diagnostics. Anti-sheep and anti-mouse secondary antibodies were from Pierce (Chester, U.K.). Anti-ubiquitin antibodies were from Calbiochem (La Jolla, CA, U.S.A.).
Protein phosphatase assays
Cell lysates were prepared 48 h post-transfection with pCMV-FLAG-PPP5 in lysis buffer containing 1 mM sodium vanadate and 1.0 μM okadaic acid. Anti-FLAG–agarose immunopellets were prepared as described above using 50 μl of anti-FLAG–agarose for the lysate from each 10-cm-diameter plate of cells. The immunopellets were washed once in lysis buffer containing 1 mM sodium vanadate and 1.0 μM okadaic acid, and then three times in lysis buffer without the phosphatase inhibitors. Protein phosphatase activity bound to the resin was determined as described in [38] in the absence of bivalent cations using casein (phosphorylated with protein kinase A and [γ-32P]ATP) as the substrate [39]. One unit of activity is the amount of enzyme which catalyses the release of 1 μmol of [32P]phosphate per min.
RESULTS
Identification of Ppp5-interacting proteins
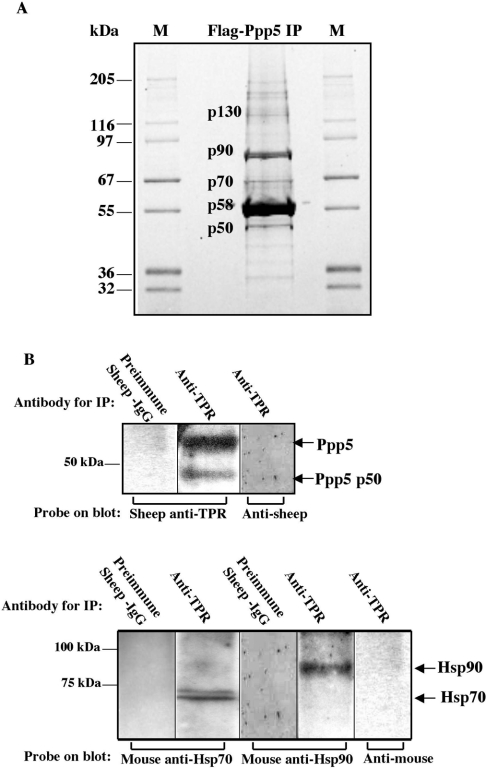
Following transfection of cDNA encoding FLAG-tagged Ppp5 into HEK-293 cells, proteins in the cell lysate were sedimented with anti-FLAG–agarose beads. Figure 1 shows a Sypro-Orange-stained gel of material eluted by the FLAG peptide from anti-FLAG–agarose. The protein bands visualized were excised, digested with trypsin, and identified by MS and comparison of their peptide ions with those from sequences of previously characterized proteins in the UCSF (University of California San Francisco) Protein Prospector database (http://prospector.ucsf.edu/). By this fingerprinting method, we identified Hsp70 as a new protein that interacts with Ppp5 (Figure 1A) and confirmed the co-sedimentation of Hsp90 that has been reported previously [7,11]. A high-molecular-mass form of Ppp5 (approx. 130 kDa) and a proteolytically cleaved form of Ppp5 (p50) were also identified, but no other protein bands were present in sufficient quantities for identification by MS. The interaction of endogenous Ppp5 with endogenous Hsp70 was examined following immunopelleting of Ppp5 from HEK-293 cell lysates with antibodies against the TPR domain of Ppp5. Figure 1(B) shows co-immunoprecipitation of endogenous Hsp70 and Hsp90 with endogenous Ppp5.
Figure 1. Detection of proteins interacting with Ppp5.
(A) Proteins bound to FLAG–Ppp5 were separated by SDS/PAGE and stained with Sypro Orange. Vector expressing FLAG–Ppp5 was transfected into HEK-293 cells. Lysates were incubated with anti-FLAG–agarose, and the adsorbed material was eluted with FLAG peptide from a column of the anti-FLAG–agarose. Proteins in the eluate were separated by SDS/PAGE, excised from the gel and digested with trypsin. Their peptides were analysed using MS, followed by identification of the proteins using the UCSF Protein Prospector database (http://prospector.ucsf.edu/). Bands providing sufficient material for identification by protein fingerprinting are labelled: p50, Ppp5 truncated at C-terminus; p58, FLAG–Ppp5; p70, Hsp70; p90, Hsp90; p130, high-molecular-mass form of Ppp5. M lanes, marker proteins of the indicated molecular masses in kDa. (B) Detection of Hsp70 and Hsp90 in anti-TPR immunopellets. Antibodies against the TPR-domain of Ppp5 coupled to Protein-G–Sepharose or control antibodies coupled to Protein-G–Sepharose were incubated with lysates from HEK-293 cells. Following centrifugation, proteins in the pellet were analysed by SDS/PAGE and subsequent immunoblotting with anti-Hsp70, anti-Hsp90 or anti-TPR antibody, followed by appropriate secondary antibodies and detection by ECL®. Note that the buffer systems in (A) and (B) are different. Ppp5 p50 in (B) migrates at the same position as p50 in (A) when the same buffer system is used.
Arachidonic acid and nocodazole cause loss of Hsp interactions with Ppp5 and increase Ppp5 activity
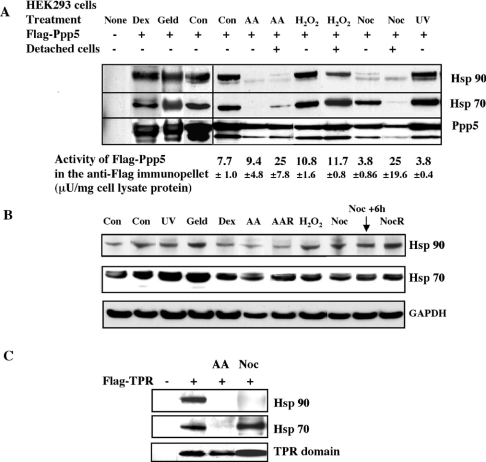
The interaction of FLAG–Ppp5 with Hsp70 and Hsp90 was examined under a variety of conditions. pCMV5-FLAG-PPP5 was transfected into HEK-293 cells, and, after 12–18 h of culture, the cells were subjected to several stimuli. FLAG–Ppp5 was immunopelleted from the cell lysates and examined for the presence of interacting proteins by SDS/PAGE and immunoblotting (Figure 2A). Arachidonic acid caused loss of interaction of FLAG–Ppp5 with Hsp70 and Hsp90 in both adherent and detached cells. Nocodazole caused loss of Hsp70 interaction in detached cells and a large decrease in Hsp90 interactions in both adherent and detached cells. The levels of Hsp70 and Hsp90 in cell extracts were not significantly altered by nocodazole treatment (Figure 2B). The presence of Hsp70 in the FLAG–Ppp5 pellet in the virtual absence of Hsp90 in adherent cells indicates that Hsp70 can bind to Ppp5 in the absence of Hsp90. In contrast with nocodazole treatment, γ-irradiation and aphidicolin (results not shown), UV and H2O2 treatments (Figures 2A and 2B) did not cause any clear reproducible effects on the binding of Hsps to FLAG–Ppp5, although minor variations could not be ruled out by this method. Similar results to those in HEK-293 cells (Figures 2A and 2B) were obtained when the treatments were carried out with MCF7 cells (results not shown). Geldanamycin, which binds to the nucleotide-binding site of Hsp90 and inhibits Hsp90 function [40], did not eliminate the interaction of FLAG–Ppp5 with Hsp90 and Hsp70 in HEK-293 cells. Since Ppp5 has been reported to bind to Hsp90 in glucocorticoid receptor complexes, we tested the effect of dexamethasone on the interaction of the Hsp complexes with Ppp5, but no differences were observed in HEK-293 cells (Figure 2A).
Figure 2. Analysis of Hsp interaction of Ppp5 under different conditions.
(A) HEK-293 cells were transfected with pCMV5-FLAG-Ppp5 and subjected to the various treatments. After the treatments, cell lysates were prepared and FLAG–Ppp5 was immunoadsorbed with anti-FLAG–agarose. Proteins from the immunopellet were examined for the presence of Hsps and by immunoblotting with anti-Hsp70 and anti-Hsp90 antibodies. The FLAG–Ppp5 in the immunopellet was also assayed for phosphatase activity using 32P-labelled casein as a substrate. The average activities from at least three independent assays±S.D. are presented. None, no transfection (untreated cells). Transfected cells: Con, untreated cells; Geld, 5 μM geldanamycin for 8 h; Dex, 500 nM dexamethasone for 8 h; AA, 500 μM arachidonic acid for 8 h; H2O2, 5 mM H2O2 for 30–60 min; Noc, 500 nM nocodazole for 18 h; UV, 200 J/m2 UVC light. Detached cells present in the culture medium after arachidonic acid, H2O2 and nocodazole treatments were also examined. (B) Examination of the levels of endogenous Hsp70 and Hsp90 in HEK-293 cell lysates after the treatments by immunoblotting with anti-Hsp70 and anti-Hsp90 antibodies. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control to check for equal loading of the samples. No clear changes in Hsp70 and Hsp90 levels were observed. AAR, archachidonic-acid-treated detached (rounded-up) cells; NocR, nocodazole-treated detached (rounded-up) cells. (C) Hsp70 interaction occurs with the TPR domain of Ppp5. HEK-293 cells were transfected with pCMV5-FLAG-TPR and subjected to treatments. Cell lysates were prepared from cells adhering to the plates. After immunoadsorption with anti-FLAG–agarose, the immunopellet was examined by immunoblotting for Hsp70 and Hsp90, and the TPR domain. AA, 500 μM arachidonic acid for 8 h; Noc, 500 nM nocodazole for 18 h. Abbreviation: U, unit.
Transfection of vector expressing the FLAG-tagged TPR domain (amino acids 2–240) of Ppp5 into HEK-293 cells, followed by sedimentation of the FLAG–TPR with anti-FLAG–agarose, revealed the presence of Hsp70 and Hsp90 in the immunopellet (Figure 2C), indicating that Hsp70, like Hsp90 [11,27], interacts with the TPR domain. Treatment of the cells with arachidonic acid caused a loss of interaction of the TPR domain with both Hsp70 and Hsp90, while treatment with nocodazole led to a loss of interaction with Hsp90 in adherent cells (Figure 2C), as found for Ppp5. These studies indicate that both nocodazole and arachidonic acid exert their effects via the TPR domain of Ppp5.
Ppp5 activity was assayed following transfection of the cells with FLAG–Ppp5 and immunoprecipitation of the latter with anti-FLAG antibodies. The activity in untreated transfected HEK-293 cells was low, confirming in vitro studies which showed that Ppp5 exists in a low-activity form where access to the catalytic domain is blocked by the TPR domain [20,21]. Treatment of cells did not lead to any very clear changes in Ppp5 activity in adherent cells, although the activities after arachidonic acid and H2O2 treatments in adherent cells were slightly higher than in untreated cells, and in cells treated with UV (Figure 2A), dexamethasone and aphidicolin (results not shown). In detached cells, treatment with arachidonic acid or nocodazole led to a more than 3-fold activation of Ppp5 when the protein phosphatase activities were assayed in immunopellets and calculated per mg of cell lysate protein (Figure 2A). However, the level of FLAG–Ppp5 in immunopellets after arachidonic acid and nocodazole treatments was less than the control levels as judged by blotting (Figure 2A). Therefore the specific activity of FLAG–Ppp5 in arachidonic-acid- and nocodazole-treated detached cells is likely to be substantially more than 3-fold the basal activity of untreated cells. More precise determination of the increases in specific activity proved difficult, due to the variable amounts of FLAG–Ppp5 in the immunopellets from nocodazole- and arachidonic-acid-treated detached cells.
Treatment of HEK-293 cells with 500 μM arachidonic acid caused detachment of some of the cells from the plates (Figure 3A). Although transient expression of FLAG–Ppp5 did not elicit cell detachment, the combined effect of arachidonic acid treatment and transient expression of FLAG–Ppp5 was observed to lead to a synergistic enhancement of detachment of the cells from the plates (Figure 3). On replating the detached cells, they were found to be viable. In order to ascertain the features of Ppp5 involved in this process, we examined the effect of mutants of Ppp5. Transient transfection of the TPR domain of Ppp5 or an inactive FLAG–Ppp5 carrying the mutation H304A (His304→Ala) in the presence of arachidonic acid was found to elicit detachment of cells from the plate, indicating that rounding up of cells and detachment is not dependent on Ppp5 activity. Ppp5-(M1–N419)–HA and FLAG–TPR do not contain the nuclear localization signal of Ppp5, demonstrating that this feature of Ppp5 is also not required. Enhanced cell detachment in the presence of arachidonic acid therefore appears to be entirely dependent on the TPR domain.
Figure 3. Investigation of the effect of expression of Ppp5 and Ppp5 mutants in HEK-293 cells treated with arachidonic acid.
(A) Accumulation of detached cells in the incubation medium. HEK-293 cell cultures were transfected with different pCMV5-FLAG-Ppp5 constructs. At 24 h after transfection, 500 μM arachidonic acid (AA) was added to the incubation medium. Cells present in the incubation medium were collected at various times, cell lysates were prepared, and the protein concentrations were measured by Coomassie Blue reagent and plotted as a function of the incubation time after addition of arachidonic acid. FLAG–TPR and FLAG–Ppp5-(H304A) are inactive mutants of Ppp5. Ppp5-(M1–N419)–HA is lacking the C-terminal nuclear localization signal. (B) Expression of the FLAG–Ppp5 constructs in the HEK-293 cells studied in (A). Samples from the detached, rounded-up (R) and adherent cells (A) were collected and, after lysis, examined for protein expression with by immunoblotting with anti-TPR antibody (S412). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Ppp5 undergoes proteolytic cleavage and increases in molecular mass
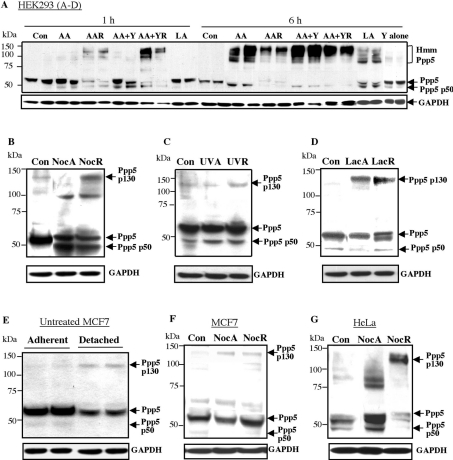
Endogenous Ppp5 was examined by blotting following arachidonic acid and nocodazole treatment (Figures 4A and 4B). In lysates from detached, as well as the adherent, HEK-293 cells, several species of Ppp5 were found with lower molecular mass than 58 kDa, including a prominent form migrating at 45–50 kDa depending on the buffer used (designated p50). Inspection of the entire blots revealed, in addition, the presence of both high-molecular-mass bands of Ppp5 (Hmm, Figure 4A), including a form at approx. 130 kDa (designated p130, Figure 4B). These bands cross-reacted with the anti-TPR antibodies, and were usually more evident in detached rather than adherent cells during arachidonic acid and nocodazole treatments. Further treatment with the Rho kinase inhibitor Y27632 enhanced the effect of arachidonic acid, but the Rho kinase inhibitor by itself did not lead to discernable effects on Ppp5 (Figure 4A). Other treatments, such as UV irradiation, did not cause any obvious changes in Ppp5 detectable by immunoblotting (Figure 4C). Since it was possible that the high-molecular-mass forms were ubiquitinated or otherwise modified before degradation, the cells were incubated with the proteasome inhibitor lactacystin. Lactacystin by itself led to the presence of an approx. 130 kDa form of Ppp5 (Figure 4D), although it did not substantially increase the p130 form of Ppp5 during nocodazole treatment (results not shown). Lactacystin also led to the production of a Ppp5 species migrating only slightly faster than 58 kDa Ppp5 (designated p56, Figure 4D). High-molecular-mass bands of approx. 130 kDa were seen following nocodazole treatment of MCF7 and HeLa cells (Figures 4E–4G). Interestingly, adherent and detached MCF7 cells in the absence of treatment showed slightly different patterns of Ppp5, with Ppp5 at 58 kDa being weaker in detached than adherent cells, and the presence of a weak p130 band being visible in detached cells (Figure 4E).
Figure 4. Appearance of the proteolytically cleaved and high-molecular-mass forms of endogenous Ppp5 after arachidonic acid and nocodazole treatments.
Cells were treated as stated below and collected in NuPAGE sample loading buffer. Electrophoresis was carried out in Mes buffer, and blots were prepared and probed with anti-TPR antibody. (A–D) HEK-293 cells. In blot (A), two lanes are shown per treatment. Hmm, high-molecular-mass forms of Ppp5. (E–F) MCF7 cells, (G) HeLa cells. Con, untreated controls; AA, 500 μM arachidonic acid; LA, 500 μM linoleic acid; Y, 10 μM Rho kinase inhibitor Y27632 present for 1–6 h as indicated. A, adherent cells; R, detached (rounded-up) cells; Noc, 1.7 μM nocodazole for 18 h; UV, 200 J/m2 UVC light; Lac, 5 μM lactacystin for 6 h; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
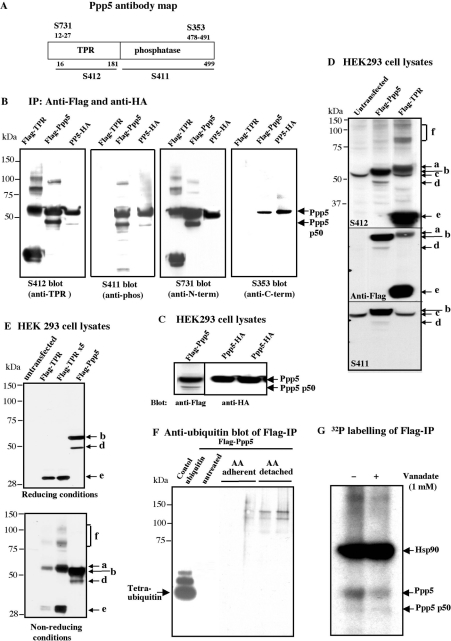
Analysis of the p50 form seen following the expression of FLAG–Ppp5 (Figure 1A) by MS revealed that it comprised the TPR domain plus the phosphatase domain minus the C-terminal region. Inspection of the data showed that no tryptic peptides C-terminal of amino acid Arg425 were detected by fingerprint analysis of p50, whereas some of these peptides were present in Ppp5. The calculated molecular masses of Ppp5-(M1–R425) and FLAG–Ppp5-(A2–R425) are 49 kDa and 50 kDa respectively. Immunoblotting of FLAG–Ppp5 and Ppp5–HA immunopellets from HEK-293 cells expressing these constructs confirmed that p50 was a form of Ppp5 cleaved at the C-terminus (Figures 5A and 5B). Unexpectedly, we found that the N-terminally tagged FLAG–Ppp5 can be cleaved to p50 in cell extracts, but Ppp5 HA-tagged at the C-terminus does not readily undergo cleavage, suggesting the native C-terminus is required to maintain the correct three-dimensional structure for proteolytic cleavage to p50 take place (Figures 5A and 5C). A p56 form of Ppp5 similar to that seen after lactacystin treatment migrating only slightly faster than full-length Ppp5 (Figure 4D) could sometimes be seen in nocodazole-treated cell lysates with anti-(TPR domain) and the anti-(phosphatase domain) antibodies, but not with an antibody raised against an N-terminal peptide (amino acids 12–27), indicating that this form was missing the extreme N-terminus (Figure 6A). It is therefore likely that the increase in lower-molecular-mass forms of Ppp5 in the region 50–56 kDa after arachidonic acid or nocodazole treatment occurs by at least two different proteolytic cleavages.
Figure 5. Analysis of in vivo modifications of the transfected FLAG-tagged construct of Ppp5 under different conditions.
(A) Map of Ppp5 with the regions recognized by the antibodies S411, S412, S353 and S731 marked. (B) FLAG–Ppp5, FLAG–TPR and Ppp5–HA constructs transfected into HEK-293 cells. Cell lysates were subjected to immunoprecipitation with anti-FLAG–agarose and anti-HA–agarose respectively; immunopellets were analysed in immunoblotting with antibodies indicated in the Figure. (C) HEK-293 cells were transfected with FLAG–Ppp5 and Ppp5–HA constructs, and lysates were immunoblotted with anti-Flag and anti-HA antibodies respectively. (D) HEK-293 cells were transfected with FLAG–Ppp5 and FLAG–TPR constructs of Ppp5. Lysates were analysed by immunoblotting with anti-TPR domain (S412), anti-FLAG and anti-phosphatase domain (S411) antibodies. Ppp5 forms depicted: a, 58 kDa form of the TPR domain; b, FLAG–Ppp5; c, endogenous Ppp5; d, FLAG–Ppp5 p50 (truncated from C-terminus); e, FLAG–TPR (29 kDa); f, >80 kDa forms of FLAG–TPR. (E) Detection of the 58 kDa and higher-molecular-mass forms of FLAG–TPR under non-reducing conditions. Lysates from HEK-293 cells that had been transfected with the FLAG–TPR construct (1 μg and 5 μg) and FLAG–Ppp5 construct (1 μg) were analysed by electrophoresis in reducing (see the Materials and methods section) and non-reducing conditions (without dithiothreitol and NuPAGE antioxidant) followed by immunoblotting with anti-FLAG antibody. (F) Investigation of ubiquitinated forms of FLAG–Ppp5. FLAG–Ppp5 was transfected into HEK-293 cells, and, after 24 h, the cells were treated with 500 μM arachidonic acid (AA) for 8 h. Anti-FLAG immunopellets from untreated adherent cells, and from treated adherent and detached cells were examined by immunoblotting with anti-ubiquitin antibodies. (G) Autoradiography of the anti-FLAG immunopellet from HEK-293 cell lysates prepared in the absence (−) and presence (+) of 1 mM vanadate and 1 μM okadaic acid. The cells were transfected with pCMV-FLAG-Ppp5 and labelled with [32P]phosphate.
Figure 6. Analysis of the accumulation of the high-molecular-mass Ppp5 and immunocytological localization of endogenous Ppp5 after treatment with nocodazole.
(A) Immunoblot analysis of MCF7 cells. Con, untreated control MCF7 cells; NA, adherent cells after 500 nM nocodazole for 18 h; NR, detached cells from nocodazole treatment; Rec, adherent cells 24 h after removal of nocodazole. After the above treatments, cells were collected in NuPAGE sample loading buffer and incubated at 70 °C for 15–30 min. Electrophoresis was in Mops buffer, and blots were prepared and probed with antibodies as indicated in the Ppp5 antibody map (Figure 5A), except that the S353 antibody is not shown, because it recognizes the C-terminus only weakly and gave several artifactual bands with lysates. (B) Indirect immunofluorescence of endogenous Ppp5 in MCF7 cells treated as in (A). Ppp5 was visualized using S731, S412 and S411 antibodies, and anti-sheep IgG-FITC secondary antibody with an inverted microscope connected to Openlab digital imaging software (Improvision). Phase-contrast images of the same cells are shown below the immunofluorescent panels. (C) Comparison of the detection of endogenous Ppp5, p50 and p130 by different antibodies. Two lysates from HEK-293 cells treated with nocodazole were immunoblotted with antibodies against the N-terminus (S731), TPR domain (S412) and phosphatase domain of Ppp5 (S411). Note the different intensities of Ppp5, p50 and p130 with the different antibodies. (D) DAPI (4,6-diamidino-2-phenylindole) staining of the detached cells merged with the signal from anti-TPR antibodies (left-hand panels); control with anti-sheep secondary antibody alone and the phase-contrast image of the same cells (right-hand panels).
Transfection of HEK-293 cells with a pCMV5-FLAG-TPR construct led to expression of the FLAG–TPR domain (29 kDa) and a form cross-reacting with anti-FLAG antibodies at 58 kDa (migrating very slightly slower than FLAG–Ppp5), as well as high-molecular-mass species (labelled a and f respectively in Figures 5D and 5E). An examination of the FLAG–TPR domain and FLAG–Ppp5 by immunoblotting after electrophoresis in reducing and non-reducing conditions demonstrated that the TPR domain of Ppp5 was predominantly a dimer (58 kDa) in non-reducing conditions (Figure 5E, lower panel, second lane from the left). Higher-molecular-mass species could also be seen (Figure 5E, lower panel, third lane from the left). In contrast, full-length Ppp5 did not form multimers in non-reducing conditions (Figure 5E, lower panel, fourth lane from the left), although very high amounts of FLAG–Ppp5 showed the presence of low levels of Ppp5 high-molecular-mass species (results not shown), probably due to the presence of FLAG–Ppp5 p50 in the Ppp5 preparations. Dimeric and multimeric species of cleaved Ppp5 therefore appear to arise by formation of cysteine linkages between some of the many cysteine residues in the TPR domain. Probing with anti-ubiquitin antibodies showed that the FLAG–TPR 58 kDa band was not ubiquitinated, although one low-abundance form of FLAG–TPR of approx. 150 kDa gave a good signal (results not shown). Mass spectrophotometric analysis of the p130 band in FLAG–Ppp5 immunopellets (Figure 1A) showed that it comprised Ppp5 peptides covering sequences to Arg441, without the C-terminal amino acids Cys442–Met499 being detected, but there was no evidence for the presence of ubiquitin peptides in the p130 trypsin digest. The data suggest that the high-molecular-mass species of FLAG–Ppp5 migrating at 100–150 kDa (Figure 5E) may be multimers of proteolytically cleaved Ppp5. After treatments which activate Ppp5, such as arachidonic acid, blotting of FLAG–Ppp5 immunopellets from HEK-293 cells with anti-ubiquitin antibodies yielded two weakly cross-reacting bands, suggesting that high-molecular-mass polyubiquitinated forms of Ppp5 are also present (Figure 5F). No cross-reaction of FLAG–Ppp5 with anti-Sumo1 antibodies was obtained.
Proteolysis of endogenous Ppp5 observed in adherent cells precedes the appearance of high-molecular-mass forms of Ppp5 observed prominently in detached cells (Figure 4). Overall, these data suggest that the N- and/or C-terminus of Ppp5 may be proteolytically cleaved following arachidonic acid or nocodazole treatment, leading to several ∼50 kDa forms and an increase in activity. Some of the cleaved Ppp5 species may form multimers stabilized by disulphide bonds. In addition, some high-molecular-mass forms of proteolytically cleaved Ppp5 may be ubiquitinated, and their activity curtailed by proteolytic degradation in proteasomes. Analysis of HEK-293 cells cultured in the presence of [32P]phosphate with or without arachidonic acid treatment showed that, although Hsp90 was phosphorylated on serine, threonine and tyrosine residues (phosphoamino acid analysis; results not shown), Ppp5 contained only trace levels of 32P. No increase in level of phosphotyrosine, phosphoserine and phosphothreonine was detected in Ppp5 with phospho-specific antibodies (Sigma) in response to nocodazole and aphidicolin treatments. Thus we have no evidence for the phosphorylation of Ppp5 occurring before proteolytic activation (Figure 5G).
Activation of Ppp5 may involve translocation of Ppp5 from the nucleus to the cytoplasm
Our previous studies have demonstrated that Ppp5 was mostly nuclear in untreated HeLa cells, but is found in both the cytoplasm and nucleus [4,5,28]. A similar mostly nuclear localization of endogenous Ppp5 was noted in untreated MCF7 cells (Figure 6). After nocodazole treatment, Ppp5 was more abundant in the cytoplasm than the nucleus in both adherent and detached cells. This relocation of Ppp5 was apparent with the anti-N-terminal peptide antibody (S731) and the anti-TPR antibody (S412) (Figure 6B). DAPI (4′,6-diamidino-2-phenylindole) staining of the DNA confirms the presence of the nucleus in the detached cells, where Ppp5 is mainly in the cytoplasm (Figure 6D). The C-terminal peptide antibody (S353) was not sufficiently sensitive for immunocytological analyses. Relocation of Ppp5 from the nucleus to the cytoplasm on nocodazole treatment was less apparent than with the anti-(phosphatase domain) antibodies (Figure 6B). However, it should be noted that there is differential recognition of the different forms of Ppp5 by the two antibodies, with the anti-(phosphatase domain) antibody showing higher affinity for full-length Ppp5 (p58) relative to the p50 and p130 forms of Ppp5 than does the anti-TPR domain antibody (Figure 6C). Thus the high ratio of nuclear/cytoplasmic stain with the anti-(phosphatase domain) antibody supports the idea that full-length Ppp5 is mostly nuclear and translocates to the cytoplasm upon proteolytic cleavage. Lactacystin enhanced the relocation of Ppp5 to the cytoplasm (results not shown). This alteration in subcellular localization of Ppp5 is consistent with cleavage of the C-terminal region, which has been shown previously to encompass a nuclear localization signal [5]. Cleavage of the N-terminal region would not be expected to alter the subcellular localization of Ppp5.
DISCUSSION
Ppp5 is involved in stress responses that may lead to cell-cycle arrest
The yeast two-hybrid system has been employed extensively to search for proteins that interact with Ppp5 [9,10,14–19], but this method may identify sections of proteins that do not normally interact with Ppp5 in vivo. We therefore took a different approach of identifying native proteins that could be found associated with FLAG-tagged Ppp5 in mammalian cell lysates, and then specifically eluting the FLAG–Ppp5 complexes using FLAG peptide. By this method, Hsp70 and Hsp90 were the only major interacting proteins identified. Hsp70, not known previously to bind to Ppp5, was found to interact with the TPR domain of Ppp5, as found previously for Hsp90 [27]. We then searched for conditions that caused loss of Hsp binding to Ppp5. Of the reagents we tested, arachidonic acid and the microtubule-depolymerizing agent nocodazole were the most effective in causing loss of Hsp70 and Hsp90 interactions with Ppp5, particularly in cells that had rounded-up and detached from the plates, because, in the case of nocodazole treatment, they were undergoing a metaphase cell-cycle arrest. Nocodazole, like arachidonic acid, was found to exert its action via an effect on the TPR domain of Ppp5. Both agents, arachidonic acid at slightly higher than physiological levels and nocodazole, which causes depolymerization of microtubules resulting in damage to the mitotic spindle, are likely to lead to significant cellular stress. Our finding that the endogenous Ppp5 binds to endogenous Hsp70 and Hsp90, and the fact that a major role of these Hsps is response to cellular stress [41], points to a function for Ppp5 in stress-related processes.
Nocodazole also causes activation of the spindle-damage checkpoint at metaphase [42]. This checkpoint has two arms in mammalian cells, which are thought to monitor the attachment of microtubules to kinetochores and the generation of tension that results from bipolar attachment of sister chromatids. In response to unattached kinetochores, a Mad2 (mitotic arrest deficient 2)dependent arm prevents Cdc20 from activating the APC/C (cyclosome) and degrading securin and mitotic cyclins, thereby preventing sister chromatid separation and anaphase. In response to lack of spindle tension, Bub2 (budding uninhibited by benzimidazole 2) protects Clb2 (cyclin B2) from degradation by APC–Cdh1 (Cdc20 homologue), thereby preventing cytokinesis and continued cell-cycle progression. Activation of Ppp5 may dephosphorylate or maintain certain proteins in their dephosphorylated forms to arrest cell-cycle progression. Alternatively, or in addition, since several components become phosphorylated when the spindle-damage checkpoint is activated by nocodazole, Ppp5 may be required to dephosphorylate one or possibly several components of the checkpoint pathway to allow cell-cycle progression to continue. However, at present, we do not have any direct evidence for the function of Ppp5 in metaphase arrest. Other studies have implicated Ppp5 in a p53-dependent cell-cycle arrest at G1 in response to glucocorticoids [8,12], S-phase cell-cycle arrest in response to ionizing irradiation [19] and in DNA-repair processes after ionizing radiation [18].
Since Ppt1 is a non-essential gene in S. cerevisiae [4], it is unlikely that its mammalian orthologue Ppp5 is directly involved in essential cellular processes or metabolic pathways unless its function is redundant with that of another protein serine/threonine phosphatase. Stress signals are not essential in optimal cell environments, and are only used in adverse conditions. The cellular stress may then sometimes retard entry into mitosis to allow repair processes to operate and thereby ensure genomic stability. A function in such stress pathways would explain why deletion of the PPT1 gene in S. cerevisiae is not detrimental in a variety of media and conditions tested [4]. Depletion of Ppp5 in Drosophila Schneider 2 cells using RNAi (RNA interference) does not affect cell growth or survival in routine culture conditions [43]. The advantages of responding to stress appropriately and/or recovering from the stress when the triggering insult has been removed would explain why Ppt1 or its orthologue Ppp5 has been conserved throughout eukaryotic evolution. The data in Figure 4(E), which show that the detached MCF7 cells have a slightly different Ppp5 pattern on immunoblotting with a decrease in full-length Ppp5 and an increase in p130, support the concept that a proportion of the detached cells will be damaged and may therefore be responding to stress and/or checkpoint arrest.
Since human Ppp5 was found to interact with ASK1 in a yeast two-hybrid analysis, and ASK1 is activated in response to H2O2 treatment, Ppp5 was proposed to play a role in oxidative stress [9]. Ppp5 was thought to be required for negative regulation of ASK1 via dephosphorylation of a phosphothreonine residue within the activation loop of ASK1. It is therefore interesting that our preliminary results found that, although H2O2 did not fully dissociate Ppp5 from Hsp70 and Hsp90, high-molecular-mass forms of Ppp5 were sometimes observed in HEK-293 cell lysates after H2O2 treatment (results not shown), consistent with a role for Ppp5 in recovery from oxidative stress. It is also possible that other forms of stress which cause cell-cycle or growth arrest may activate Ppp5 without complete dissociation from Hsps, consistent with a role for Ppp5 in other stress-related processes [8,12,17–19].
The finding that the main proteins binding to Ppp5 are Hsp90 and Hsp70, and the presence of a TPR domain in Ppp5, raises the question of whether Ppp5 acts as a co-chaperone for the Hsps. Co-chaperones influence the interaction of client proteins with Hsp90, and several known co-chaperones bind to Hsp90 through their TPR domains [44]. Reagents such as arachidonic acid and nocodazole, which we have shown to interfere with the binding of Hsp90 to Ppp5, would then be expected to interfere with some of the functions of Hsp90. A decrease in Hsp90-mediated folding of proteins in mitogenic signalling cascades may lead to cell-cycle arrest [44,45]. Alternatively, or in addition, Ppp5 might be important in maintaining the correct level of phosphorylation of Hsp90, which has been linked to the chaperoning function of Hsp90 [46].
Mechanism of activation of Ppp5
The likely participation of Ppp5 in stress responses that may be involved in cell-cycle arrest indicates that it will be important to determine the pathway and mechanism of activation of Ppp5. Our results indicate that Ppp5 is maintained in a low-activity form bound to Hsp70 and/or Hsp90 until it is required. The amphipathic groove visible in the crystal structure of the TPR domain of Ppp5 was predicted to bind target proteins [25]. Mutation of basic residues (Lys32, Arg74, Lys97 and Arg101) with side chains pointing into this groove show that this region is responsible for binding to the acidic C-terminus of Hsp90 [27]. Hsp70 and Hsp 90 form multichaperone complexes through the adaptor Hop (Hsp-organizing protein), which has distinct TPR domains (each comprising three TPR motifs) that bind to the acidic C-termini (EEVD) of these two Hsps [47]. Hsp70 may therefore be present in Ppp5 immunopellets through association with Hsp90. However, in the studies described in the present paper, the virtually complete loss of Hsp90 binding to Ppp5 without loss of Hsp70 binding (Figure 2) suggests that Hsp70 binds to Ppp5 independently of Hsp90. By comparison with the TPR domains of Hop that bind Hsp70 and Hsp90, the TPR domain of Ppp5 comprising three TPR motifs could only directly bind to either Hsp70 or Hsp90. Consequently the data indicate that Ppp5 may form complexes with Hsp70 that are independent of complex formation with Hsp90.
Loss of binding of Hsps following treatment of cells with arachidonic acid or nocodazole correlates with activation of Ppp5. The activity of bacterially expressed Ppp5 can be stimulated substantially by proteolysis [20,21], the precise level of stimulation depending on the substrate used and the basal activity of the bacterially expressed Ppp5, which may vary substantially in different preparations, probably because trace proteolysis activates the enzyme and leads to higher basal levels. Ppp5 was found to be activated more than 25-fold towards casein by trypsin proteolysis, which removes the TPR domain, leaving an active phosphatase domain fragment (Ser160–Met499), and this was similar to its activation by arachidonic acid [20]. These studies indicated that full-length Ppp5 may be essentially inactive, and that displacement of the inhibitory TPR domain by proteolysis or lipid binding can lead to activation of the enzyme. Kang et al. [22] found that mutation of Glu76 in the loop between the two α-helices of TPR domain 2 resulted in a 10-fold elevation in basal activity. Sinclair et al. [21] found that subtilisin proteolysis produced a phosphatase domain fragment that additionally had ten residues cleaved from the C-terminus, and showed that this fragment Ppp5 (Thr171–Met489) and truncated Ppp5 (Met1–Pro486) exhibited specific activities towards several substrates that were 4–14-fold higher than the basal activity of Ppp5 and comparable with those of lipid-stimulated Ppp5. The data suggest that the C-terminal 13 amino acids act in a co-ordinated manner with the N-terminal TPR domain to suppress the activity of Ppp5 and mediate its activation by lipid.
In the present study, following treatment of cells with arachidonic acid or nocodazole, endogenous 58 kDa Ppp5 appears to be proteolytically cleaved, yielding a prominent fragment, termed p50, as well as lower levels of other fragments, close in size to p58. We have shown by mass-spectrophotometric analysis and immunoblotting that a significant fraction of N-terminally FLAG-tagged Ppp5 expressed in mammalian cells is cleaved at the C-terminus to yield a p50 fragment (Figures 1A and 1B). In contrast, immunoblotting of endogenous Ppp5 after arachidonic acid or nocodazole treatments reveals proteolytic cleavage at the N-terminus as well as at the C-terminus. The occurrence of several endogenous Ppp5 fragments of different sizes supports the concept that there may be more than one proteolytic cleavage that leads to activation of Ppp5. The C-terminal 80 amino acids of Ppp5 have been shown to encode an unusual nuclear localization signal that is probably located in the highly conserved section Phe476–Asn491 [5]. Proteolysis at the C-terminus may therefore be expected to increase the cytoplasmic location of Ppp5, providing an explanation for the increased cytoplasmic immunostaining of Ppp5 observed after nocodazole treatment in the present study, as well as in other situations [9,10,27]. Cleavage of the N-terminus only could lead to an active form of Ppp5 that would be retained in the nucleus.
High-molecular-mass forms of endogenous Ppp5 are formed after activation of Ppp5 by proteolysis in response to arachidonic acid and nocodazole (Figure 4). Some high-molecular-mass species of Ppp5 are ubiquitinated (Figure 5F), supporting the concept that degradation of ubiquitinated forms via the proteasome occurs consecutively to activation by proteolysis. However, expression of FLAG–Ppp5 produces a 130 kDa species that by mass-spectrophotometric analysis does not appear to be ubiquitinated and is likely to be a proteolytically cleaved FLAG–Ppp5 dimer. Although it is possible that this form of FLAG–Ppp5 arises artifactually from the high level of expression of FLAG–Ppp5, it may also be that proteolytically cleaved endogenous Ppp5 may form dimers or multimers, as well as undergoing ubiquitination. These polymeric species may be stabilized in non-reducing or even in partially reducing electrophoretic conditions by the formation of a cysteine bond.
Our model for Ppp5 function would therefore be that during or immediately after synthesis Ppp5 binds to Hsp70. Maturation of Ppp5 may then allow displacement of Hsp70 by Hsp90 via formation of multi-chaperone complexes containing Hsp70, Hop and Hsp90. Ppp5 is stored in a low-activity state bound to Hsp90 in the nucleus. In response to nocodazole and possibly other stresses, some of which cause cell-cycle or growth arrest, Ppp5 dissociates from the Hsps and is activated by proteolytic cleavage (Scheme 1). Activation of Ppp5 by proteolysis does not exclude the possibility of some prior modification, such as phosphorylation, but we do not have any evidence for such an event. Proteolysis may occur at the N- or C-terminus. When proteolysis removes the C-terminal nuclear localization signal, Ppp5 relocates to the cytoplasm (in the absence of nuclear membrane breakdown), possibly by diffusion, since at present there is no evidence for a nuclear export signal in Ppp5. Proteolytically cleaved Ppp5 may also form dimers and multimers stabilized by disulphide bonds, which conceivably act as a store of active monomers. Active Ppp5 may then dephosphorylate proteins required in their unphosphorylated forms for activation of/recovery from stress processes that may involve cell-cycle arrest. The active Ppp5 may then be modified by polyubiquitination and destroyed by proteolytic degradation via the proteasome. Other studies have shown that Ppp5 may interact with gluticocorticoid receptor complexes [11], and that Ppp5 may be activated by interaction with other proteins [9,18]. The recognition that Ppp5 may be involved in stress-activated checkpoint arrest or recovery suggests that an inhibitor of Ppp5 or its activation system may act favourably in concert with microtubule-targeting drugs to kill cancer cells more effectively.
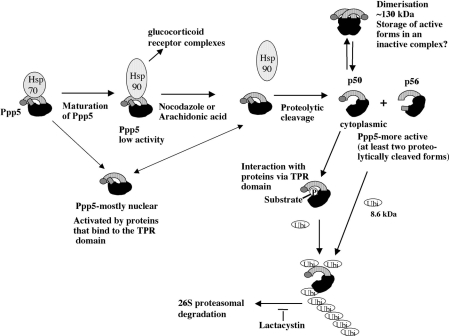
Scheme 1. Schematic representation of the proposed model for the in vivo regulation of Ppp5.
Ppp5 comprises a short N-terminal section (grey), a TPR domain (striped), a phosphatase catalytic domain (black) and a C-terminal region (hatched) that includes an unusual nuclear localization sequence. The N-terminal half of Ppp5, including the TPR domain and the C-terminal region, are autoinhibitory. Proteolytic cleavage may remove the C-terminal hatched region or the N-terminal grey section. Newly synthesized Ppp5 forms a complex with Hsp70. Via multichaperone complexes, Hsp90 may displace Hsp70, and Ppp5 bound to Hsp90 may move to the nucleus in a low-activity form. In response to nocodazole, arachidonic acid and possibly other stress stimuli, Ppp5 (58 kDa) dissociates from the Hsps and is proteolytically cleaved at either the C-terminus (p50) or the N-terminus (p56). Cleavage at the C-terminus allows Ppp5 to translocate to the cytoplasm. A small fraction of proteolytically cleaved Ppp5 may form dimers (or multimers) by interaction of the TPR domains, which may serve as a store for the production of active monomers. Proteolytic cleaved Ppp5 monomers dephosphorylate phosphoprotein substrate(s). The phosphate moiety of the substrate may initially bind to the TPR domain, allowing the phosphatase domain to catalyse the cleavage of phosphate from the substrate. Active Ppp5 is then polyubiquitinated and destroyed via the proteasome. Other studies suggest that Ppp5 may be activated in glucocorticoid receptor–Hsp90 complexes [7], other Hsp90 complexes [17] or by interaction of its TPR domain with other proteins [9,10,13,18,19].
Acknowledgments
This work was funded by the Medical Research Council, U.K.
References
- 1.Cohen P. T. W. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem. Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva A. V., Kutuzov M. A. RdgC/PP5-related phosphatases: novel components in signal transduction. Cell Signalling. 1999;11:555–562. doi: 10.1016/s0898-6568(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 3.Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol. Metab. 2001;12:28–32. doi: 10.1016/s1043-2760(00)00335-0. [DOI] [PubMed] [Google Scholar]
- 4.Chen M. X., McPartlin A. E., Brown L., Chen Y. H., Barker H. M., Cohen P. T. W. A novel human protein serine/threonine which possesses four tetratricopeptide repeat (TPR) motifs and localizes to the nucleus. EMBO J. 1994;13:4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthwick E. B., Zeke T., Prescott A. R., Cohen P. T. W. Nuclear localization of protein phosphatase 5 is dependent on the carboxy-terminal region. FEBS Lett. 2001;491:279–284. doi: 10.1016/s0014-5793(01)02177-9. [DOI] [PubMed] [Google Scholar]
- 6.Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M.-S., Silverstein A. M., Pratt W. B., Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J. Biol. Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 8.Zuo Z., Urban G., Scammell J. G., Dean N. M., McLean T. K., Aragon I., Honkanen R. E. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]
- 9.Morita K., Saitoh M., Tobiume K., Matsuura H., Enomoto S., Nishitoh H., Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y., Katoh H., Mori K., Negishi M. Gα12 and Gα13 interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr. Biol. 2002;12:1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein A. M., Galigniana M. D., Chen M.-S., Owens-Grillo J. K., Chinkers M., Pratt W. B. Protein phosphatase 5 is a major component of glucocorticoid receptor–hsp90 complexes with properties of an FK506-binding immunophilin. J. Biol. Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- 12.Zuo Z., Dean N. M., Honkanen R. E. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21WAF1/Cip1 and mediate growth arrest. J. Biol. Chem. 1998;273:12250–12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- 13.Urban G., Golden T., Aragon I. V., Cowsert L., Cooper S. R., Dean N. M., Honkanen R. E. Identification of a functional link for the p53 tumor suppressor protein in dexamethasone-induced growth suppression. J. Biol. Chem. 2003;278:9747–9753. doi: 10.1074/jbc.M210993200. [DOI] [PubMed] [Google Scholar]
- 14.Ollendorf V., Donoghue D. J. The serine/threonine phosphatase PP5 interacts with CDC16 and CDC27, two tetratricopeptide repeat-containing subunits of the anaphase-promoting complex. J. Biol. Chem. 1997;272:32011–32018. doi: 10.1074/jbc.272.51.32011. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S., Sancar A. Human blue-light photoreceptor hCRY2 specifically interacts with protein serine/threonine phosphatase 5 and modulates its activity. Photochem. Photobiol. 1997;66:727–731. doi: 10.1111/j.1751-1097.1997.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 16.Lubert E. J., Hong Y., Sarge K. D. Interaction between protein phosphatase 5 and the A subunit of protein phosphatase 2A. J. Biol. Chem. 2001;276:38582–38587. doi: 10.1074/jbc.M106906200. [DOI] [PubMed] [Google Scholar]
- 17.Shao J., Hartson S. D., Matts R. L. Evidence that protein phosphatase 5 functions to negatively modulate the maturation of the Hsp90-dependent heme-regulated eIF2α kinase. Biochemistry. 2002;41:6770–6779. doi: 10.1021/bi025737a. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler T., Chen B. P., Harper R., Morotomi-Yano K., Huang B. C., Meek K., Cleaver J. E., Chen D. J., Wabl M. DNA–PKCs function regulated specifically by protein phosphatase 5. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1247–1252. doi: 10.1073/pnas.0307765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali A., Zhang J., Bao S., Liu I., Otterness D., Dean N. M., Abraham R. T., Wang X. F. Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev. 2004;18:249–254. doi: 10.1101/gad.1176004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M. X., Cohen P. T. W. Activation of protein phosphatase 5 by limited proteolysis or the binding of fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair C., Borchers C., Parker C., Tomer K., Charbonneau H., Rossie S. The tetratricopeptide repeat domain and a C-terminal region control the activity of Ser/Thr protein phosphatase 5. J. Biol. Chem. 1999;274:23666–23672. doi: 10.1074/jbc.274.33.23666. [DOI] [PubMed] [Google Scholar]
- 22.Kang H., Sayner S. L., Gross K. L., Russell L. C., Chinkers M. Identification of amino acids in the tetratricopeptide repeat and C-terminal domains of protein phosphatase 5 involved in autoinhibition and lipid activation. Biochemistry. 2001;40:10485–10490. doi: 10.1021/bi010999i. [DOI] [PubMed] [Google Scholar]
- 23.Skinner J., Sinclair C., Romeo C., Armstrong D., Charbonneau H., Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J. Biol. Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey A. J., Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–5632. doi: 10.1021/bi016090h. [DOI] [PubMed] [Google Scholar]
- 25.Das A. K., Cohen P. T. W., Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein–protein interactions. EMBO J. 1998;15:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young J. C., Obermann W. M. J., Hartl F. U. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J. Biol. Chem. 1998;273:18007–18010. doi: 10.1074/jbc.273.29.18007. [DOI] [PubMed] [Google Scholar]
- 27.Russell L. C., Whitt S. R., Chen M.-S., Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J. Biol. Chem. 1999;274:20060–20063. doi: 10.1074/jbc.274.29.20060. [DOI] [PubMed] [Google Scholar]
- 28.Brown L., Borthwick E. B., Cohen P. T. W. Drosophila protein phosphatase 5 is encoded by a single gene that is most highly expressed during embryonic development. Biochim. Biophys. Acta. 2000;1492:470–476. doi: 10.1016/s0167-4781(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri M. Cloning and characterization of a novel serine/threonine protein phosphatase type 5 from Trypanosoma brucei. Genes Dev. 2001;266:1–13. doi: 10.1016/s0378-1119(01)00367-5. [DOI] [PubMed] [Google Scholar]
- 30.Lindenthal C., Klinkert M. Q. Identification and biochemical characterization of a protein phosphatase 5 homologue from Plasmodium falciparum. Mol. Biochem. Parasitol. 2002;120:257–268. doi: 10.1016/s0166-6851(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen M. X., Chen Y. H., Cohen P. T. W. Polymerase chain reactions using Saccharomyces, Drosophila and human DNA predict a large family of protein serine/threonine phosphatases. FEBS Lett. 1992;306:54–58. doi: 10.1016/0014-5793(92)80836-6. [DOI] [PubMed] [Google Scholar]
- 32.Jeong J. Y., Johns J., Sinclair C., Park J. M., Rossie S. Characterization of Saccharomyces cerevisiae protein Ser/Thr phosphatase T1 and comparison to its mammalian homolog PP5. BMC Cell Biol. 2003;4:3. doi: 10.1186/1471-2121-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatzkan E., Yarden O. ppt-1, a Neurospora crassa PPT/PP5 subfamily serine/threonine protein phosphatase. Biochim. Biophys. Acta. 1997;1353:18–22. doi: 10.1016/s0167-4781(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 34.Meek S., Morrice N., MacKintosh C. Microcystin affinity purification of plant protein phosphatases: PP1C, PP5 and a regulatory A-subunit of PP2A. FEBS Lett. 1999;457:494–498. doi: 10.1016/s0014-5793(99)01093-5. [DOI] [PubMed] [Google Scholar]
- 35.Becker W., Kentrup H., Klumpp S., Schultz J. E., Joost H. G. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J. Biol. Chem. 1994;269:22586–22592. [PubMed] [Google Scholar]
- 36.Carnegie G. K., Sleeman J. E., Morrice N., Hastie C. J., Peggie M. W., Philp A., Lamond A. I., Cohen P. T. W. Protein phosphatase 4 interacts with the survival of motor neurons complex and enhances the temporal localisation of snRNPs. J. Cell Sci. 2003;116:1905–1913. doi: 10.1242/jcs.00409. [DOI] [PubMed] [Google Scholar]
- 37.Hastie C. J., Carnegie G. K., Morrice N., Cohen P. T. W. A novel 50 kDa protein forms complexes with protein phosphatase 4 and is located at centrosomal microtubule organizing centres. Biochem. J. 2000;347:845–855. [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen P. T. W., Browne G. J., Delibegovic M., Munro S. Assay of protein phosphatase 1 complexes. Methods Enzymol. 2003;366:135–144. doi: 10.1016/s0076-6879(03)66012-x. [DOI] [PubMed] [Google Scholar]
- 39.MacKintosh C., Moorhead G. Assay and purification of protein (serine/threonine) phosphatases. In: Hardie D. G., editor. Protein Phosphorylation: a Practical Approach. Oxford: Oxford University Press; 1999. pp. 153–181. [Google Scholar]
- 40.Roe S. M., Prodromou C., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999;42:260–262. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 41.Nollen E. A. A., Morimoto R. I. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J. Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 42.Musacchio A., Hardwick K. G. The spindle checkpoint; structural insights into dynamic signalling. Nat. Rev. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- 43.Silverstein A. M., Barrow C. A., Davis A. J., Mumby M. C. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young J. C., Moarefi I., Hartl F. U. Hsp90: a specialized but essential protein folding tool. J. Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmbrecht K., Zeise E., Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y.-G., Gilmore R., Leone G., Coffey M. C., Weber B., Lee P. W. K. Hsp90 phosphorylation is linked to its chaperoning function. J. Biol. Chem. 2001;276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
- 47.Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H., Hartl F. U., Moarefi I. Structure of the TPR domain–peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]