Abstract
The two COX (cyclo-oxygenase) isoenzymes COX-1 and -2 catalyse the initial step in the conversion of arachidonic acid into PG (prostaglandin) hormones. The identification of an mRNA transcript encoding a splice variant of human COX-1 was reported more than a decade ago [Diaz, Reginato and Jimenez (1992) J. Biol. Chem. 267, 10816–10822], yet catalytic activity and tissue expression of the corresponding spliced protein remained uncharacterized. The splice variant lacks amino acids 396–432, corresponding to the last 37 amino acids of exon 9 of the gene encoding COX-1. These amino acids form a loop at one side of the peroxidase active site of the protein. We expressed the full-length and spliced COX-1 cDNAs in COS-7 and Sf9 insect cells, and determined the PG-forming activity using incubations with radiolabelled arachidonic acid and HPLC analyses. When expressed in either system, abundant PG formation was observed with the full-length COX-1, whereas the spliced protein did not form any detectable product. Peroxidase activity was readily detected in microsomes prepared from COS-7 cells transfected with COX-1 but not with the splice variant. In reverse transcriptase–PCR experiments, we detected the mRNA for the alternatively spliced and full-length COX-1 in human brain, tonsil and colon tissue, yet we were unable to detect expression of the spliced protein in the same tissues using immunoprecipitation and Western-blot analyses. We conclude that, whereas the mRNA transcript for the spliced COX-1 is present in various human tissues, the corresponding protein is either not formed or subject to rapid proteolytic degradation.
Keywords: arachidonic acid, cyclo-oxygenase 1 (COX-1), expression, prostaglandin, prostaglandin H synthase (PGH synthase), splice variant
Abbreviations: ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid); COX, cyclo-oxygenase; HETE, hydroxyeicosatetraenoic acid; PG, prostaglandin; RACE, rapid amplification of cDNA ends; RP, reversed phase; RT, reverse transcriptase; UTR, untranslated region
INTRODUCTION
The COX (cyclo-oxygenase) isoenzyme COX-1 plays fundamental roles in the generation of PGs (prostaglandins) in homoeostasis and several other physiological functions including gastric protection and control of renal blood flow [1,2]. The door remains open, also, to potential roles for COX-1 as a primary player in pathogenesis; for example, COX-1 was implicated recently in a specific instance of carcinogenesis in the human ovary [3]. As is the case with both COX isoenzymes, COX-1 is a bifunctional protein with separate active sites that catalyse the oxygenase and the peroxidase reactions in the transformation of arachidonic acid to the PG endoperoxide, PGH2 [4]. The two enzymic activities are mechanistically linked through the haem group located in the peroxidase active site [5,6]. In the COX active site, arachidonic acid is doubly oxygenated to PGG2, which is subsequently reduced at the peroxidase site to the corresponding hydroxide, PGH2 [5–7]. PGH2 is the common precursor to all PG hormones, including thromboxane and prostacyclin [1,8].
In 1992, an alternatively spliced transcript of the human PTGS1 (prostaglandin synthase-1) gene was discovered in cloning experiments using a human lung fibroblast cDNA library by Diaz et al. [9]. Subsequent expression analysis revealed the presence of the spliced mRNA transcript in fetal and adult tibial cartilage, and in dermal, lung and synovial fibroblasts. In the same study, it was shown that treatment of cultured human lung fibroblasts with transforming growth factor β1, interleukin 1β and tumour necrosis factor α caused a 1.6–2.6-fold up-regulation of the spliced mRNA when compared with the intact COX-1 transcript [9]. Lack of 111 nt in this splice variant does not change the reading frame, but results in a shorter, but otherwise intact, polypeptide chain. The missing 37 amino acids include Asn-409, a glycosylation site required for correct protein folding [10]. The absence of Asn-409 in the spliced transcript led to the (unproven) assumption that the corresponding protein might lack enzymic activity [9]. This is not a certainty, as we describe herein that the two ‘ends’ of the polypeptide to be rejoined across the missing exon lie close to each other in the three-dimensional structure of the COX-1 protein.
The splice variant reported by Diaz et al. is different from a second, more recently discovered variant in dog brain that includes extra coding sequence by incorporation of intron 1 into the mRNA transcript, and that was given the (mis)nomer COX-3 [11]. The latter is not expressed as a functional protein in human, rat or mouse as the corresponding additional intron 1 sequence in the mRNA transcript spans 94 (human) or 98 (rat and mouse) nt, thus shifting the coding sequence out of frame [12–14].
In the spliced mRNA transcript of human COX-1 detected by Diaz et al. [9], the last 111 nt of exon 9 corresponding to amino acids 396–432 are absent. The question of tissue expression and catalytic activity of the spliced COX-1 protein has remained unaddressed to date. The initial analyses of mRNA expression pointed towards regulation of the splice variant being different from that of the full-length protein [9], thus opening up the possibility of a distinct COX enzyme that differs from COX-1 and -2. We detected the mRNA in the course of RT (reverse transcriptase)–PCR experiments with human cDNAs and were prompted to further investigate its potential significance. In the study reported here, we have cloned and expressed the spliced COX-1 cDNA to investigate its catalytic activities and analysed the expression of mRNA and protein in various human tissues.
EXPERIMENTAL
Cloning of full-length and spliced COX-1 cDNAs
Full-length and spliced human COX-1 cDNAs were cloned by PCR from human tonsil cDNA using a forward primer with a 5′ BamHI site and a reverse primer encoding a 3′ NotI site [15,16]. Initially, the clones were sequenced in the pCR2.1 vector (Invitrogen), and then subcloned into the pcDNA3.1 vector (Invitrogen) and the pVL1393 baculovirus transfer vector (BD Biosciences) using the BamHI and NotI restriction sites.
Expression in COS-7 and Sf9 cells
Full-length and spliced human COX-1 cDNAs in the pcDNA3.1 vector were transiently transfected into COS-7 cells using FuGENE6 (Roche) as described previously [16]. Cells were harvested 48 h post-transfection by scraping and washed once with PBS. Sf9 insect cells were infected with virus and grown for 72 h according to the manufacturer's instructions (BD Biosciences). Cells were harvested by centrifugation, washed with PBS and stored at −80 °C until use.
Incubations and product analyses
COS-7 and Sf9 cells were disrupted by sonication on ice in incubation buffer (100 mM Tris/HCl, 0.5 mM phenol, pH 8.0 and 1 μM haematin). Equal amounts of cells expressing full-length or spliced COX-1 were used for incubations with 50 μM [1-14C]arachidonic acid in a total volume of 100 μl for 10 min at room temperature (25 °C). After protein precipitation by the addition of 250 μl of methanol and 125 μl of methylene chloride, the samples were extracted using 100 mg of Oasis HLB cartridges (Waters, Milford, MA, U.S.A.) as described earlier [16,17].
Product formation was analysed by RP (reversed phase)-HPLC using a Waters Symmetry C18 5 μm column (0.46 cm×25 cm) eluted at a flow rate of 1 ml/min with a stepwise gradient of the following solvents: acetonitrile/water/acetic acid in the ratio 37.5:62.5:0.01 (by vol.) for 15 min, next in the ratio 70:30:0.01 (by vol.) for 15 min and finally with methanol. Before HPLC analysis, unlabelled standards of PGD2, PGE2 and PGF2α (2 μg each) and HETEs (hydroxyeicosatetraenoic acids; 0.2 μg each) were added to facilitate UV detection of the peaks. Chromatography was monitored using an Agilent 1100 series diode array detector coupled on-line with a Packard A140 Radiomatic liquid-scintillation detector.
Preparation of microsomes
Eight confluent 100 mm culture plates, each of COS-7 cells transiently transfected with the full-length or spliced human COX-1 cDNA in pcDNA3.1 or with vector alone, were used. Cells were scraped and harvested by centrifugation. After sonication in 10 ml of 100 mM Tris/HCl buffer (pH 7.4), cells were centrifuged at 1000 g for 10 min, and the supernatant was centrifuged again at 10000 g for 10 min. Microsomes were pelleted by centrifugation at 100000 g for 60 min. The supernatant was removed and the microsomes were dissolved in 100 μl of 100 mM Tris/HCl buffer (pH 7.4) by vortex-mixing and passing through a glass homogenizer on ice. Protein content was determined using the Bradford protein assay (Bio-Rad).
Peroxidase activity
Peroxidase activity was measured spectrophotometrically by following the increase in absorbance at 417 nm using a PerkinElmer PE35 spectrophotometer. To a microcuvette (1 cm path length) containing 500 μl of 100 mM Tris/HCl, 0.5 mM phenol (pH 8.0), haematin (1 μM final) and 200 μM ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)], the microsome preparations (30 μg of protein each) from transiently transfected COS-7 cells were added. After 1 min of equilibration, the reaction was started by the addition of 1 μl of a solution of 20 mM H2O2 in water. The reaction was recorded at room temperature for 10 min.
Analysis of tissue expression
The frontal, temporal and cerebellar cortex of human brain from two subjects each were used. Tissue was collected less than 6 h post mortem. Colon tissue consisted of matching pairs of normal and tumour tissue samples from four patients. Total RNA was extracted from tissue using TRI Reagent (Molecular Research Center, Cincinnati, OH, U.S.A.) following the manufacturer's instructions. cDNA was synthesized using 20 μg of total RNA from brain and colon in each reaction using an oligo(dT) adaptor primer (5′-ATGAATTCGGTACCCGGGATCCTTTTTTTTTTTTTTTTT-3′) with and without the addition of murine MLV RT (Promega) following a standard method [18]. Several samples of tonsil cDNA prepared with the oligo(dT) adaptor primer were available from a previous study [15]. RT–PCR experiments for detection of the spliced and full-length COX-1 transcripts were conducted in 50 μl reactions using Amplitaq Gold polymerase (PerkinElmer) and standard reaction conditions. The forward and reverse primers were 5′-AGTGGCTATTTCCTGCAG-3′ and 5′-GAGACTCCCTGATGACAT-3′ respectively. The following temperature programme was used: 95 °C for 10 min (1 cycle); 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s (30 cycles); 72 °C for 7 min. PCR products were separated using gel electrophoresis (3% agarose) and visualized with ethidium bromide. Gels were destained, washed and blotted on to a Hybond-N+ nylon membrane (Amersham Biosciences). The membrane was probed with a 32P-labelled oligonucleotide, 5′-AGGCATGAGGGGGTGCCA-3′, prepared by Nick-translation using [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs). Specifically bound radioactivity was visualized using a phosphoimager or by exposure to film.
Immunoprecipitation and Western-blot analyses
Immunoprecipitation was performed using human brain tissue and lysates from transiently transfected COS-7 cells. Brain tissue (100–150 mg) or cellular pellets from a 100 mm culture plate were each homogenized by sonication on ice in 500 μl of a buffer containing 50 mM Tris/HCl (pH 7.9), 150 mM NaCl, 5 mM EDTA, 2 mM NaF, 1 mM diethyldithiocarbamate, 1% Nonidet P40, 0.5% deoxycholate, 0.1% SDS, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml soya bean trypsin inhibitor, 1 mM PMSF and 0.5 mM dithiothreitol. The homogenates were centrifuged at 10000 g for 10 min at 4 °C. The protein content of the supernatant was determined using the Bradford protein assay. An aliquot of 1 mg of protein of each sample was incubated with 30 μl of pretreated Protein G–agarose (Pierce) for 30 min at 4 °C. For pretreatment, 150 μl of a 1:1 slurry of Protein G–agarose was mixed with 850 μl of homogenization buffer containing 2.5 mg of BSA for 1 h at 4 °C. The protein slurry was centrifuged (1 min at 13000 g), washed four times with homogenization buffer and finally resuspended by adding 75 μl of homogenization buffer. After preclearing with Protein G–agarose, the samples were centrifuged (1 min at 13000 g), and the supernatants were incubated with 20 μl of an agarose conjugate of goat polyclonal COX-1 antibody (Santa Cruz Biotechnology) for 3 h at 4 °C. The samples were centrifuged (1 min at 13000 g), washed with 500 μl of homogenization buffer and finally eluted with 20 μl of SDS/PAGE loading buffer for 5 min at 70 °C. Each sample (10 μl) was separated on a 10% SDS/polyacrylamide gel and blotted electrophoretically on to a 0.2 μm nitrocellulose membrane. Human COX-1 and the splice variant were expressed in COS-7 cells, and the crude cellular homogenates were loaded as authentic standards.
Colon tissue (normal and tumour) was homogenized in PBS using a bead-beater, and the protein concentration was determined using the Bradford protein assay. Total protein (25 μg) was separated using a 10% SDS/polyacrylamide gel and blotted electrophoretically on to a 0.2 μm nitrocellulose membrane.
For Western-blot detection, a monoclonal antibody raised against purified ovine COX-1 (Cayman Chemical, Ann Arbor, MI, U.S.A.) was used in a 1:2000 dilution. Specifically bound protein was detected using a goat anti-mouse IgG alkaline phosphatase-conjugated secondary antibody (Jackson ImmunoResearch) and Nitro Blue Tetrazolium and 5-bromo-4-chloroindol-3-yl phosphate as chromogenic substrates. Alternatively, a sheep anti-mouse IgG horseradish peroxidase-coupled antibody was used (Amersham Biosciences) in combination with an ECL® Western blotting detection reagent kit (Amersham Biosciences).
3′-RACE (rapid amplification of cDNA ends)
The 3′-UTR (untranslated region) of intact human COX-1 and the splice variant were cloned using cDNA prepared from human colon tumour tissue with the oligo(dT) adaptor primer in a 3′-RACE experiment. In the first round of PCR, the upstream primer was 5′-TGTGACCTGCTGAAGGCTGAG-3′ and the downstream primer was 5′-ATGAATTCGGTACCCGGGATCC-3′ using standard reaction conditions and the Expand High Fidelity polymerase (Roche) and the following temperature programme: 94 °C for 1 min (1 cycle); 94 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min (30 cycles); 72 °C for 7 min. For the second round of PCR, 1 μl of a 20-fold dilution of the first-round PCR was used with three different upstream primers, 5′-AGTGGCTATTTCCTGCAG-3′ (OP3950), 5′-CTGACTCCTTCAAGATCG-3′ (RACE1), 5′-GACTCCTTCAAGATCGGTG-3′ (RACE2), whereas the downstream primer for all reactions was 5′-ATGAATTCGGTACCCGGG-3′. The reaction conditions and temperature programme were identical with the first round of PCR. Prominent bands at approx. 1600 and 1400 bp respectively were obtained depending on the different upstream primers (OP3950 or RACE1/RACE2). The bands were excised from a 1.2% agarose gel and cloned into the pCR2.1TOPO vector (Invitrogen) for subsequent sequencing reactions.
In vitro translation assay
mRNA was isolated from total RNA of three colon tissue samples (2 tumour, 1 normal) and from COS-7 cells transfected with fulllength or spliced COX-1 cDNAs or with vector control (pcDNA3.1). The mRNA purification kit from Amersham Biosciences was used according to the manufacturer's instructions. mRNA (1 μg of each) was translated into protein using the Retic Lysate IVT in vitro translation kit (Ambion, Austin, TX, U.S.A.), supplemented with 10 μCi of [35S]methionine per reaction following the manufacturer's instructions. A control reaction without the addition of mRNA was included. In vitro translated COX-1 protein was immunoprecipitated as described above, and 50% of the precipitate was analysed by SDS/PAGE. After the gel was dried, radiolabelled proteins were detected by exposure to a phosphoimager storage screen.
RESULTS
PCR cloning
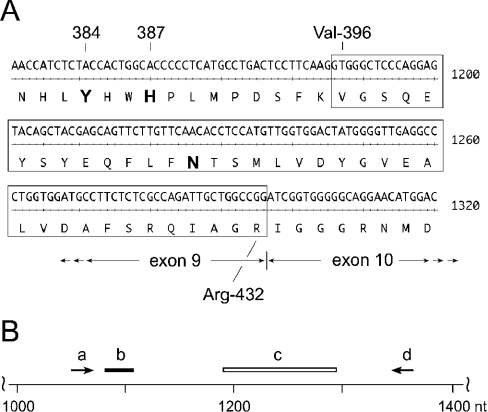
In cloning experiments using cDNA prepared from human tonsils, we detected a COX-1 clone that migrated on agarose gel electrophoresis at approx. 100 nt smaller than the expected size of ≈1.8 kb for the open reading frame. The clone was sequenced and found to be missing 111 bp from position 1186 to 1296 of the open reading frame, corresponding to 37 amino acids from residues 396 to 432 (Figure 1). This cDNA was identical with a human COX-1 splice variant reported by Diaz et al. [9].
Figure 1. Partial nucleotide and amino acid sequence of human COX-1.
(A) Nt 1141–1320 of the human COX-1 cDNA are shown. The 111 nt (encoding 37 amino acids from position 396 to 432) missing in the alternatively spliced human COX-1 transcript are boxed. The catalytically important residues, Tyr-384 (Y384) and His-387 (H387), are shown in boldface. Asn-409 (N409; boldface) is a consensus glycosylation site in human COX-1. (B) Schematic location of oligonucleotides. The primers used in RT–PCR experiments correspond to nt 1054–1071 (upstream, a) and nt 1346–1363 (downstream, d). The oligonucleotide probe (b) used for detection of the RT–PCR amplification products is located from nt 1081 to 1107. The spliced out 111 nt (1186–1296) are represented by a box (c).
COX and peroxidase activity
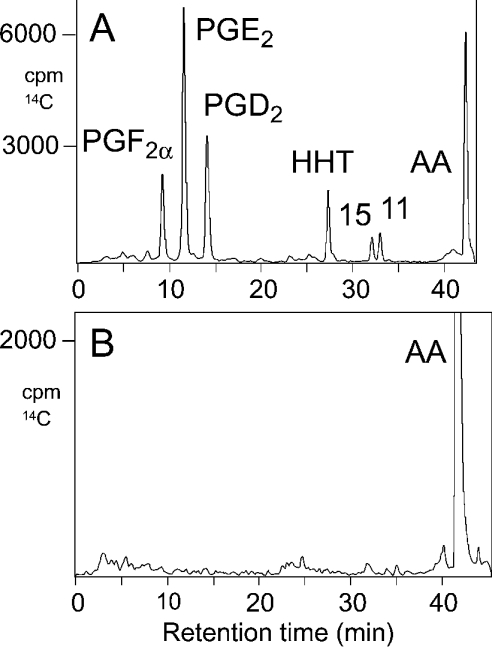
The intact and spliced cDNAs were subcloned into the pcDNA3.1 and pVL1393 expression vectors for expression in COS-7 and Sf9 insect cells respectively. In a previous study [16], we have found that transient transfection into COS-7 cells gave higher expression levels for COX-1 than using vaccinia-virus-mediated co-expression of the T7 RNA polymerase gene in HeLa cells. Western analyses confirmed efficient expression of the spliced and full-length protein in both systems, as well as in HeLa cells (results not shown). The COX activity of both proteins was determined by incubating COS-7 and Sf9 cell homogenates with [14C]arachidonic acid. Product formation was analysed using RP-HPLC and on-line detection of radioactivity by liquid-scintillation counting. Full-length COX-1 expressed in COS-7 and Sf9 cells formed the expected mixture of PGE2, PGD2 and PGF2α together with the by-products 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid, 15- and 11-HETE (Figure 2A). When the cDNA of the splice variant was expressed in either COS-7 or Sf9 cells, we were unable to detect formation of any COX-derived product, including the HETE by-products. A representative chromatogram is shown in Figure 2(B). Similar negative results were obtained consistently on repeated expression, and also on varying substrate concentrations. In incubations with 14C-labelled linoleic, α-linolenic and eicosapentaenoic acids, no product formation was observed with the recombinant spliced COX-1.
Figure 2. RP-HPLC analysis of products formed by recombinant full-length and spliced human COX-1.
The cDNAs for full-length (A) and spliced (B) human COX-1 were transiently transfected into COS-7 cells. Incubations with 50 μM [14C]arachidonic acid and product extraction were performed as described in the Experimental section. Products were analysed by RP-HPLC using a Waters Symmetry C18 column (0.46 cm×25 cm) eluted at a flow rate of 1 ml/min with a stepwise gradient of the following solvents: acetonitrile/water/acetic acid (37.5:62.5:0.01, by vol.) for 15 min, acetonitrile/water/acetic acid (70:30:0.01, by vol.) for 15 min and finally with methanol. Peaks were detected using a diode array detector coupled on-line with a liquid-scintillation counter. Radiolabelled products were identified by co-chromatography with added unlabelled standards. 11, 11-HETE; 15, 15-HETE; AA, arachidonic acid; HHT, 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid.
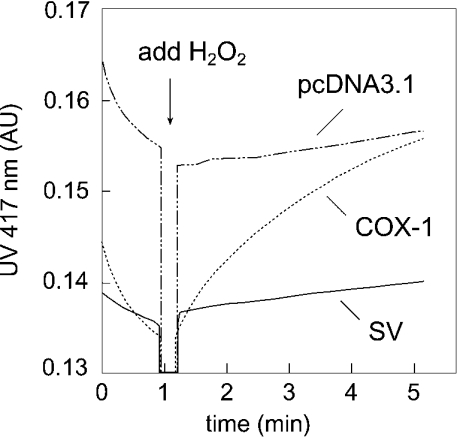
We next analysed the peroxidase activity of the two enzymes using microsomes prepared from COS-7 cells transiently transfected with either full-length or spliced COX-1 cDNA, or with vector alone (Figure 3). Using the substrates H2O2 and ABTS in a spectrophotometric assay, we readily detected peroxidase activity of the full-length COX-1 as an increase in the absorbance at 417 nm [4 mAU·(mg of protein)−1·min−1, where mAU stands for milli-absorbance units]. Using microsomes from COS-7 cells transfected with the splice variant, we were unable to detect any measurable increase in the absorbance at 417 nm over the vector control (Figure 3). Western-blot analyses confirmed that both the full-length and the spliced COX-1 were present in the microsomal fraction. We conclude that the spliced COX-1 protein is devoid of cyclo-oxygenase and peroxidase activity under conditions where the corresponding activities of the intact COX-1 are readily detected.
Figure 3. Peroxidase activity of recombinant full-length and spliced human COX-1.
Peroxidase activity was measured spectrophotometrically as the increase in absorbance at 417 nm due to the oxidation of the substrate ABTS. The reaction was started by the addition of 1 μl of a solution of 20 mM H2O2 in water to the cuvette containing incubation buffer, microsome preparation, ABTS and haem as described in the Experimental section. Microsomes were prepared from COS-7 cells transiently transfected with cDNAs of full-length (COX-1) and spliced COX-1 (SV), or with vector control (pcDNA3.1). Equal amounts of protein were used in the assays.
Tissue expression
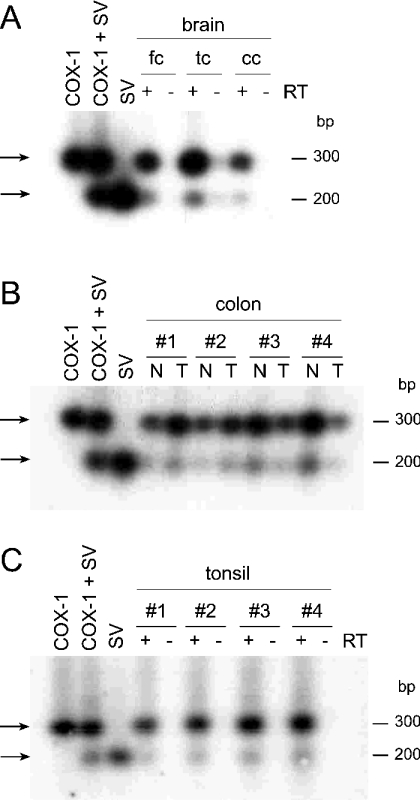
Expression of the alternatively spliced and full-length COX-1 mRNA transcripts and protein was analysed in human brain, colon and tonsil tissue. RNA and protein were extracted from the same tissue samples for expression analysis using RT–PCR and Western immunoblotting. For RT–PCR experiments, we used a set of oligonucleotide primers that cover the spliced region and yield amplification products of ≈300 bp for the full-length and ≈200 bp for the spliced mRNA (Figure 1B, primers ‘a’ and ‘d’). PCR products were visualized by ethidium bromide staining, and after transfer to a nylon membrane by hybridization with a specific 32P-labelled oligonucleotide probe that can detect both transcripts (Figure 1B, oligonucleotide ‘b’).
The expected size PCR amplification products for both transcripts were detected in the human brain, colon and tonsil tissues analysed (Figure 4). The frontal, temporal and cerebellar cortex of human brain obtained from two subjects were analysed. In the RT–PCRs, prominent amplification products were observed for RNA from only one of the subjects (shown in Figure 4A). Positive PCR bands for full-length or spliced COX-1 were observed in all of the three brain regions analysed from this subject. The signals for the full-length mRNA were approx. 10–20-fold more abundant than the spliced mRNA. Control cDNAs prepared without the addition of RT gave no bands. In four matching pairs of normal and tumour colon tissue, the spliced mRNA was detected in all eight samples at approx 5–10% of the abundance of the full-length mRNA (Figure 4B). No obvious differences in the abundance of mRNA of either full-length COX-1 or the splice variant were observed between normal and tumour samples. Four tonsil cDNA samples were analysed, and, again, the presence of the full-length and spliced COX-1 transcripts was detected in a ratio of approx. 10–20 to 1, without obvious variation in abundance between the samples (Figure 4C).
Figure 4. Analysis of full-length and spliced COX-1 mRNA expression in human tissue using RT–PCR.
RT–PCR analysis of (A) human brain tissue (fc, frontal cortex; tc, temporal cortex; cc, cerebellar cortex), (B) matching pairs of normal (N) and tumour (T) tissue from four different human colon samples, (C) tonsil tissue from four patients. cDNA (1 μl) was used in the PCRs with a pair of oligonucleotide primers that yield a product of ≈300 and 200 bp for the full-length and spliced COX-1 mRNAs respectively. PCR products were visualized by hybridization with a 32P-labelled oligonucleotide probe. In the positive controls, 10 pg of each template in the pcDNA3.1 vector were used. RNA was extracted from the tissues using TRI Reagent, and 20 μg of total RNA were reverse-transcribed using murine MLV RT and an oligo(dT) adaptor primer. SV, splice variant.
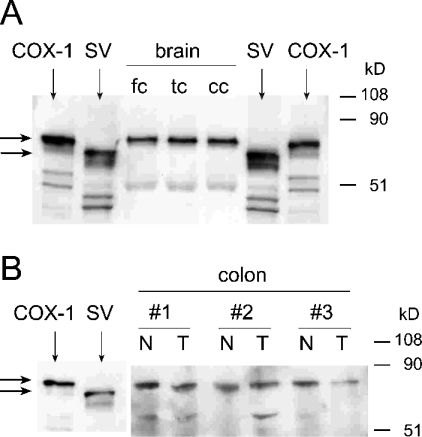
Expression of the corresponding full-length and spliced COX-1 proteins was analysed by Western blotting using the brain and colon tissue samples that had been found to express the spliced mRNA transcripts (Figure 5). For Western-blot detection, a commercial mouse monoclonal antibody raised against purified ovine COX-1 was used. The antibody recognized the full-length as well as the spliced COX-1 proteins. Because of low expression levels of COX-1 in human brain, the samples (1 mg of protein) were concentrated by immunoprecipitation using an agarose-conjugated goat polyclonal antibody directed against a peptide within the C-terminal 50 amino acids of human COX-1. The ability of the antibody to immunoprecipitate both the intact and spliced COX-1 protein was confirmed using COS-7 cell homogenates transiently transfected with the corresponding cDNAs (results not shown). In the immunoprecipitated proteins, a band corresponding to native COX-1 was readily detected in the three human brain regions analysed (frontal, temporal and cerebellar cortex), whereas no band was visible at the electrophoretic mobility of the splice variant, even on overexposure of the blot (Figure 5A). Measurement of the relative signal intensities showed that there was an up to 100 times stronger signal in the COX-1 band, meaning that even 5–10% relative abundance of the splice variant would have been detectable. As shown above, the spliced mRNA had been readily identified by RT–PCR in these samples (Figure 4A). From the matching pairs of normal and tumour tissue from human colon, 25 μg of total protein was separated by SDS/PAGE and analysed by Western blotting. Full-length COX-1 protein was equally abundant in normal and tumour tissues, but the splice variant was undetectable in the samples, although the spliced mRNA was readily detected in the RT–PCR experiments (Figures 4B and 5B). We next analysed human platelets, a prototypical site of expression of COX-1, but we were unable to detect expression of the spliced protein by Western blotting (results not shown).
Figure 5. Western-blot analysis of COX-1 expression in human brain and colon tissue.
Western-blot analyses of (A) human brain tissue (fc, frontal cortex; tc, temporal cortex; cc, cerebellar cortex) and (B) three matching pairs of normal (N) and tumour (T) tissue from human colon. The identical tissue samples as in Figures 4(A) and 4(B) were used. In (A), 1 mg of brain tissue protein was used for immunoprecipitation, and half of the pellet obtained was analysed by SDS/PAGE; in (B), 25 μg of tissue protein was analysed. Authentic standards for COX-1 and the splice variant (SV) were prepared by transient transfection of the cDNAs into COS-7 cells. The blots were probed with a monoclonal antibody raised against purified ovine COX-1 (Cayman Chemical) in a 1:2000 dilution. In (B), the last six lanes of the membrane were exposed 5-fold compared with the first two lanes.
Characterization of the spliced COX-1 mRNA transcript
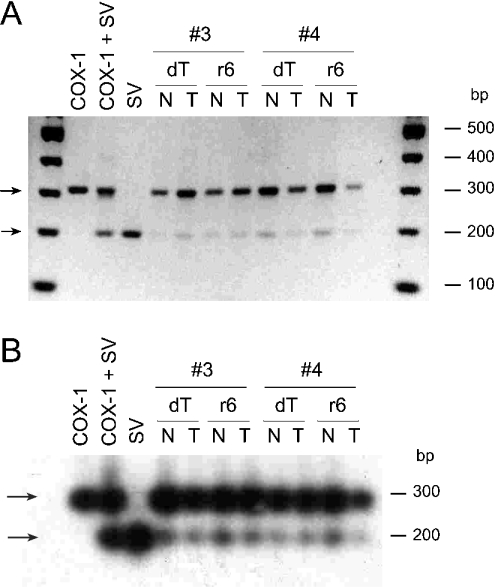
The 3′-UTR of the splice variant reported by Diaz et al. [9] was derived from a cDNA library, and contained only the first 638 bp of a total of 749 bp of the 3′-UTR of COX-1 reported by Funk et al. [19]. This opened up the possibility that the spliced transcript is lacking a poly(A)+ (polyadenylated) tail which could have a negative effect on translation efficiency. In our cloning experiments and tissue expression analyses by RT–PCR, we detected the spliced mRNA in cDNA samples prepared with an oligo(dT) adaptor primer, indicating the presence of a poly(A)+ tail at the 3′-end of the spliced mRNA. We compared the abundance of the spliced transcript in cDNAs prepared in parallel from human colon tissue (normal and tumour) using either the oligo(dT) adaptor primer or a random hexamer primer for cDNA synthesis (Figure 6). The RT–PCR amplification products for the full-length transcript and the splice variant were equally abundant in the corresponding samples regardless of the method of cDNA synthesis used (Figure 6).
Figure 6. Detection of full-length and spliced COX-1 mRNA in cDNA prepared using an oligo(dT) adaptor primer and a random hexamer primer.
RT–PCR analysis of full-length and spliced COX-1 mRNAs was performed in cDNA prepared with an oligo(dT) adaptor primer (dT) or with a random hexamer primer (r6). RNA from matching pairs of normal (N) and tumour (T) tissue from colon samples 3 and 4 was used for cDNA synthesis. RT–PCR primers and conditions were identical to those in Figure 4. Amplification products were detected in (A) by ethidium bromide staining and in (B) by hybridization with a 32P-labelled oligonucleotide probe after blotting to a nylon membrane. In the positive controls, 10 pg of each template in the pcDNA3.1 vector was used.
To resolve directly the issue of whether the 3′-UTR of the spliced mRNA has a poly(A)+ tail, we cloned the 3′-UTR using a RACE technique with upstream primers that were either specific for the spliced sequence or that would amplify the full-length sequence as well. We sequenced four clones of the spliced and two clones of the full-length COX-1 3′-UTR and found that all were identical with the sequence reported by Funk et al. [19] except for two minor differences. All sequences lack one nucleotide at position 413 of the reported 3′-UTR, and all sequences have an additional 11 bp sequence (uuucuaaaguu) instead of the last two nucleotides in front of the poly(A)+ tail. We conclude that there are no apparent differences between the 3′-UTR sequences of the spliced and intact COX-1 mRNAs that could account for the lack of translation into protein.
In vitro translation assay
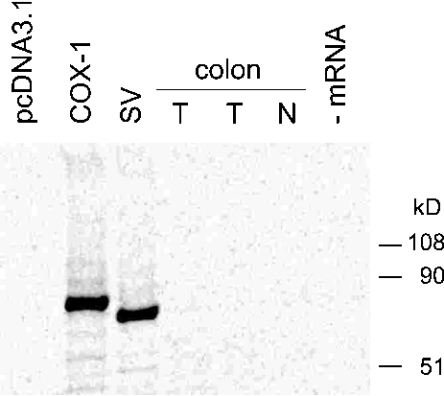
To gain insight into the reason for the apparent absence of the spliced protein in samples that contain the corresponding mRNA, we analysed whether the spliced mRNA transcript is functionally intact and can be translated into protein using an in vitro translation system. mRNA was extracted from colon tissue (normal and tumour) and from COS-7 cells transiently transfected with the spliced and intact COX-1 cDNAs or with vector control (pcDNA3.1). The mRNAs were in vitro translated using a reticulocyte lysate kit, and 35S-radiolabelled COX-1 proteins were immunoprecipitated and analysed by SDS/PAGE. Two distinct bands of the correct size were obtained for the full-length and spliced COX-1 proteins using mRNA from the transfected cells (Figure 7). Neither of the colon tissue mRNA samples formed a radiolabelled protein co-migrating with the full-length or the spliced COX-1 standards. A probable explanation why colon mRNA was not translated into protein is the presence of a 20-nt segment in the 3′-UTR of the COX-1 mRNA that silences translation, as well as a recently discovered cis element within the first 100 nt of the 5′-UTR that has a negative effect on translational efficiency in the rabbit reticulocyte lysate [20,21]. Both elements are absent in the spliced and full-length cDNA constructs.
Figure 7. In vitro translation assay.
The in vitro translation assay was performed as described in the Experimental section. mRNA was extracted from transfected COS-7 cells (pcDNA3.1, COX-1 and SV) and from normal (N) and tumour (T) tissue from human colon. Equal amounts of mRNA were added to rabbit reticulocyte lysates, and a control without addition of mRNA was included. 35S-radiolabelled proteins were detected using a phosphoimager.
DISCUSSION
Several important reasons warranted the characterization of the catalytic activity and tissue expression of this splice variant of human COX-1. The assumption put forward in the original report that the alternatively spliced COX-1 protein would lack catalytic activity was not proven experimentally [9]. The second reason relates to the lack of data on tissue expression of the spliced COX-1 protein. The original findings by Diaz et al. had implied differences in the transcriptional regulation of the splice variant compared with COX-1 [9], and we initially sought to confirm this at the protein level. The question of specific expression of the spliced COX-1 protein needed attention because commercially available antibodies used, for example, in immunohistochemical studies could not differentiate between the full-length and the spliced proteins. The appearance of double bands for COX on Western analysis are usually attributed to differences in glycosylation, yet the expression of a second transcript remains a viable alternative. Finally, it was compelling to speculate the existence of a novel COX enzyme with regulation and expression different from COX-1 and -2, and identical or maybe altered oxygenase or peroxidase activity.
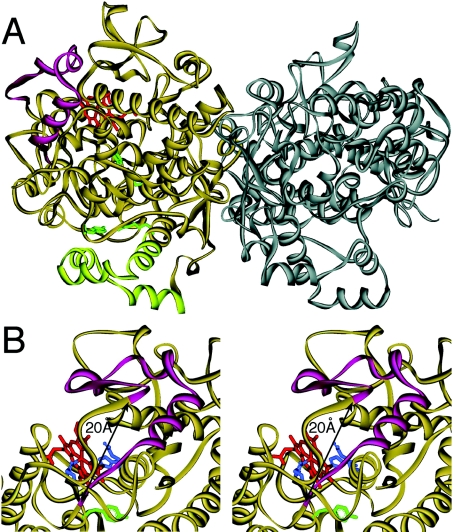
The amino acids deleted in this splice variant of human COX-1 (396–432) form a loop that constitutes one side of the peroxidase active site of the protein, as exemplified in the three-dimensional structure of ovine COX-1 (Figure 8) [22]. The α-carbon atoms of residues Lys-395 and Ile-433 (human COX-1 sequence) are only approx. 20 Å (1 Å=0.1 nm) from each other [23]. In the spliced protein lacking the 37 residues in between, the two bordering/flanking residues Lys-395 and Ile-433 are connected to each other in the protein backbone. The close proximity of the two residues on the surface of the enzyme suggested to us that the absence of the 37 amino acids could be overcome during folding of the spliced protein, eventually without deleterious effects on the catalytic activities. On the other hand, it should be noted that the proximal haem ligand His-387 is only eight amino acids away from the splice site. Disturbance of the sequence close to the haem ligand could possibly shift this residue away from its normal location and impair haem binding. In addition, the spliced protein lacks Asn-409, an N-glycosylation site that is necessary for correct folding, but not for catalytic activity [10]. Given these considerations, together with an inspection of the structural features, it appears to be an open question whether the spliced protein would retain catalytic activity or not, or whether it might eventually have different activity.
Figure 8. Three-dimensional structure of ovine COX-1 illustrating the location of amino acids Val-396 to Arg-432.
A ribbon diagram of the crystal structure of the ovine COX-1 homodimer is shown [22]. (A) The 37 amino acids from Val-396 to Arg-432 (magenta) are located on the far side of the dimer interface forming a loop near the haem binding (peroxidase) site. The four membrane binding helices at the bottom are shown in light green, four critical oxygenase active site residues (Arg-120, Tyr-355, Tyr-385 and Ser-530; ovine COX-1 numbering) are in green and the haem group in the peroxidase active site is depicted in red. (B) Stereo view of the haem binding site. Compared with (A), the protein is turned by approx. 30° to the right. The distance between the α-carbon atoms of Lys-395 and Ile-433 is approx. 20 Å. The distal (Gln-203 and His-207) and proximal (His-388) haem ligands are in blue and Tyr-385 is in green.
We used two different expression systems, transient transfection into COS-7 cells and baculovirus-mediated expression in Sf9 insect cells, to investigate the oxygenase and peroxidase catalytic activities of the recombinant spliced human COX-1. Both systems are suitable for robust expression of the human COX-1 isoenzyme [16,24]. In the activity assays, we found that the spliced COX-1 protein did not exhibit any COX or peroxidase activity under conditions where the full-length protein was highly active. COX is active as a homodimer and therefore we had also considered the possibility that the spliced protein might represent a dominant negative form of COX-1 that would render a heterodimer of full-length and spliced protein catalytically inactive. To test this hypothesis, we co-transfected the full-length and spliced cDNAs into COS-7 cells. When we analysed the product formation by the co-transfected cell homogenates, we did not observe a reduction in activity that would have supported this hypothesis (results not shown).
One explanation for the lack of catalytic activity of the alternatively spliced COX-1 protein could be the inability of the peroxidase active site of the enzyme to bind the haem cofactor. Peroxidase activity is necessary to spark the oxygenation reaction by oxidizing Tyr-384 to the corresponding tyrosyl radical which in turn performs the initial hydrogen abstraction at C-13 of the arachidonic acid substrate [6]. Thus lack of haem binding in the splice variant could explain both the absence of peroxidase and of oxygenase activity. A second possibility is that absence of the glycosylation site Asn-409 impaired protein folding and rendered the resulting spliced enzyme non-functional. As with all negative results, there is also the possibility that we failed to find the right conditions in the assays, or that some of the reagents used are more active towards one form of the enzyme than the other. This possibility was tested for the antibodies we have used and found not to be the case.
We analysed the expression of COX-1 and the splice variant in human brain, colon and tonsil tissue samples. We selected brain tissue because previous studies have implicated the potential existence of a COX isoform in human brain that would be different from COX-1 and -2 [25,26]. In addition, we analysed normal and cancerous human colon tissue. In human intestinal mucosa, COX-1 derived PGs are important regulators of tissue integrity and homoeostasis [27]. In colon cancer, up-regulated COX-2 expression is involved in the progression of the disease [28]. Furthermore, transcriptional regulation of the splice variant by cytokines and growth factors was described in the original report [9]. Therefore we investigated the possibility of expression of the splice variant in matching pairs of normal and cancerous human colon samples. We detected the spliced mRNA in all tissue samples analysed at approx. 5–10% of the abundance of the full-length mRNA. We did not observe differences in the expression levels of intact and spliced mRNAs between normal and tumour colon tissue. A consistent finding was that while an abundant signal for the full-length COX-1 protein was observed in all the tissue samples analysed, we did not detect expression of the spliced protein in any of the samples, even though matching RT–PCR experiments had shown the presence of the spliced mRNA transcript. We were unable to detect expression of the spliced protein in any of the tissue samples analysed.
In conclusion, we have demonstrated that the alternatively spliced transcript of human COX-1 lacking 37 amino acids near the peroxidase active site is present at the mRNA level but cannot be detected as a protein in human tissue. Furthermore, the recombinant spliced protein lacks catalytic activity. The present study has resolved several important open questions, and as a consequence of our findings, it is our view that this splice variant can be disregarded as a player and as a confounding factor in eicosanoid metabolism in human tissues.
Acknowledgments
We thank Dr T. Montine (University of Washington, Seattle, WA, U.S.A.) and Dr R. DuBois (Vanderbilt University, Nashville, TN, U.S.A.) for providing the brain and colon samples respectively, and Dr D. Dixon and Dr M. Melner for helpful suggestions. This work was supported by National Institutes of Health grant GM-53638.
References
- 1.Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Smith W. L., DeWitt D. L., Garavito R. M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R. A., Tejada L. V., Tong B. J., Das S. K., Morrow J. D., Dey S. K., DuBois R. N. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- 4.Garavito R. M., Malkowski M. G., DeWitt D. L. The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat. 2002;68–69:129–152. doi: 10.1016/s0090-6980(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 5.van der Donk W. A., Tsai A. L., Kulmacz R. J. The cyclooxygenase reaction mechanism. Biochemistry. 2002;41:15451–15458. doi: 10.1021/bi026938h. [DOI] [PubMed] [Google Scholar]
- 6.Rouzer C. A., Marnett L. J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 7.Smith W. L., Song I. The enzymology of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat. 2002;68–69:115–128. doi: 10.1016/s0090-6980(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 8.Funk C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 9.Diaz A., Reginato A. M., Jimenez S. A. Alternative splicing of human prostaglandin G/H synthase mRNA and evidence of differential regulation of the resulting transcripts by transforming growth factor beta 1, interleukin 1 beta, and tumor necrosis factor alpha. J. Biol. Chem. 1992;267:10816–10822. [PubMed] [Google Scholar]
- 10.Otto J. C., DeWitt D. L., Smith W. L. N-glycosylation of prostaglandin endoperoxide synthases-1 and -2 and their orientations in the endoplasmic reticulum. J. Biol. Chem. 1993;268:18234–18242. [PubMed] [Google Scholar]
- 11.Chandrasekharan N. V., Dai H., Roos K. L. T., Evanson N. K., Tomsik J., Elton T. S., Simmons D. L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinchuk J. E., Liu R. Q., Trzaskos J. M. COX-3: in the wrong frame in mind. Immunol. Lett. 2003;86:121. doi: 10.1016/s0165-2478(02)00268-7. [DOI] [PubMed] [Google Scholar]
- 13.Kis B., Snipes J. A., Isse T., Nagy K., Busija D. W. Putative cyclooxygenase-3 expression in rat brain cells. J. Cereb. Blood Flow Metab. 2003;23:1287–1292. doi: 10.1097/01.WCB.0000090681.07515.81. [DOI] [PubMed] [Google Scholar]
- 14.Shaftel S. S., Olschowka J. A., Hurley S. D., Moore A. H., O'Banion M. K. COX-3: a splice variant of cyclooxygenase-1 in mouse neural tissue and cells. Mol. Brain Res. 2004;123:136. doi: 10.1016/j.molbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C., Keeney D. S., Boeglin W. E., Brash A. R. Detection and cellular localization of 12R-lipoxygenase in human tonsils. Arch. Biochem. Biophys. 2001;386:268–274. doi: 10.1006/abbi.2000.2217. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C., Boeglin W. E., Prusakiewicz J. J., Rowlinson S. W., Marnett L. J., Samel N., Brash A. R. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and 2. A critical role for serine 530 and valine 349. J. Biol. Chem. 2002;277:478–485. doi: 10.1074/jbc.M107471200. [DOI] [PubMed] [Google Scholar]
- 17.Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- 18.Brash A. R., Boeglin W. E., Chang M. S., Shieh B.-H. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J. Biol. Chem. 1996;271:20949–20957. doi: 10.1074/jbc.271.34.20949. [DOI] [PubMed] [Google Scholar]
- 19.Funk C. D., Funk L. B., Kennedy M. E., Pong A. S., Fitzgerald G. A. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 1991;5:2304–2312. [PubMed] [Google Scholar]
- 20.Duquette M., Laneuville O. Translational regulation of prostaglandin endoperoxide H synthase-1 mRNA in megakaryocytic MEG-01 cells. Specific protein binding to a conserved 20-nucleotide cis element in the 3′-untranslated region. J. Biol. Chem. 2002;277:44631–44637. doi: 10.1074/jbc.M207007200. [DOI] [PubMed] [Google Scholar]
- 21.Laneuville O., Gosselin D., Guerbaz M. Proc. 8th Int. Conf. on Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation and Related Diseases. Chicago, IL: 2003. A cis element in the open reading frame of prostaglandin endoperoxide H synthase-1 (PGHS-1) mRNA with a negative effect on translation efficiency. September, Abstract 20. [Google Scholar]
- 22.Picot D., Loll P. J., Garavito R. M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature (London) 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 23.Malkowski M. G., Ginell S. L., Smith W. L., Garavito R. M. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science. 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 24.Smith T., Leipprandt J., DeWitt D. Purification and characterization of the human recombinant histidine-tagged prostaglandin endoperoxide H synthases-1 and -2. Arch. Biochem. Biophys. 2000;375:195–200. doi: 10.1006/abbi.1999.1659. [DOI] [PubMed] [Google Scholar]
- 25.Willoughby D. A., Moore A. R., Colville-Nash P. R. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet. 2000;355:646–648. doi: 10.1016/S0140-6736(99)12031-2. [DOI] [PubMed] [Google Scholar]
- 26.Simmons D. L., Botting R. M., Robertson P. M., Madsen M. L., Vane J. R. Induction of an acetaminophen-sensitive cyclooxygenase with reduced sensitivity to nonsteroid antiinflammatory drugs. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3275–3280. doi: 10.1073/pnas.96.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–175. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 28.Sheng H., Shao J., Kirkland S. C., Isakson P., Coffey R. J., Morrow J., Beauchamp R. D., DuBois R. N. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J. Clin. Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]