Abstract
Human DCs (dendritic cells) express surface CD83 upon activation. Comparing the surface induction of CD83 with the upregulation of CD40, CD80 and CD86 during LPS (lipopolysaccharide)-induced DC maturation showed that CD83 induction occurred more rapidly. Despite the lack of CD83 on immature DCs, it was detected in these cells by Western blotting and flow cytometry. Indirect immunofluorescence revealed CD83 inside immature DCs in perinuclear regions. CD83 was absent on monocytes and macrophages, but it was detected inside these cells and found to be rapidly surface-expressed upon LPS-induced activation. Whereas CD83 expression on activated DCs was sustainable, its expression on monocytes and macrophages was transient. Optimal interleukin-4 co-stimulation during DC generation from monocytes was found to be essential for stable CD83 surface expression. CD83 was detected as 37 and 50 kDa forms in transfected 293T cells. Macrophages and immature DCs expressed the 37 kDa form, whereas mature DCs predominantly expressed the 50 kDa form. In monocytes, CD83 was detected as a 22 kDa detergent-insoluble form. The rapid CD83 surface induction on DCs and macrophages was blocked by brefeldin A, but not by cycloheximide, showing that fresh CD83 synthesis was not essential. Tunicamycin inhibited the expression of the 50 and 37 kDa CD83 forms, and also blocked CD83 surface expression on DCs and macrophages. PNGase F (peptide N-glycosidase F) digestion reduced the 37 and 50 kDa CD83 forms to 28 kDa. In summary, monocytes, macrophages and immature DCs contain preformed intracellular CD83, and its rapid surface expression upon activation is post-translationally regulated in a process involving glycosylation.
Keywords: CD83, dendritic cell, macrophage, monocyte, tunicamycin
Abbreviations: APC, antigen-presenting cell; (im/m)DC, (immature/mature) dendritic cell; (G)M-CSF, (granulocyte)/macrophage-colony-stimulating factor; IFN-α, interferon-α; IL-4, interleukin-4; LPS, lipopolysaccharide; PE, phycoerythrin; PNGase F, peptide N-glycosidase F
INTRODUCTION
Monocyte differentiation into macrophages is regulated by M-CSF (macrophage colony-stimulating factor) or GM-CSF (gra nulocyte/M-CSF) [1]. In vitro, monocytes differentiate into DCs (dendritic cells) under the dual stimulation of GM-CSF and IL-4 (interleukin-4) [2–4]. Macrophages are major tissue scavengers, and are important in the activation and regulation of host immunity [5,6]. DCs are highly specialized APCs (antigen-presenting cells) which perform antigen capturing at the immature stage [i.e. as immature DCs (imDCs)] and presentation upon maturation [i.e. as mature (m)DCs] [7,8]. CD14+ monocytes differentiate into macrophages, which remain CD14+, but monocyte-derived DCs lack or have little surface CD14 [2]. Surface CD83 is a hallmark of mDCs [9], which also exhibit increased surface expression of MHC, co-stimulatory, adhesion and activation molecules [3,4,7,8].
CD83 is a member of the IgSF (immunoglobulin superfamily) of receptors, consisting of a single polypeptide of 187 amino acids with a single V-type, Ig-like N-terminal extracellular domain and a short (39 amino acids) C-terminal cytoplasmic domain [10,11]. Although its functions on DCs remain unclear, studies have indicated roles of CD83 in the modulation of antigen presentation and CD4+ T cell generation. The human T51 B lymphoblastoid cells that were transfected to express CD83 became more potent in allogeneic activation of CD8+ T cells, and the activated T cells exhibited increased cytotoxicity [12]. Both B cells and DCs release soluble CD83 upon activation [13,14]. Like siglecs (sialic-acid-binding, immunoglobulin-like lectins), the soluble form of CD83 binds to sialic acid residues on a 72 kDa monocyte surface protein [15,16], but it lacks the inhibitory motif found in the cytoplasmic domains of some siglecs [15]. Immobilized CD83 was shown to promote the activation of CD8 T cells in peripheral blood mononuclear cells through monocytes [12]. It also binds to activated CD8+ T cells, although it is not clear whether sialic acid residues on the T cells are involved [16]. Soluble CD83 was also shown to bind to DCs, and the binding was shown to inhibit DC maturation and immunostimulation [17]. Recently, Senechal et al. [18] showed that mDCs infected by human cytomegalovirus lost surface CD83, and thus released CD83 could effectively inhibit DC-elicited T cell activation. In mice, CD83 is also abundantly expressed on thymic epithelial cells [19]. CD83-deficient mice showed reduced CD4+ T cell generation, suggesting that CD83 regulates T cell differentiation in the thymus [18].
In the present study, we have examined CD83 expression during the course of LPS (lipopolysaccharide)-induced DC maturation, leading to the detection of preformed intracellular CD83 in monocytes, macrophages as well as imDCs. In a previous study [20], preformed intracellular CD83 has been detected in imDCs by flow cytometry. However, in another study, CD83 was found to be absent in imDCs by Western blotting [21]. Therefore whether imDCs express preformed intracellular CD83 or not has been controversial. Using multiple methods, we have demonstrated the presence of preformed CD83 in imDCs, monocytes and macrophages. We have also shown both that the preformed CD83 was rapidly induced on the cell surface upon LPS stimulation and the requirement for asparagine (Asn)-linked glycosylation for CD83 surface expression on these cells.
EXPERIMENTAL
Reagents and cell cultures
The following mouse monoclonal antibodies were obtained from Ancell Corporation (Bayport, MN, U.S.A.): CD14 (FITC), CD40 (FITC) and CD86 (FITC). The anti-CD83 (clone HB15e; PE, phycoerythrin) and anti-CD80 (PE) antibodies were purchased from BD Pharmingen (San Diego, CA, U.S.A.), and another CD83 antibody (clone HB15a) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, U.S.A.). LPS (Escherichia coli O55:B5) was obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). Recombinant human GM-CSF, M-CSF and IL-4 were purchased from R&D Systems, Inc. (McKinley Place N.E., MN, U.S.A.). Cycloheximide, brefeldin A and tunicamycin were purchased from Sigma. THP-1 cells (A.T.C.C.) were cultured at 37 °C in the presence of 5% CO2 in RPMI 1640 medium supplemented with 10% (v/v) bovine calf serum (HyClone), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate and 0.0012% (v/v) 2-mercaptoethanol (complete RPMI). Human embryonic-kidney 293T cells (A.T.C.C.) were cultured in Dulbecco's modified Eagle's medium with the same supplements.
Isolation of monocytes and culturing of DCs and macrophages
Monocytes were isolated from healthy adult blood donors (National University Hospital Blood Donation Centre), essentially as described previously [22]. Briefly, PBMCs (peripheral blood mononuclear cells) were isolated from buffy coats using Ficoll-Paque Plus (Amersham Biosciences) and, after washing, allowed to adhere to tissue culture plates for 2 h at 37 °C. Non-adherent cells were removed by washing, and the adherent monocytes were harvested. Thus isolated monocytes routinely have approx. 95% purity. The isolated monocytes were cultured at 1×106/ml in complete RPMI. To generate DCs, monocytes were cultured for 6 days in the presence of GM-CSF (20 ng/ml) and IL-4 (20 ng/ml, or otherwise stated) with one-half of the medium being replaced by fresh medium every other day. Macrophages were cultured from isolated monocytes in the presence of M-CSF (20 ng/ml). To activate monocytes with LPS, the cells were cultured in the presence of LPS (0.5 μg/ml) and GM-CSF (1 ng/ml) following a specified 48 h time course. To activate DCs or macrophages, LPS was added to the cultured cells on day 6 following a 48 h time course. In some experiments, DCs and macrophages were preincubated with cycloheximide (10 μg/ml), brefeldin A (10 μg/ml) or tunicamycin (10 μg/ml) for 30 min before stimulation with LPS (0.5 μg/ml), and the cells were stimulated for 6 h with LPS in the presence of these inhibitors and stained for CD83. Monocytes and macrophages were also activated with LPS (0.5 μg/ml) for 6 h in the presence of IL-4 (20 ng/ml) before staining for surface CD83.
The CD83 expression vector
cDNA encoding human CD83 was obtained by reverse transcriptase-PCR using RNA isolated from mDCs and a pair of CD83-specific primers (5′→3′, cggggtaccaccatgtcgcgcggcctcc/gaagggccctgctcataccagttctgtc). The PCR product was cloned into the KpnI/ApaI site of the pcDNA3.1 plasmid (Invitrogen) to generate the phCD83 expression vector. The construct was verified by sequencing from both directions.
Transfection
293T cells were subcultured in 24-well tissue-culture plates, and then were transfected with the phCD83 vector or, as a control, the pcDNA3.1 plasmid using the GenePORTER 2 reagent (Gene Therapy Systems, La Jolla, CA, U.S.A.) [23]. The cells were cultured for 24 h, before analyses by flow cytometry and Western blotting. In some experiments, 293T cells were transfected in the presence of tunicamycin or cycloheximide, and CD83 expression was examined in these cells by flow cytometry and Western blotting.
Flow cytometry
Monocytes, macrophages, DCs or transfected 293T cells were washed, resuspended in cold complete RPMI and then divided into 50 μl aliquots. Each aliquot was incubated with a specific or isotype control antibody for 30 min on ice. The cells were washed in FACSwash [PBS containing 2.5% (v/v) bovine calf serum and 0.05% (w/v) NaN3] and resuspended in 1% (w/v) cold paraformaldehyde in PBS, pH 7.6. To detect intracellular CD83, the washed cells were fixed for 10 min in 1% (w/v) paraformaldehyde in PBS, pH 7.6, and then stained after permeabilization for 10 min with 0.2% (w/v) saponin in FACSwash and blocking for 30 min in 20% (v/v) goat serum. The stained cells were examined on a FACSCalibur, and results were analysed using the CellQuest software (BD Biosciences).
Confocal microscopy
Cells were washed and allowed to attach to glass coverslips coated with polylysine. The cells were fixed in 3.7% (w/v) formaldehyde for 20 min, and permeabilized for 10 min in 0.2% (w/v) saponin. The cells were blocked for 30 min in 20% (v/v) goat serum (in PBS) and then stained for 1.5 h with a CD83 antibody (HB15a). After washing, the cells were stained further with biotin-conjugated goat anti-mouse IgG, and then with streptavidin–FITC. The cells were washed and then incubated for 15 min with propidium iodide (10 μg/ml) and RNAse A (200 μg/ml). The cells were then washed and mounted for analyses. Phase contrast and fluorescent images were captured using a LSM510 laser-scanning microscope and the Zeiss LSM Image Browser software.
Western blotting
Western blotting was performed essentially as previously described [22]. Cells were washed in PBS and then lysed on ice in a lysis buffer containing 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Nonidet P40 and the Com plete™ protease-inhibitor cocktail (Roche). Protein concentration was determined using the bicinchoninic acid reagent (Pierce Chemical Company, Rockford, IL, U.S.A.). The lysates (50 μg of protein) were separated on SDS/12.5% (w/v) PAGE gels and electroblotted on to a nitrocellulose membrane. The blots were blocked with 5% (w/v) non-fat milk in the TBST buffer [50 mM Tris/HCl (pH 7.5)/150 mM NaCl/0.1% (v/v) Tween 20] and were then probed with an anti-CD83 antibody (clone HB15a) overnight at 4 °C. The blots were washed in TBST buffer and probed further with biotin-conjugated anti-mouse IgG and then horseradish peroxidase–streptavidin (Dako). For monocytes, insoluble materials after cell lysis were also separated by SDS/PAGE and subjected to CD83 Western blotting. Signals were developed by ECL (enhanced chemiluminescence; Amersham Biosciences).
PNGase F (peptide N-glycosidase F) digestion of asparagine (Asn)-linked carbohydrate structures
Human embryonic kidney 293T cells (2×106) were transfected with 4 μg of the phCD83 vector and then lysed as above (100 μl). Upon removal of insoluble materials by centrifugation, the soluble fraction was made 0.5% (w/v) with SDS and 1% (v/v) with 2-mercaptoethanol and then denatured for 10 min at 100 °C. After the addition of Nonidet P40 to 1% (v/v) and sodium phosphate, pH 7.5, to 50 mM, 1 unit of PNGase F (New England Biolabs, Schwalbach, Germany) was added to 20 μl of lysate and incubated for 1 h at 37 °C. As a control, the 293T cell lysate was similarly processed, except that PNGase was omitted. The samples were then separated on SDS/12.5% (w/v) polyacrylamide gels and analysed by Western blotting using the HB115a CD83 antibody. This experiment was repeated twice with similar results.
RESULTS
CD83 is preformed inside imDCs and rapidly induced on the cell surface upon DC activation
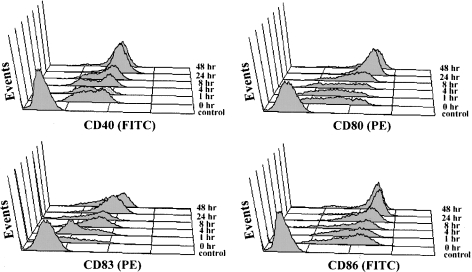
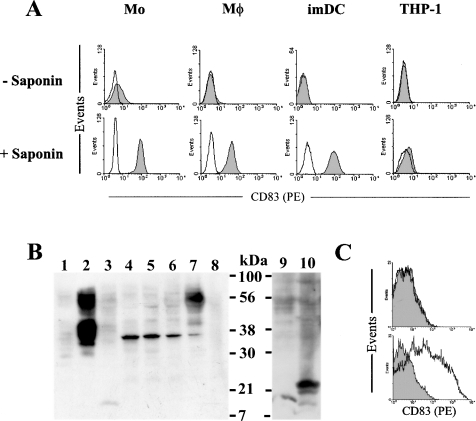
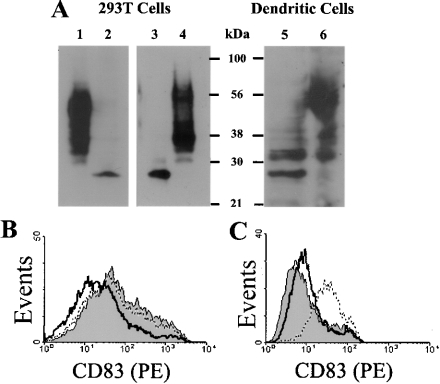
Upon activation, DCs increase surface expression of MHC and other molecules involved in antigen presentation, e.g. CD40, CD54, CD80 and CD86 [7,8]. CD83 is expressed on activated human DCs as a specific maturation marker. How CD83 surface induction is regulated is not clear. Examining the kinetics of CD83 expression on LPS-activated DCs, following a 48 h time course (i.e. 1, 4, 8, 24 and 48 h), showed that CD83 was rapidly induced on DCs, i.e. within 4 h (Figure 1). In contrast, the up-regulation of CD40, CD80 and CD86 was only detectable after 8–24 h. The rapid surface induction of CD83 was consistent with CD83 mRNA being detected in imDCs [21]. However, whether imDCs contained preformed intracellular CD83 was controversial [20,21]. In the present study, imDCs were permeabilized with saponin and then stained with a CD83 antibody (clone HB15e). As summarized in Figure 2(A), although CD83 was absent on the surface of imDCs, it was clearly detected inside the cells. As a control, CD83 was not detectable on or inside THP-1 cells, which was known to lack CD83 mRNA (Figure 2A, and results not shown).
Figure 1. Comparison of surface CD83 induction with the up-regulation of CD40, CD80 and CD86 during DC maturation.
ImDCs were cultured and activated with LPS (0.5 μg/ml) for up to 48 h, and the expression of CD40, CD80, CD83 and CD86 was examined by flow cytometry at a series of time points, i.e. 1, 4, 8, 24 and 48 h. Isotype IgG was used as a control in each experiment. ‘Control’ represents imDC stained with isotype IgG. Signals obtained with isotype IgG at the different time points were aligned with the ‘control’ and were not shown. The full y-axes in each histogram represent 128 events. These results are representative of three similar experiments.
Figure 2. Detection of intracellular CD83 in monocytes, macrophages and imDCs.
(A) Monocytes (Mo), macrophages (MΦ) and imDCs were fixed with paraformaldehyde and permeabilized with saponin. Upon blocking with 20% (v/v) goat serum, the cells were stained with a PE-labelled CD83 antibody (clone HB15e; filled histograms) or, as a control, isotype IgG (open histograms). The monocytic THP-1 cells were used as controls. (B) Monocytes (lane 3), resting macrophages (lane 4), LPS-activated macrophages (lane 5), imDCs (lane 6) and mDCs (lane 7) were lysed with Nonidet P40, and the soluble fractions were analysed by Western blotting using the HB15a CD83 antibody (Santa Cruz Biotechnology). Aliquots (50 μg) of each sample were separated by SDS/PAGE. Also included in the blots was the soluble fraction of THP-1 cells (lane 8), and 293T cells transfected with the pcDNA3.1 plasmid (lane 1) or the phCD83 expression vector (lane 2). The transfected cells were cultured for 24 h. For monocytes, both the soluble (lane 9) and insoluble (lane 10) fractions were subjected to Western blot analysis. (C) Expression of CD83 by 293T cells: 293T cells were transfected with the phCD83 expression vector (lower panel) or, as a control, with the pcDNA3.1 plasmid (upper panel). The cells were stained with a PE-labelled CD83 antibody (HB15e; open histograms). The filled histograms represent signals detected with isotype IgG. All results shown are representative of at least three similar experiments.
Intracellular CD83 was detected in different molecular-mass forms
The presence of intracellular CD83 in imDCs was also demonstrated by Western blotting. The detergent-soluble fractions of the imDC and mDC lysates were subjected to Western blotting analysis using a CD83 antibody (clone HB15a). As summarized in Figure 2(B) (lanes 6 and 7), CD83 was detected in both imDCs and mDCs. It was also noted that, although a 37 kDa polypeptide was detected in both imDCs and mDCs, the intensity of this 37 kDa band was much reduced in mDCs. Instead, a 50 kDa band, which was absent in imDCs, was the dominant CD83 form in mDCs (Figure 2B, lanes 6 and 7). As a negative control, neither CD83 form was detected in the THP-1 cell lysate (Figure 2B, lane 8). When 293T cells were transfected to express CD83, both the 37 kDa and 50 kDa CD83 forms were detected by Western blotting (Figure 2B, lanes 1 and 2). As a control, neither form was detected in vector-transfected 293T cells. By flow cytometry, CD83 was detected on the surface of transfected 293T cells (Figure 2C). CD83 was apparently expressed on 293T cells in a constitutive manner, which was in contrast with the tightly regulated CD83 expression on DCs. These results also show that CD83 undergoes substantial post-translational modifications, resulting in the expression of different molecular-mass forms. How the different CD83 forms may be related to its surface expression will be discussed below.
Monocytes and macrophages contain preformed intracellular CD83
Monocytes and macrophages are closely related to DCs. The detection of preformed intracellular CD83 inside imDCs implied that, despite the lack of surface CD83 on monocytes and macrophages, CD83 could also be present inside these cells. In fact, a subpopulation of CD14+ monocytes has been shown to express surface CD83 upon activation with IFN-α (interferon-α) [24]. Therefore CD83 expression was similarly examined in monocytes and macrophages. By flow cytometry, little CD83 was detected on monocytes (Figure 2A). However, CD83 was clearly detected inside these cells upon membrane permeabilization. CD83, which was absent on macrophages, was also detected inside these cells (Figure 2A). By Western blotting, the 37 kDa CD83 form was detected in macrophages as well as in imDCs (Figure 2B, lanes 4 and 5). However, CD83 was not detected in the soluble fraction of the monocyte lysate by Western blotting (Figure 2B, lanes 3 and 9). We then examined the detergent-insoluble faction of monocytes in which a 22 kDa form of CD83 was detected (Figure 2B, lane 10). This 22 kDa CD83 form corresponds well in size to a polypeptide predicted from the CD83 amino acid sequence (187 residues). However, how this 22 kDa CD83 form may be related to the 37 and 50 kDa forms is not clear.
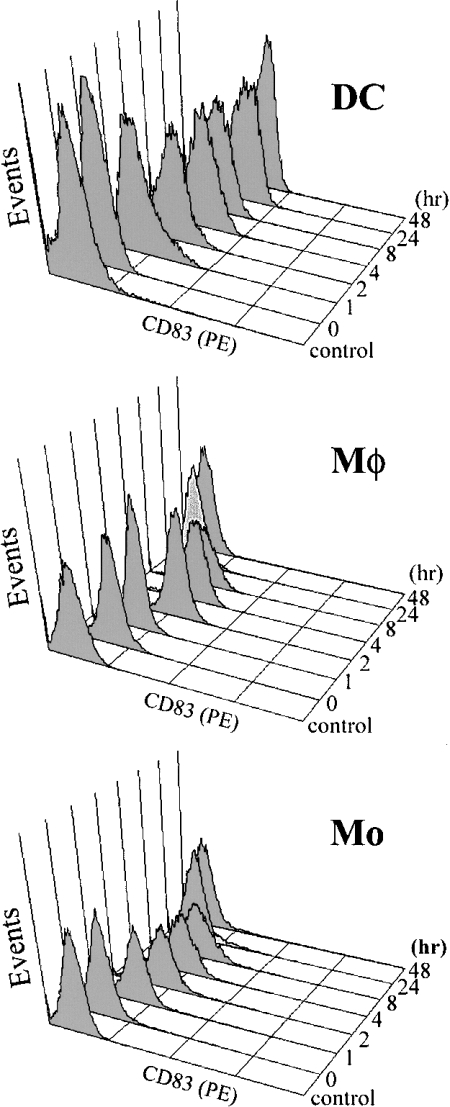
CD83 is rapidly, but transiently, induced on LPS-activated monocytes and macrophages
The presence of preformed CD83 inside monocytes and macrophages raised the possibility that CD83 could be induced on to the surface of these cells. CD83 expression was monitored by flow cytometry following a 48 h LPS-stimulated time course, i.e. 1, 2, 4, 8, 24 and 48 h. CD83 was rapidly induced on LPS-activated monocytes and macrophages, i.e. within 1 h of LPS stimulation (Figure 3). Whereas the induced CD83 expression on activated DCs was sustainable, its expression on monocytes and macrophages was transient. CD83 expression on these cells peaked 2–4 h after LPS stimulation, but it became undetectable on the surface within 24 h (Figure 3). In the case of macrophages, this was not due to the complete depletion of intracellular CD83, because CD83 was still detectable in macrophages by Western blotting after LPS stimulation for 48 h (Figure 2B, lane 5). The transient nature of CD83 expression on activated monocytes and macrophages could explain why it was not detected on these cells in most previous studies.
Figure 3. Rapid surface expression of CD83 on LPS-activated monocytes, macrophages and DCs.
The cells were stained essentially as described in Figure 1. The expression of CD83 on LPS-activated monocytes (Mo), macrophages (MΦ) and DCs was examined following a 48 h time course, i.e. 1, 2, 4, 8, 24 and 48 h, by flow cytometry using the HB15e CD83 antibody. Controls were used as in Figure 1. The full y-axes represent 64 events. These results were obtained in two or three independent experiments.
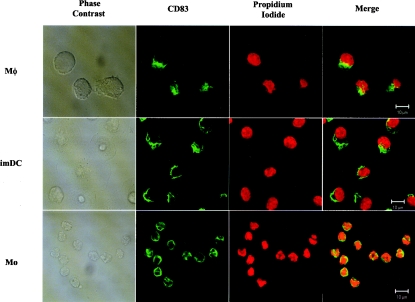
Intracellular CD83 is localized to perinuclear regions
Since CD83 was clearly detected inside monocytes, macrophages and imDCs, the subcellular location of CD83 was examined in these cells. The cells were permeabilized and then stained for intracellular CD83. The nuclei were co-stained with propidium iodide. As shown in Figure 4, CD83 was clearly detected in all three cell types. In all these cells, CD83 was found in perinuclear membrane networks resembling the endoplasmic reticulum (Figure 4). Co-staining for Golgi-97, a marker of the Golgi network, showed no co-localization with these CD83-containing compartments (results not shown). Although the nature of these CD83+ compartments remains to be defined, these results clearly confirmed the presence of preformed CD83 inside all three cell types.
Figure 4. Subcellular localization of CD83 in monocytes, macrophages and imDCs.
The cells were isolated or cultured as described in the Experimental section. Cells were washed and allowed to attach to glass coverslips that had been coated with polylysine. Upon fixation with paraformaldehyde, the cells were permeabilized with saponin. After blocking with 20% (v/v) goat serum, the cells were co-stained with a CD83 antibody (HB15a) and propidium iodide. Phase contrast and fluorescence images were captured using a LSM510 laser-scanning microscope and the Zeiss LSM Image Browser software. MΦ, macrophages; Mo, monocytes. Similar results were obtained from at least three independent experiments. Scale bars represent 10 μm.
The preformed CD83 in macrophages and DCs is surface-expressed upon LPS-induced activation
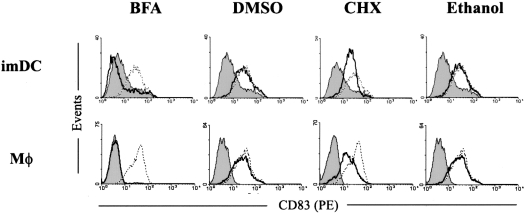
The presence of preformed CD83 inside monocytes, macrophages and DCs, and the rapid CD83 induction on the cell surface upon activation, implied that the preformed intracellular pool of CD83 was rapidly surface-translocated upon activation of these cells. To examine this possibility, DCs and macrophages were activated with LPS in the presence or absence of cycloheximide, which inhibits protein synthesis but not secretion. The rapid CD83 surface induction was examined on the activated DCs and macrophages 6 h after LPS stimulation. As shown in Figure 5, cycloheximide did not block the rapid CD83 induction on these cells. However, the rapid CD83 surface induction was effectively inhibited by brefeldin A, an inhibitor of protein secretion but not protein synthesis (Figure 5). It shows that the rapid CD83 surface induction on both cell types was not dictated by fresh CD83 synthesis, but it was essentially dependent on the integrity of the protein secretion mechanism. These results showed that the rapid CD83 expression on activated DCs, macrophages and monocytes most likely mobilizes the preformed intracellular CD83 pool.
Figure 5. The intracellular preformed CD83 is rapidly surface expressed on activated DCs and macrophages.
ImDCs and macrophages were activated for 6 h with LPS in the presence (solid line) or absence (dotted line) of brefeldin A (BFA), DMSO (the BFA solvent), cycloheximide (CHX) or ethanol (the CHX solvent), and then stained for surface CD83. Filled histograms in the upper panels are signals detected on imDCs with the CD83 antibody. The filled histograms in the lower panel are signals detected on macrophages (MΦ) with isotype IgG. These results are representative of three independent experiments.
CD83 surface expression requires glycosylation
Results so far provide no explanation as to why CD83 was found in three distinct molecular-mass forms, i.e. 22, 37 and 50 kDa. CD83 contains three potential Asn-linked glycosylation sites; therefore it is possible that differential occupation of these glycosylation sites may yield the different CD83 forms. 293T cells were then transfected with the phCD83 vector, and the lysate was treated with PNGase F. As shown in Figure 6A (lanes 1 and 2), PNGase treatment reduced the 37 and 50 kDa forms to 28 kDa, showing that the larger CD83 forms were generated as a result of Asn-linked glycosylation. The transfected 293T cells were also cultured in the presence or absence of tunicamycin, an inhibitor of Asn-linked glycosylation. In the absence of tunicamycin, CD83 was detected as two diffuse bands of 37 and 50 kDa respectively (Figure 6A, lane 4). In the presence of tunicamycin, the 50 kDa and the 37 kDa CD83 forms disappeared, leaving a discrete 28 kDa band (Figure 6A, lane 3). DCs activated in the absence of tunicamycin expressed predominantly the 50 kDa CD83 form, with the 37 kDa form being a minor component (Figure 6A, lane 6). In the presence of tunicamycin, activated DCs lost the expression of the 50 kDa form. Instead, the 28 kDa form was produced, and a slightly larger band remained (Figure 6A, lane 5). These results showed that the diffuse 37 and 50 kDa forms of CD83 were most likely to be derived from the 28 kDa form through Asn-linked glycosylation. The sequence of CD83 predicts a polypeptide of approx. 20 kDa, which is similar to the 22 kDa form detected in monocytes, but significantly smaller than the 28 kDa CD83 form. The relationship between the 22 kDa CD83 form and the 28 kDa form is not clear. We then examined whether Asn-linked CD83 glycosylation is required for its surface expression. DCs activated in the presence or absence of tunicamycin were then examined for surface CD83 expression. As shown in Figure 6(C), it was completely blocked by tunicamycin. In contrast, CD83 expression on transfected 293T cells was inhibited, but not completely blocked, by tunicamycin (Figure 6B). Therefore Asn-linked glycosylation of CD83 appears to be a key step in the regulation of its surface expression on DCs, macrophages and monocytes.
Figure 6. Surface induction of CD83 on DCs requires Asn-linked glycosylation.
(A) Transfected 293T cells were lysed, and the soluble fraction was either untreated (lane 1) or treated with PNGase F (lane 2). The samples were then separated on SDS/PAGE gels and subjected to Western blotting using the HB15a CD83 antibody. The transfected 293T cells were also cultured in the presence of tunicamycin (lane 3) or DMSO (lane 4) for 24 h, and then lysed with Nonidet P40. The soluble fraction was subjected to SDS/PAGE, followed by Western blotting using the CD83 antibody. ImDCs were activated with LPS in the presence of tunicamycin (lane 5) or DMSO (lane 6) for 6 h, and were similarly analysed by Western blotting using the CD83 antibody. The molecular-mass standards are shown, and apply to all three blots. (B) Transfected 293T cells were cultured for 24 h in the presence of tunicamycin (solid line) or DMSO (dotted line), and then stained with the CD83 antibody and analysed by flow cytometry. The filled histogram represents signals on transfected 293T cells cultured in the absence of tunicamycin and DMSO. (C) ImDCs were activated with LPS in the presence of tunicamycin (solid line) or DMSO (dotted line), or were unactivated (filled histogram) and then stained with the CD83 antibody and analysed by flow cytometry. The results are representative of two or three independent experiments.
Optimal IL-4 stimulation during monocyte differentiation confers the ability to sustain surface CD83 expression upon activation
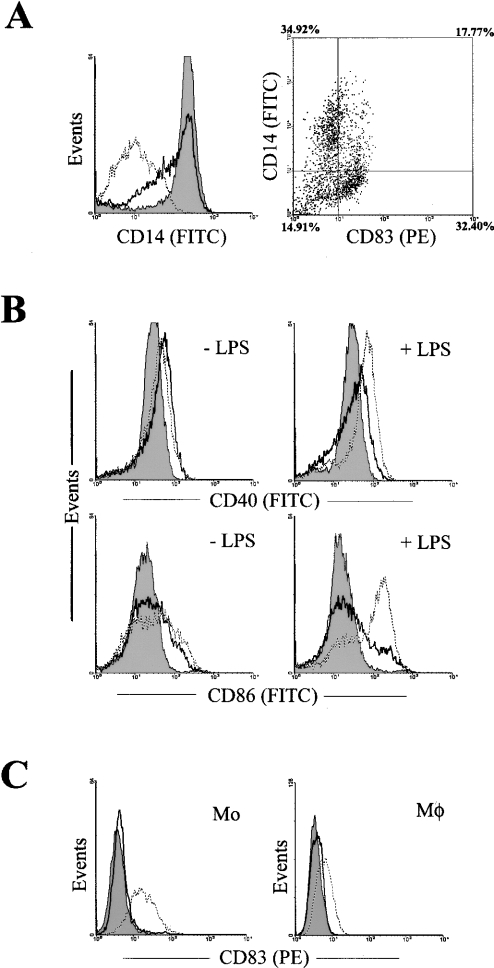
Monocytes differentiate into macrophages in the presence of GM-CSF alone, and these cells only acquire DC properties under the additional stimulation of IL-4. This implies that IL-4 is important in monocyte acquisition of the ability to sustain surface CD83 expression upon activation. In the present study, DCs are generated from monocytes in the presence of GM-CSF and IL-4, each at 20 ng/ml. Surface CD14 diminished on the cultured cells when IL-4 was used at 20 ng/ml (Figure 7A, left panel, dotted line). When IL-4 was used at 2 ng/ml, a heterogeneous population of cells was generated as judged by CD14 expression (Figure 7A, left panel, solid line). When this heterogeneous population of cells was activated with LPS and then double-stained for surface CD14 and CD83, CD14+ cells were found mostly negative for surface CD83, and CD83+ cells were mostly CD14− (Figure 7A, right panel). A minor population of CD14+/CD83+ cells was also observed (Figure 7A, right panel). In the absence of IL-4, monocytes differentiated into macrophages that were CD14+ (Figure 7A, left panel, grey-shaded histogram). Thus derived cells were found to be unable to sustain surface CD83 expression (results not shown).
Figure 7. Sustained surface CD83 expression on monocyte-derived and LPS-activated DCs relies on optimal IL-4 stimulation during monocyte differentiation.
(A) Monocytes were cultured in the presence of GM-CSF alone (filled profiles) or together with IL-4 at 2 ng/ml (solid lines) or 20 ng/ml (dotted lines). The expression of CD14 on these cells was examined by flow cytometry (left panel). Cells cultured in the presence of 2 ng/ml IL-4 (corresponding to the solid line on the left panel) were activated with LPS, and then co-stained for surface CD14 (FITC) and CD83 (PE), and the results are shown in dot–plot form (right panel). (B) CD40 and CD86 expression on monocytes cultured in the presence of GM-CSF alone (filled profiles) or together with IL-4 at 2 ng/ml (solid lines) or 20 ng/ml (dotted lines) before and after activation with LPS. Isotype controls are not shown, but these were all calibrated and positioned between 100 and 101. ‘+LPS’, LPS was added on day 6 (0.5 mg/ml) to the cells, which were then cultured for 48 h. ‘−LPS’, cells were similarly cultured for another 48 h without addition of LPS. (C) Monocytes and macrophages were activated with LPS for 6 h (dotted lines) or 24 h (solid lines) in the presence of IL-4 (20 ng/ml), and CD83 expression on these cells was detected by flow cytometry. The filled profiles represent isotype IgG controls. Mo, monocytes; MΦ, macrophages. These experiments were repeated for more than three times, yielding similar results.
The presence of IL-4 at either 2 or 20 ng/ml moderately up-regulated the surface expression of CD40 and CD86 during monocyte differentiation (Figure 7B). Whereas monocytes cultured in the presence of 20 ng/ml IL-4 could increase further surface CD40 and CD86 expression upon activation, only a small fraction of the cells cultured with 2 ng/ml IL-4 exhibited increased CD40 and CD86 expression (Figure 7B). Therefore optimal IL-4 co-stimulation together with GM-CSF is essential for monocyte acquisition of DC properties, including the ability to sustain surface CD83. The presence of IL-4 during monocyte and macrophage activation by LPS was insufficient to confer stable surface CD83 expression (Figure 7C). The ability to sustain surface CD83 expression appears to be unique to DCs. The exact mechanism that DCs have acquired during differentiation that has enabled the cells to sustain surface CD83 expression remains to be determined.
DISCUSSION
Surface CD83 expression is a hallmark of mature human DCs. Here, we have shown that CD83 was expressed as a preformed protein inside imDCs, and its surface expression is rapidly induced on activated DCs. Our detection of CD83 inside imDCs agrees with a previous study in which CD83 was detected inside permeabilized imDCs by flow cytometry [20]. However, in another study, CD83 was not detected in imDCs by Western blotting [21]. We detected CD83 in imDCs by multiple approaches, i.e. Western blotting, flow cytometry and confocal microscopy. We also detected CD83 inside monocytes and macrophages using similar methods. The cause of discrepancy between these studies is not clear. It could be due to the type of CD83 antibody used. We could not detect CD83 in imDCs and mDCs by Western blotting using the HB15e clone of CD83 antibody, although the same antibody could detect CD83 by flow cytometry and immunofluorescence microscopy.
Our observation that CD83 is expressed by monocytes and macrophages is not the first report of CD83 expression by cells other than DCs. CD83 is known to be expressed by activated human B cells [13,14], and is also induced on a subpopulation of CD2+/CD14+ monocytes upon activation with IFN-α [24]. Activated T cells also appear to express CD83 [25,26]. In mice, CD83 is expressed also by thymic epithelial cells, and has been shown to regulate the generation of CD4+ T cells [19]. In the present study, we found that CD83 is constitutively expressed inside monocytes and macrophages, as well as in imDCs. Upon activation of the cells with LPS, CD83 was also rapidly induced on the surface of these cells. However, activated monocytes and macrophages only transiently express surface CD83, which is in contrast with the stable CD83 expression on activated DCs. The mechanism by which DCs, but not monocytes and macrophages, sustain surface CD83 expression is not clear. We showed that optimal IL-4 co-stimulation during monocyte differentiation was important in the acquisition of this property. However, the presence of IL-4 during the brief period of monocyte and macrophage activation could not confer these cells the ability to sustain surface CD83 expression.
Another feature of CD83 expression is the detection of multiple molecular-mass forms by Western blotting. CD83 is apparently detected in three distinct sizes of 22, 37 and 50 kDa respectively. Only the 37 kDa form was detected in macrophages and imDCs. However, the expression of this 37 kDa CD83 form was markedly reduced in mDCs. Instead, mDCs predominantly expressed the 50 kDa form. Inhibition of Asn-linked glycosylation in 293T cells resulted in the loss of the 50 kDa and 37 kDa CD83 forms, but a 28 kDa form was generated. Removal of Asn-linked carbohydrate also reduced these larger CD83 forms to 28 kDa. The 28 kDa form is most likely the precursor of the larger forms that lack Asn-linked glycosylation. The relationship of the 22 kDa CD83, detected in the detergent-insoluble fraction of the monocyte lysate, to the larger CD83 forms is not clear. Since the sequence of CD83 (187 amino acid residues) predicts a polypeptide of approx. 20 kDa, the 22 kDa CD83 may represent the nascent CD83 polypeptide, which becomes the 28 kDa form in macrophages and DCs upon certain type of modifications. However, Asn-linked glycosylation does not appear to contribute to these modifications.
Our results clearly showed the presence of preformed intracellular CD83. The rapid CD83 surface expression on DCs and macrophages was blocked by brefeldin A, but not by cycloheximide, implying that the intracellular CD83 pool contributed to its rapid surface expression. How LPS-elicited cell signalling regulates CD83 surface expression is not clear. For macrophages and DCs, this apparently requires, or involves the regulation of, Asn-linked CD83 glycosylation. However, this regulatory mechanism does not appear to exist in 293T cells. Transfected 293T cells constitutively express surface CD83. Although tunicamycin prevented CD83 glycosylation in 293T cells, CD83 expression on 293T cells was not blocked. On the basis of these results, it is suggested that regulated CD83 surface expression upon translation is unique to APCs. CD83 expression on B cells is also inducible and transient upon LPS stimulation (results not shown).
The expression of CD83 by all professional APCs is consistent with its reported functions in the regulation of antigen presentation. Cell-surface or immobilized CD83 has been shown to enhance CD8+ T cell activation [12]. The release of soluble CD83 from activated B cells and DCs, and the ability of soluble CD83 to bind to activated CD8+ T cells, monocytes and DCs, may represent another mechanism through which CD83 regulates antigen presentation and T cell proliferation or differentiation [12,16–18]. Human cytomegalovirus appears to exploit this mechanism to evade host immunity [18]. The functional implications of transient CD83 expression on monocytes and macrophages are not clear. If the transient CD83 expression on activated monocytes, macrophages and B cells was due to shedding of surface CD83, the dominance of these cells in number, compared with DCs, reflects a major source of soluble CD83 from these cells. In this context, neutrophils have also been reported to express CD83 upon activation, and may also contribute to the generation of soluble CD83 [27]. However, it remains to be determined why CD83 expression is specifically sustained on DCs, but not on monocytes, macrophages and B cells.
Acknowledgments
We thank Joanna Mah for the preparation of buffy coats. This project is supported by a Singapore National Medical Research Council grant R-364-000-014-213.
References
- 1.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature (London) 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colonystimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender A., Sapp M., Schuler G., Steinman R. M., Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L. J., Tedder T. F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S. The macrophage. BioEssays. 1995;17:977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 8.Mellman I., Steinman R. M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L. J., Tedder T. F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]
- 10.Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 1992;149:735–742. [PubMed] [Google Scholar]
- 11.Lechmann M., Berchtold S., Hauber J., Steinkasserer A. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 2002;23:273–275. doi: 10.1016/s1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 12.Scholler N., Hayden-Ledbetter M., Dahlin A., Hellstrom I., Hellstrom K. E., Ledbetter J. A. Cutting edge: CD83 regulates the development of cellular immunity. J. Immunol. 2002;168:2599–2602. doi: 10.4049/jimmunol.168.6.2599. [DOI] [PubMed] [Google Scholar]
- 13.Kozlow E. J., Wilson G. L., Fox C. H., Kehrl J. H. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood. 1993;81:454–461. [PubMed] [Google Scholar]
- 14.Hock B. D., Kato M., McKenzie J. L., Hart D. N. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int. Immunol. 2001;13:959–967. doi: 10.1093/intimm/13.7.959. [DOI] [PubMed] [Google Scholar]
- 15.Crocker P. R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 2002;12:609–616. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 16.Scholler N., Hayden-Ledbetter M., Hellstrom K. E., Hellstrom I., Ledbetter J. A. CD83 is a sialic acid-binding Ig-like lectin (Siglec) adhesion receptor that binds monocytes and a subset of activated CD8+ T cells. J. Immunol. 2001;166:3865–3872. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 17.Lechmann M., Krooshoop D. J., Dudziak D., Kremmer E., Kuhnt C., Figdor C. G., Schuler G., Steinkasserer A. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 2001;194:1813–1821. doi: 10.1084/jem.194.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senechal B., Boruchov A. M., Reagan J. L., Hart D. N., Young J. W. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103:4207–4215. doi: 10.1182/blood-2003-12-4350. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto Y., Tu L., Miller A. S., Bock C., Fujimoto M., Doyle C., Steeber D. A., Tedder T. F. CD83 expression influences CD4+ T cell development in the thymus. Cell. 2002;108:755–767. doi: 10.1016/s0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 20.Albert M. L., Pearce S. F., Francisco L. M., Sauter B., Roy P., Silverstein R. L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruse M., Rosorius O., Kratzer F., Bevec D., Kuhnt C., Steinkasserer A., Schuler G., Hauber J. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J. Exp. Med. 2000;191:1581–1590. doi: 10.1084/jem.191.9.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao W., Bobryshev Y. V., Lord R. S. A., Oakley R. E. I., Lee S. H., Lu J. Dendritic cells in the arterial wall express C1q: potential significance in atherogenesis. Cardiovasc. Res. 2003;60:175–186. doi: 10.1016/s0008-6363(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Tay P. N., Cao W., Li W., Lu J. Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS Lett. 2002;532:171–176. doi: 10.1016/s0014-5793(02)03669-4. [DOI] [PubMed] [Google Scholar]
- 24.Di Pucchio T., Lapenta C., Santini S. M., Logozzi M., Parlato S., Belardelli F. CD2+/CD14+ monocytes rapidly differentiate into CD83+ dendritic cells. Eur. J. Immunol. 2003;33:358–367. doi: 10.1002/immu.200310010. [DOI] [PubMed] [Google Scholar]
- 25.Cramer S. O., Trumpfheller C., Mehlhoop U., More S., Fleischer B., von Bonin A. Activation-induced expression of murine CD83 on T cells and identification of a specific CD83 ligand on murine B cells. Int. Immunol. 2000;12:1347–1351. doi: 10.1093/intimm/12.9.1347. [DOI] [PubMed] [Google Scholar]
- 26.McKinsey T. A., Chu Z., Tedder T. F., Ballard D. W. Transcription factor NF-κB regulates inducible CD83 gene expression in activated T lymphocytes. Mol. Immunol. 2000;37:783–788. doi: 10.1016/s0161-5890(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamashiro S., Wang J. M., Yang D., Gong W. H., Kamohara H., Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–3963. [PubMed] [Google Scholar]