Abstract
PSP94 (prostate secretory protein of 94 amino acids), an abundant protein within semen, has reported local functions within the reproductive tract and reported systemic functions. Mechanisms of action remain poorly understood, but binding to undefined molecules within the prostate, pituitary, testis and blood may initiate some of these actions. PSP94 serum measurements, especially of bound and free forms, have potential clinical utility in prostate cancer management. Identification of the binding molecules will help in the understanding of PSP94's action, and enable further development of PSP94 serum assays. PSPBP (PSP94-binding protein) was purified from human serum by ammonium sulphate fractionation, ion-exchange and affinity chromatography. The glycosylated protein ran as two bands on SDS/PAGE (70 and 95 kDa). N-terminal sequencing yielded a 30-amino-acid sequence, identical with the translated N-terminal region of a previously published cDNA (GenBank® accession number AX136261). Reverse transcriptase PCR and plaque hybridization demonstrated PSPBP mRNA in peripheral blood leucocytes and in a prostate cDNA library. Northern blotting showed 2 kb mRNA species in prostate, testis, ovary and intestine. Immunohistochemistry demonstrated PSPBP in tissues, including pituitary and Leydig cells, supporting a role for PSP94 in hormonal control at the pituitary gonadal axis. ELISA demonstrated that PSPBP levels were significantly lower (P=0.0014) in the serum of a prostate cancer population (n=65) compared with a control population (n=70). PSPBP identification will help the understanding of PSP94's functions and facilitate ELISA development to address the clinical value of PSP94 serum assays.
Keywords: cysteine-rich secretory protein (CRISP), ELISA, immunohistochemistry, β-microseminoprotein, prostate secretory protein of 94 amino acids (PSP94), PSP94-binding protein (PSPBP)
Abbreviations: FSH, follicle-stimulating hormone; GPI, glycosylphosphatidylinositol; LH, luteinizing hormone; PBL, peripheral blood leucocyte; PSA, prostate-specific antigen; PSP94, prostate secretory protein of 94 amino acids; PSPBP, PSP94-binding protein; PTHrP, parathyroid-hormone-related protein; SCP, sperm-coating glycoprotein; TMB, 3,3′,5,5′-tetramethylbenzidine
INTRODUCTION
PSP94 (prostate secretory protein of 94 amino acids) is a major component of semen, together with PSA (prostate-specific antigen) and PAP (prostatic acid phosphatase) [1]. PSP94 has also been termed β-microseminoprotein, and PIP (prostatic inhibin peptide). A number of functions, both systemic and confined within the reproductive tract, have been postulated or demonstrated for PSP94, including a modulator of circulating FSH (follicle-stimulating hormone) levels [2], a motility inhibitor of sperm [3], a binder of immunoglobulins in the female reproductive tract [4], a growth regulator and inducer of apoptosis in prostate cancer cells in vitro and in vivo [5], and a regulator of calcium levels during hypercalcaemia of malignancy [6]. There have been several reports suggesting the existence of cell-surface-binding sites for PSP94 in prostate, pituitary and testis [7–9]. It has been suggested that these binding sites represent receptors for PSP94, and as such may mediate some of the reported actions of the molecule. As yet, no receptor has been identified, and the details of the mechanisms behind the actions of PSP94 are not fully understood.
Several groups have addressed the clinical potential of PSP94 serum measurements as a prostate cancer biomarker. Serum concentrations of normal males lie within the range 0–20 ng/ml, and the levels are often highly elevated in patients with confirmed prostate cancer [10–13]. Diagnostic, prognostic and monitoring utilities have been suggested, especially in patient cohorts inadequately served by existing clinical markers. Abrahamsson et al. [11] indicated that a proportion of the immunoreactive PSP94 within the serum of prostate cancer patients had a molecular mass higher than the native 10.7 kDa protein, and suggested that this could be the result of aggregation or due to a high-molecular-mass binder in the patients' blood. More recent work by Xuan and colleagues, involving fractionation of prostate cancer patients' sera by molecular mass and Western blotting of the denatured fractions with polyclonal anti-PSP94 antibodies, suggested that the majority of PSP94 within many of these sera was in a high-molecular-mass form of between 60 and 150 kDa [14]. Furthermore, these authors suggested that antibodies raised to free PSP94 were not efficient at detecting the high-molecular-mass form of the protein, and that previous studies looking at the utility of serum PSP94 immunoassays for prostate cancer management were simply assessing the free form of the protein [14]. As the majority of PSP94 in the serum of some prostate cancer patients was shown to be in the high-molecular-mass form, the previous studies may not have been realizing the full clinical significance of PSP94 serum measurements. In a continuation of this work, Xuan and colleagues demonstrated that the ratio of bound to free forms of PSP94 in the pre-treatment serum of patients receiving curative intent radiotherapy for prostate cancer was a significant and independent predictor of relapse-free interval [15].
In order to understand the biology and functions of PSP94 more thoroughly, and to investigate fully the clinical utility of bound and free PSP94 measurements, identification and characterization of the putative binding protein is crucial. This is the subject of the present study.
EXPERIMENTAL
Materials
Human PSP94 was prepared from human semen [16] and was radiolabelled with mono-iodinated Bolton–Hunter reagent (PerkinElmer, Woodbridge, Ontario, Canada). Sephadex G100, CNBr-activated Sepharose and the enzymic protein deglycosylation kit were obtained from Sigma-Aldrich (Oakville, Ontario, Canada). Macroprep® High Q and disposable PD10 gel-filtration columns were purchased from Bio-Rad Laboratories (Mississauga, Ontario, Canada). Gelcode® Blue stain reagent and glycoprotein staining kit were purchased from Pierce (Rockford, IL, U.S.A.). Horseradish-peroxidase-labelled and biotinylated anti-mouse antibodies, horseradish-peroxidase-linked anti-rabbit antibodies and isotype-matched control mouse IgG1 were obtained from Dako Cytomation (Mississauga, Ontario, Canada). Streptavidin–biotinylated horseradish-peroxidase-labelling reagent was purchased from Vector Laboratories (Burlingame, CA, U.S.A.). Polyclonal anti-PSP94 antibodies were raised in rabbit, and were purified by Protein A and affinity purification. Titermax® adjuvant was obtained from CytRx Corporation (Los Angeles, CA, U.S.A.). Centriprep® centrifugal concentrating devices were purchased from Millipore Corporation (Bedford, MA, U.S.A.), sequencing grade ProBlott™ membranes were from Applied Biosystems (Foster City, CA, U.S.A.), mouse monoclonal isotyping test strips were from Roche Diagnostics (Laval, Québec, Canada) and ECL® (enhanced chemiluminescence) kit was from Amersham Biosciences (Baie D'Urfé, Québec, Canada). Normal human tissue microarray slides were obtained from Zymed (San Francisco, CA, U.S.A.), Super Block® and TMB (3,3′,5,5′-tetramethylbenzidine) reagent were from ScyTek laboratories (Logan, UT, U.S.A.), Nunc Maxisorp® ELISA plates were from VWR International (Montréal, Québec, Canada). Tri-reagent was obtained from Molecular Research Center (Cincinnati, OH, U.S.A.). Multiple tissue Northern blots and the human prostate cDNA library (5′ Stretch Plus, λ Triplex library) were purchased from Clontech (Palo Alto, CA, U.S.A.), Thermoscript® RT-PCR kit was from Life Technologies (Rockville, MD, U.S.A.), QIAEX II DNA extraction kit was from Qiagen (Mississauga Ontario, Canada), cloning and expression plasmid pCR2.1, and Escherichia coli (strain TOP10) were from Invitrogen (Carlsbad, CA, U.S.A.). Total protein lysates from human prostate, pituitary and ovary were obtained from BioChain Institute (Hayward, CA, U.S.A.). All other reagents used were of analytical grade.
125I-labelled PSP94-binding assays
Human PSP94 (20 μg) in 15 μl of 100 mM sodium bicarbonate (pH 8.0) was labelled with 125I using 1 mCi of mono-iodinated Bolton–Hunter reagent at 0 °C following the manufacturer's instructions. The free iodine was separated from the iodine incorporated into the PSP94 using a PD10 disposable gel-filtration column. Typically, incorporation was approx. 60%, giving a specific activity of approx. 30 μCi/μg. In a total volume of 500 μl, the test sample [serum diluted 1:2 in PBS (10 mM sodium phosphate and 140 mM NaCl, pH 7.5), or fractions from purification trials] was incubated for 16 h at 34 °C with 50 ng of radiolabelled PSP94, with or without excess unlabelled competitor (10 μg of unlabelled PSP94) in PBS/gelatin (PBS with 0.1% Type A gelatin including 8 mM sodium azide as a preservative). The equilibrated mixture was then placed on ice, and the components were separated according to their molecular mass by gel filtration at 4 °C using a 1 cm×20 cm Sephadex G100 column equilibrated with PBS/gelatin. The radioactivity in the collected 0.5 ml fractions was measured using a rack γ counter, and the total counts in the high-molecular-mass peak (generally contained within fractions 5–12) and low-molecular-mass peak (fraction 12 onwards) were calculated. Equilibrium binding analysis was performed by adding ranges of concentrations of radiolabelled PSP94 (1×10−10−2×10−7 M) to 250 μl of normal male human serum in a total volume of 500 μl and incubation at 34 °C for 16 h. Separation of high- and low-molecular-mass components was performed using Sephadex G100 gel filtration as described above to estimate the proportions of bound and free PSP94. Non-linear least-squares regression analysis of the data was performed using the Ligand program [17] to derive estimations of the total concentration of binding protein within the serum and the affinity of the binding interaction.
PSPBP (PSP94-binding protein) purification
Pooled normal human male serum was diluted 1:2 in PBS and precipitated by adding 192 g of ammonium sulphate per litre (32%) at 4 °C with constant stirring. After 30 min, the precipitated proteins were removed by centrifugation at 5000 g for 15 min. The precipitate was discarded, and a further precipitation was performed on the supernatant by adding ammonium sulphate to 47% (an additional 94 g per litre). The precipitate was collected by centrifugation, and dissolved in 100 ml of 100 mM NaCl and 10 mM Mes, pH 6.5, per litre of diluted serum starting material. The solution was dialysed against 50 vol. of this buffer at 4 °C for 16 h using a 6–8 kDa cut-off dialysis membrane. The dialysis buffer was replaced with fresh buffer, and dialysis was continued for a further 6 h. The total protein content within the dialysed ammonium-sulphate-precipitated human serum was estimated using UV absorbance at 280 nm. (1 absorbance unit was estimated to contain approx. 1 mg/ml of protein.) A 50 mm×100 mm Macroprep High Q anion-exchange column was equilibrated with 100 mM NaCl and 10 mM Mes, pH 6.5. The column was prepared and run at room temperature (22 °C), the flow rate was maintained at 6 ml/min using a peristaltic pump, and the eluate was monitored using UV absorbance. A 4 g (total protein) sample of the dialysed fraction was applied to the column, the column was washed with 100 mM NaCl and 10 mM Mes, pH 6.5, and the bound proteins were eluted sequentially with 10 mM Mes, pH 6.5, containing 200 mM NaCl, 300 mM NaCl and 1 M NaCl respectively. The 300 mM eluate (containing the PSP94-binding activity, as determined by the radiobinding assay described above) was buffer-exchanged into PBS (using 10 kDa molecular mass cut-off Centriprep® concentrators) and concentrated to approx. 2 mg/ml (D280 of 2.0). PSP94 (5 mg) in 100 mM sodium bicarbonate, pH 8.0, was conjugated to 1 ml of swollen CNBr-activated Sepharose according to the manufacturer's protocol. Routinely, between 3 and 4 mg of PSP94 was conjugated to the 1 ml of packed gel. The partially purified binding protein preparation (the product of the ammonium sulphate precipitation and anion-exchange chromatography steps), at a concentration of approx. 2 mg/ml in PBS containing 0.05% Tween 20 and 0.05% NaN3, was incubated overnight at 34 °C on an end-to-end shaker with 5 μg/ml PSP94–Sepharose conjugate (concentration relative to PSP94) without and with a 20-fold excess of unlabelled PSP94 as a competition control. The unbound proteins were separated from the affinity matrix by rapid filtration, and were washed with at least 100 vol. (relative to the matrix volume) of ice-cold PBS. PSPBP was eluted from the PSP94 matrix by competitive elution, incubating the matrix with a 5-fold excess of unconjugated PSP94 for 1 h at 34 °C with gentle agitation. The PSPBP was separated from the unconjugated PSP94 by gel filtration at room temperature using a 1 cm×20 cm Sephadex G100 column equilibrated and run with PBS at a flow rate of 1 ml/min. The eluate was monitored using UV absorbance, and the high-molecular-mass peak containing the PSPBP–PSP94 complex was pooled.
SDS/PAGE and glycosylation analysis
Deglycosylation of the purified PSPBP was performed using an enzymic deglycosylation kit. N-linked carbohydrates were removed with PNGase F (peptide N-glycosidase F), and O-linked oligosaccharides with O-glycosidase and α-2(3,6,8,9)-neuraminidase following the manufacturer's protocol. SDS/7.5% PAGE was performed on the digested and undigested samples in parallel gels and stained with total protein and glycoprotein-specific regents (Pierce Gelcode® reagents).
N-terminal amino acid sequencing
SDS/7.5% PAGE analysis of the PSPBP was performed. The proteins on the SDS/PAGE gel were electroblotted on to sequencing grade PVDF membranes. N-terminal amino acid sequencing was performed by automated Edman degradation (Procise 494 cLC, Applied Biosystems), employing the protocol described by Hewick et al. [18].
Isolation of human PSPBP cDNA and Northern blot
Total RNA was isolated from healthy blood donor PBLs (peripheral blood leucocytes) using Tri-reagent, ethanol-precipitated and resuspended in water. RNA was reverse-transcribed into cDNA using the Thermoscript® reverse transcriptase PCR system. The cDNA was subsequently amplified by PCR using custom primer: 5′-ATGCACGGCTCCTGCAGTTTCCTGATGCTT-3′ based on the cDNA sequence from the GenBank® cDNA database (http://www.ncbi.nlm.nih.gov/) and 3′ RACE (rapid amplification of cDNA ends) adaptor primer: 5′-GCCCACGCGTCGACTAGTAC(T)17-3′. Amplified DNA was resolved by agarose gel electrophoresis, excised from the gel and concentrated using QIAEX II DNA extraction kit. Purified DNA was ligated into pCR2.1 plasmid and used to transform E. coli. Ampicillin-resistant bacterial colonies were screened for cDNA-positive inserts by restriction enzyme analysis and DNA sequence analysis.
We confirmed and verified the size of our bacterial PSPBP cDNA clones by screening a human prostate 5′-Stretch Plus cDNA library by plaque hybridization using a [32P]cDNA insert as a probe. Positive plaques were purified and assayed for PSPBP cDNA using Southern blotting, followed by DNA sequence analysis using flanking vector primers (λ Triplex) and custom internal primers.
mRNA expression in normal human tissues was assessed by hybridizing a multiple tissue Northern blot (containing 2 μg of polyadenylated RNA per lane) with a 32P-labelled PSPBP cDNA probe that spanned the PSPBP cDNA sequences 346–745.
Generation of anti-PSPBP monoclonal antibodies
Balb/c mice were immunized subcutaneously with 15 μg of PSPBP in TiterMax® adjuvant. After 21 days, the mice were boosted with a further 15 μg of PSPBP and a final intraperitoneal boost after a further 3 weeks. After 4 days, the B-lymphocytes from the spleens of the mice were harvested and fused with NS0 myeloma cells. Splenocytes (105) were plated into each well of 96-well plates in IMDM (Iscove's modified Dulbecco's medium) selection medium (with 20% foetal bovine serum, antibiotics, 100 μM sodium hypoxanthine, 0.4 μM aminopterin, 16 μM thymidine and 1 ng/ml interleukin-6). After 10 days of incubation at 37 °C, the supernatants in the wells containing visible clones were screened by ELISA for reactivity to PSP94 or PSPBP. Maxisorp® microtitre 96-well plates were coated overnight at 4 °C with either purified PSP94 (100 μl/well at 1.5 μg/ml in 0.1 M NaHCO3) or PSPBP (100 μl/well at 0.2 μg/ml in 0.1 M NaHCO3). Aliquots of 100 μl of hybridoma supernatant were incubated with the coated ELISA plates for 1 h at 34 °C before washing and applying 100 μl aliquots of a horseradish-peroxidase-linked rabbit anti-mouse antibody (1:1000) for a further 1 h, before washing the plates again and development of the peroxidase signal with TMB reagent. After 30 min, the plates were read at 630 nm in an automated spectrophotometer. The clones maintaining strong titres for the antigens were re-cloned by limiting dilution. The isotypes of the antibodies within the hybridoma supernatants were determined using antibody-isotyping test strips. Mouse IgG1 monoclonal antibodies were purified using a high-salt Protein A procedure as described in [19].
Co-precipitation of PSP94 and PSPBP from normal human serum
Normal male human serum (10 ml) diluted 1:2 in PBS or 20 ml of PBS alone, was incubated for 1 h at 22 °C with end-to-end shaking with 50 μg of 17G9 (anti-PSPBP monoclonal antibody) or pre-immune mouse IgG conjugated to 25 μl of CNBr-activated Sepharose. The matrix was washed thoroughly in PBS, and boiled for 5 min in reducing SDS/PAGE sample buffer. The proteins were resolved by 10–20% gradient SDS/PAGE, together with standard quantities of PSP94, and transferred on to PVDF membranes for Western blotting. After blocking of the membrane in Super Block® and incubation with affinity-purified rabbit polyclonal anti-(human PSP94) antibodies for 1 h at room temperature with gentle agitation (1 μg/ml in PBS, 1% BSA and 0.05% Tween 20), followed by a further incubation in horseradish-peroxidase-conjugated polyclonal goat anti-rabbit immunoglobulin antisera (at a 1:1000 dilution in PBS, 1% BSA and 0.05% Tween 20), the peroxidase signal was developed with ECL® as per the manufacturer's protocol.
PSPBP immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections (in the form of a tissue microarray containing 31 normal human tissues) were de-waxed in xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation in 80% methanol containing 0.6% H2O2 for 20 min. Antigen retrieval was performed by microwaving the sections in 10 mM sodium citrate (pH 6.0) to boiling point for 15 min. Endogenous biotin was suppressed as described previously [20], and the sections were blocked in 3% BSA in PBS before incubation with the primary antibodies (anti-PSPBP antibodies: 17G9, 2B10 and 1B11; PSP94 antibody: P1E8; control antibody: isotype-matched control IgG1) diluted in the blocking solution at a concentration of 1 μg/ml overnight at 4 °C. After washing three times for 5 min in PBS, biotinylated rabbit anti-mouse immunoglobulins at 1:400 in blocking solution were applied for 1 h at room temperature; after a further wash in PBS, the sections were incubated with a streptavidin–biotinylated horseradish peroxidase complex for a further 1 h. After washing, the sections were incubated with 0.05% (w/v) 2,4-diaminobenzidine tetrahydrochloride solution in PBS with 0.01% H2O2 for 10 min, before the sections were counterstained in haematoxylin, dehydrated and mounted.
PSPBP Western blot
Normal human whole–tissue lysates (20 μg) from testis, pituitary and ovary, and purified PSPBP (5 ng) were subjected to SDS/7.5% PAGE under reducing conditions. The proteins were transferred on to PVDF membranes for Western blotting. The membrane was blocked in Super Block®, and incubated with monoclonal anti-PSPBP (17G9) antibodies for 1 h at room temperature with gentle agitation (1 μg/ml in PBS, 1% BSA and 0.05% Tween 20). After washing, the membrane was incubated with horseradish-peroxidase-conjugated polyclonal rabbit anti-mouse immunoglobulin antisera (at a 1:1000 dilution in PBS, 1% BSA and 0.05% Tween 20), and, after a further wash, the peroxidase signal was developed with enhanced chemiluminescence as per the manufacturer's protocol.
PSPBP concentrations in prostate cancer patients' sera
A validated sandwich ELISA to measure the concentration of PSPBP within male human serum was developed under contract with Medicorp (Montréal, Québec, Canada) using the 17G9 and 3F4 anti-PSPBP monoclonal antibodies. During the process of validation, the performance of the assay was assessed with respect to sensitivity, precision, reproducibility, recovery, linearity, specificity, hook effect, drift, matrix effect and interfering substances (results not shown). The PSPBP protein ELISA was applied to 135 serum samples, prospectively collected with appropriate informed consent and under the approval of Southern Connecticut State University institutional review board, which were randomly pulled by category from a larger cohort of frozen samples collected between July 1998 and June 1999. The samples were classified into two groups: 70 controls (with a normal digital rectal examination and normal trans-rectal ultrasound) and 65 pretreatment cancers (biopsy positive).
RESULTS
PSP94-binding activity in human serum
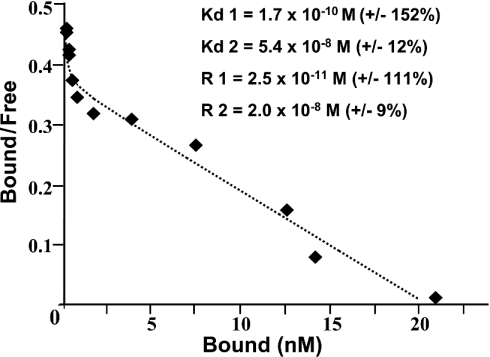
The development of an assay to detect and semi-quantitatively assess the PSPBP was necessary in order to develop a purification strategy. Incubation of 125I-labelled PSP94 with serum resulted in the formation of a high-molecular-mass complex, and specificity was confirmed by competition with excess unlabelled PSP94 (Figure 1). To establish the serum concentration of PSPBP in order to help develop a purification strategy, equilibrium binding studies were performed. Preliminary experiments suggested that equilibrium between the PSP94 and PSPBP was achieved after overnight incubation at 34 °C (results not shown). Non-linear least-squares regression analysis [17] indicated that the data were described significantly more accurately by a two-binding-site model than a single-binding-site model (P=0.02). These data, in the form of a Scatchard plot are presented in Figure 2, with the total concentration of binding sites in undiluted serum amounting to approx. 40 nM. The relative low abundance of the PSPBP compared with the total concentration of proteins within whole human serum indicated that a multi-step purification strategy was required in order to achieve the purification factor necessary to yield pure PSPBP.
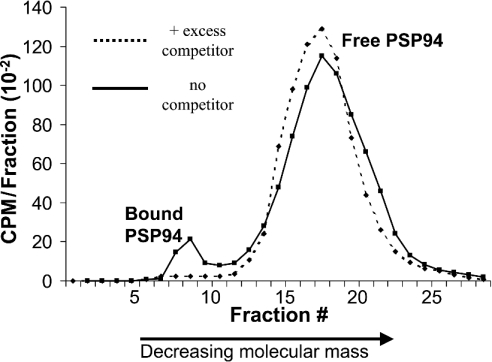
Figure 1. Detection of PSPBP in serum.
Sephadex G100 gel filtration of normal human serum incubated with 125I-labelled PSP94 with and without excess unlabelled PSP94. Note that the majority of the free PSP94 eluted later than fraction 12. In this experiment, approx. 7% of the total radioactivity was associated with the high-molecular-mass peak, and this value fell to less than 1% in the competition control.
Figure 2. PSP94–PSPBP binding kinetics.
Non-linear least-squares regression analysis (Scatchard plot) illustrating equilibrium binding of a range of concentrations of 125I-labelled PSP94 to human serum (R, binding site concentration; Kd, dissociation constant; +/−, standard errors of the estimates).
PSPBP purification
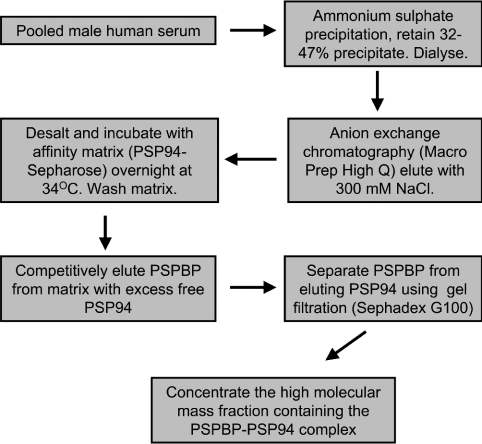
Normal human serum was separated into four fractions using increasing concentrations of ammonium sulphate. PSPBP was assessed in each fraction using the 125I-labelled PSP94-binding assay after buffer exchange and standardization of the protein content within each fraction. The PSP94-binding activity was localized in the 32–47% ammonium sulphate precipitate. Ion-exchange trials indicated that PSPBP became bound to the anion matrix at pH 6.5, and stepwise elution was performed by increasing salt concentration. The flow-through, 200 mM eluate, and 1 M eluate contained little PSP94-binding activity, with the vast majority of binding activity located within the 300 mM fraction. Preliminary investigations using affinity chromatography on whole human serum, or ammonium-sulphate-precipitated serum were not successful due to the very high concentration of total protein within the starting material. Affinity chromatography on the partially purified binding protein preparation (ammonium sulphate fractionation and ion-exchange chromatography) was more successful. Elution of the PSPBP from the affinity matrix was performed by competitive elution, as extremes of salt concentration or pH did not appear to be effective at stripping the molecule from the matrix. The excess unconjugated PSP94 used for elution was separated from the PSPBP by gel filtration. From a starting volume of 1 litre of human serum, it was possible to obtain approx. 200 μg of PSPBP. Inclusion of excess free PSP94 during the affinity-purification step resulted in no detectable PSPBP (as assessed by SDS/PAGE) binding to the affinity matrix. The process for purifying the PSPBP is summarized in Figure 3.
Figure 3. Summary of PSPBP purification.
PSPBP purification procedure from pooled male human serum. After the development of PSPBP specific monoclonal antibodies, an alternative affinity-purification procedure was developed using an antibody matrix (17G9) and acid elution (results not shown). This resulted in the purification of PSPBP uncomplexed with PSP94.
Preliminary characterization of the PSPBP
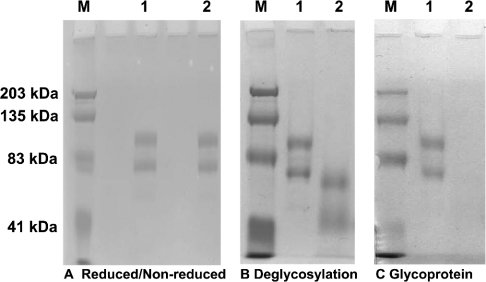
Both reducing and non-reducing SDS/PAGE analysis indicated that the PSPBP ran as two major bands (Figure 4A), with estimated molecular masses of 94.4 and 71.0 kDa. Glycoprotein staining of SDS/PAGE gels indicated that the PSPBP is glycosylated (Figure 4C), and removal of the sugar residues resulted in a reduction in the molecular masses for the high- and low-molecular-mass PSPBPs to 67.6 and 52.2 kDa respectively (Figure 4B).
Figure 4. SDS/PAGE and glycosylation.
(A) SDS/7.5% PAGE of PSPBP under reducing (lane 1) and non-reducing (lane 2) conditions. (B) SDS/7.5% PAGE of PSPBP before (lane 1) and after (lane 2) enzymic deglycosylation. (C) Parallel gel to (B), but stained with a glycoprotein-specific reagent, confirming that the PSPBP is a glycoprotein (lane 1) and confirming the absence of sugar residues after the enzymic deglycosylation process (lane 2). Lanes M represent molecular-mass standards with sizes indicated in kDa.
PSPBP identification, cloning and Northern blotting
To establish the identity of the PSPBP, N-terminal amino acid sequencing was performed on the high- and low-molecular-mass PSPBP SDS/PAGE bands. The N-terminal amino acid sequence obtained from the 94.4 kDa band was LTDEEKRLMVELHN and from the 71 kDa band, it was LTDEEKRLMVELHNLYRAQVSPTASDMLHM. In order to determine the full-length PSPBP protein sequence, we cloned the corresponding cDNA and also isolated the full-length cDNA clone from a prostate-specific phage library. Results indicated that our PSPBP cDNA was at least 1860 bp in length and contained one principal open reading frame encoding a 463-amino-acid protein (Figure 5). The mature PSPBP mRNA appears to be derived from a multiply spliced species (encoded from chromosome locus 6p21.2) with the major open reading frame of 1389 bases followed by a 486 base 3′-untranslated region. Sequence homology searches (http://www.ncbi.nlm.nih.gov/) indicate that our cDNA sequence is very similar to previously published sequences (GenBank® accession number AX136261 for the first report), with the predicted protein sequences differing by seven amino acids (Figure 5). The predicted molecular mass of this protein (47 kDa after subtraction of a 27-amino-acid signal sequence) is consistent with the experimentally derived size of the deglycosylated low-molecular-mass PSPBP. The nature of the high-molecular-mass PSPBP is currently unknown, although alternative splicing may offer an explanation.
Figure 5. Predicted amino acid sequence of PSPBP.
Predicted amino acid sequence for the PSPBP derived from the mRNA of PBLs. There are seven differences when compared with a previously published hypothetical sequence (CAC39772) and these are: Glu179→Gly, Ala232→Thr, Gly247→Glu, Pro253→Ala, Leu255→Lys, Gly256→Ala and Val288→Ala. A predicted secretory sequence cleavage site is located between amino acids 27 and 28 (S), and a predicted GPI cleavage site between amino acids 437 and 438 (GPI). The amino acids identified by N-terminal sequencing are in bold. The underlined region represents the highly conserved SCP domain.
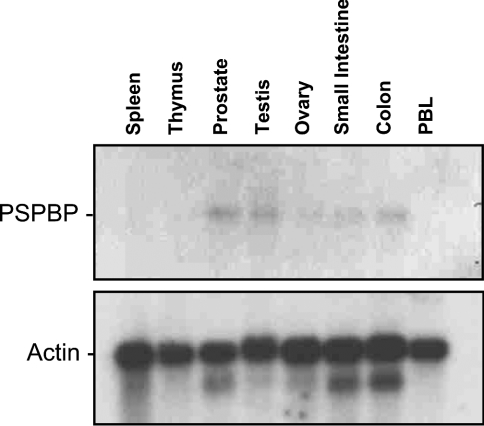
Northern blot analysis using our PSPBP cDNA probe revealed a single 2 kb mRNA species in normal prostate, testis, small intestine, colon and, to a lesser extent, ovary (Figure 6). The 2 kb mRNA band agrees well with the size of the full-length cDNA sequence.
Figure 6. PSPBP Northern blot.
Northern blot of PSPBP, demonstrating the presence of a 2 kb mRNA species in prostate, testis, ovary, small intestine and colon.
Generation of anti-PSPBP monoclonal antibodies
Immunization of mice with the PSPBP–PSP94 complex resulted in high titres for the PSPBP 1 week after the second boost and a lower titre for PSP94. A number of stable anti-PSPBP antibody-secreting clones were generated to several different epitopes of the PSPBP. All of these antibodies clearly labelled the PSPBP, but not PSP94, by Western blotting, were unreactive to PSP94 itself by ELISA assay and all were of the IgG1κ subclass (results not shown). Antibody 17G9 was used for the immunoprecipitation studies, two antibodies to independent epitopes were used for the PSPBP ELISA development (17G9 and 3F4), and three antibodies for the immunohistochemical study (17G9, 2B10 and 1B11), each recognizing an independent epitope. Several PSP94-specific monoclonal antibodies were also generated, and one of these (P1E8) was used as a positive control antibody for the immunohistochemical study.
Immunoprecipitation of PSP94 from serum using anti-PSPBP antibodies
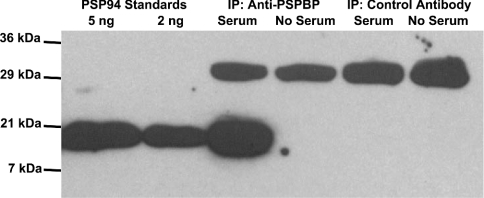
In order to establish whether the PSP94–PSPBP interaction occurs naturally within the blood, the PSPBP within normal male human serum was immunoprecipitated with an immobilized anti-PSPBP specific monoclonal antibody (17G9), and the precipitated proteins were run on SDS/PAGE and analysed by Western blotting using polyclonal rabbit anti-(human PSP94) antibodies. This Western blot is presented in Figure 7, and demonstrates clearly that PSP94 co-precipitates with the PSPBP antibody, but not with pre-immune mouse IgG, indicating that PSP94 is naturally complexed with its binding protein within human serum.
Figure 7. Co-precipitation of PSPBP and PSP94.
Immunoprecipitation showing the presence of PSP94 (detected with polyclonal anti-PSP94 antibodies) in the proteins precipitated from normal male human serum with a monoclonal antibody (17G9) specifically recognizing the PSPBP (third lane from the left). Controls included incubation of the PSPBP matrix with buffer alone (fourth lane from the left), and replacement of the anti-PSPBP matrix with a pre-immune mouse IgG matrix (fifth and sixth lanes from the left). In each of these lanes, a 30 kDa band was apparent representing cross-reaction of the horseradish-peroxidase-labelled secondary antibody with the light chain of mouse IgG.
PSPBP immunohistochemistry
In order to assess the normal tissue expression of the PSPBP, a normal tissue array was labelled immunohistochemically with three PSPBP-specific monoclonal antibodies, a PSP94-specific monoclonal antibody and an isotype-matched control monoclonal antibody. A summary of the results is described in Table 1 and photomicrographs are shown in Figure 8. In a wide range of tissues, a low proportion of cells within the connective tissue were labelled, and this was a consistent finding with the three antibodies; this pattern was most prevalent in tonsil. Other tissues demonstrated similar patterns of labelling with all three antibodies, including the pituitary gland and Leydig cells of the testis. In these tissues, the isotype-matched control antibody and the anti-PSP94 antibody gave no detectable labelling. For these reasons, we can be highly confident that the connective tissue, pituitary and Leydig cell labelling represents PSPBP. Other tissues, such as the kidney, parathyroid gland, stomach and liver, were labelled with only one or two of the antibodies. While this may represent genuine PSPBP expression, and differences between the antibody labelling due to differences in antibody affinity or epitope accessibility, we should be more cautious in the interpretation of these results. PSP94 expression with the P1E8 antibody was detected in prostate (Figure 8) and less intense labelling in the islet cells of the pancreas. There was no evidence for PSP94 and PSPBP co-expression in any cell types.
Table 1. Immunohistochemistry of PSPBP in normal human tissues.
−, +, ++ and +++ represent absent, weak, moderate and strong labelling respectively; mAb, monoclonal antibody.
| Tissue | Binding protein (mAb 17G9) | mAbs 1B11 and 2B10 – concordance with 17G9 |
|---|---|---|
| Lung | − | Yes |
| Skin | − | Yes |
| Muscle | − | 2B10 +/−, 1B11 −ve |
| Heart | − | Yes |
| Stomach | Lower area of gland ++ | 2B10 yes, 1B11 −ve |
| Oesophagus | − | Yes |
| Small intestine | Granular+epithelium | 2B10 and 1B11 −ve |
| Colon | − | Yes |
| Liver | Granular + | 2B10 and 1B11 −ve |
| Spleen | − | Yes |
| Pancreas | Focal+<5% of cells | 2B10 and 1B11 −ve |
| Salivary gland | Focal+ | 2B10 and 1B11 −ve |
| Pituitary gland | 5% Cells ++ | Yes |
| Adrenal gland | − | Yes |
| Thyroid gland | Granular+5% cells | 2B10 and 1B11 −ve |
| Parathyroid gland | ++ Principal cells | 1B11 +/−, 2B10 −ve |
| Thymus gland | − | Yes |
| Tonsil | +++ Trabeculae | Yes |
| Bone marrow | − | Yes |
| Uterus | − | Yes |
| Cervix | − | Yes |
| Ovary | − | Yes |
| Kidney | Cytoplasmic+5% cells | 2B10 −ve, 1B11 ++ |
| Prostate gland | 5% Stromal area + | Yes |
| Testis | ++ Leydig cells | Yes |
| Omentum | − | Yes |
| Peripheral nerve | − | Yes |
| Cerebral cortex | 5% Area + | 1B11 Yes, 2B10 −ve |
| Cerebellum | − | Yes |
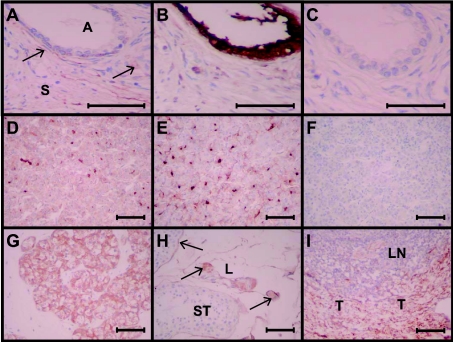
Figure 8. PSPBP immunohistochemistry in normal human tissues.
Photomicrographs illustrating immunohistochemical labelling of normal tissues with PSPBP and control antibodies. (A, B and C) Normal human prostate labelled with 17G9 (anti-PSPBP), P1E8 (anti-PSP94) and isotype-matched control antibodies respectively. Areas labelled A are prostatic glands or acini, and S represents the fibromuscular stroma. The arrows in (A) represent labelling of specific cells within the stroma. The entire epithelium in (B) is labelled with the anti-PSP94 antibody, and an absence of labelling with the control antibody (C) indicates specificity. (D, E and F) are sections of pituitary labelled with 17G9 and 1B11 anti-PSPBP antibodies and the control isotype-matched antibody respectively. Note the similar labelling with the two anti-PSPBP antibodies (D and E). (G, H and I) are all labelled with the 17G9 antibody and are sections of parathyroid gland, testis and tonsil respectively. In (G), the principal cells of the parathyroid gland are labelled; in (H), the seminiferous tubule (ST) membrane and the Leydig cells (L) label with the 17G9 antibody (arrows); in (I), the connective tissue within the trabeculae (T) of the tonsil express PSPBP, but not the lymphoid tissue (LN). Scale bar, 50 μm.
PSPBP Western blot
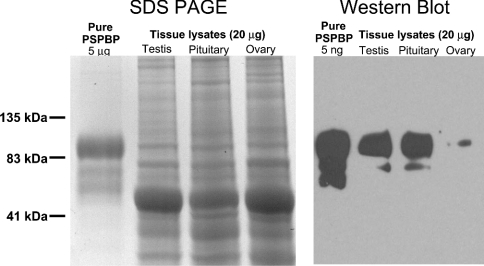
In order to confirm that the immunohistochemical data was genuinely reflecting PSPBP expression, a Western blot was performed on whole human tissue lysates from testis, pituitary and ovary (Figure 9). PSPBP was detected by Western blotting in testis and pituitary, and trace amounts in ovary. The high-molecular-mass form of the protein appeared to be more prevalent in the tissue extracts than in the serum-extracted PSPBP. The PSPBP levels detected by Western blot are in concordance with those seen by immunohistochemistry, albeit no expression was visualized in ovary.
Figure 9. PSPBP Western blot.
Reducing SDS/7.5% PAGE and PSPBP Western blot of human whole tissue lysates using the 17G9 anti-PSPBP monoclonal antibody. PSPBP is present in testis, pituitary and, to a lesser extent, ovary.
PSPBP concentrations in prostate cancer patients' sera
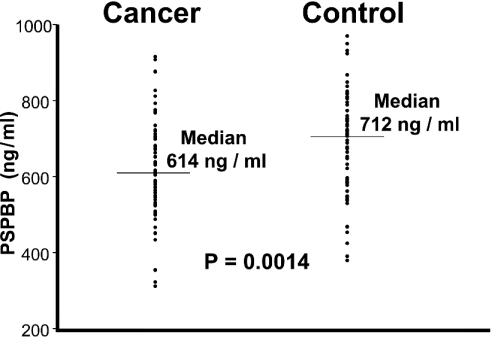
Serum PSP94 is known to be elevated in a proportion of prostate cancer patients when compared with controls. In order to determine whether the PSPBP is influenced by or may have a role in prostate cancer, the PSPBP was quantified by validated ELISA in the sera of newly diagnosed prostate cancer patients and in non-cancer controls. The characteristics of the two populations are described in Table 2. The PSPBP levels within the two groups were not normally distributed, so the groups were compared by non-parametric means, showing a significantly lower median serum PSPBP concentration in the cancer group (Figure 10). The presence of prostate cancer, and the likely alterations of PSP94 in the blood of this population may have influenced the synthesis and processing of the PSPBP within the cancer group.
Table 2. Demographics of cancer and non-cancer population.
| Cancer | Non-cancer | ||
|---|---|---|---|
| Age (years) | Mean | 67.2 | 60.3 |
| Median | 67.3 | 60.1 | |
| Range | 51.0–80.9 | 49.9–81.2 | |
| Total PSA (ng/ml) | Mean | 19.36 | 1.46 |
| Median | 6.64 | 1.02 | |
| Range | 1.08–422 | 0.32–4.98 | |
| PSPBP (ng/ml) | Mean | 623 | 724 |
| Median | 614 | 713 | |
| Range | 316–1014 | 384–2072 |
Figure 10. PSPBP levels in prostate cancer patients' sera.
PSPBP measurements in newly diagnosed prostate cancer patients and in patients without prostate cancer. Serum measurements were made using a sandwich ELISA and two monoclonal antibodies recognizing different epitopes of the PSPBP (3F4 immobilized, and 17G9 conjugated with horseradish peroxidase). The control population has significantly higher PSPBP levels than the cancer group (Kruskall–Wallis test, P=0.0014), indicating that the PSPBP may have involvement in or a relationship to prostate cancer.
DISCUSSION
Previous studies have indicated that PSP94 binds to unspecified proteins in blood [11,14] and at the cell surface [7–9]. Identification and characterization of these PSPBPs may help in the understanding of some of the mechanisms behind the many reported functions of PSP94. In the present study, we have purified from blood and identified by N-terminal amino acid sequencing a PSPBP. Furthermore, we have demonstrated by Northern blot and immunohistochemical analysis that both PSPBP mRNA and protein are present in a range of normal tissue types.
Homology searches indicate that the human PSPBP shares 58% identity with the predicted amino acid sequences encoded by a hypothetical mouse cDNA (GenBank® accession number BAB03453). This protein and a similar hypothetical rat protein (XP_215351) are likely to represent the rodent homologues of the human PSPBP. No experimental data are published describing the function of the human PSPBP or these hypothetical rodent proteins.
Comparison of the PSPBP amino acid sequence with the Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) indicates that a region within the human PSPBP (Figure 5) and homologous rodent proteins, have amino acid sequence similarity (of up 40% identity) with a defined family of proteins harbouring the SCP (sperm coating glycoprotein)-like extracellular domain [21]. This group of proteins is also known as the CRISP (cysteine-rich secretory protein) family. The conserved domain within this family of proteins is present in SCP-1, also known as AEG-1 (acidic epididymal glycoprotein) [21]; TPX-1 (testis-specific protein) [22]; GliPR (glioma pathogenesis-related protein), also known as RTVP-1 (related to testis-specific, vespid and pathogenesis proteins) [23]; insect and snake venoms [24]; and plant pathogenesis proteins of the PR-1 (pathogenesis-related 1) family [25]. The mechanisms behind the actions of these proteins are not fully understood [26]. The PSPBP can be considered to be a member of this family, with a function assigned through experimentation. We do not yet know whether PSP94 binding is a feature common to other proteins with SCP domains. Interestingly, previous reports indicate that specific binding sites for PSP94 are present in spermatid plasma membranes [9], and PSP94 has been shown to associate with the surface of sperm [3], an interaction potentially mediated by SCP-1.
Post-translational-modification-prediction software [27] confirms the signal cleavage site, and provides strong evidence that the PSPBP can attach to the cell surface through a GPI (glycosylphosphatidylinositol) anchor, with a cleavage site at between amino acids 437 and 438. This suggests that the PSPBP can exist as a cell-surface glycoprotein in addition to its role as a secreted blood protein. GPI-anchored proteins are known to have diverse functions, including roles in signal transduction [28]. The putative membrane bound form of the PSPBP may act as a cell-surface receptor for PSP94, and, as such, may be integrally involved in many of the reported functions of PSP94.
Immunohistochemical analysis with a panel of anti-PSPBP monoclonal antibodies demonstrates that PSPBP is expressed in a range of tissue types (Figure 8). Labelling of a small proportion of cells within connective tissues was a feature common to several tissue types. Non-connective tissues were also labelled by one or more antibodies. The pituitary gland exhibited similar labelling with each of the anti-PSPBP antibodies. Previous studies have demonstrated that PSP94 binds specifically to plasma membranes derived from the pituitary [8], and injections of PSP94 to castrated rats results in a reduction in the levels of circulating FSH [2], presumably by some direct action of PSP94 on the pituitary. The demonstration of PSPBP within pituitary cells suggests a mechanism whereby PSP94 may exert its effect on FSH secretion.
Leydig cells within the testis also exhibited labelling with each of the three anti-PSPBP antibodies. Leydig cells are responsible for testosterone production in response to LH (luteinizing hormone), which itself is secreted from the pituitary gland. Furthermore, early studies have indicated that testosterone administration to normal human males induces a 5-fold increase in circulating PSP94 [29]. Similarly, administration of hCG (human chorionic gonadotropin) to normal human males, which stimulates the LH receptors on the Leydig cells and subsequent testosterone spike, results in a 40-fold increase in the serum levels of PSP94 [30]. The dramatic rise in serum PSP94 concentrations in response to elevated testosterone levels may constitute part of a feedback mechanism to restore androgen homoeostasis initiated by the binding of PSP94 to PSPBP at the surface of the Leydig cells.
One of the anti-PSPBP antibodies (17G9) clearly labelled the principal cells of the parathyroid gland. Previous studies have suggested a role of PSP94 in the control of serum calcium levels [6]. Administration of PSP94 to rats harbouring prostate cancer cells transfected with full-length PTHrP (parathyroid hormone related protein) (MatLyLu-PTHrP), a prostate cancer model characterized by malignancy-associated hypercalcaemia, resulted in a reduction in tumour volume, a normalization of plasma calcium levels, with a corresponding reduction in PTHrP levels. The mechanism behind the action of PSP94 on serum calcium levels in this model is unclear, but a role of PSP94 in calcium control at the parathyroid gland cannot be ruled out.
With the identification of PSPBP, it is now possible to develop and characterize antibodies specific for the free and bound forms of PSP94. These reagents will allow the development of immunoassays to quantify the various forms of PSP94 and PSPBP in human serum. Clinical applicability of these assays, particularly in areas of prostate cancer management poorly served by existing cancer markers, can be addressed. As a first step in this process, we assessed PSPBP in the serum of a small cohort of prostate cancer patients in comparison with a urologically referred non-cancer control group, and demonstrated that the circulating PSPBP level was reduced in the cancer group when compared with the controls. This suggests that the PSPBP may indeed have some relationship to prostate carcinogenesis, possibly as a result of abnormal levels of circulating PSP94 within the blood of the cancer population.
PSA is another abundantly secreted prostatic protein. PSA serum assays are well established tools for prostate cancer diagnosis and monitoring. PSA is also known to exist in the blood both free and complexed to high-molecular-mass molecules, in particular to α-1-antichymotrypsin and α2-macroglobulin. Serum assays that differentiate between free and total forms of PSA are reported to have greater diagnostic value in prostate cancer than assays that measure the total form of PSA alone [31]. Underlying the apparent similarities between PSA and PSP94 and their binding molecules are important differences. PSA is a potent protease, and is inactivated by binding very tightly to the highly abundant broad-specificity protease inhibitors in the blood. PSP94 does not have demonstrated protease activity, PSPBP is a low-abundance blood protein and the interaction has defined kinetics. It is therefore likely that the PSP94–PSPBP interaction has a specific function related to the reported actions of PSP94 itself.
Further work will establish the functional relationship between PSP94 and PSPBP, and establish the clinical utility of serum assays for free and total PSP94 and for PSPBP.
Acknowledgments
We thank Dr Maureen O'Connor, Beatrice Paulroc, France Dumas and Anne Marcil from the Biotechnology Research Institute in Montreal for assistance with the protein purification method development, N-terminal amino acid sequencing and monoclonal antibody generation. Dr Ann Chambers' (London Regional Cancer Centre, London, Ontario, Canada) valuable contribution during the early stages of this project is gratefully acknowledged. This work was supported by the Canadian Institute of Health Research-(University-Industry)-Procyon Biopharma Inc. grant (UOP-63722). J.W.X. is a recipient of CIHR-UI-Procyon Scientist salary award. J.R.R., J.E.T, C.P., C.M., M.T.R., J.W., S.V.G. and K.A. are current or past employees of Procyon Biopharma Inc. Procyon Biopharma Inc. has a financial interest in the subject matter discussed in the present paper.
References
- 1.Lilja H., Abrahamsson P. A. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12:29–38. doi: 10.1002/pros.2990120105. [DOI] [PubMed] [Google Scholar]
- 2.Sheth A. R., Arabatti N., Carlquist M., Jornvall H. Characterization of a polypeptide from human seminal plasma with inhibin (inhibition of FSH secretion)-like activity. FEBS Lett. 1984;165:11–15. doi: 10.1016/0014-5793(84)80004-6. [DOI] [PubMed] [Google Scholar]
- 3.Chao C. F., Chiou S. T., Jeng H., Chang W. C. The porcine sperm motility inhibitor is identical to β-microseminoprotein and is a competitive inhibitor of Na+,K+-ATPase. Biochem. Biophys. Res. Commun. 1996;218:623–628. doi: 10.1006/bbrc.1996.0110. [DOI] [PubMed] [Google Scholar]
- 4.Hirano M., Kamada M., Maeda N., Yamamoto S., Aono T., Koide S. S. Presence of immunoglobulin binding factor on human sperm surface as sperm coating antigen. Arch. Androl. 1996;37:163–170. doi: 10.3109/01485019608988518. [DOI] [PubMed] [Google Scholar]
- 5.Garde S. V., Basrur V. S., Li L., Finkelman M. A., Krishan A., Wellham L., Ben-Josef E., Haddad M., Taylor J. D., Porter A. T., Tang D. G. Prostate secretory protein (PSP94) suppresses the growth of androgen-independent prostate cell line (PC3) and xenografts by inducing apoptosis. Prostate. 1999;38:118–125. doi: 10.1002/(sici)1097-0045(19990201)38:2<118::aid-pros5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Shukeir N., Arakelian A., Kadhim S., Garde S., Rabbani S. A. Prostate secretory protein (PSP-94) decreases tumor growth and hypercalcemia of malignancy in a syngenic in vivo model of prostate cancer. Cancer Res. 2003;63:2072–2078. [PubMed] [Google Scholar]
- 7.Phadke M., Vijayalakshmi S., Sheth A. R. Evidence for the presence of specific receptors for inhibin in human prostate. Ind. J. Exp. Biol. 1982;20:419–420. [PubMed] [Google Scholar]
- 8.Vanage G. R., Sheth A. R. Binding characteristics of inhibin to rat pituitary plasma membranes. Ind. J. Exp. Biol. 1982;20:445–447. [PubMed] [Google Scholar]
- 9.Dandekar S. P., Sheth A. R., Ghosh D. Presence of specific receptor binding sites for inhibin in rat spermatids. Andrologia. 1983;15:274–278. doi: 10.1111/j.1439-0272.1983.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang C. L., Brassil D., Rozzell M., Schellhammer P. F., Wright G. L., Jr Comparison of prostate secretory protein with prostate specific antigen and prostatic acid phosphatase as a serum biomarker for diagnosis and monitoring patients with prostate carcinoma. Prostate. 1993;23:201–212. doi: 10.1002/pros.2990230303. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson P. A., Andersson C., Bjork T., Fernlund P., Lilja H., Murne A., Weiber H. Radioimmunoassay of β-microseminoprotein, a prostatic-secreted protein present in sera of both men and women. Clin. Chem. 1989;35:1497–1503. [PubMed] [Google Scholar]
- 12.Teni T. R., Sheth A. R., Kamath M. R., Sheth N. A. Serum and urinary prostatic-inhibin-like peptide (PIP) in benign prostatic hyperplasia and carcinoma of the prostate. Cancer Lett. 1988;43:9–14. doi: 10.1016/0304-3835(88)90205-4. [DOI] [PubMed] [Google Scholar]
- 13.von der Kammer H., Krauhs E., Aumüller G., Scheit K. H. Characterization of a monoclonal antibody specific for prostatic secretory protein of 94 amino acids (PSP94) and development of a two-site binding enzyme immunoassay for PSP94. Clin. Chim. Acta. 1990;187:207–219. doi: 10.1016/0009-8981(90)90106-3. [DOI] [PubMed] [Google Scholar]
- 14.Wu D., Guo Y., Chambers A. F., Izawa J. I., Chin J. L., Xuan J. W. Serum bound forms of PSP94 (prostate secretory protein of 94 amino acids) in prostate cancer patients. J. Cell. Biochem. 1999;76:71–83. doi: 10.1002/(sici)1097-4644(20000101)76:1<71::aid-jcb8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Bauman G., Xuan J. W., Chin J., Sakai H., Guo Y., Garde S., Fraser J. E., Venketesan V. M. PSP94: evaluation of prognostic utility in patients treated with radiotherapy for non-metastatic prostate cancer. Prostate J. 2000;2:94–101. [Google Scholar]
- 16.Baijal-Gupta M., Fraser J. E., Clarke M. W., Xuan J. W., Finkelman M. A. A new scalable purification procedure for prostatic secretory protein (PSP94) from human seminal plasma. Protein Expression Purif. 1996;8:483–488. doi: 10.1006/prep.1996.0128. [DOI] [PubMed] [Google Scholar]
- 17.Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 18.Hewick R. M., Hunkapillar M. W., Hood L. E., Dreyer W. J. A gas–liquid solid phase peptide and protein sequenator. J. Biol. Chem. 1981;256:7990–7997. [PubMed] [Google Scholar]
- 19.Harlow E., Lane D. Antibodies: a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1988. Immunoaffinity purification; p. 510. [Google Scholar]
- 20.Reeves J. R., Cooke T. G., Fenton Lee D., McNicol A. M., Ozanne B. W., Richards R. C., Walsh A. Localization of EGF receptors in frozen tissue sections by antibody and biotinylated EGF-based techniques. J. Histochem. Cytochem. 1994;42:307–314. doi: 10.1177/42.3.8308248. [DOI] [PubMed] [Google Scholar]
- 21.Mizuki N., Kasahara M. Mouse submandibular glands express an androgen-regulated transcript encoding an acidic epididymal glycoprotein-like molecule. Mol. Cell. Endocrinol. 1992;89:25–32. doi: 10.1016/0303-7207(92)90207-m. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara M., Gutknecht J., Brew K., Spurr N., Goodfellow P. N. Cloning and mapping of a testis-specific gene with sequence similarity to a sperm-coating glycoprotein gene. Genomics. 1989;5:527–534. doi: 10.1016/0888-7543(89)90019-0. [DOI] [PubMed] [Google Scholar]
- 23.Murphy E. V., Zhang Y., Zhu W., Biggs J. The human glioma pathogenesis-related protein is structurally related to plant pathogenesis-related proteins and its gene is expressed specifically in brain tumors. Gene. 1995;159:131–135. doi: 10.1016/0378-1119(95)00061-a. [DOI] [PubMed] [Google Scholar]
- 24.Lu G., Villalba M., Coscia M. R., Hoffman D. R., King T. P. Sequence analysis and antigenic cross-reactivity of a venom allergen, antigen 5, from hornets wasps and yellow jackets. J. Immunol. 1993;150:2823–2830. [PubMed] [Google Scholar]
- 25.Dixon D. C., Cutt J. R., Klessig D. F. Differential targeting of the tobacco PR-1 pathogenesis-related proteins to the extracellular space and vacuoles of crystal idioblasts. EMBO J. 1991;10:1317–1324. doi: 10.1002/j.1460-2075.1991.tb07650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szyperski T., Fernandez C., Mumenthaler C., Wuthrich K. Structure comparison of human glioma pathogenesis-related protein GliPR and the plant pathogenesis-related protein P14a indicates a functional link between the human immune system and a plant defense system. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2262–2266. doi: 10.1073/pnas.95.5.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronegg J., Buloz D. Detection/prediction of GPI cleavage site (GPI-anchor) in a protein (DGPI) 1999 http://129.194.185.165/dgpi/
- 28.Ikezawa H. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 2002;25:409–417. doi: 10.1248/bpb.25.409. [DOI] [PubMed] [Google Scholar]
- 29.Hurkadli K. S., Arbatti S., Mehta S., Sheth A. R. Effect of administration of a single dose of testosterone oenanthate on human serum and seminal plasma inhibin concentration. Andrologia. 1983;15:350–354. doi: 10.1111/j.1439-0272.1983.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 30.Hurkadli K. S., Arbatti N. J., Mehta S., Sheth A. R. Serum inhibin levels after administration of hCG. Arch. Androl. 1984;12:45–48. doi: 10.3109/01485018409161147. [DOI] [PubMed] [Google Scholar]
- 31.Stenman U. H., Leinonen J., Alfthan H., Rannikko S., Tuhkanen K., Alfthan O. A complex between prostate-specific antigen and α1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]