Abstract
Most G-protein-coupled receptors that undergo agonist-dependent internalization require the presence of specific cytoplasmic-tail residues to initiate interactions with proteins of the endocytic machinery. Here we show that the UT receptor (urotensin II receptor) undergoes internalization, and that specific serine residues of the receptor's cytoplasmic tail participate in this process. We first observed a time-dependent increase in internalization of the UT receptor expressed in COS-7 cells following binding of the agonist urotensin II. This sequestration was significantly reduced in the presence of sucrose, demonstrating that the agonist-activated UT receptor is internalized in part by clathrin-coated pits. Moreover, the sequestered receptor was co-localized in endocytic vesicles with β-arrestin1 and β-arrestin2. To assess whether specific regions of the receptor's cytoplasmic tail were involved in internalization, five UT receptor mutants were constructed. In four constructs the receptor's cytoplasmic tail was truncated at various positions (UTΔ367, UTΔ363, UTΔ350 and UTΔ336), and in the other four adjacent serine residues at positions 364–367 were replaced by Ala (Mut4S). Each mutant, except UTΔ367, demonstrated a significantly reduced internalization rate, thereby revealing the importance of specific serine residues within the cytoplasmic tail of the UT receptor for its ability to be internalized efficiently.
Keywords: β-arrestin, G-protein-coupled receptor, internalization, site-directed mutagenesis, urotensin II, urotensin II receptor
Abbreviations: β-arrestin2-381T, β-arrestin2 that is truncated at residue 381; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; ERK, extracellular-signal-regulated kinase; FBS, fetal bovine serum; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor; GRK, G-protein-coupled receptor kinase; MAPK, mitogen-activated protein kinase; Mut4S, UT receptor in which four adjacent serine residues at positions 364–367 are replaced by Ala; UII, urotensin II; UT receptor, urotensin II receptor; UTΔ367 (etc.), truncation mutant comprising residues 1–367 of the UT receptor (etc.); YFP, yellow fluorescent protein
INTRODUCTION
UII (urotensin II), a cyclic undecapeptide isolated originally from the caudal neurosecretory system of teleost fish [1], has since been identified in many classes of vertebrates, including humans [2]. Studies have shown that UII has potent haemodynamic effects, acting both as a vasodilator [3] as well as a vasoconstrictor [4].
UII is the endogenous ligand for the GPCR (G-protein-coupled receptor) GPR14, now known as the UT receptor [5]. UII binds to the UT receptor with high affinity, which leads to the phospholipase C-dependent production of inositol phosphates [6] and increased levels of cytoplasmic Ca2+ [5]. The UT receptor is found in the central nervous system and is widely expressed in human tissues, including the left atrium and ventricle of the heart, smooth muscle cells of the coronary artery and aorta, as well as endothelial cells from several vascular beds [2,7]. Compelling evidence has implicated the UII/UT system in the pathophysiology of hypertension, heart failure, and cardiac fibrosis and hypertrophy [8].
Upon stimulation by agonists, GPCRs transfer information to intracellular second messengers via their coupling to heterotrimeric G-proteins, resulting in the activation of a diverse variety of effector systems. GPCR function is then regulated by several processes, including desensitization, internalization, recycling and down-regulation. Most GPCRs exhibit homologous desensitization following prolonged agonist stimulation. Agonist-activated receptors are rapidly phosphorylated by GRKs (GPCR kinases) [9], which in turn promote the binding of β-arrestins. The β-arrestins preclude receptor–G-protein interaction, leading to functional uncoupling of the G-protein from its associated receptor, and to a rapid down-regulation of the receptor's effector systems. The β-arrestin–receptor complex is sequestered in clathrin-coated pits, thereby facilitating endocytosis and eventual targeting towards various intracellular compartments [10]. Following internalization in endosomal compartments, some GPCRs, such as the β2-adrenergic receptor [11], recycle towards the plasma membrane, while others, such as the δ-opioid receptor [12] or the vasopressin V2 receptor [13], are targeted for proteolysis to lysosomes or the proteasome respectively.
The rat UT receptor belongs to the peptide subfamily of the rhodopsin-like family of GPCRs, and is composed of 386 residues. The receptor's cytoplasmic tail possesses serine/threonine residues that are potential phosphorylation sites for various kinases. Our goal was to determine whether the UT receptor undergoes internalization following binding of the agonist UII and to verify the putative involvement of specific residues of the receptor's C-terminal tail in this process. Our results indicate that a specific cluster of serine residues in the cytoplasmic tail of the UT receptor is involved in facilitating internalization of this receptor.
EXPERIMENTAL
Materials
BSA and bacitracin were from Sigma. The cDNA encoding the rat UT receptor (GPR14) subcloned in the mammalian expression vector pcDNA3 was a gift from Dr Brian O'Dowd (University of Toronto, Canada). DMEM (Dulbecco's modified Eagle's medium), FBS (fetal bovine serum), penicillin/streptomycin and oligonucleotide primers were purchased from Gibco Life Technologies (Gaithersburg, MD, U.S.A.). FuGENE™-6 and protease inhibitor cocktail were purchased from Roche (Roche Molecular Biochemicals, Mannheim, Germany). Rabbit polyclonal antibodies directed against phosphorylated and active forms of p42/p44 MAPK (mitogen-activated protein kinase) and the Phototope™-HRP Detection Kit (enhanced chemiluminescence) immunodetection system were purchased from New England Biolabs (Mississauga, Canada).
UII was synthesized in our laboratory using Fmoc (fluoren-9-ylmethoxycarbonyl)-protected amino acids and Wang resin. The synthetic peptides were purified to homogeneity by reverse-phase HPLC separation. Cysteine residues were protected by acetamidomethyl groups, which were removed by using mercury acetate. Disulphide bond formation was achieved by DMSO oxidation. The purity and identities of the peptides were determined by TLC and MS. 125I-labelled UII (specific radioactivity 400 Ci/mmol) was prepared with Iodo-Gen™ as described by Fraker and Speck [14]. Briefly, 25 μl of 1 mM peptide solution was incubated with 20 μg of Iodo-Gen (Pierce Chemical Co.), 65 μl of 100 mM borate buffer, pH 7.8, and 1 mCi of Na125I for 30 min at room temperature. The labelled peptides were purified by HPLC on a C18 column (10 μm; Allteck Associates Inc.) with a 35–45% (v/v) acetonitrile gradient. The specific radioactivity of the labelled hormones was determined by self-displacement and saturation binding analysis.
PCR mutagenesis
Mutant UT receptor cDNAs were constructed by oligonucleotide-directed mutagenesis (Expand High Fidelity PCR System; Roche) using the rat UT receptor cDNA inserted into pcDNA3 (Invitrogen Inc.) as a template. Two sets of forward and reverse oligonucleotides were used (Life Technologies Inc.) to introduce stop codons at Gln368, Ser364, Ser351 and Ser337 (to generate UTΔ367, UTΔ363, UTΔ350 and UTΔ336 respectively), and to replace the serine residues at positions 364–367 by alanines (to generate Mut4S). PCR products were subcloned using the KpnI and XbaI sites of pcDNA3.1 after digestion by the same restriction enzymes. For UT–GFP (UT receptor–green fluorescent protein) and Mut4S–GFP conjugates, rat UT receptor or Mut4S cDNA inserted into pcDNA3.1 was amplified and PCR products were subcloned using the EcoRI and BamHI sites of pEGFP-N1 (BD Biosciences Clontech, Mississauga, ON, Canada) after digestion by the same restriction enzymes. Mutagenesis was confirmed by automated nucleotide sequencing.
Cell culture and transfections
COS-7 and HEK-293 cells were grown in DMEM containing 10% (v/v) FBS, 100 i.u./ml penicillin and 100 μg/ml streptomycin at 37 °C. COS-7 cells were plated at 1×106 cells per 100 mm culture dish. When the cells reached 70% confluency, they were transfected with 4 μg of plasmid DNA and 8 μl of FuGENE™-6 as described by the manufacturer. Transfected cells were grown for 48 h before performing binding assays. HEK-293 cells were plated on 22 mm2 coverslips in 6-well plates at a density of 2.5×105 cells. When cells reached 70% confluency, they were transfected with 1 μg of plasmid DNA and 2 μl of FuGENE™-6 as described by the manufacturer. Transfected cells were grown for 48 h before collecting images using a confocal microscope (Zeiss LSM-510 META). When using the UT–GFP construct, the efficiency of transfection of COS-7 and HEK-293 cells was found to be approx. 30% and 15% respectively.
Binding experiments
COS-7 cells were grown for 48 h post-transfection in 100 mm culture dishes, washed once with PBS, and subjected to one freeze–thaw cycle. Broken cells were then gently scraped in washing buffer (20 mM Tris/HCl, pH 7.4, 5 mM MgCl2), centrifuged at 2500 g for 15 min at 4 °C, and resuspended in binding buffer (20 mM Tris/HCl, pH 7.4, 5 mM MgCl2, 0.1% BSA, 0.01% bacitracin). For saturation binding assays, broken cells (10–15 μg of protein) were incubated for 1 h at room temperature in binding buffer containing increasing concentrations of 125I-UII (0.15–20 nM; 50 Ci/mmol) in a final volume of 0.5 ml. Bound radioactivity was separated from free ligand by filtration through GF/C filters pre-soaked for at least 3 h in binding buffer. Non-specific binding was measured in the presence of 1 μM unlabelled UII. Receptor-bound radioactivity was evaluated by γ-radiation counting. Kd and Bmax values were evaluated by Scatchard plot analysis. Peptide stability was evaluated by TLC, and no degradation was observed after incubation of the peptide with COS-7 cell membranes for up to 2 h.
Measurement of agonist-induced inositol phosphate production
Inositol phosphate accumulation was determined as described in [15]. Briefly, COS-7 cells were seeded in six-well plates, transfected, and labelled for 16 h in serum-free, inositol-free DMEM containing 10 μCi/ml myo-[3H]inositol (Amersham Pharmacia Biotech). Cells were washed twice with PBS/0.1% (w/v) dextrose and then incubated in stimulation buffer (DMEM containing 25 mM Hepes, 10 mM LiCl and 0.1% BSA, pH 7.4) for 30 min at 37 °C. Inositol phosphate production was induced with 100 nM UII for 10 min at 37 °C in stimulation buffer. Incubations were terminated by the addition of ice-cold perchloric acid (final concentration 5%, v/v). Water-soluble inositol phosphates were then extracted with an equal volume of a 1:1 (v/v) mixture of 1,1,2-trichlorotrifluoroethane and tri-n-octylamine. The samples were mixed vigorously and centrifuged at 2500 g for 30 min. The upper phase containing inositol phosphates was applied to an AG1-X8 resin column (Bio-Rad). Inositol phosphates were eluted sequentially by the addition of an ammonium formate/formic acid solution of increasing ionic strength. Fractions containing inositol phosphates were collected and measured in a liquid scintillation counter.
Protein expression and immunoblotting
Cells were lysed in SDS sample buffer (62.5 mM Tris/HCl, pH 6.8, 2.3% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.005% Bromophenol Blue, 1 mM PMSF), and proteins (40 μg) were separated by SDS/PAGE on 10% (w/v) polyacrylamide gels. Proteins were detected immunologically following electrotransfer on to nitrocellulose membranes. Blots were then incubated with antibodies directed against phosphorylated or unphosphorylated forms of p42/p44 MAPK in blocking solution overnight at 4 °C and then with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1000) in blocking solution for 1 h. Blots were visualized using the Phototope™-HRP Detection Kit enhanced chemiluminescence system. Protein concentrations were measured using a modified Lowry procedure with BSA as standard [16].
125I-UII internalization assay
Internalization was evaluated as described previously [17]. Briefly, transfected COS-7 cells were incubated for various periods of time at 37 °C in internalization buffer (25 mM Hepes, pH 7.4, DMEM, 0.1% BSA) containing 0.2 nM 125I-UII. Internalization was stopped by washing the cells three times with ice-cold PBS. Cells were then incubated for 120 min in 1 ml of ice-cold acidic solution (150 mM NaCl, 50 mM acetic acid, pH 3). The supernatant containing the acid-released radioactivity was collected for analysis. Cells were then solubilized with 0.1 M NaOH and their acid-resistant radioactive content was evaluated. Internalization was calculated from the ratio of acid-resistant binding to total binding (acid-resistant plus acid-released). Non-specific binding was measured in the presence of 1 μM unlabelled UII.
Confocal microscopy
HEK-293 cells expressing UT–GFP or Mut4S–GFP and β-arrestin1–YFP (yellow fluorescent protein) or β-arrestin2–YFP were stimulated at 37 °C with UII for the indicated periods of time and then fixed for 15 min at room temperature with a solution of 4% (w/v) paraformaldehyde in PBS. Cover slips were mounted with GelTol Aqueous Mounting Medium (Immunon). Confocal images were collected on a Zeiss laser-scanning confocal microscope (LSM-510 META) using a 63× oil immersion lens. In cells expressing only UT–GFP, the fluorescence was visualized in the single-track mode with laser excitation (488 nm) and emission (LP 505) filter sets. In cells expressing both GFP and YFP fusion proteins, images were collected using the lambda mode. In this mode, the closely overlapping emission spectra of GFP and YFP were separated to produce cross-talk-free images of individual fluorescent proteins. Lambda images were unmixed with the Zeiss LSM-510 META image processing software using the individual spectra of the YFP- and the GFP-expressing proteins as references.
Data analysis
Results were collected in triplicate and are presented as means±S.D. Binding curves, binding capacity (Bmax) and Kd values were derived from the Kell program (Biosoft, Ferguson, MO, U.S.A.), which uses a weighted non-linear curve-fitting routine.
RESULTS
Characterization of 125I-UII internalization
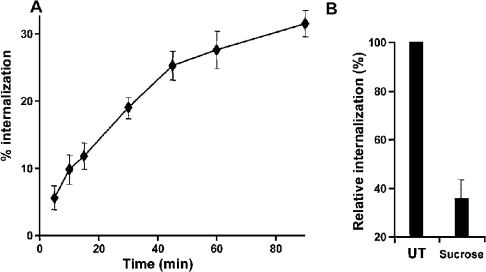
To determine whether the UT receptor undergoes agonist-induced internalization, we assessed the kinetics of 125I-UII internalization. Figure 1(A) shows the time course for the internalization of 125I-UII in COS-7 cells transiently expressing the UT receptor. During incubation at 37 °C, the acid-resistant binding of 125I-UII to the wild-type receptor increased to a value representing 31±1.9% of total specific binding within 90 min of stimulation. The half-time of internalization was 20 min. This uptake of radioactive material is consistent with internalization of the hormone–receptor complex.
Figure 1. Internalization of 125I-UII by the wild-type UT receptor in transiently transfected COS-7 cells.
Cells expressing the wild-type UT receptor were incubated with 125I-UII (0.2 nM) at 37 °C for different periods of time (A). The effect of 0.45 M sucrose on receptor internalization was evaluated after 90 min (B). Internalization was stopped by washing the cells three times with ice-cold PBS. Acid-resistant binding was evaluated as indicated in the Experimental section. Data are expressed as a percentage of total binding for each time point, and represent means± S.D. of triplicate values. Internalization was calculated from the ratio of acid-resistant binding to total binding (acid-resistant plus acid-released). Non-specific binding was measured in the presence of 1 μM unlabelled UII. These results are representative of three independent experiments.
To better characterize this process, we evaluated the effects of hyperosmolar sucrose, a known inhibitor of clathrin-mediated endocytosis, on the internalization of 125I-UII. COS-7 cells expressing the wild-type UT receptor were pretreated with medium or with 0.45 M sucrose to block clathrin-mediated internalization before stimulation with UII; sucrose was present continuously during the experiment. The kinetics of 125I-UII internalization were studied after a 90 min treatment. As shown in Figure 1(B), sucrose significantly blocked the internalization of 125I-UII in cells expressing the UT receptor, with a decrease of 65% relative to non-treated cells, indicating that receptor-specific internalization can be mediated by clathrin-coated vesicles.
Trafficking of the agonist-activated UT receptor
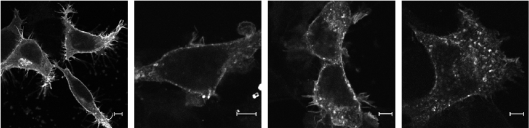
We used functional UT–GFP to visualize intracellular trafficking of the receptor in transfected HEK-293 cells. UT–GFP has the GFP in its C-terminal portion, and this modification does not affect ligand binding, receptor expression or the receptor's capacity to activate phospholipase C (results not shown). In the absence of agonist (0 min), UT–GFP was localized at the plasma membrane (Figure 2). After a 5 min stimulation of the UT receptor with 100 nM UII, we observed a punctate pattern of UT–GFP at the plasma membrane. Longer exposure to agonist (15 min) led to the localization of UT–GFP within endocytic vesicles. These vesicles, which were first detected within 5 min of agonist addition, subsequently grew in size and number, and were still observed after a 45 min treatment with agonist. These results demonstrate that agonist-activated UT receptors are internalized and localized ultimately to endocytic vesicles.
Figure 2. Intracellular trafficking of UT–GFP.
HEK-293 cells were transiently transfected with UT–GFP. Shown are representative confocal microscopic images of UT–GFP fluorescence in HEK-293 cells treated with agonist for (from left to right) 0, 5, 15 and 45 min at 37 °C. Scale bars represent 5 μm. Experiments were repeated three times, with similar results.
Involvement of C-terminal tail residues in UT receptor internalization
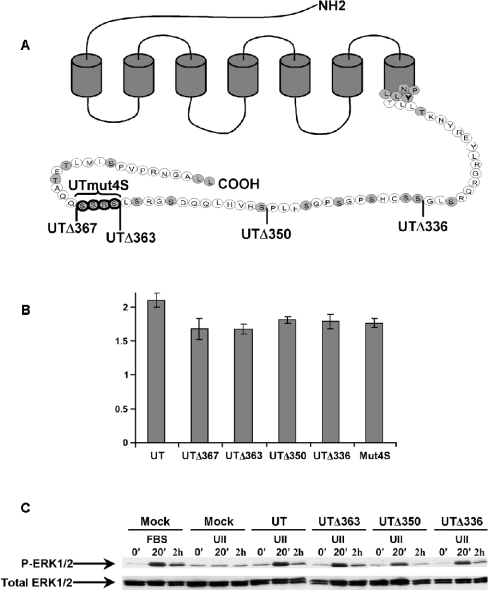
To assess whether the cytoplasmic C-terminal tail of the UT receptor plays a role in its internalization, we initially constructed three truncation mutants in which the last 23 (UTΔ363), 36 (UTΔ350) or 50 (UTΔ336) residues were removed (Figure 3A). These truncations removed many potential phosphorylation sites from the cytoplasmic tail; among these is a cluster of four adjacent serine residues at positions 364–367. To first characterize the pharmacological properties of the truncation mutants, binding analysis was performed using increasing concentrations of 125I-UII (saturation kinetics) in transiently transfected COS-7 cells. We found no significant differences in Kd values (4 nM) between the wild-type UT receptor and mutated receptors (Table 1). Moreover, the binding capacities of both the wild-type and mutant receptors were similar, with values ranging between 0.5 and 1.0 nM. These results demonstrate that the cytoplasmic deletions do not affect the affinity of the UT receptor for UII, and nor do they alter the receptor's capacity for being expressed at the cell surface.
Figure 3. Functional characterization of UT receptors.
(A) Schematic representation of the UT receptor showing full-length (wild-type), C-terminal deletion mutations and point mutations of the UT receptor. Mutant cDNAs were ligated into pcDNA3.1 as described in the Experimental section. Shown is the wild-type UT receptor (386 aa), sequentially C-terminally deleted mutant receptors UTΔ367 (−19 aa), UTΔ363 (−23 aa), UTΔ350 (−36 aa) and UTΔ336 (−50 aa), and a mutant receptor in which the serine cluster replaced by alanines (Mut4S). (B) Inositol phosphate production was evaluated in COS-7 cells transfected with wild-type or mutant receptors. Inositol phosphate production was induced with 100 nM UII for 10 min at 37 °C in stimulation buffer. The phase containing inositol phosphates was applied to an AG1-X8 resin column (Bio-Rad). Inositol phosphates were eluted sequentially by the addition of an ammonium formate/formic acid solution of increasing ionic strength [36]. Fractions containing inositol phosphates were collected and counted for radioactivity in a liquid scintillation counter. (C) Effect of UT receptor truncation on MAPK activation. COS-7 cells transiently transfected with either wild-type or truncated receptor were serum-starved overnight and then treated with 10% (v/v) FBS as a positive control or 100 nM UII at 37 °C for 0 min, 20 min or 2 h in DMEM and lysed. Equal amounts of protein were resolved by SDS/PAGE. Activated MAPK was identified by immunoblotting using phospho-MAPK specific antibody, which labelled bands of 44 and 42 kDa (ERK1 and ERK2 respectively). The total amount of MAPK was shown to be the same in all samples on parallel blots that were probed with antibody against total MAPK. Experiments were repeated three times, with similar results.
Table 1. Affinities and expression of UT receptor mutants expressed in transiently transfected COS-7 cells.
Saturation-binding studies with membranes expressing wild-type and mutated receptors were carried out as described in the Experimental section. Bmax and Kd values were derived from the Kell program (Biosoft), which uses a weighted non-linear curve-fitting routine.
| Receptor | Kd (nM) | Bmax (nM) |
|---|---|---|
| Wild-type | 4.17±0.51 | 0.63±0.14 |
| UTΔ367 | 4.49±0.64 | 0.49±0.17 |
| UTΔ363 | 4.28±0.40 | 0.55±0.23 |
| UTΔ350 | 4.65±0.73 | 0.53±0.33 |
| UTΔ336 | 4.93±1,07 | 0.68±0.39 |
| Mut4S | 4.71±0.16 | 1.01±0.58 |
The functional properties of the transiently transfected wild-type and mutant receptors were also evaluated by measurement of UII-induced inositol phosphate production (Figure 3B). Cells were incubated for 10 min with 0.1 μM UII and total inositol phosphate accumulation was measured. In each experiment, receptor densities were assessed by binding assay to adjust the inositol phosphate production to receptor expression. As shown in Figure 3(B), stimulation of COS-7 cells expressing wild-type or mutant receptors with UII caused a significant accumulation of total inositol phosphates (between 1.7- and 2.1-fold increase above the basal level), which demonstrated that deletions in the cytoplasmic tail did not significantly affect the functional properties of the receptor. Another property of the UT receptor is its capacity to activate the ERK1/2 (extracellular-signal-regulated kinase 1/2) pathway [18]. We measured the effect of C-terminal truncation of the UT receptor on ERK1/2 activation by immunoblotting with antibodies against the phosphorylated, active forms of ERK1/2. Addition of UII to mock-transfected cells did not induce ERK1/2 activation (Figure 3C). However, addition of UII to COS-7 cells transfected with the wild-type receptor led to a robust activation of ERK1/2 after 20 min, and phospho-ERK1/2 was still observed 2 h following UII addition. Mutant receptors UTΔ363, UTΔ350 and UTΔ336 gave similar responses to the wild-type receptor at each time point measured. These results demonstrate that mutations in the cytoplasmic tail of the UT receptor do not affect its capacity to activate the ERK1/2 pathway.
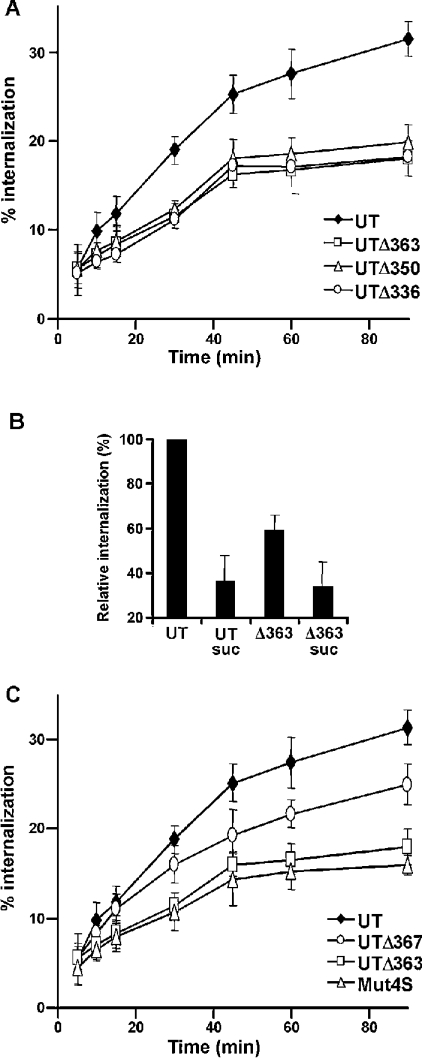
We then studied the internalization kinetics of the truncated UT receptors over a 90 min period. Removal of the last 23 residues from the cytoplasmic tail yielded a mutant receptor (UTΔ363) with significantly impaired internalization kinetics (Figure 4A). Indeed, the maximal acid-resistant binding of 125I-UII to UTΔ363 was 18±2% at 90 min. Removal of the last 23 residues of the cytoplasmic tail of the UT receptor thus reduced its internalization by 42% relative to the wild-type receptor after 90 min of stimulation (Figure 4A). Removal of either 36 or 50 residues of the cytoplasmic tail had no further effect on the internalization rate of the UT receptor compared with that of UTΔ363 (Figure 4A). In order to verify whether the residual internalization observed with mutant receptor UTΔ363 was attributable to the clathrin-coated vesicle pathway, the kinetics of 125I-UII internalization were studied after 90 min of stimulation with UII in transiently transfected COS-7 cells expressing the mutant UTΔ363 receptor in the absence or in the presence of 0.45M sucrose. As shown in Figure 4(B), sucrose significantly blocked the internalization of 125I-UII in cells expressing the UTΔ363 receptor to the same extent as with the wild-type receptor, indicating that the residual receptor-specific internalization of 125I-UII is also mediated by clathrin-coated vesicles. These results demonstrate that the last 23 residues of the cytoplasmic tail of the UT receptor contain determinant(s) that are important for its internalization, and that the residual internalization observed with truncated receptors is also dependent on the clathrin-coated vesicle pathway.
Figure 4. Effects of C-terminal truncations on UT receptor internalization.
COS-7 cells expressing wild-type UT receptor (◆), UTΔ363 (□), UTΔ350 (Δ) or UTΔ336 (○) (A), and COS-7 cells expressing wild-type UT receptor (◆), UTΔ367 (○), UTΔ363 (□) or Mut4S (Δ) (B), were incubated with 125I-UII (0.2 nM) at 37 °C for different periods of time. (C) The effects of 0.45 M sucrose (suc) on internalization of the wild-type receptor and UTΔ363 were evaluated after 90 min. Internalization was stopped by washing the cells three times with ice-cold PBS. Acid-resistant binding was evaluated as indicated in the Experimental section. Values are expressed as a percentage of total binding for each time point, and represent means±S.D. of triplicate values. Internalization was calculated from the ratio of acid-resistant binding to total binding (acid-resistant plus acid-released). Non-specific binding was measured in the presence of 1 μM unlabelled UII. These results are representative of three independent experiments.
To assess the importance of the serine cluster present in the last 23 residues of the cytoplasmic tail of the UT receptor, we constructed, expressed and evaluated the internalization kinetics of the mutant receptors UTΔ367 and Mut4S. These mutations respectively removed all residues after the serine cluster and replaced this cluster by alanines. The binding properties and functional characterization of these mutant receptors showed no significant differences when compared with the wild-type receptor (Table 1 and Figure 3). As shown in Figure 4(C), deletion of the last 19 residues or replacement of the four adjacent serines by alanines attenuated internalization relative to the wild-type receptor. The maximal acid-resistant binding of 125I-UII to UTΔ367 and Mut4S was 25±1% and 16±1% respectively within 90 min of stimulation. Removal of the serine cluster present within the last 23 residues of the cytoplasmic tail of the UT receptor thus reduced its internalization by 49% relative to the native receptor after 90 min of stimulation (Figure 4C). These data demonstrate the importance of the four adjacent serine residues located in the last 23 residues of the cytoplasmic tail of the UT receptor in its internalization, but also demonstrated that the last 19 residues have a role in this process.
Trafficking of β-arrestins with agonist-activated wild-type and Mut4S receptors
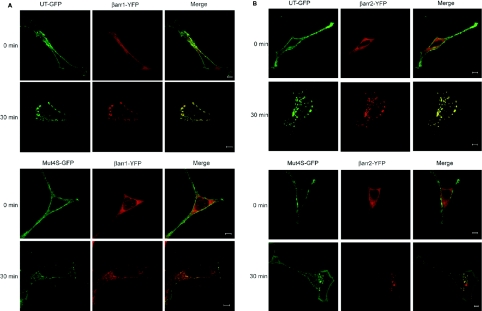
We used either functional UT–GFP or Mut4S–GFP and β-arrestin1–YFP or β-arrestin2–YFP conjugates to visualize the intracellular trafficking of the receptors and the association of β-arrestins with the agonist-occupied receptors in transfected HEK-293 cells. Similar to UT–GFP, Mut4S–GFP has the GFP moiety in its C-terminal portion, and this modification does not affect ligand binding, receptor expression or the receptor's capacity to activate phospholipase C (results not shown). In the absence of agonist (0 min), UT–GFP and Mut4S–GFP were localized at the plasma membrane (Figures 5A and 5B), while β-arrestin1–YFP and β-arrestin2–YFP were distributed uniformly in the cytoplasm of the cells, as indicated by the homogeneous β-arrestin1–YFP and β-arrestin2–YFP fluorescence. Upon agonist stimulation (30 min), there was an extensive redistribution of cell surface UT–GFP and cytoplasmic β-arrestin1–YFP and β-arrestin2–YFP to large perinuclear structures (Figures 5A and 5B, merged images). These vesicles, which were first detected within 5 min of agonist addition (results not shown), qualitatively grew in size and number after 30 min of agonist treatment (Figures 5A and 5B). Agonist activation of Mut4S–GFP also promoted the co-localization of the mutant receptor with β-arrestin1–YFP and β-arrestin2–YFP, but in smaller vesicles than those observed with the wild-type receptor. Moreover, whereas the fluorescence of UT–GFP disappeared totally from the plasma membrane after agonist treatment, the fluorescence of Mut4S–GFP was still detectable after the same period of UII treatment. Next, we verified whether Mut4S–GFP co-localizes with β-arrestins in large vesicles after prolonged periods of time following agonist stimulation. Following 90 min of UII stimulation, UT–GFP was detected with β-arrestin2–YFP in large vesicles, whereas Mut4S–GFP was still observed in small vesicles (results not shown). This demonstrates that, in the absence of the serine cluster, Mut4S is not found in large vesicles.
Figure 5. Co-localization of β-arrestin1 and β-arrestin2 to agonist-activated UT or Mut4S receptors.
HEK-293 cells were transiently transfected with either UT–GFP or Mut4S–GFP plus either β-arrestin1–YFP or β-arrestin2–YFP cDNAs. Shown are representative confocal microscopic images of either UT–GFP or Mut4S–GFP and β-arrestin1–YFP (A) or β-arrestin2–YFP (B) fluorescence (illustrated as red pseudocolour) in HEK-293 cells treated with agonist for 30 min at 37 °C. Scale bars represent 5 μm. Experiments were repeated three times, with similar results.
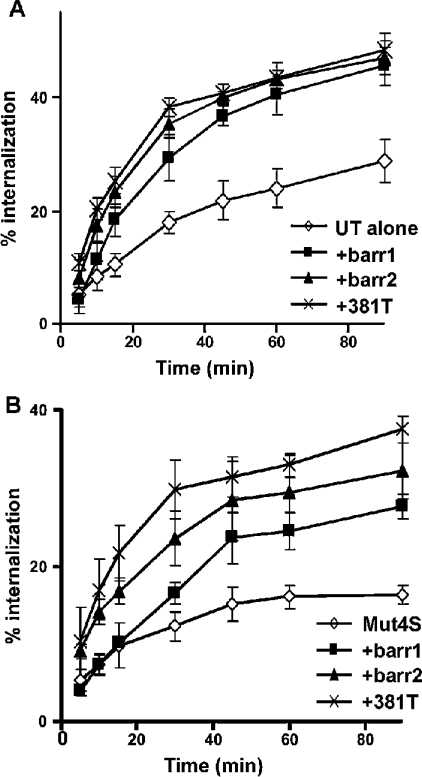
To examine the effects of β-arrestin in more detail, COS-7 cells were co-transfected with UT or Mut4S receptors plus β-arrestin1, β-arrestin2 or β-arrestin2-381T (β-arrestin2 that is truncated at residue 381), and internalization assays were performed after a time course of agonist exposure (Figure 6). β-Arrestin2-381T acts similarly to β-arrestin2-(1–382), which has been shown to exhibit phosphorylation-independent receptor binding due to the removal of key regulatory residues [19]. In vitro, this mutant binds to the agonist-activated β2-adrenergic receptor regardless of its phosphorylation status. We found that co-expression of β-arrestin1, β-arrestin2 or β-arrestin2-381T increased the internalization of the UT receptor to the same extent and at each time point. These results confirm the importance of β-arrestin for internalization of the UT receptor. Co-transfection of β-arrestin1, β-arrestin2 or β-arrestin2-381T increased the internalization rate of Mut4S in COS-7 cells (Figure 6B), demonstrating the role of β-arrestins in Mut4S internalization. Interestingly, the phosphorylation-independent β-arrestin2-381T increased the rate of internalization of Mut4S to a greater extent than did β-arrestin1 or β-arrestin2, suggesting that the specific serine cluster present in the C-terminal tail of the UT receptor is phosphorylated following UII activation.
Figure 6. Effects of overexpression of β-arrestin1, β-arrestin2 and truncated β-arrestin2-381T on the internalization of wild-type UT receptor and Mut4S.
COS-7 cells expressing the wild-type UT receptor (A) or the Mut4S mutant (B) either alone (◇) or with β-arrestin1 (■; barr1), β-arrestin2 (▲) or β-arrestin2-381T (×) were incubated with 125I-UII (0.2 nM) at 37 °C for different periods of time. Internalization was stopped by washing the cells three times with ice-cold PBS. Data are expressed as a percentage of total binding for each time point, and represent means±S.D. for three independent experiments. Internalization was calculated from the ratio of acid-resistant binding (internalized) to total binding (acid-resistant plus acid-released). Non-specific binding was measured in the presence of 1 μM unlabelled UII.
DISCUSSION
Agonist activation of GPCRs not only initiates the receptor-mediated signal transduction cascade, but also triggers the activation of cellular mechanisms that lead to receptor desensitization and internalization.
In the present study, we show that stimulation by UII promotes the internalization of its cognate UT receptor. This appears to take place via clathrin-coated pits, since hypertonic sucrose, which has been used extensively to block clathrin-mediated internalization of numerous GPCRs, inhibits this process [20]. However, it has been observed for the M2 muscarinic acetylcholine receptor that, although sucrose blocked internalization of the receptor, neither clathrin nor caveolae were involved in this process [21]. We therefore cannot exclude the possibility that UT receptor may also be internalized by other atypical pathways.
We also demonstrate that the maximal UT receptor internalization obtained after 90 min of stimulation by UII was 31%, with a t1/2 value of 20 min. These values differ from those reported for other rhodopsin-like receptors. For example, the maximal internalization of the angiotensin type 1A receptor was reported to be 60%, and was reached after 10 min of stimulation [17]. Internalization percentages of 70% in 20 min, 80% in 10 min and 90% in 30 min have been observed for the vasopressin V2 receptor [22], the neurokinin 1 receptor [23,24] and the neurotensin receptor [25] respectively, with t1/2 values for all receptors of <7 min. Moreover, the internalization kinetics observed with the UT receptor were correlated with confocal imaging of the UT–GFP receptor construct, whereby a punctate pattern of fluorescence was observed at the membrane of transfected cells after 5 min of agonist stimulation, but only after 15 min of activation did we observe fluorescence associated with endocytic vesicles. Although overexpression of β-arrestin1 or β-arrestin2 did lead to a greater extent of receptor internalization, it did not significantly improve the t1/2. These relatively slow internalization kinetics of the UT receptor may be explained by the receptor being a poor substrate for GRK family members, thereby leading to a low state of phosphorylation and a weaker interaction with β-arrestins.
The role of different intracellular domains and residues of GPCRs in triggering internalization has been evaluated in several studies using site-directed mutagenesis. For some GPCRs, such as the β2-adrenergic receptor, deletions within the third intracellular loop alter agonist-induced internalization [26]. For the M3 muscarinic acetylcholine receptor, mutation of serine- and threonine-rich domains present in either the third intracellular loop or the C-terminal tail of the receptor abolishes agonist-induced internalization [27,28]. Moreover, many examples showing the importance of the C-terminal tail of GPCRs in their internalization have been described [29]. Since the C-terminal tail plays a critical role in the agonist-induced regulation of a number of GPCRs, we chose in the present study to investigate the role of this region of the UT receptor in agonist-induced internalization.
We produced and expressed truncated UT receptors in order to identify which portion and/or specific determinants are involved in the internalization process. Our results show that deleting up to 50 residues of the cytoplasmic tail (UTΔ336) did not affect binding capacity or the efficiency with which the receptors were able to translocate to the cell surface. The UTΔ336 mutant was functional in its capacity to activate phospholipase C and the ERK pathway. The mechanisms by which GPCRs control the activity of MAPKs vary according to receptor and cell type, but fall broadly into one of three categories: (1) signals initiated by classical G-protein effectors, e.g. protein kinase A and protein kinase C, (2) signals initiated by cross-talk between GPCRs and classical receptor tyrosine kinases, e.g. ‘transactivation’ of EGF (epidermal growth factor)-like receptors, and (3) signals initiated by direct interactions between β-arrestins and components of the MAPK cascade, e.g. β-arrestin scaffolds [30]. Since deletion of the UT receptor C-terminal tail reduces internalization without affecting either phospholipase C or ERK activation, and UT receptor internalization involves β-arrestin association, this would suggest that ERK activation by the UT receptor in COS-7 cells is mediated by the classical G-protein effectors or by transactivation of the EGF receptor, and possibly not by β-arrestin scaffolds. Additional deletions of the UT receptor would be needed to address this point further.
Although C-terminal deletions did not affect the functional properties of the UT receptor, our data indicate that four adjacent serine residues present in the last portion of the C-terminal tail are involved in internalization of the receptor. Indeed, removal of the last 23 residues (UTΔ363) or replacement of this cluster by four alanines (Mut4S) significantly reduced agonist-induced internalization by 49% relative to the wild-type receptor after 90 min of agonist activation. Removal of 36 and 50 residues from the C-terminal tail, which also removed a total of 15 out of 17 potential phosphoacceptor sites within this domain, did not further affect the rate of internalization of the UT receptor compared with that observed with the UTΔ363 mutant, suggesting that residues located between positions 336 and 363 do not contribute significantly to the internalization process. It also suggests that other regions of the receptor probably participate in UT receptor internalization. Mutant UTΔ367, which retains the serine cluster, is also internalized less efficiently than the wild-type receptor. This would suggest that the last 19 residues of the C-terminal tail contain motifs that, although not crucial to the process, participate in receptor internalization, possibly by facilitating the association of proteins involved in the internalization machinery.
Following agonist-induced activation of GPCRs, a common mechanism of regulation involves kinase-dependent phosphorylation of receptors that uncouples them from the heterotrimeric G-protein and promotes a rapid desensitization of signalling [31]. Phosphorylated C-terminal tails of GPCRs become good docking sites for β-arrestin binding, which contributes to keep the receptor uncoupled from the G-protein and to target activated receptors toward clathrin-coated vesicles for internalization in endosomes. On the basis of their interaction with β-arrestins and their internalization patterns, GPCRs can be divided into two distinct classes [32,33]. Class A receptors bind β-arrestin2 with higher affinity than β-arrestin1 and do not interact with visual arrestin. In contrast, class B receptors bind both β-arrestin isoforms with similar high affinity and also interact with visual arrestin. In the latter case, β-arrestin forms a stable complex with receptors that co-localize in endocytic vesicles, whereas class A–β-arrestin complexes dissociate at or near the plasma membrane. The ability of class B receptors to bind β-arrestin family members with such high affinity appears to be mediated in part by clusters of phosphorylated residues in the receptor C-terminal tail that is absent from class A receptors [32,34].
The UT receptor possesses such a serine cluster located in its C-terminal tail. Moreover, the use of a functional UT–GFP construct and either β-arrestin1–YFP or β-arrestin2–YFP allowed us to show that agonist-activated UT receptors form a stable endocytic complex with β-arrestin1 as well as with β-arrestin2, and that this complex co-localizes to a considerable number of large vesicles, thus classifying UT as a class B receptor. Previous reports have shown how specific serine/threonine clusters in C-terminal tails of class B GPCRs serve as the primary site for agonist-induced receptor phosphorylation [35]. Without this motif, the receptor–β-arrestin interaction is less stable, and the agonist-occupied receptor dissociates from β-arrestin at or near the plasma membrane. Although the UT receptor possesses characteristics of class B receptors, replacement of the serine clusters by alanine did not totally abrogate stable receptor–β-arrestin2 complex formation. Indeed, in cells transfected with Mut4S, small vesicles (as opposed to larger ones found with the wild type) containing β-arrestins were observed, coupled with a basal level of fluorescence, indicating the presence of the mutant receptor at the cell surface. Therefore Mut4S maintains its ability to associate with β-arrestin1 as well as β-arrestin2 in intracellular vesicles, despite removal of the potentially phosphorylated serine cluster docking site, an essential condition for stable receptor–β-arrestin associations of other class B GPCRs. This pattern of receptor trafficking is reminiscent of the substance P receptor, whereby removal of all the serine/threonine clusters in the C-terminal tail did not prevent the receptor–arrestin complex from trafficking, albeit weakly, into endocytic vesicles [35].
In view of our results, it is possible that the internalization defect of Mut4S revolves around the absence of the phosphorylated serine cluster, which prevents the high-affinity interaction between β-arrestin and the receptor. The robust and persistent co-localization of agonist-activated UT–GFP with β-arrestin1–YFP and β-arrestin2–YFP in large perinuclear vesicles may highlight a more stable association between the UT receptor and β-arrestins. However, the Mut4S–GFP construct, although fully functional, would not allow a stable receptor–β-arrestins association, and subsequently would not enable the receptor–β-arrestin complex to be localized to large endosomal vesicles. Moreover, the fact that the overexpression of β-arrestin1, β-arrestin2 and especially the phosphorylated-receptor-independent β-arrestin2 (β-arrestin2-381T) all increased the extent of internalization of the Mut4S receptor favour this hypothesis. This outcome could be explained by the fact that overexpression of β-arrestins with Mut4S would compensate for the decrease in receptor affinity for β-arrestin.
In summary, we demonstrate that stimulation by UII promotes internalization of UT receptor. The serine cluster in the C-terminal tail of UT seems to be necessary for its efficient internalization and targeting into large vesicles.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (to E.E., G.G. and R.L.), the Heart and Stroke Foundation (to R.L.) and CIHR grant MOP-49447 (to S.A.L.). M.S. holds a Fonds de la Recherche en Santé du Québec (FRSQ) fellowship. S.A.L. holds a Canada Research Chair in Molecular Endocrinology. E.E. holds a J. C. Edwards Chair in Cardiovascular Research. R.L. is a Senior Scholar of the FRSQ.
References
- 1.Pearson D., Shively J. E., Clark B. R., Geschwind I. I., Barkley M., Nishioka R. S., Bern H. A. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas S. A., Dhanak D., Johns D. G. From ‘gills to pills’: urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol. Sci. 2004;25:76–85. doi: 10.1016/j.tips.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Stirrat A., Gallagher M., Douglas S. A., Ohlstein E. H., Berry C., Kirk A., Richardson M., MacLean M. R. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- 4.Douglas S. A., Sulpizio A. C., Piercy V., Sarau H. M., Ames R. S., Aiyar N. V., Ohlstein E. H., Willette R. N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames R. S., Sarau H. M., Chambers J. K., Willette R. N., Aiyar N. V., Romanic A. M., Louden C. S., Foley J. J., Sauermelch C. F., Coatney R. W., et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature (London) 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 6.Saetrum Opgaard O., Nothacker H., Ehlert F. J., Krause D. N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 7.Douglas S. A., Ohlstein E. H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2000;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- 8.Douglas S. A. Human urotensin-II as a novel cardiovascular target: ‘heart’ of the matter or simply a fishy ‘tail’? Curr. Opin. Pharmacol. 2003;3:159–167. doi: 10.1016/s1471-4892(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 9.Pitcher J. A., Freedman N. J., Lefkowitz R. J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 10.Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 11.von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J. Biol. Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 12.Tanowitz M., Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J. Biol. Chem. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 13.Martin N. P., Lefkowitz R. J., Shenoy S. K. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J. Biol. Chem. 2003;278:45954–45959. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- 14.Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 15.Lanctot P. M., Leclerc P. C., Escher E., Leduc R., Guillemette G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry. 1999;38:8621–8627. doi: 10.1021/bi9830516. [DOI] [PubMed] [Google Scholar]
- 16.Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 17.Laporte S. A., Servant G., Richard D. E., Escher E., Guillemette G., Leduc R. The tyrosine within the NPXnY motif of the human angiotensin II type 1 receptor is involved in mediating signal transduction but is not essential for internalization. Mol. Pharmacol. 1996;49:89–95. [PubMed] [Google Scholar]
- 18.Ziltener P., Mueller C., Haenig B., Scherz M. W., Nayler O. Urotensin II mediates ERK1/2 phosphorylation and proliferation in GPR14-transfected cell lines. J. Recept. Signal Transduction Res. 2002;22:155–168. doi: 10.1081/rrs-120014593. [DOI] [PubMed] [Google Scholar]
- 19.Kovoor A., Celver J., Abdryashitov R. I., Chavkin C., Gurevich V. V. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J. Biol. Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 20.Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roseberry A. G., Hosey M. M. Internalization of the M2 muscarinic acetylcholine receptor proceeds through an atypical pathway in HEK293 cells that is independent of clathrin and caveolae. J. Cell Sci. 2001;114:739–746. doi: 10.1242/jcs.114.4.739. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer R., Kirsch J., Fahrenholz F. Agonist and antagonist-dependent internalization of the human vasopressin V2 receptor. Exp. Cell Res. 1998;244:327–339. doi: 10.1006/excr.1998.4159. [DOI] [PubMed] [Google Scholar]
- 23.Sanders M. A., LeVine H., III Desensitization of the neurokinin 1 receptor is mediated by the receptor carboxy-terminal region, but is not caused by receptor internalization. J. Neurochem. 1996;67:2362–2372. doi: 10.1046/j.1471-4159.1996.67062362.x. [DOI] [PubMed] [Google Scholar]
- 24.Garland A. M., Grady E. F., Payan D. G., Vigna S. R., Bunnett N. W. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem. J. 1994;303:177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermans E., Octave J. N., Maloteaux J. M. Receptor mediated internalization of neurotensin in transfected Chinese hamster ovary cells. Biochem. Pharmacol. 1994;47:89–91. doi: 10.1016/0006-2952(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 26.Cheung A. H., Sigal I. S., Dixon R. A., Strader C. D. Agonist-promoted sequestration of the beta 2-adrenergic receptor requires regions involved in functional coupling with Gs. Mol. Pharmacol. 1989;35:132–138. [PubMed] [Google Scholar]
- 27.Moro O., Lameh J., Sadee W. Serine- and threonine-rich domain regulates internalization of muscarinic cholinergic receptors. J. Biol. Chem. 1993;268:6862–6865. [PubMed] [Google Scholar]
- 28.Yang J., Williams J. A., Yule D. I., Logsdon C. D. Mutation of carboxyl-terminal threonine residues in human m3 muscarinic acetylcholine receptor modulates the extent of sequestration and desensitization. Mol. Pharmacol. 1995;48:477–485. [PubMed] [Google Scholar]
- 29.Krupnick J. G., Benovic J. L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 30.Luttrell L. M. Activation and targeting of mitogen-activated protein kinases by G-protein-coupled receptors. Can. J. Physiol. Pharmacol. 2002;80:375–382. doi: 10.1139/y02-045. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 32.Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 33.Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Barak L. S., Anborgh P. H., Laporte S. A., Caron M. G., Ferguson S. S. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J. Biol. Chem. 1999;274:10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- 35.Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis. J. Biol. Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 36.Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]