Abstract
Background
Dyslipidemia is a common complication of type 2 diabetes mellitus (T2DM), which is the leading cause of morbidity and mortality in developed countries, including Saudi Arabia. Injectable glucagon-like peptide-1 receptor agonists (GLP-1RA) are recent therapies found to be effective in treating dyslipidemia in diabetic patients by acting similarly to the body's internal glucagon peptide hormone, regulating blood sugar levels and reducing triglycerides and low-density lipoprotein (LDL) levels.
Aim of the study
This retrospective cohort study aimed to investigate the effect of GLP-1RA on the lipid profile of dyslipidemia diabetic patients who were not controlled using statins.
Methods
Data were collected from the medical records of male and female diabetic patients with dyslipidemia (uncontrolled by statins) who were administered to the diabetic center of King Salman Bin Abdulaziz Hospital in Riyadh, Saudi Arabia, and received GLP-1RA (dulaglutide) from June 2023 to August 2023. The primary endpoint was the change in triglycerides and LDL-C levels after three months of using dulaglutide, and the secondary endpoints included the change in body weight, BMI, and HbA1c%. Descriptive analysis was conducted to present numerical and categorical data. The Wilcoxon signed-rank test was used to compare numerical data before and after using dulaglutide. Ethical considerations were taken into account by ensuring anonymous data collection and obtaining IRB approval before data collection.
Results
The study included 102 patients with a median (interquartile range (IQR)) age of 59 (14) years. Females constituted 55.2% of the population. Obesity (96.1%), hypertension (71.6%), and retinopathy (13.7%) were the most commonly reported comorbidities. The study showed a significant reduction in body weight, BMI, HbA1c, hemoglobin, mean corpuscular volume (MCV), serum LDL-C, and triglyceride concentrations after three months of using dulaglutide (p<0.001).
Conclusion
Our study results confirm the positive effect of the GLP-1RA (dulaglutide) on the lipid profile of diabetic patients with dyslipidemia uncontrolled by statins.
Keywords: statins, diabetes mellitus, dyslipidemia, dulaglutide, glp-1ra
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic disease affecting 387 million people worldwide, and it is estimated to reach 600 million by 2035 [1]. Locally, in the Kingdom of Saudi Arabia, the incidence of T2DM is 32.8%, and it is also predicted to reach 45.36% by 2030 [2]. In fact, the World Health Organization (WHO) ranked Saudi Arabia as the second country in the Middle East with a high prevalence of T2DM [3]. T2DM is defined as a metabolic syndrome with deficient insulin signaling in adipose tissues and skeletal muscles, resulting in exhaustion of the beta cells in the pancreatic islets and chronic hyperglycemia [4,5].
Several chronic conditions are associated with uncontrolled T2DM, such as dyslipidemia, cardiovascular diseases, myocardial and cerebral infarctions, neuropathy, retinopathy, and nephropathy [6]. As a common metabolic comorbidity of T2DM, dyslipidemia (abnormal plasma lipids and lipoprotein levels) accounts for high mortality rates in developed countries, including Saudi Arabia [7,8].
T2DM management involves various interventions, including lifestyle changes and medications such as sulfonylureas, thiazolidinediones, glucosidase inhibitors, dipeptidyl peptidase 4 (DPP-4) inhibitors, metformin, and insulin therapies, all of which contribute to substantial improvements in blood glucose [4]. However, focusing on treatment strategies that address T2DM comorbidities is found to be more efficient [9]. Glucagon-like peptide-1 receptor agonists (GLP-1RA) mimic the pancreatic glucagon peptide hormone, regulating blood sugar levels and activating insulin production [4]. Moreover, incorporating GLP-1RA into the treatment regimen of diabetic patients could be useful in cases such as metformin intolerance or contraindication, patients with sustained hemoglobin A1c greater than 1.5% above the target range over three months, and patients with chronic kidney diseases or heart failure [10].
GLP-1RAs were found to be effective in reducing total cholesterol, low-density lipoprotein (LDL), and triglyceride (TG) levels by blocking their absorption from the small intestine, inhibiting hepatic production, and inactivating very low-density lipoprotein (VLDL) production. Thus, they lead to effective weight loss and improvements in dyslipidemia. However, there are several knowledge gaps regarding the efficacy of GLP-1RAs in this regard, including the underlying pathophysiological mechanism and the relation between statins and GLP-1RA in LDL-C reduction [11]. Therefore, this study aimed to determine the effect of three months of dulaglutide treatment on the lipid profile of T2DM patients with dyslipidemia uncontrolled by statins.
Materials and methods
Study design and participants
This observational retrospective cohort study was conducted at the diabetic center of King Salman Bin Abdulaziz Hospital in Riyadh, Saudi Arabia. The study included both male and female patients aged over 18 with T2DM and dyslipidemia uncontrolled by statins (atorvastatin), even at the maximum tolerable dose. Data were collected from the medical records of eligible subjects who initiated GLP-1RA therapy (dulaglutide) in June 2023, with follow-up data available for three months (until August 2023). Patients with incomplete medical records were excluded from the study.
Data collection
At baseline (before initiation of dulaglutide therapy), demographic data (age, gender, body weight, height, body mass index (BMI), nationality, and educational level), clinical data (comorbidities, diabetic complications, and antidiabetic medications), and laboratory data (HbA1c, hemoglobin level, mean corpuscular volume (MCV) level, serum TGs, and LDL) were collected. After three months of follow-up, data on body weight, height, BMI, and laboratory data were collected.
Statistical analysis
Statistical analysis was conducted using the computer program IBM SPSS Statistics for Windows, Version 26 (Released 2019; IBM Corp., Armonk, New York). Baseline data were reported using descriptive statistics. The normality of distribution for continuous variables was analyzed using the Shapiro-Wilk test. The mean and standard deviation (SD) were calculated for parametric data, while the median and IQR were calculated for nonparametric data. Categorical variables were expressed as absolute numbers and valid percentages. Wilcoxon signed-rank test was used to compare numerical data collected after three months of follow-up to baseline values. Statistical significance was considered when the p-value was less than 0.05.
Results
The analysis included data from the medical records of 102 T2DM patients with dyslipidemia not controlled by statins who were prescribed dulaglutide 1.5 mg for at least three months. The mean (SD) age of the included patients was 60.4 (8.8) years. More than half (37, 55.2%) were females, and the vast majority (52, 92.9%) were of Saudi nationality. The most common comorbidities among the studied patients were obesity (98, 96.1%), hypertension (73, 71.6%), and retinopathy (14, 13.7%) (Table 1).
Table 1. Baseline demographic characteristics of the patients (n=102).
SD: standard deviation; IQR: interquartile range
| Parameters | Category | Number | Percentage |
| Age (years) | Mean (SD) | 60.4 (8.8) | - |
| Median (IQR) | 59 (14) | - | |
| Gender (n=67) | Female | 37 | 55.2 |
| Male | 30 | 29.4 | |
| Nationality (n=56) | Saudi | 52 | 92.9 |
| Non-Saudi | 4 | 7.1 | |
| Educational level (n=47) | Undergraduate | 19 | 40.4 |
| Graduate | 28 | 59.6 | |
| Comorbidities (n=102) | Obesity | 98 | 96.1 |
| Hypertension | 73 | 71.6 | |
| Retinopathy | 14 | 13.7 | |
| Myocardial infarction | 10 | 9.8 | |
| Cerebral infarction | 5 | 4.9 | |
| Nephropathy | 3 | 2.9 | |
| Others | 3 | 3 |
All included patients were prescribed dulaglutide 1.5 mg/0.5 mL for at least three months. In addition to dulaglutide, the most commonly used antidiabetic medications were metformin (92.2%), empagliflozin (15.7%), and sitagliptin (8.8%). After three months of using dulaglutide, the data showed a statistically significant reduction in all assessed parameters.
The median (IQR) body weight of the studied patients at baseline, 91 (15.5) kg, significantly decreased (p<0.001) to 87.3 (13.8) by 5 (3.75) kg after three months of using dulaglutide with median (IQR) decrease value of 5 (3.75) kg and a median (IQR) percent reduction of 5.6% (2.7). In addition, the median (IQR) BMI at baseline, 33.8 (5.2) kg/m², significantly decreased (p<0.001) to 31.1 (4.7) kg/m² after three months. The median (IQR) reduction in BMI was 3 (2) kg/m², with a median (IQR) percent reduction of 8.7% (4.3).
The same as body weight and BMI, the median (IQR) HbA1c at baseline, 8.9% (0.5), significantly decreased (p<0.001) to 8.2% (0.7) after three months of follow-up. The median (IQR) change in HbA1c was -0.9% (0.7), with a median (IQR) percent reduction of 10.1% (7.8).
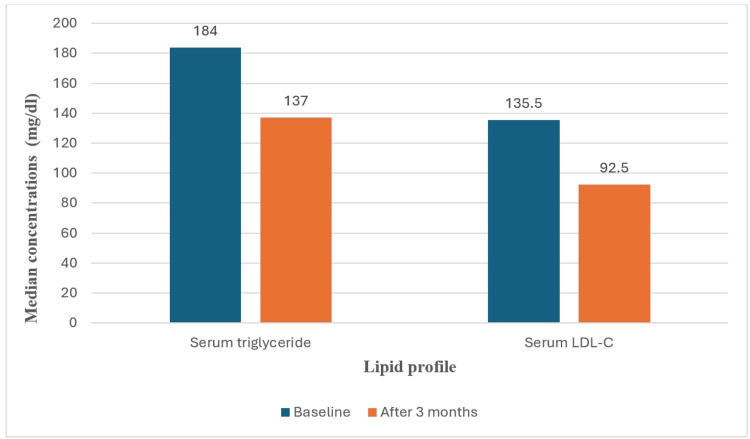
Regarding the lipid profile, the median (IQR) serum TG level at baseline was 184 (80) mg/dL, which significantly decreased (p<0.001) to 137 (79.3) mg/dL after three months of follow-up with a median (IQR) reduction of 20 (61.8) mg/dL and a median (IQR) percent reduction of 20.2% (31.5).
In addition to the reduction in TG levels, the median (IQR) LDL-C at baseline, 135.5 (74) mg/dL, was significantly decreased (p<0.001) to 92.5 (56) mg/dL after three months of follow-up. The median (IQR) reduction in LDL-C was -25.5 (56) mg/dL, with a median (IQR) percent reduction of 22.76% (40.5).
Regarding hemoglobin level, there was a statistically significant decrease (p<0.001) in the median (IQR) value at baseline, 13 (3) g/dL, to reach 12 (4) g/dL after three months. The median (IQR) change in hemoglobin was -0.5 (1.5) g/dL, with a median (IQR) percent reduction of 3.8%. The median (IQR) MCV at baseline was 90 (5) fL, which significantly decreased (p<0.001) to 86 (11) fL after three months with a median (IQR) change of -3 (9) fL and a median (IQR) percent reduction of 3.2% (11). More details are provided in Table 2 and Figure 1.
Table 2. Laboratory variables at the initiation of dulaglutide treatment and after the three-month follow-up period.
IQR: interquartile range; BMI: body mass index; HbA1c: hemoglobin A1C; MCV: mean corpuscular volume; LDL-C: low-density lipoprotein cholesterol
| Variable | Baseline Median (IQR) | Median (IQR) After the Three-Month Follow-Up | Change Median (IQR) | % Change Median (IQR) | p-value |
| Weight (kg) | 91 (15.5) | 87.3 (13.8) | -5 (3.75) | -5.6 (2.7) | <0.001 |
| BMI (kg/m2) | 33.8 (5.2) | 31.1 (4.7) | -3 (2) | -8.7 (4.3) | <0.001 |
| HbA1c (%) | 8.9 (0.5) | 8.2 (0.7) | -0.9 (0.7) | -10.1 (7.8) | <0.001 |
| Hemoglobin (g/dL) | 13 (3) | 12 (4) | -0.5 (1.5) | -3.8 (12) | <0.001 |
| MCV (fL) | 90 (5) | 86 (11) | -3 (9) | -3.2 (11) | <0.001 |
| Serum triglyceride (mg/dL) | 184 (80) | 137 (79.3) | -20 (61.8) | -20.2 (31.5) | <0.001 |
| LDL-C (mg/dL) | 135.5 (74) | 92.5 (56) | -25.5 (56) | -22.76 (40.5) | <0.001 |
Figure 1. Change in the lipid profile after three months of using dulaglutide.
LDL-C: low-density lipoprotein cholesterol
Discussion
During the last decade, significant efforts have been made to improve the lipid profile, body weight, and blood sugar levels in T2DM patients. Despite the use of multiple antidiabetic and lipid-lowering therapies, GLP-1RA, such as dulaglutide, is considered a substantial solution that enhances the quality of life of suffering patients [11]. This study could add to the body of knowledge and show possible efficient management of dual metabolic syndromes, diabetes, and dyslipidemia; therapy could decrease inadequate patient adherence to multiple medications.
Several studies have investigated the long-term effect of GLP-1RA on the lipid profile [12-14]. However, this retrospective cohort study aimed to assess the short-term effect of dulaglutide on the lipid profile of dyslipidemia diabetic patients in the diabetic center of King Salman Bin Abdulaziz Hospital in Riyadh, Saudi Arabia.
Despite the short-term duration, the addition of dulaglutide in this study to the dyslipidemia diabetic patients who are already following atorvastatin and antidiabetic medications showed successful improvements in many parameters. In our study, the mean age was 60.4 ± 8.81 years, similar to the previous study, which obtained a mean age of 63.8 years [12]. Clearly, this is the most common mean age of T2DM patients since the literature reports that the mean age at diagnosis of diabetes is 54.60 ± 9.48 years [15].
In accordance with several studies [16-18], our study findings regarding body weight and BMI showed a significant reduction (p<0.001) by 5 kg and 3 kg/m2, respectively, quite surprisingly, in only three months of follow-up after the administration of dulaglutide, along with those of Ishigaki et al., which found that the body weight decreased by 1.3 kg and the BMI by 0.5 kg/m2 after six months of administration of GLP-1RA treatment [12]. However, this may be a result of the large sample size (932) compared to ours, which can lead to some variations in the results.
Additionally, a similar study conducted in Saudi Arabia showed a significant decrease in BMI and body weight for patients following statins after initiating GLP-1RA [13]. The underlying mechanism is well documented. The GLP-1RA increases satiety, lowers appetite, delays gastric emptying, and reduces intestinal motility by increasing insulin secretions [4].
Regarding glycemic control, using dulaglutide along with other antidiabetic medications revealed a clinically significant reduction (p<0.001) in the HbA1c levels by 0.9% compared to the findings of Ishigaki et al., who found a significant decrease in HbA1c by 0.45% after using dulaglutide after six months of follow-up [12]. This is consistent with Ajabnoor et al.'s findings [13] and opposing Alanazi et al.'s findings [16], which did not find a significant reduction in HbA1c. This is attributed to the reported effect of GLP-1RA on improving beta cell functions, blocking glucagon secretion, and enhancing insulin sensitivity [19].
Furthermore, this study found a significant marked reduction (p<0.001) in the serum TG concentrations by 20 mg/dL and LDL-C by 25.5 mg/dL after following dulaglutide for three months. Similarly, Ishigaki et al. revealed a significant decrease in TG concentrations by 10.8 mg/dL and LDL-C concentrations by 2.2 mg/dL after administration of GLP-1RA for six months [12]. In contrast, a study conducted by Ajabnoor et al. found no association between GLP-1RA therapy and lipid profile changes [13]. However, our study findings align with those of other studies [20,21].
Another potential finding is a significant reduction in hemoglobin levels and MCV; in other words, dulaglutide could be associated with anemia in dyslipidemia diabetic patients as an adverse effect. A similar pattern of results was observed in a case report conducted in Saudi Arabia regarding a 30-year-old male who demonstrated acute hemolytic anemia following semaglutide administration. His anemia improved once he stopped taking semaglutide [22]. In addition, these results tied well with a study that found that patients who received SGLT2 inhibitors had a lower prevalence of composite anemia compared to those who received GLP-1RA. However, the mechanism of hemoglobin reduction associated with GLP-1RA is still unknown [15]. Nonetheless, we cannot neglect the effect of statins taken by the patients, as Ahn et al. revealed a significant fivefold increase in the risk of iron deficiency anemia following the administration of statins to the patients [23].
Strengths and limitations
The main strength of the study is that it collects real-world data on various metabolic parameters from patients' medical records, which enhances the generalizability of the results. One of the limitations of the current study is that it is a single-center study with a relatively small sample size and a short duration of follow-up. In addition, the retrospective nature of the study could include a risk of selection bias, the lack of a control group, the potential presence of confounding factors, and missing/incomplete data records. We recommended conducting future multicenter comparative studies with a larger sample size to get in-depth information about the safety and efficacy of dulaglutide in improving the lipid profile of patients with T2DM.
Conclusions
In the current study, using GLP-1RA (dulaglutide) for three months was associated with a significant reduction in body weight, BMI, HbA1c, hemoglobin, MCV, serum LDL-C, and TG levels in T2DM patients with dyslipidemia who were not controlled by statins. Adding dulaglutide to the antidiabetic and antihyperlipidemic treatment regimen could potentially improve the lipid profile, aid in body weight reduction, and consequently enhance the quality of life of these patients. In addition to showing efficient management of dual metabolic syndromes, diabetes, and dyslipidemia, it could decrease inadequate patient adherence to multiple medications.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board of King Saud Medical City issued approval H1RI-25-Jun24-03. Data were collected anonymously, and confidentiality was maintained throughout all stages of the research.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Odai M. Albahli, Saqib Ali, Fahad Alblaihi, Abdulaziz A. Aljaman
Acquisition, analysis, or interpretation of data: Odai M. Albahli, Saqib Ali, Fahad Alblaihi, Abdulaziz A. Aljaman
Drafting of the manuscript: Odai M. Albahli, Saqib Ali, Fahad Alblaihi, Abdulaziz A. Aljaman
Critical review of the manuscript for important intellectual content: Odai M. Albahli, Saqib Ali, Fahad Alblaihi, Abdulaziz A. Aljaman
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 5th Edition. Amsterdam, Netherlands: International Diabetes Federation; 2011. [Google Scholar]
- 2.Prevalence and future prediction of type 2 diabetes mellitus in the Kingdom of Saudi Arabia: a systematic review of published studies. Meo SA. https://pubmed.ncbi.nlm.nih.gov/27339576/ J Pak Med Assoc. 2016;66:722–725. [PubMed] [Google Scholar]
- 3.AlDawish MA, Robert AA. Handbook of Healthcare in the Arab World. Cham, Switzerland: Springer; 2021. Diabetes mellitus in Saudi Arabia: challenges and possible solutions; pp. 1083–1100. [Google Scholar]
- 4.Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. Pirro V, Roth KD, Lin Y, et al. J Clin Endocrinol Metab. 2022;107:363–378. doi: 10.1210/clinem/dgab722. [DOI] [PubMed] [Google Scholar]
- 5.The physiology and pharmacology of incretins in type 2 diabetes mellitus. Holst JJ. Diabetes Obes Metab. 2008;10:14–21. [Google Scholar]
- 6.Cardiovascular complications of diabetes: from microvascular to macrovascular pathways. Zakir M, Ahuja N, Surksha MA, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.45835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Diabetes Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 8.Prevalence and associated factors of dyslipidemia among adults with type 2 diabetes mellitus in Saudi Arabia. Alzaheb RA, Altemani AH. Diabetes Metab Syndr Obes. 2020;13:4033–4040. doi: 10.2147/DMSO.S246068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The real DAPSI: a real-world retrospective study on assessing the efficacy and safety of a fixed-dose combination of dapagliflozin and sitagliptin in the Indian population. Bhattacharjee R, Rai M, Joshi P, Prasad A, Birla A. Cureus. 2023;15:0. doi: 10.7759/cureus.46767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. Hunt B, Malkin SJ, Moes RG, Huisman EL, Vandebrouck T, Wolffenbuttel BH. BMJ Open Diabetes Res Care. 2019;7:0. doi: 10.1136/bmjdrc-2019-000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liraglutide in type 2 diabetes: from pharmacological development to clinical practice. Rossi MC, Nicolucci A. https://pubmed.ncbi.nlm.nih.gov/19848045/ Acta Biomed. 2009;80:93–101. [PubMed] [Google Scholar]
- 12.Glucagon-like peptide-1 receptor agonist utilization in type 2 diabetes in Japan: a retrospective database analysis (JDDM 57) Ishigaki Y, Strizek A, Aranishi T, Arai N, Imaoka T, Cai Z, Maegawa H. Diabetes Ther. 2021;12:345–361. doi: 10.1007/s13300-020-00977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The possible effect of the long-term use of glucagon-like peptide-1 receptor agonists (GLP-1RA) on Hba1c and lipid profile in type 2 diabetes mellitus: a retrospective study in Kauh, Jeddah, Saudi Arabia. Ajabnoor GM, Hashim KT, Alzahrani MM, et al. Diseases. 2023;11:50. doi: 10.3390/diseases11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The effect of GLP-1 analogues on lipid profile in type 2 diabetic patients-a retrospective observational study. Lončar A, Blaslov K, Bulum T, Duvnjak L. https://www.researchgate.net/publication/309062052_The_effect_of_GLP-1_analogues_on_lipid_profile_in_type_2_diabetic_patients_-_A_retrospective_observational_study Diabetology. 2015;44:59–66. [Google Scholar]
- 15.Association between age at diagnosis of type 2 diabetes and cardiovascular diseases: a nationwide, population-based, cohort study. Hu C, Lin L, Zhu Y, et al. Front Endocrinol (Lausanne) 2021;12:717069. doi: 10.3389/fendo.2021.717069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effect of glucagon-like peptide-1 agonist (liraglutide) on weight and glycemic control among adults with type 2 diabetes mellitus attending primary care center at Security Forces Hospital in Riyadh, Saudi Arabia. Alanazi NK, Ghoraba MA. J Family Med Prim Care. 2020;9:3933–3936. doi: 10.4103/jfmpc.jfmpc_361_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liraglutide effects on glycemic control and weight in patients with type 2 diabetes mellitus: a real-world, observational study and brief narrative review. Yousef CC, Thomas A, Matar MA, et al. Diabetes Res Clin Pract. 2021;177:108871. doi: 10.1016/j.diabres.2021.108871. [DOI] [PubMed] [Google Scholar]
- 18.Relationship between exposure to liraglutide and weight loss: a cross-sectional study in Riyadh, Saudi Arabia. Alrowais SS, Baghdadi LR. https://srb.sdl.edu.sa/esploro/outputs/journalArticle/Relationship-between-exposure-to-liraglutide-and/9951362708331 Int J Clin Exp Med. 2021;14:2435–2445. [Google Scholar]
- 19.Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. Thomas MK, Nikooienejad A, Bray R, et al. J Clin Endocrinol Metab. 2021;106:388–396. doi: 10.1210/clinem/dgaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glucagon-like peptide-1 receptor agonists reduced the low-density lipoprotein cholesterol in Japanese patients with type 2 diabetes mellitus treated with statins. Hasegawa Y, Hori M, Nakagami T, Harada-Shiba M, Uchigata Y. J Clin Lipidol. 2018;12:62–69. doi: 10.1016/j.jacl.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Ratner RE, Maggs D, Nielsen LL, et al. Diabetes Obes Metab. 2006;8:419–428. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 22.Acute hemolytic anemia following semaglutide injection: a case report. Saleh AK. World Fam Med J. 2022;20 [Google Scholar]
- 23.Possible link between statin and iron deficiency anemia: a South Korean nationwide population-based cohort study. Ahn J, Lee S, Won S. Sci Adv. 2023;9:6194. doi: 10.1126/sciadv.adg6194. [DOI] [PMC free article] [PubMed] [Google Scholar]