Abstract
Post-translational thio-acylation of a fusion protein between the α2A-adrenoceptor and the α subunit of the G protein Go1 is both dynamic and regulated by agonist binding. Incorporation of [3H]palmitate into the fusion protein was reduced substantially in the presence of the agonist adrenaline. This was dependent on the concentration of adrenaline and correlated with occupancy of the ligand binding site. Both the receptor and G-protein elements of the fusion construct incorporated [3H]palmitate but this occurred more rapidly for the G-protein element and regulation of acylation by the agonist occurred only for the G protein. The kinetics of de-palmitoylation of the α2A-adrenoceptor–Gαo1 fusion were accelerated markedly by agonist. Again, this reflected modulation of the G protein but not of the receptor. Agonist-induced regulation of the kinetics of thio-acylation of the G protein was abolished, however, in a mutant unable to bind guanosine 5′-[γ-[35S]thio]triphosphate ([35S]GTP[S]) in response to adrenaline. Despite the dynamic nature of the post-translational acylation and its regulation by agonist, the ability of adrenaline to activate the G protein, monitored by stimulation of the binding of [35S]GTP[S] to such fusion constructs, was unaffected by the palmitoylation potential of either the receptor or G-protein element.
Keywords: acylation, G protein, G protein-coupled receptor (GPCR), signal transduction
Abbreviations: DMEM, Dulbecco's modified Eagle's medium; GPCR, G protein-coupled receptor; [35S]GTP[S], guanosine 5′-[γ-[35S]thio]triphosphate
INTRODUCTION
Addition of saturated fatty acids such as palmitate to proteins is a common post-translational modification [1–2]. Palmitoylation usually [1–2], but not exclusively [3], occurs via thio-ester linkage to cysteine residues. As this linkage is relatively easily reversed, such thio-acylation is recognized to be potentially dynamic and may be subject to regulation [1]. Acylation of G protein α subunits plays a key role in targeting and anchoring what are essentially soluble polypeptides to membranes and specialized membrane sub-domains [3–5], and removal of palmitate can result in the release of a G protein from the membrane. Although there are seven transmembrane element polypeptides, many class A, rhodopsin-family G protein-coupled receptors (GPCRs) are also targets for post-translational acylation [1]. In the bulk of, but not all [6–7], cases such modification occurs only at one or more cysteine residues in the C-terminal tail of the GPCR. By extrapolation from studies on rhodopsin [8–9] this modification allows anchorage of the C-terminus to the membrane and defines the end of the region often known generically as the ‘fourth intracellular loop’. The atomic-level structure of bovine rhodopsin indicates this region to be a helix running parallel to the plasma membrane [9] and conservation of sequence and structure suggests this will likely be the case for other related GPCRs. A significant number of GPCRs have been demonstrated to be targets for palmitoylation and in many cases mutation of the sites of palmitoylation has been shown to alter downstream signalling or the regulation of the receptor [6–7,10–15]. Furthermore, alterations in the regulation of palmitoylation of both GPCRs and cognate G proteins have been noted following addition of receptor agonists [16–19]. It is thus of interest to know if regulation of GPCR and G protein acylation is co-ordinated and if acylation of one partner modulates palmitoylation of the other. One means to examine the inter-relationship between palmitoylation of a GPCR and a G protein is to take advantage of fusion proteins in which the two polypeptides are expressed from a single open reading frame [20]. Such constructs have become widely used to examine many aspects of the interactions between these two protein classes, including the regulation of palmitoylation. Employing both a fusion protein between the β2-adrenoceptor and the α subunit of Gs and a variant incorporating a thrombin cleavage site between the sequences of the two polypeptides, Loisel et al. [18] demonstrated that palmitoylation of the constructs was regulated by addition of appropriate agonists and that both elements of the fusion were targets for palmitoylation. In a related study, Stevens et al. [19] showed that agonists produced co-ordinated regulation of the acylation status of both the α1b-adrenoceptor and the α subunit of G11.
One of the earliest demonstrations of the palmitoylation of a GPCR employed the α2A-adrenoceptor [11–12]. However, the measured half-life of acylation of this GPCR was not substantially shorter than the half-life of the protein [12]. Furthermore, although the addition of agonist was reported to enhance turnover of palmitate, the effect was modest [12]. Herein, we have examined the effects of agonist on the palmitoylation of both the GPCR and G-protein elements of various forms of α2A-adrenoceptor–Gαo1 fusion proteins [21]. Palmitoylation of both elements of the construct is dynamic. However, only for the G protein but not the GPCR is the acylation regulated by agonist binding. This regulation requires that the G protein can be activated. Despite the capacity of agonist to regulate G-protein palmitoylation the acylation status of the G protein does not inherently alter the effectiveness of information transfer from GPCR to G protein. This is in marked contrast to activation of members of the Gs and Gq/G11 protein sub-families.
EXPERIMENTAL
Materials
All materials for tissue culture were supplied by Life Technologies Inc. (Paisley, Scotland, U.K.). [9,10-3H]palmitic acid and [3H]RS-79948-197 [21–22] were from Amersham Biosciences (Little Chalfont, Bucks., U.K.) and guanosine 5′-[γ-[35S]thio]triphosphate ([35S]GTP[S]; 1250 Ci/mmol) was from PerkinElmer Lifesciences (Great Shelford, Cambridge, U.K.). Oligonucleotides were purchased from Cruachem (Glasgow, Strathclyde, U.K.). Receptor ligands were purchased from RBI (Gillingham, Kent, U.K.). The production and characterization of antiserum ON1 was described by Mullaney and Milligan [23]. All other chemicals were from Sigma (Poole, Dorset, U.K.) and were of the highest grade available.
Fusion proteins
The pertussis toxin-resistant α2A-adrenoceptor–G-protein fusion proteins used throughout this study were prepared as described previously [21–22]. In brief, Cys351 of rat Gαo1 was mutated to isoleucine by site-directed mutagenesis and then used to create the α2A-adrenoceptor–Gα fusion proteins using the porcine α2A-adrenoceptor in pcDNA3 as described by Wise and Milligan [22]. Further alterations to generate the thio-acylation-resistant variants employed standard PCR-based mutagenesis techniques. PCR reactions were carried out on a Hybaid Omnigene thermal cycler in a total volume of 100 μl containing 100 ng of DNA template, 0.25 mM dNTPs (dATP, dCTP, dGTP, dTTP), 50 pmol of sense and anti-sense oligonucleotide primers, 1×Pfu thermophilic buffer, and 2.5 units of Pfu polymerase. A set of mutation primers to incorporate the desired mutations, as well as a set of primers to allow amplification of the full length construct were employed. The full length primers were: sense 5′-GCT ACC CGT CCA GCT CAA CGG TGC C-3′, anti-sense 5′-CGT CAC ACA CCA TCT TGG AGT CTG C-3′. The specific mutation primers were: C442A α2A-adrenoceptor–Go1α sense 5′-GCC TTC AAG AAG ATC CTC GCA CGT GGG GAC AGG AAA CGG-3′, anti-sense 5′-CCG TTT CCT GTC CCC ACG TGC GAG GAT CTT CTT GAA GGC-3′ (the mutated residues are shown in bold in the above sequences and the position of a newly created restriction site, Eco72I, is underlined), and α2A-adrenoceptor-C3SGo1α sense 5′-GGA AAC GGA TCG CCA TGG GAA GTA CTC TGA GCG CAG AGG AGA GA-3′, anti-sense 5′-TCT CTC CTC TGC GCT CAG AGT ACT TCC CAT GGC GAT CCG TTT CC-3′ (again the mutated residues are shown in bold and the position of a newly created restriction site, ScaI, is underlined).
To create C442A α2A-adrenoceptor-C3SGo1α, the C442Aα2A-adrenoceptor–Go1α construct was used as the DNA template. The primers used to create α2A-adrenoceptor–C3SGo1α were then employed as before to incorporate the second mutation. This mutant contains both of the new restriction sites, Eco72I, and ScaI.
Cell maintenance
HEK293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% newborn calf serum and 1% L-glutamine in a 37 °C humidified 5% CO2 atmosphere. Confluent cells were passaged at 1:10 by the addition of 1 ml of trypsin to detach cells. The cells were pipetted gently with 9 ml of fresh medium to resuspend evenly. For cells to be used in palmitoylation experiments the culture dishes were pre-treated with poly-D-lysine (1:10 dilution with sterile water, treated for 30 min then poly-D-lysine was removed and the dishes allowed to air-dry).
Transfections
HEK293T cells were transiently transfected at approximately 80% confluency in 10 cm dishes. DNA (10 μg) and 30 μl of lipofectAMINE™ reagent were mixed gently with 450 μl of OptiMEM (Gibco) and incubated for 45 min. During this period, cell monolayers were washed with OptiMEM and medium replaced with 9.5 ml of OptiMEM. The DNA–lipofectAMINE mix was added dropwise to the plates and returned to the incubator for 3 h. The transfection medium was replaced with fresh DMEM and the cells analysed in the following 24–48 h.
Preparation of cell membranes
Cells were grown to confluency in either 75 cm2 or 150 cm2 flasks, the medium discarded and cells harvested by scraping using disposable cell scrapers. The cells were resuspended, washed using 2×10 ml of ice-cold PBS and spun for 5 min at 3000 g in a refrigerated centrifuge. The supernatant was discarded, the pellet resuspended in 1 ml of TE buffer (10 mM Tris, 5 mM EDTA, pH 7.5, at 4 °C) and the mixture homogenized by 30 strokes of a chilled glass-on-glass Dounce homogenizer. The homogenate was spun for 6 min at 1500 g resulting in two fractions. The upper supernatant was removed and spun at 50000 g for 30 min and the lower fraction was discarded. The resultant pellet was resuspended in 300 μl of TE buffer and, following determination of protein concentration, diluted to 1 μg/μl and stored at −80 °C until required.
[3H]RS-79948-197 binding studies
To determine the levels of expression of the various α2A-adrenoceptor–G-protein fusion proteins the specific binding of the high affinity α2-adrenoceptor antagonist [3H]RS-79948-197 [21–22] was measured. Binding assays were initiated by the addition of 1 μg of protein, to an assay buffer (75 mM Tris/HCl, 5 mM EDTA, 12.5 mM MgCl2, pH 7.5) containing [3H]RS-79948-197 (0–3 nM). Non-specific binding was determined by the addition of 100 μM idazoxan. The samples were incubated at 30 °C for 40 min then bound ligand was separated from free by vacuum filtration through GF/C filters. The filters were washed three times in TE buffer, and bound ligand measured by liquid scintillation spectrometry. In competition-binding experiments, varying concentrations of adrenaline (10 nM–1 mM) competed with a single near saturating concentration of [3H] RS-79948-197.
Palmitoylation assays
Cells were labelled with 0.5 mCi/ml [9,10-3H]palmitic acid in DMEM supplemented with 2 mM L-glutamine, 5% (v/v) dialysed newborn calf serum, and 5 mM pyruvic acid at 37 °C in a 5% CO2 humidified atmosphere. In chase experiments non-radioactive palmitic acid was added at 100 μM. After incubation for the appropriate time in the presence and absence of adrenaline, reactions were terminated by the addition of 200 μl of 1% (w/v) SDS. Cells were sheared by passage through a 25-gauge needle and proteins in the samples denaturated by a 5 min incubation at 100 °C. After chilling to 4 °C, 800 μl of solubilization buffer [1% (v/v) Triton X-100, 10 mM EDTA, 100 mM NaH2PO4, 10 mM NaF, 50 mM Hepes (pH 7.2)] was added, and the samples pre-cleared by incubation for 1 h at 4 °C with 100 μl Pansorbin (Calbiochem, La Jolla, CA, U.S.A.). After equalizing samples for total cell protein the pre-cleared supernatants were then incubated for 16 h at 4 °C with protein A–Sepharose and 10 μl of antiserum ON1 [23]. Immune complexes were isolated by centrifugation, washed three times with immunoprecipitation buffer [1% (v/v) Triton X-100, 100 mM NaCl, 50 mM NaH2PO4, 100 mM NaF, 50 mM Hepes (pH 7.2) plus 0.5% SDS]. Samples were eluted from the protein A–Sepharose by the addition of electrophoresis buffer containing 20 mM DTT and heating to 80 °C for 3 min. Analysis was by SDS/PAGE, using 10% (w/v) polyacrylamide resolving gels, which were then transferred onto PVDF membranes and subjected to autoradiography.
Immunoblotting
Cell lysates or membranes were subjected to SDS/PAGE, using 10% (w/v) polyacrylamide resolving gels then transferred onto nitrocellulose membranes. The nitrocellulose was blocked in 5% milk powder (prepared in PBS/0.2% Tween 20) overnight and washed three times with PBS/0.2% Tween 20 over a 30 min period. Incubation with the primary antibody was in 3% milk powder prepared in PBS/0.2% Tween 20 for 1 h at room temperature, followed by three washes in PBS/0.2% Tween 20 over a 30 min period. Secondary antibody was incubated in 3% milk powder in PBS/0.2% Tween 20 for 1 h at room temperature, again followed by 3 washes over 30 min with PBS/0.2% Tween 20. The nitrocellulose was then incubated with a 50:50 (v/v) mixture of ECL® reagents (Amersham) for 2 min prior to exposure to and development of X-ray film.
[35S]GTP[S]-binding assays
[35S]GTP[S]-binding experiments were initiated by the addition of cell membranes containing 10 fmol of the various fusion constructs to an assay buffer {20 mM Hepes (pH 7.4), 3 mM MgCl2, 100 nM NaCl, 1 μM guanosine 5′-diphosphate, 0.2 mM ascorbic acid, 50 nCi of [35S]GTP[S]} in the presence or absence of varying concentrations of adrenaline. Non-specific binding was determined in the same conditions but with the addition of 100 μM GTP[S]. Reactions were incubated for 2.5 min at 30 °C and were terminated by the addition of 0.5 ml of ice-cold buffer, containing 20 mM Hepes (pH 7.4), 3 mM MgCl2 and 100 mM NaCl. The samples were centrifuged at 16000 g for 15 min at 4 °C, and the resulting pellets were resuspended in solubilization buffer (100 mM Tris, 200 mM NaCl, 1 mM EDTA, 1.25% Nonidet P-40) plus 0.2% SDS. Samples were precleared with Pansorbin, followed by immunoprecipitation with ON1 antiserum. Finally, the immunocomplexes were washed twice with solubilization buffer, and bound [35S]GTP[S] was measured by liquid-scintillation spectrometry.
RESULTS
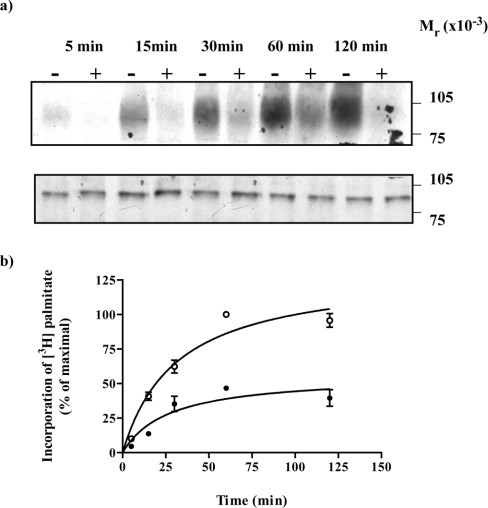
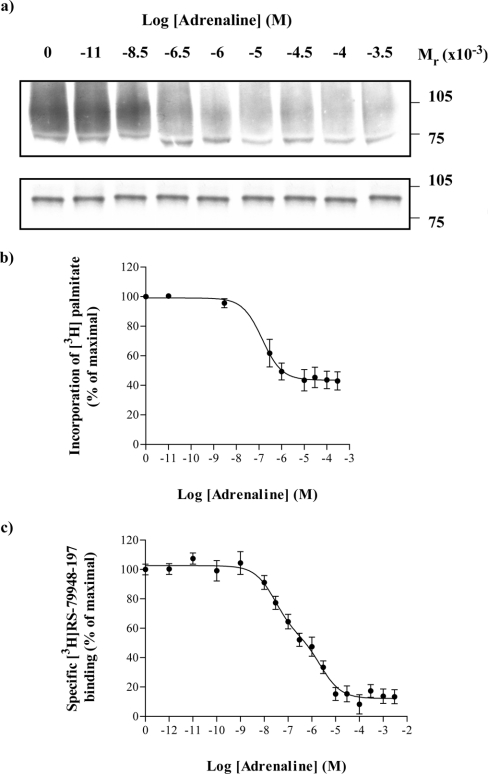
A fusion protein in which the α subunit of a pertussis toxin-resistant variant (Cys351→Ile) of Go1 was attached in-frame to the C-terminal tail of the porcine α2A-adrenoceptor [24] was expressed transiently in HEK293T cells. [9,10-3H]Palmitic acid was added to the cells in the presence or absence of the adrenoceptor agonist adrenaline (100 μM) for times varying between 5–120 min. Cell lysates were generated and immunoprecipitated using an antiserum (ON1) that identifies the N-terminal region of Gαo1 [23]. Subsequent to SDS/PAGE and autoradiography, in the absence of adrenaline, radioactivity was incorporated into a band with apparent molecular mass of some 89 kDa (Figure 1a, upper panel). This occurred in a time-dependent manner with maximal incorporation being achieved between 60–120 min (Figure 1). In the presence of adrenaline, incorporation of [3H]palmitate into the fusion protein was substantially reduced over this time scale (Figures 1a upper panel and 1b). In parallel with these studies, samples of the cell lysates were resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (Figure 1a lower panel). These studies demonstrated that equal amounts of the α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein were present in each sample and that degradation of the fusion protein was not significant over this time period. Equivalent results were obtained when the samples were immunoprecipitated with a second antiserum, OC1 that identifies the C-terminal decapeptide of Gαo1 [23] (results not shown). The effects of adrenaline were concentration-dependent. When labelling of the α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein with [3H]palmitate was allowed to proceed for 30 min in the presence of varying concentrations of adrenaline, half-maximal reduction in incorporation of [3H]palmitate into the immunoprecipitated fusion protein was obtained with 1.4±0.2×10−8 M adrenaline (Figures 2a and 2b). As the calculated affinity (corrected IC50=2.6±0.6×10−8 M) of adrenaline to bind to the α2A-adrenoceptor–(Cys351Ile)-Gαo1 fusion protein (Figure 2c) was similar it suggests that binding of the agonist to the receptor was directly responsible for the regulation of [3H]palmitoylation.
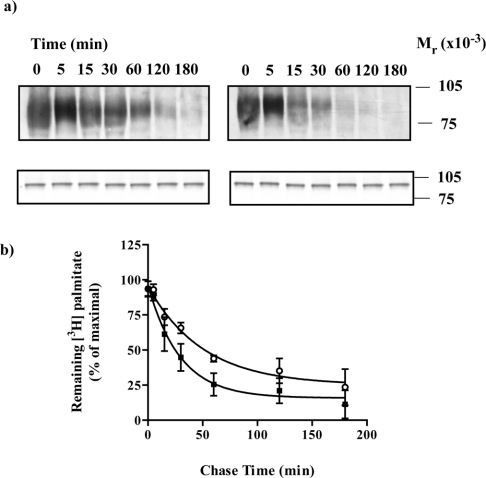
Figure 1. Incorporation of [3H]palmitate into an α2A-adrenoceptor–Gαo1 fusion protein is reduced substantially by adrenaline.
An α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein was expressed in HEK293T cells. Cells were incubated with [3H]palmitate for the indicated times in the absence (−) or presence (+) of 100 μM adrenaline. Samples were harvested and cell lysates produced. (a) Cell lysates were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (upper panel) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (lower panel). (b) Autoradiographs akin to (a, upper panel) were quantitated. Open symbols (absence), closed symbols (presence) of adrenaline. Data are means±S.E.M., n=3.
Figure 2. Adrenaline regulates incorporation of [3H]palmitate into an α2A-adrenoceptor–Gαo1 fusion protein in a concentration-dependent manner.
An α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein was expressed in HEK293T cells. Cells were incubated with [3H]palmitate for 30 min in the presence of varying concentrations of adrenaline. Samples were harvested and cell lysates produced. These were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (a, upper panel) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (a, lower panel). The effect of adrenaline was quantified in three such experiments and presented as means±S.E.M. (b). The ability of adrenaline to compete with [3H]RS-79948-197 for binding to the α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein was also assessed (c) to allow calculation of the binding affinity of adrenaline.
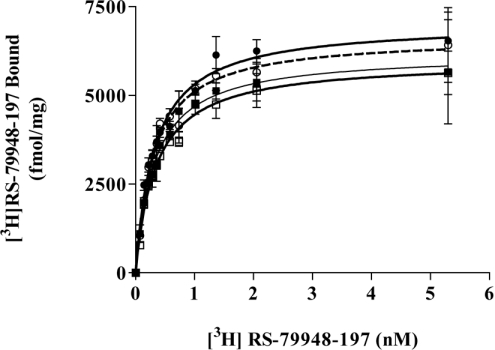
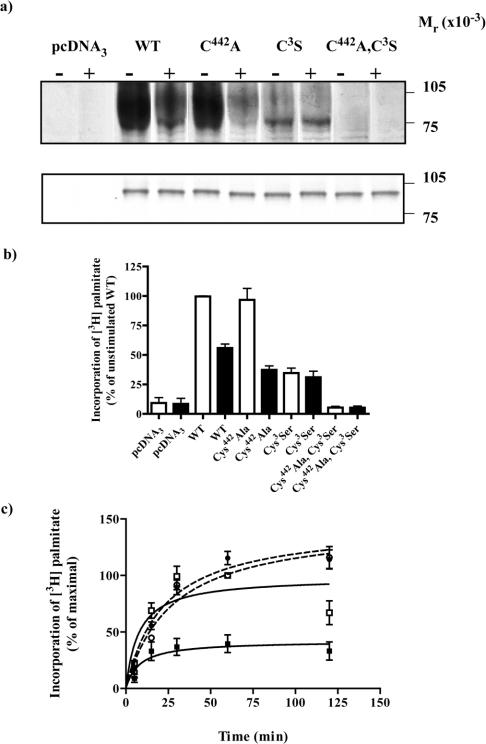
Both the α2A-adrenoceptor [11] and Gαo1 [24] contain previously characterized sites for post-translational thio-acylation. It was thus unclear if the incorporation of [3H]palmitate into the α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein occurred within the GPCR, the G-protein element, or both. The site of palmitoylation within the α2A-adrenoceptor is Cys442 and in Gαo1-Cys3. Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1, α2A-adrenoceptor–Cys3Ser (Cys351Ile)Gαo1 and Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 forms of the fusion protein were thus generated to address this issue. Following transfection of HEK293T cells the expression levels of each of these constructs and their affinity to bind the α2-adrenoceptor antagonist [3H]RS-79948-197 was assessed. There was no systematic difference in either the affinity of these constructs to bind [3H]RS-79948-197 or in expression levels of the three mutant fusion proteins compared with the wild-type, with each being expressed in the region of 6 pmol·mg−1 membrane protein and with estimated Kd=0.32±0.03×10−9 M (Figure 3). Despite this, small variations in expression levels of the individual constructs were noted in parallel transfections and were compensated for routinely by immunoprecipitation from amounts of the cell lysates containing equal amounts of each fusion protein as assessed by the specific binding of a single, near saturating concentration of [3H]RS-79948-197. Following labelling of cells with [3H]palmitate for 30 min in the absence of adrenaline, immunoprecipitation, SDS/PAGE and autoradiography, the wild-type, Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1, α2A-adrenoceptor–Cys3Ser(Cys351Ile)-Gαo1 but not the Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351-Ile)Gαo1 forms of the fusion protein incorporated [3H]palmitate (Figure 4a, upper panel). The lack of incorporation of [3H]palmitate into Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 confirmed that all the detected dynamic thio-acylation reflects modification at these two locations. However, with the same period of exposure to [3H]palmitate the extent of incorporation of radioactivity into the other three constructs was not equal (Figure 4b), despite parallel immunoblots confirming that the loading of the individual constructs was the same (Figure 4a, lower panel). Incorporation of [3H]palmitate into the α2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 fusion protein was substantially lower than for either the wild-type fusion protein or, indeed, the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 form. By contrast, the amount of incorporation of [3H]palmitate into the wild-type and Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 was not substantially different (Figure 4b). Furthermore, although the presence of adrenaline substantially reduced incorporation of [3H]palmitate into both the wild-type and the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Go1α fusion protein it did not do so for the α2A-adrenoceptor-Cys3Ser(Cys351Ile)Go1α fusion protein (Figure 4b). The extent of inhibition of [3H]palmitoylation of the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Go1α fusion protein produced by adrenaline (62.7±5.8%, mean±S.E.M., n=3) was significantly greater (P=0.01) than for the wild-type fusion protein (44.1±5.7% mean±S.E.M., n=3). To explore these differences further, time courses of the incorporation of [3H]palmitate into the α2A-adrenoceptor–Cys3Ser(Cys351Ile)Go1α and Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 fusion proteins were performed and showed that radiolabelling of the G-protein element of the fusion (t1/2=8.2±1.3 min, mean±S.E.M., n=3) occurred significantly (P<0.001) more rapidly than incorporation of [3H]palmitate into the receptor segment of the fusion (t1/2=27.4±2.9 min, mean±S.E.M., n=3) (Figure 4c). Data from time courses of labelling of the wild-type fusion protein with [3H]palmitate were not sufficient to estimate whether distinct rapid and less rapid phases were present that might correspond to incorporation into the receptor and G-protein elements. In accord with the data of Figures 4(a) and 4(b), adrenaline did not alter the amount or rate (t1/2=22.3±1.1 min, mean±S.E.M., n=3, P=0.37) of [3H]palmitate incorporation into the α2A-adrenoceptor–Cys3Ser(Cys351Ile)Go1α fusion protein and although the amount of incorporation of [3H]palmitate into the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 fusion was significantly reduced at all times points measured, the presence of adrenaline did not alter the rate (t1/2=8.3±2.0 min, mean±S.E.M., n=3, P=0.87) of labelling (Figure 4c).
Figure 3. Mutation of the potential thio-acylation sites in an α2A-adrenoceptor–Gαo1 fusion protein does not alter expression or binding affinity for an antagonist ligand.
HEK293T cells were transfected to express α2A-adrenoceptor–(Cys351Ile)Gαo1 (○), (Cys442Ala)α2A-adrenoceptor–(Cys351Ile)Gαo1 (□), α2A-adrenoceptor–(Cys3Ser)(Cys351Ile)Gαo1 (●) or (Cys442Ala)α2A-adrenoceptor–(Cys3Ser)(Cys351Ile)Gαo1 (■) fusion proteins. Membranes were prepared and the specific binding of varying concentrations of [3H]RS-79948-197 assessed.
Figure 4. Both the receptor and the G-protein elements of an α2A-adrenoceptor–Gαo1 fusion protein are targets for palmitoylation.
HEK293T cells were transfected with empty vector (pcDNA3) or to express α2A-adrenoceptor–(Cys351Ile)Gαo1 (WT), Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 (C442A), α2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 (C3S) or Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)-Gαo1 (C442A,C3S) fusion proteins. Cells were incubated with [3H]palmitate for 30 min in the absence (−) or presence (+) of 100 μM adrenaline. Samples were harvested and cell lysates produced. These were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (a, upper panel) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (a, lower panel). (b) Autoradiographs as in the upper panel of (a) were scanned and signals quantitated in the area of the film shown. Open bars, absence of adrenaline; filled bars, presence of adrenaline. Data are means±S.E.M., n=3. c. Cells expressing the C442A (squares) or the C3S (circles) constructs were incubated with [3H]palmitate for varying times in the absence (open symbols) or presence (filled symbols) of 100 μM adrenaline. Samples were then prepared as in (a). Data are means±S.E.M. n=3. Analyses of t1/2 of labelling are detailed in the Results section.
These studies indicated clear differences in the characteristics of palmitoylation, and the effects of adrenaline on this, in the GPCR and G-protein elements of the fusion proteins. Thio-acylation has long been recognized to have the potential for dynamic regulation [1,5]. Despite this, in early studies on the α2A-adrenoceptor it was reported that the half-life of [3H]palmitoylation of this GPCR was very similar to the half-life of the protein [12]. The dynamics of de-acylation of a polypeptide can be studied via experiments performed in pulse-chase format. Following transfection of HEK293T cells with the α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein and labelling of the cells with [3H]-palmitate for 30 min, the radiolabel was removed and replaced with non-radioactive palmitate (100 μM). Samples were taken for analysis at times up to 180 min. [3H]palmitate was removed from the immunoprecipitated fusion protein with t1/2=35±9 min (mean±S.E.M., n=3) (Figure 5). When the chase was conducted in the presence of adrenaline, removal of [3H]palmitate from the fusion protein was substantially more rapid (P<0.05), with t1/2=20±3 min, demonstrating that agonist enhances de-palmitoylation of the fusion protein. To assess the contribution of the G protein to these effects, HEK293T cells were transfected to express the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein in which only the G-protein element is a target for thio-acylation. Using the same protocol the rate of disappearance of [3H]palmitate from immunoprecipitated samples was again rapid (t1/2=37±5 min) and accelerated (t1/2=17±2 min, P<0.05) by the presence of agonist (Figure 6). Again, parallel immunoblots of cell lysates confirmed equal loading of the gel lanes. Equivalent experiments were then performed with the α2A-adrenoceptor–Cys3Ser (Cys351Ile)Gαo1 fusion protein in which only the GPCR element can be a target for palmitoylation. Again, a time-dependent reduction in the presence of [3H]palmitate was observed, indicating dynamic de-palmitoylation of the receptor with a similar half-life (t1/2=27±4 min) as noted for the G protein but, by contrast, this was not altered (t1/2=29±2 min) by the presence of adrenaline (Figure 7).
Figure 5. Adrenaline increases the rate of de-palmitoylation of an α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein.
HEK293T cells were transfected to express an α2A-adrenoceptor–(Cys351Ile)Go1 fusion protein. Cells were incubated with [3H]palmitate for 30 min, washed and then excess non-radioactive palmitate (100 μM) was added in the absence (a, left-hand panel; b, open symbols) or presence (a, right-hand panel; b, filled symbols) of adrenaline (100 μM). Samples were harvested at varying times and cell lysates produced that were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (a, upper panels) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (a, lower panels). A representative experiment is shown. Experiments akin to (a) were quantitated and the loss of [3H]palmitate with time is presented as means±S.E.M., n=3 (b).
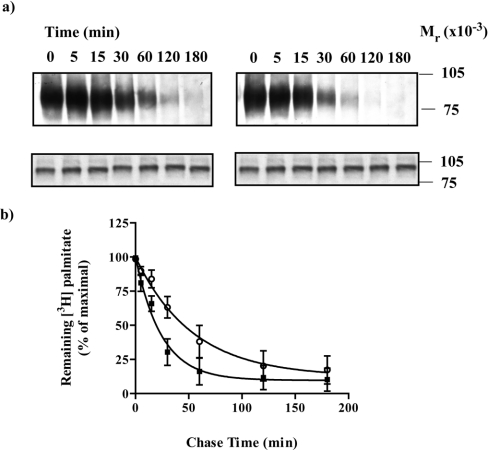
Figure 6. Adrenaline increases the rate of de-palmitoylation of Gαo1.
HEK293T cells were transfected to express the Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1 fusion protein. Cells were incubated with [3H]palmitate for 30 min, washed and then excess non-radioactive palmitate (100 μM) was added in the absence (a, left-hand panel; b, open symbols) or presence (a, right-hand panel; b, filled symbols) of adrenaline (100 μM). Samples were harvested at varying times and cell lysates were produced that were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (a, upper panels) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (a, lower panels). A representative experiment is shown. Experiments akin to (a) were quantitated and the loss of [3H]palmitate is presented as means±S.E.M., n=3 (b).
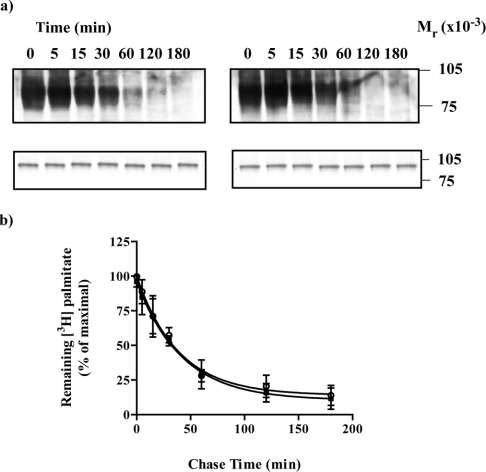
Figure 7. Adrenaline does not alter the rate of de-palmitoylation of the α2A-adrenoceptor.
HEK293T cells were transfected to express the α2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 fusion protein. Cells were incubated with [3H]palmitate for 30 min, washed and then excess non-radioactive palmitate (100 μM) was added in the absence (a, left-hand panel; b, open symbols) or presence (a, right-hand panel; b, filled symbols) of adrenaline (100 μM). Samples were harvested at varying times and cell lysates were produced that were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (a, upper panels) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (a, lower panels). A representative experiment is shown. Experiments akin to (a) were quantitated and the loss of [3H]palmitate is presented as means±S.E.M., n=3 (b).
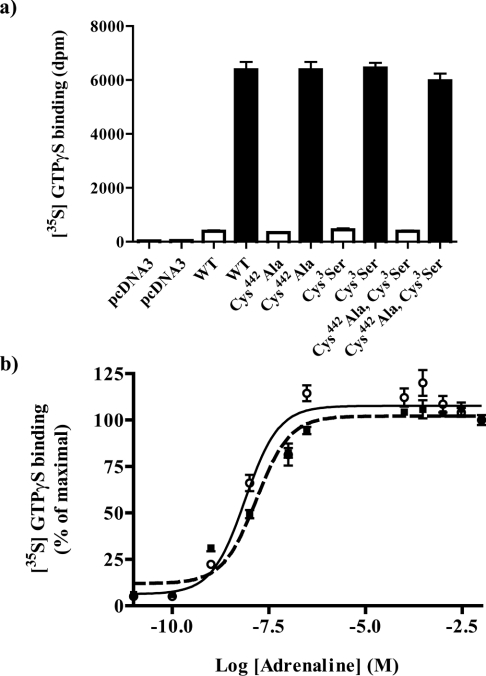
It has been suggested that the acylation potential and status of either the GPCR or the G protein can alter effective information transfer from GPCR to G protein [25]. We assessed this for the effect of adrenaline. Membranes expressing each of the four α2A-adrenoceptor–(Cys351Ile)Gαo1 fusion proteins were used to measure the binding of [35S]GTP[S] in the absence and presence of 100 μM adrenaline. Samples were subsequently immunoprecipitated with antiserum ON1 and counted. In each case adrenaline resulted in a large increase in [35S]GTP[S] binding but neither basal [35S]GTP[S] binding nor the maximal effect of adrenaline were different between the four individual fusion proteins (Figure 8a). Equally, when the potency of adrenaline to enhance the binding of [35S]GTP[S] to either the α2A-adrenoceptor–(Cys351Ile)Gαo1 (7.7±2.7×10−9 M) or Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)Gαo1 (1.5±0.5×10−8 M) fusion proteins was measured, this was again not significantly different (Figure 8b).
Figure 8. The palmitoylation potential of α2A-adrenoceptor–Go1α fusion proteins does not modify agonist-stimulated binding of [35S]GTP[S].
HEK293T cells were transfected with empty vector (pcDNA3) or to express α2A-adrenoceptor–(Cys351Ile)Go1α (WT), Cys442Alaα2A-adrenoceptor–(Cys351Ile)Go1α (Cys442-Ala), α2A-adrenoceptor–Cys3Ser(Cys351Ile)Go1α (Cys3Ser) or Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)Go1α (Cys442Ala, Cys3Ser) fusion proteins. Membranes were prepared and samples containing 10 fmol of [3H]RS-79948-197 binding sites were used to measure basal (open bars) and 100 μM adrenaline-stimulated binding of [35S]GTP[S] (a). Parallel assays monitored the capacity of varying concentrations of adrenaline to enhance binding of [35S]GTP[S] (b) to α2A-adrenoceptor–(Cys351Ile)Go1α (open symbols) and Cys442Alaα2A-adrenoceptor–Cys3Ser(Cys351Ile)Go1α (filled symbols). At the termination of incubation, samples were immunoprecipitated with antiserum ON1 and counted. Data are means±S.E.M., n=3.
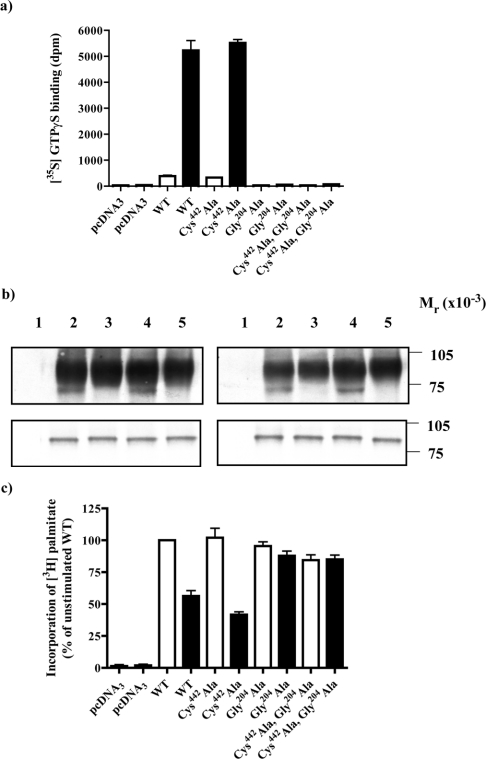
The wild-type and Cys442Alaα2A-adrenoceptor–(Cys351Ile)-Gαo1 fusion proteins were further modified to encode a Gly204→Ala mutation within the G-protein element of the constructs. This Gly is totally conserved in the α subunit of heterotrimeric G proteins. Such glycine to alanine mutations prevent effective exchange of GTP for GDP and hence the G protein is unable to adopt the active conformation. As anticipated, addition of adrenaline prevented binding of [35S]GTP[S] to these constructs (Figure 9a). We therefore used these forms of the fusions to assess if activation was required to produce agonist-regulation of G-protein palmitoylation. Although incorporation of [3H]palmitate was produced, unlike the wild-type α2A-adrenoceptor–(Cys351Ile)Gαo1 and Cys442Alaα2A-adrenoceptor–(Cys351Ile)Gαo1fusion proteins, there was no effect of adrenaline on the palmitoylation status of wild-type α2A-adrenoceptor–Gly204Ala(Cys351Ile)Gαo1 or the Cys442Alaα2A-adrenoceptor – Gly204Ala (Cys351Ile)G αo1 fusions (Figures 9b and 9c), indicating that G-protein activation is required to produce agonist regulation of G-protein palmitoylation.
Figure 9. Adrenaline does not regulate the palmitoylation of an α2A-adrenoceptor–Go1α fusion protein in which the G protein cannot bind [35S]GTP[S].
HEK293T cells were transfected with empty vector (pcDNA3; lanes 1, panel b) or to express either α2A-adrenoceptor–(Cys351Ile)Go1α (WT; lanes 2, panel b), Cys442Alaα2A-adrenoceptor–(Cys351Ile)Go1α (Cys442Ala; lanes 3, panel b), α2A-adrenoceptor–Gly204Ala(Cys351Ile)Go1α (Gly204Ala; lanes 4, panel b) or Cys442Alaα2A-adrenoceptor–Gly204Ala(Cys351Ile)Go1α (Cys442-Ala, Gly204Ala; lanes 5, panel b) fusion proteins. (a) Membranes were prepared from these cells and samples containing 10 fmol of [3H]RS-79948-197 binding sites used to measure basal (open bars) and 100 μM adrenaline-stimulated binding of [35S]GTP[S] (filled bars). (b) In parallel, cells were incubated with [3H]palmitate for 30 min in the absence (left-hand panels) or presence (right-hand panels) of 100 μM adrenaline. Samples were harvested and cell lysates produced. These were either immunoprecipitated with antiserum ON1 prior to SDS/PAGE and autoradiography for 1 month (upper panels) or resolved directly by SDS/PAGE and immunoblotted with antiserum ON1 (lower panels). (c) Experiments akin to (b) were quantitated and incorporation of [3H]palmitate in the absence (open bars) and presence (filled bars) of adrenaline is presented as means±S.E.M., n=3.
DISCUSSION
The porcine α2A-adrenoceptor was one of the first GPCRs demonstrated to be a target for post-translational palmitoylation [11–12]. Furthermore, mutation of Cys442 in the C-terminal tail of this receptor abolished incorporation of [3H]palmitate, indicating this as the sole site for modification [11]. In these previous studies, however, it was reported that the half-life of [3H]palmitate on the GPCR was many hours and indeed was similar to the half-life of the protein [12]. Although this is not unique, in that recent studies have reported that the serotonin 5-HT1A receptor is stably palmitoylated [26], this appears quite different from many other protein targets for thio-acylation where the fatty acid is turned over rapidly, allowing proteins to undergo many cycles of acylation and de-acylation during their lifetime [1–2]. Furthermore, although the presence of agonist was reported to stimulate de-palmitoylation of the α2A-adrenoceptor, the effect was modest and de-acylation remained a slow process [12]. Given the predominance of examples of rapid regulation of protein thio-acylation we wished to re-examine regulation of α2A- adrenoceptor palmitoylation.
As with many other GPCRs, antisera able to efficiently immunoprecipitate the α2A-adrenoceptor are not widely available. Furthermore, in preliminary experiments, even following N-terminal epitope tagging effective immunoprecipitation of the isolated α2A-adrenoceptor remained problematic. In recent times, fusion proteins in which the N-terminus of a G protein α subunit is linked in-frame to the C-terminal tail of a GPCR have been used widely to explore many aspects of receptor and G-protein interaction and function [27]. For fusions between the β2-adrenoceptor and the α subunit of Gs [18,25] and the α1b-adrenoceptor and the α subunit of G11 [19], palmitoylation of both elements of the fusion constructs has been observed. We thus decided to re-explore the regulation of palmitoylation of the α2A-adrenoceptor and, in parallel, the α subunit of the cognate G protein Go1 using this strategy. In all the constructs employed the G-protein element of the fusion was modified by replacement of the pertussis toxin-sensitive cysteine with isoleucine. In previous studies on the related G protein, Gi1, the effectiveness of activation by the α2A-adrenoceptor was correlated highly with hydrophobicity of the amino acid at this site [28] and we [29] and others [30] now use the isoleucine-containing versions of Gi-family G proteins routinely. A further attraction in the use of Go1-containing fusion proteins for these studies was that we have previously generated antisera able to immunoprecipitate this polypeptide quantitatively [31] and thus, by extension, fusion proteins incorporating it. Furthermore, although in co-expression studies only a fraction of the expressed G protein is likely to be activated by a GPCR, and only this fraction is therefore anticipated to regulate acylation status in an agonist-dependent manner, use of the fusion protein defines that all of the G protein is regulated by agonist-occupancy of the receptor and thus effects of receptor activation on G protein thio-acylation would be expected to be maximized.
Following expression of the α2A-adrenoceptor–(Cys351Ile)-Go1α fusion protein in HEK293T cells this construct incorporated [3H]palmitate. This incorporation was substantially lower when the α2A-adrenoceptor agonist adrenaline was present during the labelling period. This is akin to the effect of agonist on palmitoylation of a β2-adrenoceptor–Gsα fusion protein [18] but distinct from that observed for an α1b-adrenoceptor–G11α fusion protein where agonist enhanced the kinetics of palmitoylation [19]. However, as both Cys442 of the receptor sequence and Cys3 of the G protein are previously characterized sites of [3H]acylation, this initial study could not determine whether the effect of agonist was on both elements of the fusion. Forms of the fusion proteins were generated in which Cys442 of the receptor was converted to alanine, Cys3 of the G protein to serine, or which incorporated both of these changes. Importantly, none of these alterations changed either how effectively the construct was expressed or the binding affinity of an α2-adrenoceptor antagonist. The fusion containing both the Cys442→Ala and the Cys3→Ser mutations did not incorporate [3H]palmitate, confirming these sites as the only ones for dynamic post-translational acylation in these constructs. Both the Cys442Alaα2-adrenoceptor–Go1α and the α2-adrenoceptor–Cys3SerGo1α fusion proteins did incorporate [3H]palmitate, demonstrating that both the receptor and G-protein elements are targets for dynamic acylation. Interestingly and unexpectedly, they were not equivalent. For equal amounts of the two, the α2-adrenoceptor–Cys3SerGo1α fusion, in which only the single site in the GPCR can be modified, incorporated significantly less [3H]palmitate within a 30 min period than the Cys442Alaα2-adrenoceptor–Go1α fusion in which only the G protein can be a target. These observations suggest that acylation of the GPCR segment is slower than of the G protein. Indeed, when we explored this directly, the half-time of [3H]palmitate incorporation into the receptor was some three times slower than into the G protein. If these experiments had been performed following co-expression of GPCR and G protein, differences in the observed extent of incorporation of [3H]palmitate could not have been interpreted because two separate antisera would have been required and these, unavoidably, would be likely to have different immunoprecipitation efficiencies.
All experiments on the extent and dynamics of acylation must consider the reversibility of the process [1]. Thus, less incorporation of [3H]palmitate into the GPCR element in direct-labelling studies is also consistent with the initial removal of non-radioactive palmitate being slower at this site, thus restricting the subsequent incorporation of [3H]palmitate. In de-palmitoylation studies, adrenaline accelerated removal of palmitate from the G protein but not from the receptor. This is indicative that although the acylation cycle of the G protein is regulated by agonist, the acylation cycle of the GPCR is not. This is in distinct contrast to studies with an α1b-adrenoceptor-G11α fusion protein where agonist-enhanced labelling of both the GPCR and G-protein elements [19]. Activation of G proteins is often associated with alteration in palmitoylation [4–5] and to test this directly we also employed fusion proteins incorporating a form of Go1α that is unable to exchange GDP for GTP and is therefore unable to attain the active state. Although dynamic, in that this form of the G protein did incorporate [3H]palmitate, acylation of this form of the G protein was not regulated by agonist. A number of other studies have suggested that activation of a G protein can regulate palmitoylation. Loisel et al. [18] also demonstrated agonist block of incorporation of [3H]palmitate into a fusion protein, in this case between the β2-adrenoceptor and Gsα. Although they raised the prospect of this reflecting decreased re-palmitoylation following de-palmitoylation, they also noted that in pulse-chase experiments the agonist caused more rapid removal of the [3H]palmitate. This combination of events was taken to reflect an inability of the β2-adrenoceptor–Gsα fusion protein to be re-palmitoylated in the presence of agonist. They did, however, observe enhanced re-palmitoylation when the studies were performed with the isolated β2-adrenoceptor and concluded that desensitization or other turn-off processes might be required for re-palmitoylation [18]. The basis for the distinct differences between the data reported here and for a β2-adrenoceptor–Gsα fusion protein is difficult to ascertain. However, the α2-adrenoceptor is known to be more resistant to agonist-induced internalization than the β2-adrenoceptor and the relatively short C-terminal tail of the α2-adrenoceptor does not contain hydroxy amino acids that might be sites for phosphorylation. Previous studies have suggested an interplay between agonist-induced phosphorylation and regulation of palmitoylation of the β2-adrenoceptor [32].
Agonist activation of the α2-adrenoceptor–Go1α fusion proteins could be monitored easily by measuring basal and adrenaline stimulation of the binding of [35S]GTP[S]. There were no obvious differences in this between the various fusion proteins employed in this study and this is consistent with previous reports that the palmitoylation potential of the α2-adrenoceptor does not alter G-protein activation [12]. This is not universal. A series of reports have noted alterations in G-protein mediated signalling associated with the expression of acylation-resistant forms of GPCRs [10,15,33–35] and in certain GPCRs such mutants also modulate phosphorylation and interactions with arrestins [36–37]. Futhermore, although the thio-acylation potential of neither the α2-adrenoceptor nor Go1α in the fusion proteins altered the extent or potency of agonist-mediated G-protein activation this is not an inherent feature of such fusion proteins. Ugur et al. [25] have noted previously that a β2-adrenoceptor–Cys3AlaGsα fusion protein, in which the G protein cannot be thio-acylated, is only about half as effective in causing agonist-mediated stimulation of adenylyl cyclase as an equivalent fusion containing the wild-type G protein. This reflects poor GPCR–G protein contacts in the mutated form as the thio-acylation-resistant variant formed less high affinity agonist binding sites [25]. Equally, following fusion between the α1b-adrenoceptor and its cognate G protein G11α, either mutational or chemical de-palmitoylation of the G protein but not of the receptor caused a 50% reduction in agonist-mediated binding of [35S]GTP[S], although the effects of mutation and chemical de-palmitoylation were not additive (Novotny, J. and Milligan, G., unpublished data).
Although concerns are sometimes expressed about the detailed function of GPCR–G-protein fusions it is important to note that the intrinsic GTPase activity of the G protein functions to deactivate this construct as expected [22] and that regulators of G protein signalling are effective GTPase activating proteins for this construct [21]. Furthermore, the G protein–β/γ complex interacts effectively and can be co-immunoprecipitated with α2A-adrenoceptor–Go1α fusion proteins [38]. Thus, the basic features and regulation of interactions between the α2A-adrenoceptor, the G protein α subunit and its interacting proteins are preserved in the fusion proteins. The production of a version of the α2A-adrenoceptor–Go1α fusion protein that interacts very poorly with the G protein–β/γ complex [38] may allow analysis of whether the interactions with β/γ also determine the effectiveness and regulation of G protein α subunit palmitoylation. This would be consistent with a range of other studies that have indicated a complex interplay in which β/γ interactions are important for membrane delivery and positioning of G protein α subunits to allow them to act as targets for palmitoylation [39–41]. The ‘fusion’ proteins we have employed in these studies do not necessarily mimic the physiological situation. They do, however, provide a very useful model system to explore aspects of GPCR–G protein interactions and their regulation. It is now common to link proteins of interest to the C-terminal tail of GPCRs. By far the most common has been the use of fluorescent proteins to visualize receptor distribution and to monitor potential protein–protein interactions [42]. However, as well as these, proteins as diverse as regulators of G protein signalling [43], β-arrestins [44] and caveolins [45] as well as G protein α subunits [42] have been fused to various receptors. Even with epitope tagging of the receptor, we were unable to achieve immunoprecipitation efficiency compatible with the generation of high-quality data on the dynamics of palmitoylation when we expressed the isolated receptor in cells. The fusion approach therefore allowed us to reach perhaps more anticipated but certainly very different conclusions on the dynamics of thio-acylation of this receptor than are currently available in the literature [11–12].
The effect of mutation of potential palmitoylation sites is markedly variable between different receptors. Some of the reported variation includes effects on cell surface delivery of the receptor, the coupling to G proteins, the effects on phosphorylation by various kinases and the regulation of agonist-induced internalization. The current data provide different conclusions on the kinetics of acylation and its regulation by agonist than previous work on the α2A-adrenoceptor and demonstrates that agonist occupancy and activation of an α2A-adrenoceptor–Go1α fusion protein regulates the palmitoylation status of the G protein but not the receptor. This effect is produced, at least partially, via agonist-induced enhancement of G protein de-palmitoylation. Given the differences in results obtained with related experiments for both the β2- and α1b-adrenoceptors, simple and universal rules on the regulation of thio-acylation may be difficult to define, even for closely related receptors. These will have to be analysed on a case by case basis.
Acknowledgments
E. B. thanks the Biotechnology and Biosciences Research Council for a studentship.
References
- 1.Qanbar R., Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol. Ther. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 2.Bijlmakers M. J., Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 3.Kleuss C., Krause E. Gαs is palmitoylated at the N-terminal glycine. EMBO J. 2003;22:826–832. doi: 10.1093/emboj/cdg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedegaertner P. B. Lipid modifications and membrane targeting of Gα. Biol. Signals Recept. 1998;7:125–135. doi: 10.1159/000014538. [DOI] [PubMed] [Google Scholar]
- 5.Chen C. A., Manning D. R. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 6.Chen C., Shahabi V., Xu W., Liu-Chen L. Y. Palmitoylation of the rat μ opioid receptor. FEBS Lett. 1998;441:148–152. doi: 10.1016/s0014-5793(98)01547-6. [DOI] [PubMed] [Google Scholar]
- 7.Hawtin S. R., Tobin A. B., Patel S., Wheatley M. Palmitoylation of the vasopressin V1a receptor reveals different conformational requirements for signaling, agonist-induced receptor phosphorylation, and sequestration. J. Biol. Chem. 2001;276:38139–38146. doi: 10.1074/jbc.M106142200. [DOI] [PubMed] [Google Scholar]
- 8.Moench S. J., Moreland J., Stewart D. H., Dewey T. G. Fluorescence studies of the location and membrane accessibility of the palmitoylation sites of rhodopsin. Biochemistry. 1994;33:5791–5796. doi: 10.1021/bi00185a017. [DOI] [PubMed] [Google Scholar]
- 9.Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science (Wasington, D.C.) 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 10.O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human β2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J. Biol. Chem. 1989;264:7564–7569. [PubMed] [Google Scholar]
- 11.Kennedy M. E., Limbird L. E. Mutations of the α 2A-adrenergic receptor that eliminate detectable palmitoylation do not perturb receptor-G-protein coupling. J. Biol. Chem. 1993;268:8003–8011. [PubMed] [Google Scholar]
- 12.Kennedy M. E., Limbird L. E. Palmitoylation of the α 2A-adrenergic receptor. Analysis of the sequence requirements for and the dynamic properties of α 2A-adrenergic receptor palmitoylation. J. Biol. Chem. 1994;269:31915–31922. [PubMed] [Google Scholar]
- 13.Loisel T. P., Adam L., Hebert T. E., Bouvier M. Agonist stimulation increases the turnover rate of β2AR-bound palmitate and promotes receptor depalmitoylation. Biochemistry. 1996;35:15923–15932. doi: 10.1021/bi9611321. [DOI] [PubMed] [Google Scholar]
- 14.Ponimaskin E. G., Heine M., Joubert L., Sebben M., Bickmeyer U., Richter D. W., Dumuis A. The 5-hydroxytryptamine(4a) receptor is palmitoylated at two different sites, and acylation is critically involved in regulation of receptor constitutive activity. J. Biol. Chem. 2002;277:2534–2546. doi: 10.1074/jbc.M106529200. [DOI] [PubMed] [Google Scholar]
- 15.Miggin S. M., Lawler O. A., Kinsella B. T. Palmitoylation of the Human Prostacyclin Receptor. Functional implications of palmitoylation and isoprenylation. J. Biol. Chem. 2003;278:6947–6958. doi: 10.1074/jbc.M210637200. [DOI] [PubMed] [Google Scholar]
- 16.Mumby S. M., Kleuss C., Gilman A. G. Receptor regulation of G-protein palmitoylation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedegaertner P. B., Bourne H. R. Activation and depalmitoylation of Gsα. Cell (Cambridge, Mass.) 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 18.Loisel T. P., Ansanay H., Adam L., Marullo S., Seifert R., Lagace M., Bouvier M. Activation of the β(2)-adrenergic receptor-Gα(s) complex leads to rapid depalmitoylation and inhibition of repalmitoylation of both the receptor and Gα(s) J. Biol. Chem. 1999;274:31014–31019. doi: 10.1074/jbc.274.43.31014. [DOI] [PubMed] [Google Scholar]
- 19.Stevens P. A., Pediani J., Carrillo J. J., Milligan G. Coordinated agonist regulation of receptor and G-protein palmitoylation and functional rescue of palmitoylation-deficient mutants of the G protein G11α following fusion to the α1b-adrenoreceptor: palmitoylation of G11α is not required for interaction with β/γ complex. J. Biol. Chem. 2001;276:35883–35890. doi: 10.1074/jbc.M103816200. [DOI] [PubMed] [Google Scholar]
- 20.Milligan G. Insights into ligand pharmacology using receptor-G-protein fusion proteins. Trends Pharmacol. Sci. 2000;21:24–28. doi: 10.1016/s0165-6147(99)01404-2. [DOI] [PubMed] [Google Scholar]
- 21.Cavalli A., Druey K. M., Milligan G. The regulator of G protein signaling RGS4 selectively enhances α 2A-adreoreceptor stimulation of the GTPase activity of Go1α and Gi2α. J. Biol. Chem. 2000;275:23693–23699. doi: 10.1074/jbc.M910395199. [DOI] [PubMed] [Google Scholar]
- 22.Wise A., Milligan G. Rescue of functional interactions between the α2A-adrenoreceptor and acylation-resistant forms of Gilα by expressing the proteins from chimeric open reading frames. J. Biol. Chem. 1997;272:24673–24678. doi: 10.1074/jbc.272.39.24673. [DOI] [PubMed] [Google Scholar]
- 23.Mullaney I., Milligan G. Identification of two distinct isoforms of the guanine nucleotide binding protein, Go, in neuroblastoma x glioma hybrid cells. Independent regulation during cyclic AMP-induced differentiation. J. Neurochem. 1990;55:1890–1898. doi: 10.1111/j.1471-4159.1990.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 24.Grassie M. A., MacCallum J. F., Guzzi F., Magee A. I., Milligan G., Parenti M. The palmitoylation status of the G-protein Go1α regulates its avidity of interaction with the plasma membrane. Biochem. J. 1994;302:913–920. doi: 10.1042/bj3020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugur O., Onaran H. O., Jones T. L. Partial rescue of functional interactions of a nonpalmitoylated mutant of the G-protein Gα(s) by Fusion to the β-adrenergic receptor. Biochemistry. 2003;42:2607–2615. doi: 10.1021/bi026470i. [DOI] [PubMed] [Google Scholar]
- 26.Papoucheva E., Dumuis A., Sebben M., Richter D. W., Ponimaskin E. G. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J. Biol. Chem. 2004;279:3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- 27.Milligan G. The use of receptor-G protein fusion proteins for the study of ligand activity. Receptors Channels. 2002;8:309–317. [PubMed] [Google Scholar]
- 28.Bahia D. S., Wise A., Fanelli F., Lee M., Rees S., Milligan G. Hydrophobicity of residue351 of the G-protein Gi1α determines the extent of activation by the α2A-adrenoceptor. Biochemistry. 1998;37:11555–11562. doi: 10.1021/bi980284o. [DOI] [PubMed] [Google Scholar]
- 29.Benians A., Leaney J. L., Milligan G., Tinker A. The dynamics of formation and action of the ternary complex revealed in living cells using a G-protein gated K+ channel as a biosensor. J. Biol. Chem. 2003;278:10851–10858. doi: 10.1074/jbc.M212299200. [DOI] [PubMed] [Google Scholar]
- 30.Jeong S. W., Ikeda S. R. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca2+ channel modulation in sympathetic neurons. Proc. Natl. Acad. Sci. U.S.A. 2000;97:907–912. doi: 10.1073/pnas.97.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgoussi Z., Carr C., Milligan G. Direct measurements of in situ interactions of rat brain opioid receptors with the guanine nucleotide binding protein Go. Mol. Pharmacol. 1993;44:62–69. [PubMed] [Google Scholar]
- 32.Moffett S., Rousseau G., Lagace M., Bouvier M. The palmitoylation state of the β(2)-adrenergic receptor regulates the synergistic action of cyclic AMP-dependent protein kinase and β-adrenergic receptor kinase involved in its phosphorylation and desensitization. J. Neurochem. 2001;76:269–279. doi: 10.1046/j.1471-4159.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 33.Horstmeyer A., Cramer H., Sauer T., Muller-Esterl W., Schroeder C. Palmitoylation of endothelin receptor A. Differential modulation of signal transduction activity by post-translational modification. J. Biol. Chem. 1996;271:20811–20819. doi: 10.1074/jbc.271.34.20811. [DOI] [PubMed] [Google Scholar]
- 34.Pizard A., Blaukat A., Michineau S., Dikic I., Muller-Esterl W., Alhenc-Gelas F., Rajerison R. M. Palmitoylation of the human bradykinin B2 receptor influences ligand efficacy. Biochemistry. 2001;40:15743–15751. doi: 10.1021/bi011600t. [DOI] [PubMed] [Google Scholar]
- 35.Munshi U. M., Peegel H., Menon K. M. Palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur. J. Biochem. 2001;268:1631–1639. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson G., Watterson K. R., Palmer T. M. Subtype-specific regulation of receptor internalization and recycling by the C-terminal domains of the human A1 and rat A3 adenosine receptors: consequences for agonist-stimulated translocation of arrestin3. Biochemistry. 2002;41:14748–14761. doi: 10.1021/bi0262911. [DOI] [PubMed] [Google Scholar]
- 37.Charest P. G., Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances β-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J. Biol. Chem. 2003;278:41541–41551. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- 38.Bertaso F., Ward R. J., Viard P., Milligan G., Dolphin A. C. Mechanism of action of Gq to inhibit Gβγ modulation of Cav2.2 calcium channels probed by use of receptor-Gα tandems. Mol. Pharmacol. 2003;63:832–843. doi: 10.1124/mol.63.4.832. [DOI] [PubMed] [Google Scholar]
- 39.Iiri T., Backlund P. S., Jr, Jones T. L., Wedegaertner P. B., Bourne H. R. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evanko D. S., Thiyagarajan M. M., Wedegaertner P. B. Interaction with Gβγ is required for membrane targeting and palmitoylation of Galpha(s) and Galpha(q) J. Biol. Chem. 2000;275:1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- 41.Evanko D. S., Thiyagarajan M. M., Siderovski D. P., Wedegaertner P. B. Gβγ isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Gαs and Gαq. J. Biol. Chem. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 42.Milligan G., Feng G. J., Ward R. J., Sartania N., Ramsay D., McLean A. J., Carrillo J. J. G protein-coupled receptor fusion proteins in drug discovery. Curr. Pharm. Des. 2004;10:1989–2001. doi: 10.2174/1381612043384295. [DOI] [PubMed] [Google Scholar]
- 43.Bahia D. S., Sartania N., Ward R. J., Cavalli A., Jones T. L., Druey K. M., Milligan G. Concerted stimulation and deactivation of pertussis toxin-sensitive G proteins by chimeric G protein-coupled receptor-regulator of G protein signaling 4 fusion proteins: analysis of the contribution of palmitoylated cysteine residues to the GAP activity of RGS4. J. Neurochem. 2003;85:1289–1298. doi: 10.1046/j.1471-4159.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 44.Martini L., Hastrup H., Holst B., Fraile-Ramos A., Marsh M., Schwartz T. W. NK1 receptor fused to β-arrestin displays a single-component, high-affinity molecular phenotype. Mol. Pharmacol. 2002;62:30–37. doi: 10.1124/mol.62.1.30. [DOI] [PubMed] [Google Scholar]
- 45.Guzzi F., Zanchetta D., Cassoni P., Guzzi V., Francolini M., Parenti M., Chini B. Localization of the human oxytocin receptor in caveolin-1 enriched domains turns the receptor-mediated inhibition of cell growth into a proliferative response. Oncogene. 2002;21:1658–1667. doi: 10.1038/sj.onc.1205219. [DOI] [PubMed] [Google Scholar]