Abstract
Cryptococcus neoformans is an important human opportunistic pathogen and a facultative intracellular parasite, particularly in HIV-infected individuals. Little is known about metal ion transport in this organism. C. neoformans encodes a single member of the Nramp (natural resistance-associated macrophage protein) family of bivalent cation transporters, known as Cramp, which we have cloned and expressed in Xenopus laevis oocytes and Spodoptera frugiperda Sf 21 insect cells. Cramp induces saturable transport of a broad range of bivalent transition series cations, including Mn2+, Fe2+, Co2+ and Ni2+. Maximal cation transport occurs at pH 5.5–6.0, consistent with the proton gradient-based energetics of other Nramp orthologues. Mn2+ transport is diminished in the presence of 140 mM Na+, compatible with a Na+ slippage mechanism proposed for the Saccharomyces cerevisiae Nramp orthologue Smf1p. Cramp resembles Smf1p with respect to predicted membrane topology, substrate specificity and pH dependence, but differs in terms of its apparent affinity for Mn2+ and negligible inhibition by Zn2+. Cramp is the first Nramp orthologue from a fungal pathogen to be functionally characterized. Insights afforded by these findings will allow the formulation of new hypotheses regarding the role of metal ions in the pathophysiology of cryptococcosis.
Keywords: bivalent cation, Cryptococcus neoformans, insect cell, intracellular parasite, manganese, Nramp
Abbreviations: Nramp, natural resistance-associated macrophage protein; TMD, transmembrane domain
INTRODUCTION
Cryptococcus neoformans causes one of the most important opportunistic infections in immunocompromised individuals, particularly those infected with HIV or immunosuppressed by cancers or drugs [1]. Although it can also disseminate widely to different organs [2], this fungal pathogen kills mainly by causing a meningoencephalitis which is difficult to treat. As with tuberculosis, the AIDS pandemic has resulted in an explosive increase in the incidence of cryptococcal infections [3,4], which now account for between 13% and 44% of all deaths in AIDS patients in sub-Saharan Africa [5–7].
C. neoformans is a facultative intracellular pathogen that is well adapted to grow and multiply or to remain latent in macrophages [8]. The acidic pH of the cryptococcal phagolysosome favours fungal growth [9,10]. Other factors of importance in the intracellular survival of C. neoformans remain to be defined. Metal ion homoeostasis is critical to the survival of other intracellular pathogens and is linked to the function of a ubiquitous family of bivalent cation transporters, the Nramp (natural resistance-associated macrophage protein) family, that are expressed in pathogenic organisms, such as Mycobacterium tuberculosis [11], M. leprae [12] and Salmonella spp. [13], as well as in host cells [14–16].
Nramp orthologues in both prokaryotes and mammals mediate the transport of transition metals, driven by proton gradients [11,13,17,18]. However, fungal Nramp orthologues have only been functionally characterized in Saccharomyces [19,20], and nothing is known of their activity in fungal pathogens such as C. neoformans.
We hypothesized that an Nramp orthologue encoded by C. neoformans, known as Cramp, functions as a proton-dependent transition-metal transporter. To test this hypothesis, we have functionally characterized Cramp by heterologous expression in Xenopus laevis oocytes and Spodoptera frugiperda Sf 21 cells, and defined its substrate preferences and proton dependence. These studies provide novel insights into the complex metal-dependent functions of multiple systems in an important intracellular pathogen.
EXPERIMENTAL
Culture of C. neoformans and extraction of total RNA
The acapsular, mutant strain CAP67 of C. neoformans (isogenic with serotype D strain B3501; a gift from Dr E. Jacobson, Medical College of Virginia, Richmond, VA, U.S.A.) was recovered from 15% (v/v) glycerol stocks stored at −80 °C. Fungi were maintained on Sabouraud dextrose agar at 30 °C, and harvested after 4 days of growth. Total fungal RNA was isolated using the FAST RNA Red Kit (Bio 101, Vista, CA, U.S.A.) and a Hybaid Ribolyser (Hybaid) [21]. Reverse transcription–PCR was carried out using Superscript™ II RNase H− reverse transcriptase (Life Technologies Inc.).
Determination of the cDNA sequence of Cramp
A TBLASTN search of the C. neoformans JEC21 genome database (http://www-sequence.stanford.edu/group/C.neoformans) using the Saccharomyces cerevisiae Smf1p sequence as the query identified a region encoding several conserved Nramp motifs. Reverse transcription–PCR was carried out on total C. neoformans CAP67 RNA using a reverse primer, C2 (5′-CGTGAGGCATGACAGTAGC-3′), and a series of forward primers located progressively further upstream, with 35 cycles of 30 s at 94 °C, 30 s at 59 °C and 2 min at 72 °C. Sequencing of the resulting amplicons permitted identification of the most upstream in-frame ATG codon before the occurrence of an in-frame stop codon within contiguous sequence. This was taken to be the probable translational start site. The translational stop signal was identified in similar fashion using a forward primer, C1 (5′-GTGTGGCTTATATCGATCCAGG-3′), and reverse primers progressively more downstream from C2 until the first in-frame stop codon (TAA) was encountered.
Cloning of expression constructs
The full-length Cramp sequence was amplified by reverse transcription–PCR from oligo(dT)-primed first-strand C. neoformans cDNA, using specific primers C17 (5′-GAAGATCTGCCACCATGAACAGGAATTGCAGT-3′; forward), containing a BglII site (underlined), and C18 (5′-GCTCTAGATTATCCGTTCCCAAGACA-3′) or C19 (5′-GCTCTAGATCACAGGTCTTCTTCAGAAATAAGCTTTTGTTCTCCGTTCCCAAGACATAG-3′) (reverse), containing XbaI sites (underlined) and, in C19, sequence encoding the Myc epitope [22] (also underlined), to permit generation of a c-Myc-tagged version of the protein.
Two Xenopus oocyte expression constructs (pLZC18#2 and pLZC19#12) were generated by cloning the two versions of the full-length Cramp open reading frames (native and c-Myc-tagged) into the BglII and XbaI sites of pLZ-5, a derivative of pSK-II (Promega Corp.), incorporating the 5′- and 3′-untranslated regions of the X. laevis β-globin gene flanking the polycloning site. For expression studies in Sf 21 cells, the open reading frames encoding the native and c-Myc fusion constructs were released from pLZC18#2 and pLZC19#12 by digestion with BglII and KpnI, and subcloned into pBacPAK8 (BD Biosciences Clontech), to yield pBacPAK8-Cn#1 and pBacPAK8Cc#2 respectively. The full-length coding sequences of all constructs were sequence-verified.
Expression and immunolocalization of Cramp in X. laevis oocytes
X. laevis oocytes were injected with Cramp cRNA (15 ng in 50 nl of water) or a corresponding volume of RNase-free water as described previously [11]. Immunolocalization and Fe2+ uptake assays were performed after 72–96 h of incubation in Barth's solution at 19 °C [23]. Oocytes embedded in OCT mounting medium (Tissue Tek) on aluminium foil were snap-frozen in liquid nitrogen-cooled 2-methylbutane (Sigma Chemical Co.). Sections (10 μm thickness) were cut on a cryostat, fixed in acetone (−20 °C, 20 min) and blocked (1 h) in PBS containing 4% (w/v) BSA. After incubation (25 °C) with mouse monoclonal anti-Myc antibody (1:100 in PBS/0.4% BSA; Invitrogen Life Technologies), the sections were washed three times (PBS/0.4% BSA) and incubated (1 h) with FITC-conjugated goat anti-mouse IgG (1:100 in PBS/0.4% BSA; Jackson ImmunoResearch Laboratories Inc.), washed three times in PBS and mounted for fluorescence microscopy.
Fe2+ uptake studies in X. laevis oocytes
Fe2+ uptake assays were performed in batches of 10–15 oocytes after a 48–96 h incubation in Barth's solution. Oocytes were then incubated for 90–150 min (25 °C) in standard uptake medium (100 mM choline chloride, 2 mM KCl, 0.5 mM CaCl2 · 6H2O, 0.5 mM MgCl2, 0.5 mM ascorbic acid, 8 mM glucose, 10 mM Hepes, 10 mM Mes) [24] containing 55Fe2+ (5 μM in 30 μM total Fe2+; Nycomed Amersham plc), and Fe2+ uptake was quantified by scintillation counting (Wallac Microbeta Plus) as described previously [11]. Uptake of 55Fe2+ was linear in this time frame (results not shown).
Sf 21 cell transfection and recombinant virus clone isolation
Spodoptera frugiperda Sf 21 cells (BD Biosciences, Clontech) were co-transfected with linearized baculovirus DNA (BD Biosciences, Clontech) together with pBacPAK8-Cc#2, according to the BacPAK™ Baculovirus expression system user manual (BD Biosciences, Clontech) [25]. Recombinant virus clones were isolated from plaques, subjected to two rounds of amplification and titred by plaque assay. Expression of Myc-tagged Cramp was assessed by immunocytochemical staining of recombinant-virus-infected cells grown on cover slips, using mouse anti-Myc antibody (Invitrogen Corp.) and FITC-conjugated AffiniPure goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.). All subsequent experiments were carried out using a single, highly expressing recombinant virus clone (C#1).
Transport assays in Sf 21 insect cells
Adherent monolayers of Sf 21 cells in 24-well tissue culture plates (4×105 cells/well in 300–400 μl of complete medium) were infected at a multiplicity of infection of 3 with recombinant virus clone C#1 and incubated for 48 h (27 °C) prior to each transport experiment. Cell monolayers infected with wild-type baculovirus served as negative controls. Transport assay methodology was modified from that of Miyasaka et al. [26]. Adherent cells were washed twice in 0.5 ml of transport buffer (140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, 10 mM Hepes, 2 mM Mes) at pH 6.0 and incubated (19–25 °C) for 5–40 min in pH-adjusted transport buffer containing a mixture of the required bivalent cation radioisotope [132 nM 54MnCl2 (Perkin Elmer Life Sciences Inc.) or 100 nM 57CoCl2 (ICN) or 330 nM 63NiCl2 (Perkin Elmer Life Sciences Inc.)] and the corresponding unlabelled cation to give a total concentration of between of 100 nM and 100 μM as required. After incubation, cells were washed three times in ice-cold transport buffer and lysed in 0.5 ml of lysis solution (0.2 M NaOH, 0.5% SDS). Metal ion uptake was quantified by scintillation counting (LS-6500; Beckman-Coulter) and normalized to units of pmol of metal ion uptake/h per 106 cells.
Inhibition studies were carried out by including appropriate concentrations of bivalent cation inhibitors as chloride salts, diluted from freshly prepared stocks. Where Fe2+ was use as a substrate or inhibitor, a 50-fold molar excess of ascorbic acid was included to minimize oxidation to Fe(III).
Statistical analyses
Pair-wise comparisons between groups were performed using Student's t tests, or Mann–Whitney U tests where data were not normally distributed. Means of multiple groups were compared using one-way ANOVA with the Bonferroni correction. Other data were modelled using non-linear regression methods.
RESULTS
Cramp sequence analysis
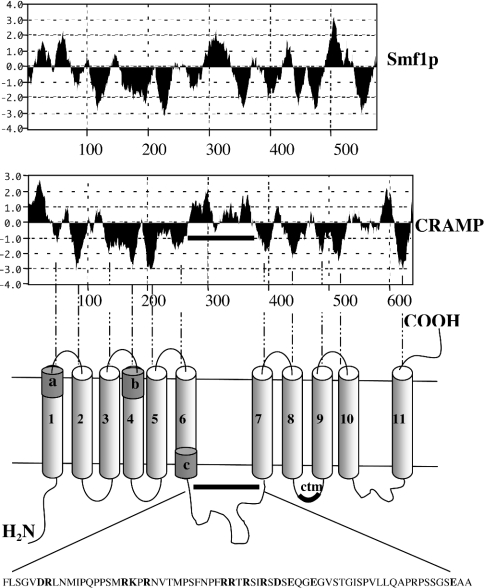
The complete genomic sequence of C. neoformans B3501 reveals that Cramp is the sole Nramp orthologue in this organism, in contrast with the genome of S. cerevisiae, which encodes three paralogous proteins. Amplification of Cramp from cDNA indicates that Cramp is transcribed in free-living C. neoformans. The Cramp gene incorporates nine introns and encodes a 637-amino-acid protein with a calculated molecular mass of 69.4 kDa. Two topology algorithms, TMPRED (http://www.ch.embnet.org/software/TMPRED_form.html) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM/), predicted the existence of 11 putative TMDs (transmembrane domains), consistent with deductions based on Kyte–Doolittle hydrophilicity plots [27] and with the experimentally determined topology of the Escherichia coli MntH protein [28]. This membrane topology is similar to that of the three S. cerevisiae orthologues (Smf1p, Smf2p and Smf3p), with the exception of a much longer hydrophilic region between TMD6 and TMD7 that contains a large number of charged residues (Figure 1). The Cramp sequence is quite divergent with respect to other Nramp proteins, but appears to be most closely related to other yeast orthologues, with amino acid identities of 29%, 34% and 30% with S. cerevisiae Smfp1, Smfp2 and Smfp3 respectively, and of 32–35% with orthologues in other fungi. These low degrees of similarity suggest that it is unlikely that lateral transfer of an ancestral Cramp gene has occurred recently. Comparison with Salmonella enterica serovar Typhimurium MntH and human Nramp2 reveals comparable degrees of identity (both 24%). In spite of these low overall degrees of similarity, other regions are well conserved, including the DPGN (Asp-Pro-Gly-Asn) and MPH (Met-Pro-His) motifs, the functionally critical glycine in TMD4 and the ‘consensus transport motif’ region [29] in the hydrophilic loop between TMDs 8 and 9 (Figure 1).
Figure 1. Predicted membrane topology of Cramp, mapped to the hydrophilicity plot.
The predicted membrane topology is based on the TMPRED and TMHMM algorithms, and the Kyte–Doolittle method (with window size of 15 amino acids) was used for hydrophilicity plots. The hydrophilicity plot for S. cerevisiae Smf1p is included at the top for comparison. The horizontal bar indicates the extended hydrophilic loop between TMDs 6 and 7 present in Cramp but absent from Smf1p (and, indeed, from most other Nramp orthologues). Charged residues are highlighted in bold. The locations of selected conserved motifs are indicated as follows: a, DPGN motif; b, functionally important glycine residue in other orthologues; c, MPH motif; ctm, consensus transport motif.
Cramp localizes to the plasma membrane in X. laevis oocytes and induces pH-dependent Fe2+ uptake
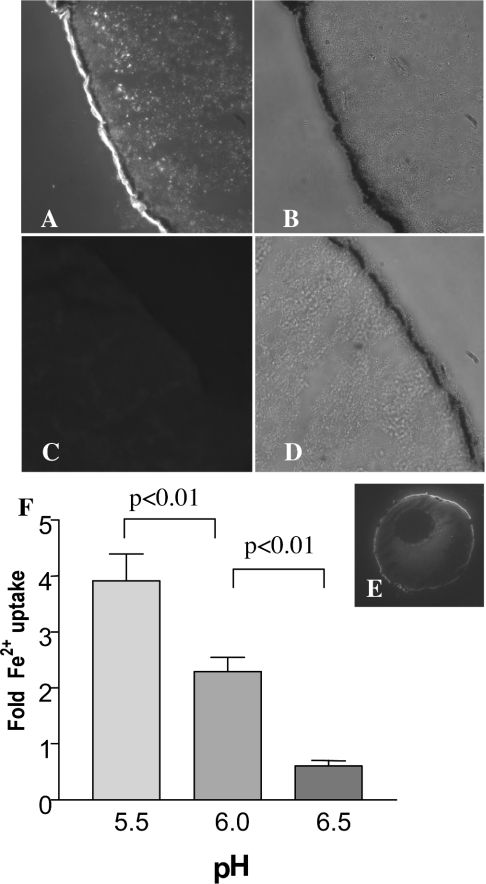
We assessed the expression of Cramp by means of immunocytochemical staining of Myc-tagged Cramp, using an anti-Myc antibody. The data shown in Figures 2(A)–2(E) confirm expression and localization of Cramp–Myc to the oocyte plasma membrane.
Figure 2. Immunolocalization of Cramp–Myc in Xenopus laevis oocyte frozen sections.
Oocytes were injected with Cramp cRNA (A, B) or an equivalent volume of RNase-free water (C, D). (A) Localization of Cramp–Myc to a rim immediately above the pigment-granule layer, probably corresponding to the plasma membrane, as well as to a population of intracytoplasmic vesicles. (E) Lower-magnification view of the same oocyte; (B, D) the same sections under white light illumination. Each image was collected under identical exposure conditions. (F) Cramp-mediated Fe2+ uptake by Xenopus oocytes as a function of pH. Columns show the fold uptake of Fe2+ in Cramp-injected oocytes compared with water-injected controls (12 oocytes per set). Error bars show S.E.M.
In medium containing 55Fe2+, we observed pH-sensitive stimulation of 55Fe2+ uptake in Cramp-expressing oocytes compared with water-injected controls over the pH range 5.5–6.5 (Figure 2F). We also observed low, but reproducible, stimulation of 54Mn2+ uptake by Cramp-expressing oocytes (approx. 2-fold compared with water-injected controls; results not shown). We observed comparable effects for both native and Myc-tagged Cramp (results not shown), indicating that the Myc tag did not alter function in this assay. Accordingly, we utilized the Myc-tagged construct in this and subsequent experiments. Next we used the insect cell expression system to obtain higher levels of functional expression in order to characterize Cramp function.
Expression of Cramp in Spodoptera frugiperda Sf 21 insect cells
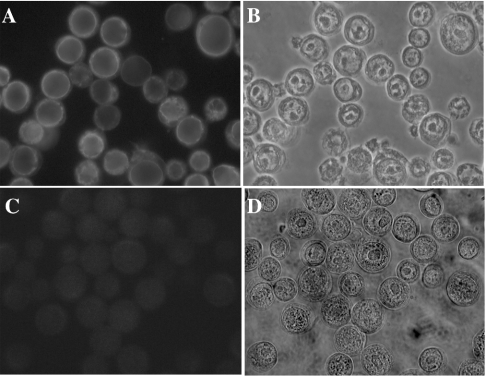
We achieved high-level expression of Cramp by co-transfecting Spodoptera frugiperda Sf 21 insect cells with modified baculovirus and a transfer vector construct containing the Cramp open reading frame. Immunocytochemical staining using an anti-Myc antibody revealed conspicuous staining for Cramp in a predominantly perinuclear location, as well as a faint peripheral rim, the latter being consistent with plasma membrane localization (Figure 3). A single, highly expressing baculovirus clone was selected and expanded for subsequent transport assays.
Figure 3. Expression of Cramp–Myc in Spodoptera frugiperda Sf 21 cells.
(A, B) Sf 21 cells infected with recombinant baculovirus encoding Cramp–Myc, immunostained with an anti-Myc antibody (FITC channel and light-field images respectively). (C, D) Control cells infected with wild-type baculovirus. Not all cells are expressing Cramp, as only approx. 70% of cells in the monolayer were infected by recombinant baculovirus.
Cramp-induced Mn2+ transport in Sf 21 cells
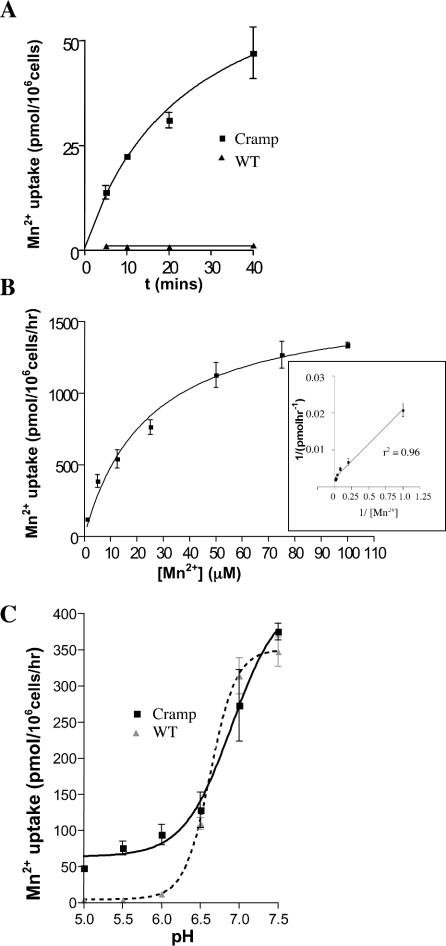
We measured 54Mn2+ accumulation by Sf 21 cells expressing Cramp in comparison with control cells transfected with non-recombinant baculovirus. At an extracellular pH of 5.5, Cramp induced significant accumulation of 54Mn2+ (up to 38-fold compared with controls), which was approximately linear for incubations of up to around 15 min. Control cells exhibited no time-dependent accumulation of Mn2+ over this period (Figure 4A). An incubation time of 15 min was used for subsequent transport assays. Mn2+ transport exhibited saturation kinetics (Figure 4B), with a Km of 24±4 μM and a Vmax of 1700±102 pmol/h.
Figure 4. Transport kinetics and effect of pH on Cramp-mediated Mn2+ accumulation by insect cells.
(A) Mn2+ accumulation at pH 5.5 by Cramp-transfected Sf 21 cells compared with control cells (WT) infected with non-recombinant baculovirus (total [Mn2+] 1 μM). Mn2+ uptake is expressed in units of pmol/h per 106 cells. Cramp induced up to 38-fold increases in Mn2+ uptake compared with controls. Cramp-mediated Mn2+ uptake is approximately linear over the time frame 0–15 min. (B) Mn2+ accumulation by Cramp-expressing Sf 21 cells (pH 5.5) as a function of total Mn2+ concentration. The inset shows a Lineweaver–Burk plot of these data. A Km of 24±4 μM and a Vmax of 1700±102 pmol/h were estimated by non-linear regression. (C) pH profiles for Mn2+ transport by Cramp-expressing and control (WT) Sf 21 cells. Essentially all Mn2+ transport between pH 5.0 and 6.0 is mediated by Cramp. At pH 6.5 and above, endogenous Mn2+ transport is apparent in control cells. Superimposition of the curves for Cramp-transfected and control cells shows that Cramp does not contribute any significant additional Mn2+ uptake at pH 6.5 and above, indicating that Cramp-mediated Mn2+ uptake is abrogated at pH values in excess of 6.5. Maximal Cramp activity was seen at pH 5.5–6.0. Mn2+ transport at pH 5.0 was significantly lower than at pH 5.5/6.0 (P=0.05). Error bars show S.E.M.
To measure the pH dependence of Cramp-induced Mn2+ uptake, we first undertook transport assays with Cramp-expressing cells incubated in transport medium buffered at pH values in the range 5.0–7.5. An abrupt accentuation of Mn2+ uptake was observed at pH values above 6.5. However, control cells exhibited the same substantial Mn2+ uptake above this pH, with negligible uptake below pH 6.5, consistent with uptake by an endogenous transporter (with properties different from those of Cramp) above pH 6.5 (Figure 4C). We could not block this activity with sodium orthovanadate (50–100 μM) or excess Pb2+ (500 μM), Zn2+ (500 μM), or Ca2+ (10 mM) (results not shown). We therefore obtained an indirect measure of Mn2+ transport attributable to Cramp by subtracting uptakes obtained with control cells from those of Cramp-expressing cells at corresponding pH values. Maximal Cramp-induced Mn2+ uptake was seen at pH 5.5–6.0, with minimal activity at values above this, consistent with pH–activity profiles of other Nramp orthologues.
Na+ and Cl− dependence of Cramp-induced Mn2+ transport in Sf 21 cells
Because bivalent cation transport by several other Nramp orthologues is modulated by the presence of Na+ and Cl− ions [19,20,30], we measured Mn2+ transport at pH 5.5 in the presence and absence of 140 mM Na+ or Cl− (substituting 140 mM choline chloride or sodium gluconate respectively for NaCl in the uptake medium). We observed a small (∼1.3-fold) but statistically significant (P<0.0001) increase in Mn2+ uptake in the absence of Na+, but no influence of Cl− (P=0.27) (results not shown).
Transport of other bivalent cations
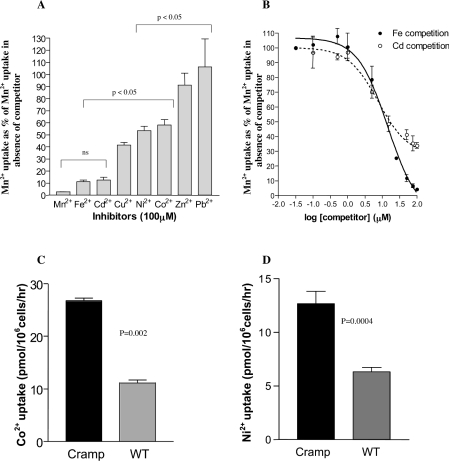
To test whether Cramp transports other bivalent cations, we screened a range of heavy metal cations for their ability to compete with Cramp-mediated Mn2+ transport when present in 100-fold molar excess (Figure 5A) at pH 5.5. Fe2+ and Cd2+ were the strongest competitors, inhibiting Mn2+ transport by approx. 90%. Cu2+ inhibited transport by approx. 60%, while Ni2+, Co2+, Zn2+ and Pb2+ competed less strongly (in that order). Because the most pronounced competition was seen with Fe2+ and Cd2+, we derived inhibition constants for these cations (Figure 5B), from which it was possible to calculate Ki values of 14.0±1 μM for Fe2+ and 7.5±1.2 μM for Cd2+, comparable with the Km for Mn2+. We also demonstrated direct transport of 57Co2+ and 63Ni2+ (Figures 5C and 5D), although fold increases in uptake compared with control cells were significantly lower than those for Mn2+, consistent with their relatively weak inhibition of Mn2+ uptake. It was not possible to measure Cramp-dependent Fe2+ transport in Sf 21 cells, as significant endogenous Fe2+ uptake was seen at both pH 5.5 and pH 7.5 (more marked at the latter pH), masking any contribution from Cramp (results not shown).
Figure 5. Substrate specificity of Cramp.
(A) Effects of potential bivalent cation competitors on Cramp-mediated Mn2+ transport in Sf 21 cells. Cramp-transfected cells were incubated for 15 min at pH 5.5 in the presence of 1 μM total Mn2+ and a 100-fold excess (100 μM) of the indicated non-radioactive bivalent cation chloride salts. Results are expressed as a percentage of the uptake seen with 1 μM Mn2+ in the absence of competitor. There were no significant differences in uptake between competitor conditions within each bracketed group. (B) Inhibition curves for Fe2+ and Cd2+. Mn2+ transport at pH 5.5 (1 μM total Mn2+) was measured at a series of Fe2+ and Cd2+ concentrations and expressed as a percentage of the Mn2+ transport observed in the absence of the competitor. Negligible endogenous Mn2+ transport occurs at pH 5.5 in Sf 21 cells. (C, D) Cramp-mediated Co2+ and Ni2+ transport. Transport was measured at pH 5.5 in the presence of 1 μM total Co2+ or Ni2+. WT, control cells infected with non-recombinant baculovirus.
DISCUSSION
The Nramp orthologue of C. neoformans functionally resembles orthologues in non-pathogenic yeast (Smf1p and Smf2p), since they all transport similar cations in a proton-dependent fashion. Direct evidence for Fe2+ transport in Xenopus oocytes and of Mn2+, Ni2+ and Co2+ transport in Sf 21 insect cells, with maximal activities at pH 5.5–6.0, are features of all yeast pumps studied [19,20,31]. Some of these transport characteristics are also shown by orthologues of Nramp encoded by bacterial (including mycobacterial) pathogens, as well as mammalian equivalents. The pH range for optimal transport by Cramp is plausibly encountered by some classes of intracellular pathogens (including cryptococci) adapted to an intra-phagosomal existence within macrophages [10,32].
The kinetic characterization of Mn2+ transport suggests that this may be a physiologically important substrate, not only because of its higher affinity for Cramp compared with other bivalent cations, but also because of the importance of Mn2+ in many crucial enzymic processes of cryptococci. As with other orthologues, competition studies also suggest that the transport of other metals (Ni2+, Co2+, Cu2+ and Cd2+) is mediated by Cramp, and that any of these transport properties may become important in the varying microenvironment encountered by a pathogen capable both of free-living and of intracellular development.
The study of Cramp in an alternative heterologous system (Xenopus oocytes) was invaluable in confirming specificity for uptake of Fe2+, because this property is obscured by the high endogenous Fe2+ uptakes manifested by insect cells. Nonetheless, kinetic characterization of Fe2+ in Xenopus was precluded by relatively low levels of functional expression and/or a low signal-to-noise ratio for Fe2+ transport (approx. 4-fold activity above control values at pH 5.5; see Figure 2). This difference in signal-to-noise ratios in the measurement of Fe2+ and Mn2+ transport highlights the probable abundance of Fe2+ transporters in biological systems, contrasting with the relative paucity of Mn2+ transporters. In both expression systems, expression of the transporter was confirmed by indirect immunofluorescence assays (Figures 2 and 3).
Despite sharing many common functional and structural features with other orthologues, Cramp is also distinguishable in several ways. For example, Zn2+ does not compete for Mn2+ uptake by Cramp. In contrast, although it is not transported by yeast Smf1p or by mammalian Nramp1 or Nramp2, Zn2+ competitively inhibits Mn2+ uptake with good affinity when these other eukaryotic orthologues are expressed in oocytes [20]. In bacteria, Zn2+ is a weak inhibitor of Salmonella MntH [13] and is actually transported by M. tuberculosis Mramp, when expressed in oocytes [11]. This type of comparative analysis will help in identifying the determinants of cation specificity and already points to mutagenesis studies for experimental verification.
Smf1p when expressed in Xenopus oocytes exhibits decreased Mn2+ transport when univalent cations (Na+, Li+, Rb+) are present in the extracellular medium [19,20]. The presence of an associated inwardly directed Na+ current, which is more pronounced at higher pH values, suggests that ‘slippage’ of these univalent cations may occur through the mechanism mediating proton symport with the primary (transition metal) substrate. This type of mechanism may protect an organism from transition-metal toxicity when environmental concentrations of bivalent cations are excessive. To determine whether the presence of Na+ affected Mn2+ transport by Cramp, we observed the effect on Mn2+ transport of substituting 140 mM choline chloride for NaCl in the transport medium. Although the detailed kinetics of the effect were not measured and the experiments were carried out at pH 5.5 (due to endogenous transport at higher pH values), a small but highly reproducible inhibition of Mn2+ transport by Na+ was observed. This is consistent with observations for yeast Smf1p. It has been reported that bivalent cation transport by Nramp2 is influenced by the presence of Cl− [30]. We examined the influence of this anion on Mn2+ transport at pH 5.5, but observed no significant effect of eliminating Cl− from the transport medium.
The fact that Cramp can transport a given cation does not, of course, imply that this process is physiologically relevant. Cramp appears to be able to transport Fe2+, Co2+, Ni2+, Cu2+ and Mn2+. However, the estimated Ki for Fe2+ is ∼14 μM, a value far in excess of the submicromolar concentrations of free Fe2+ likely to be encountered in the extracellular or cytoplasmic environments in a mammalian host. Similar considerations apply to the concentrations of Co2+, Ni2+ and Cu2+ at which any significant transport by Cramp is likely. However, the concentrations of these cations in the macrophage phagolysosome or cryptococcal vacuole are unknown, and it is conceivable that Cramp may be adapted to carry out the physiologically significant transport of one or more of these metal ions in these micro-environments. Conversely, although the apparent Km for Mn2+ of 24 μM is also high in comparison with those of other Nramp orthologues such as yeast Smf1p (∼1.9 μM) [20] or bacterial MntH (0.1 μM) [13], intracytoplasmic concentrations of Mn2+ in this range are quite plausible. Lactobacillus plantarum, for example, maintains intracellular Mn2+ concentrations as high as 35 mM [33], and most cells can tolerate much higher concentrations of free Mn2+ than of free Fe2+ [34]. In common with several other Mn2+ transporters (e.g. MntH and SitABCD in Salmonella typhimurium [13,35]), Cramp may transport Cd2+ with an affinity similar to that for Mn2+. However, this property may reflect a fortuitous similarity in electronic structure and ionic radius, as the symmetrical 4d10 configuration of Cd2+ has thermodynamic stability comparable with that of Mn2+.
Little is known about metal ion homoeostasis in Cryptococcus. There is clearly a requirement for trace metals. Iron is required for oxidoreductase enzymes (such as those involved in the electron transport chain) in almost all organisms. Copper is a component of laccase, an established cryptococcal virulence determinant involved in melanin biosynthesis [36], with a possible role in the scavenging of toxic hydroxyl radicals [37]. It is also a catalytic component of Cu/Zn-superoxide dismutase, another contributor to resistance to oxidative stress. Manganese is a catalytic component of a further class of superoxide dismutases, and is also a cofactor in a variety of metabolic enzyme reactions. In S. cerevisiae, mitochondrial Mn-superoxide dismutase acquires its cofactor through a delivery system involving the Nramp Smf2p, underlining the potential significance of Nramp orthologues in intracellular manganese trafficking [38].
We and others have previously proposed a model in which competition between pathogen and host for limiting concentrations of essential bivalent cations in the phagosomal micro-environment might be important in relation to intracellular survival [17,39,40]. In Nramp1(+) and Nramp1(−) congenic murine macrophages infected with C. neoformans or Candida albicans, a functional Nramp1 protein was associated with greater fungicidal activity at early time points (∼6 h) for unopsonized fungi, but no differences in phagocytic capacity were apparent [41,42]. Nramp1(+) cells infected with cryptococci exhibited greater lipopolysaccaride-induced secretion of tumour necrosis factor α and greater enhancement of anti-cryptococcal activity in response to interferon-γ and chloroquine than Nramp1(−) cells.
A competition model involving Cramp would be consistent with a plasma membrane location for this transporter, whereby the direction of the pH gradient would be expected to stimulate the Cramp-mediated uptake of metal ions into the fungal cell. The vacuolar membrane of Cryptococcus is another possible location for Cramp, where it might play a role in the mobilization of vacuolar metal ion stores, by analogy with the proposed role of Smf3p in S. cerevisiae [31]. However, it is difficult to extrapolate from localization studies in heterologous expression systems to firm conclusions about which membrane system(s) Cramp might occupy in Cryptococcus. It will therefore be important to carry out localization studies of native Cramp in C. neoformans to test the plausibility of these hypotheses. Phenotypic characterization of a Cramp knockout mutant both in relation to intracellular survival (under Mn2+-limiting and -replete conditions) and with respect to virulence in appropriate animal models will help to define further the roles of Cramp and Mn2+ in the physiology of this important opportunistic pathogen. The functional insights afforded by the present study now permit the formulation of specific experimental designs to address these issues.
Acknowledgments
D. A. is supported by a Wellcome Trust Advanced Clinical Fellowship (grant reference no. GR063634MA). This research was also supported by a National Institutes of Health grant GM61748 to M. M. We are indebted to Dr Michael Romero (Department of Physiology and Biophysics, Case Western Reserve University) for valuable discussions and advice on growth of insect cells.
References
- 1.Perfect J. R., Casadevall A. Cryptococcosis. Infect. Dis. Clin. North Am. 2002;16:837–874. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 2.Perfect J. R. Cryptococcosis. Infect. Dis. Clin. North Am. 1989;3:77–102. [PubMed] [Google Scholar]
- 3.Archibald L. K., McDonald L. C., Rheanpumikankit S., Tansuphaswadikul S., Chaovanich A., Eampokalap B., Banerjee S. N., Reller L. B., Jarvis W. R. Fever and human immunodeficiency virus infection as sentinels for emerging mycobacterial and fungal bloodstream infections in hospitalized patients ≥15 years old, Bangkok. J. Infect. Dis. 1999;180:87–92. doi: 10.1086/314836. [DOI] [PubMed] [Google Scholar]
- 4.Hakim J. G., Gangaidzo I. T., Heyderman R. S., Mielke J., Mushangi E., Taziwa A., Robertson V. J., Musvaire P., Mason P. R. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–1407. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 5.French N., Gray K., Watrea C., Nakiyingi J., Lugada E., Moore M., Lalloo D., Whitworth J. A., Gilks C. F. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Okongo M., Morgan D., Mayanja B., Ross A., Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 natural history cohort in Uganda. Int. J. Epidemiol. 1998;27:698–702. doi: 10.1093/ije/27.4.698. [DOI] [PubMed] [Google Scholar]
- 7.Corbett E. L., Churchyard G., Charalambos S., Samb B., Moloi V., Clayton T. C., Grant A. D., Murray J., Hayes R. J., De Cock K. M. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin. Infect. Dis. 2002;34:1251–1258. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 8.Goldman D. L., Lee S. C., Mednick A. J., Montella L., Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 2000;68:832–838. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmesser M., Kress Y., Novikoff P., Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitz S. M., Nong S. H., Seetoo K. F., Harrison T. S., Speizer R. A., Simons E. R. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 1999;67:885–890. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agranoff D., Monahan I. M., Mangan J. A., Butcher P. D., Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J. Exp. Med. 1999;190:717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeve I., Hummel D., Nelson N., Voss J., Hummell D. Overexpression, purification, and site-directed spin labeling of the Nramp metal transporter from Mycobacterium leprae. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8608–8613. doi: 10.1073/pnas.142287699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehres D. G., Zaharik M. L., Finlay B. B., Maguire M. E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 14.Cellier M., Shustik C., Dalton W., Rich E., Hu J., Malo D., Schurr E., Gros P. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukocyte Biol. 1997;61:96–105. doi: 10.1002/jlb.61.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Hackam D. J., Rotstein O. D., Zhang W., Gruenheid S., Gros P., Grinstein S. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 1998;188:351–354. doi: 10.1084/jem.188.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenheid S., Pinner E., Desjardins M., Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 18.Jabado N., Jankowski A., Dougaparsad S., Picard V., Grinstein S., Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X. Z., Peng J. B., Cohen A., Nelson H., Nelson N., Hediger M. A. Yeast SMF1 mediates H(+)-coupled iron uptake with concomitant uncoupled cation currents. J. Biol. Chem. 1999;274:35089–35094. doi: 10.1074/jbc.274.49.35089. [DOI] [PubMed] [Google Scholar]
- 20.Sacher A., Cohen A., Nelson N. Properties of the mammalian and yeast metal-ion transporters DCT1 and Smf1p expressed in Xenopus laevis oocytes. J. Exp. Biol. 2001;204:1053–1061. doi: 10.1242/jeb.204.6.1053. [DOI] [PubMed] [Google Scholar]
- 21.Casadevall A., Perfect J. R. Cryptococcus neoformans, chapter 12. Washington, DC: ASM Press; 1998. Diagnosis and laboratory techniques; pp. 381–405. [Google Scholar]
- 22.Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colman A. Translation of eukaryotic messenger RNA in Xenopus oocytes. In: Hames B. D., Higgins S. J., editors. Transcription and Translation – A Practical Approach, chapter 2. Oxford: IRL Press; 1984. pp. 271–302. [Google Scholar]
- 24.Gutierrez J. A., Yu J., Rivera S., Wessling-Resnick M. Functional expression, cloning and characterization of SFT, a stimulator of Fe transport. J. Cell Biol. 1997;139:895–905. doi: 10.1083/jcb.139.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitts P. A., Possee R. D. A method for producing recombinant baculovirus expression vectors at high frequency. BioTechniques. 1993;14:810–817. [PubMed] [Google Scholar]
- 26.Miyasaka T., Kaminogawab S., Shimizub M., Hisatsunea T., Reinachc P. S., Miyamoto Y. Characterization of human taurine transporter expressed in insect cells using a recombinant baculovirus. Protein Expression Purif. 2001;23:389–397. doi: 10.1006/prep.2001.1505. [DOI] [PubMed] [Google Scholar]
- 27.Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 28.Courville P., Chaloupka R., Veyrier F., Cellier M. F. Determination of the transmembrane topology of Escherichia coli Nramp ortholog. J. Biol. Chem. 2004;279:3318–3326. doi: 10.1074/jbc.M309913200. [DOI] [PubMed] [Google Scholar]
- 29.Cellier M., Prive G., Belouchi A., Kwan T., Rodrigues V., Chia W., Gros P. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10089–10093. doi: 10.1073/pnas.92.22.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999;18:4361–4371. doi: 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portnoy M. E., Liu X. F., Culotta V. C. Saccharomyces cerevisiae expresses three functionally distinct homologues of the nramp family of metal transporters. Mol. Cell. Biol. 2000;20:7893–7902. doi: 10.1128/mcb.20.21.7893-7902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinai A. P., Joiner K. A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu. Rev. Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 33.Archibald F. Manganese: its acquisition by and function in the lactic acid bacteria. Crit. Rev. Microbiol. 1986;13:63–109. doi: 10.3109/10408418609108735. [DOI] [PubMed] [Google Scholar]
- 34.Kehres D. G., Maguire M. E. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 35.Kehres D. G., Janakiraman A., Slauch J. M., Maguire M. E. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002;184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas S. D., Bennett J. E., Kwon-Chung K. J., Perfect J. R., Williamson P. R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L., Tewari R. P., Williamson P. R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 1999;67:6034–6039. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luk E. E., Culotta V. C. Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J. Biol. Chem. 2001;276:47556–47562. doi: 10.1074/jbc.M108923200. [DOI] [PubMed] [Google Scholar]
- 39.Agranoff D., Krishna S. Metal ion homeostasis and intracellular parasitism. Mol. Microbiol. 1998;28:403–412. doi: 10.1046/j.1365-2958.1998.00790.x. [DOI] [PubMed] [Google Scholar]
- 40.Supek F., Supekova L., Nelson H., Nelson N. Function of metal-ion homeostasis in the cell division cycle, mitochondrial protein processing, sensitivity to mycobacterial infection and brain function. J. Exp. Biol. 1997;200:321–330. doi: 10.1242/jeb.200.2.321. [DOI] [PubMed] [Google Scholar]
- 41.Blasi E., Colombari B., Mucci A., Cossarizza A., Radzioch D., Boelaert J. R., Neglia R. Nramp1 gene affects selective early steps in macrophage-mediated anti-cryptococcal defense. Med. Microbiol. Immunol. (Berlin) 2001;189:209–216. doi: 10.1007/s004300100066. [DOI] [PubMed] [Google Scholar]
- 42.Puliti M., Radzioch D., Mazzolla R., Barluzzi R., Bistoni F., Blasi E. Influence of the Bcg locus on macrophage response to the dimorphic fungus Candida albicans. Infect. Immun. 1995;63:4170–4173. doi: 10.1128/iai.63.10.4170-4173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]