Abstract
The predominant (>90%) low-molecular-mass polyphenol was isolated from the leaves of the resurrection plant Myrothamnus flabellifolius and identified to be 3,4,5 tri-O-galloylquinic acid using 1H and 13C one- and two-dimensional NMR spectroscopy. The structure was confirmed by mass spectrometric analysis. This compound was present at high concentrations, 44% (by weight) in hydrated leaves and 74% (by weight) in dehydrated leaves. Electron microscopy of leaf material fixed with glutaraldehyde and caffeine demonstrated that the polyphenols were localized in large vacuoles in both hydrated and dehydrated leaves. 3,4,5 Tri-O-galloylquinic acid was shown to stabilize an artificial membrane system, liposomes, against desiccation if the polyphenol concentration was between 1 and 2 μg/μg phospholipid. The phase transition of these liposomes observed at 46 °C was markedly diminished by the presence of 3,4,5 tri-O-galloylquinic acid, suggesting that the presence of the polyphenol maintained the membranes in the liquid crystalline phase at physiological temperatures. 3,4,5 Tri-O-galloylquinic acid was also shown to protect linoleic acid against free radical-induced oxidation.
Keywords: desiccation; liposome; MS; Myrothamnus flabellifolius; NMR spectroscopy; 3,4,5 tri-O-galloylquinic acid
Abbreviations: AAPH, 2,2′-azobis(2-amidino-propane) dihydrochloride; ESI, electrospray ionization; HMBC, heteronuclear mutliple bond correlation; HSQC, heteronuclear single-quantum coherence; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; RT, reverse transcriptase; RWC, relative water content; TFA, trifluoroacetic acid
INTRODUCTION
Certain species of plants have evolved the remarkable ability to survive regular periods of dehydration to an air-dry state [1]. These plants are collectively called resurrection plants and Southern Africa harbours a rich diversity of them [1]. Myrothamnus flabellifolius is one of the largest of these plants [2] and occurs in a vast area stretching from Namibia in the west, through Botswana, to Zimbabwe and the northern parts of South Africa in the east [3]. The plant grows between rocky outcrops with very shallow soils [4] and for approximately half of the year it exists in a dehydrated quiescent state [5]. It has been proposed that the leaves possess a high tannin content [6]. This correlates with the difficulty in performing molecular biological manipulations with nucleic acid extracts of this plant [7] as well as with reports that it is an important Southern African medicinal plant displaying anti-asthmatic properties [8].
No detailed chemical analysis has been performed to elucidate the chemical structure(s) of the main polyphenol(s) present. In the present study, we report on the structure of the main polyphenol present in hydrated and dehydrated leaves of M. flabellifolius. In addition, we present data suggesting that this polyphenol protects the membranes against desiccation and also against free radical-induced damage.
METHODS
Plant material and dehydration/rehydration treatments
M. flabellifolius plants were collected near Outjo (Namibia) and from Vaalwater (Limpopo Province, South Africa). Whole plants from the latter location were transported to Cape Town and maintained in a glasshouse as described previously [9]. These were dehydrated by withholding water for approx. 2 weeks until the plants had reached an air-dry state, with approx. 12.5% RWC (relative water content). Plants were maintained in the dry state for at least 2 weeks before rehydrating by soil watering. Leaves were sampled at various stages during dehydration and rehydration for treatments outlined below. Water content was determined gravimetrically by oven drying at 70 °C for 48 h and RWC was calculated as described previously [10].
Polyphenol purification
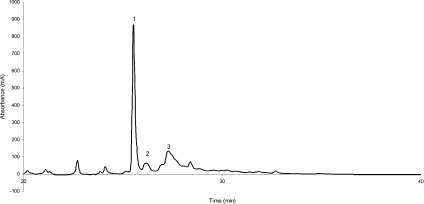
Leaves (5 g) from dehydrated plants were ground to a fine powder and extracted using the serial reflux extraction at 60 °C with 100 ml of heptane (twice) followed by 70% methanol (twice). The latter extracts were pooled to yield approx. 90% of the total extractable UV absorbing material in this fraction. Preliminary preparative separation of the main polyphenols from M. flabellifolius was performed using isocratic cellulose column chromatography using 5% acetic acid as the eluant. Selected column fractions were further purified using low-pressure liquid chromatography in 0.1% TFA (trifluoroacetic acid) using Waters RP-C18 resin. Fractions were eluted using a linear gradient between 0.1% TFA and 100% acetonitrile and 0.1% TFA for 40 min at a flow rate of 0.7 ml/min. Final purification was by preparative HPLC using a Higgins Analytical RP-C18 column and using the same buffer system but at a flow rate of 2.5 ml/min. Elution (Figure 1) yielded approx. 2 mg of an amorphous white powder (peak 1) after freeze-drying.
Figure 1. Elution profile of polyphenols eluted from the C18 HPLC column using a 0–100% gradient of acetonitrile in 0.1% TFA.
Peaks 1–3 were pooled and freeze-dried before analysis. The absorbance is shown in milli-absorbance units (mA).
Analytical techniques
ESI (electrospray ionization) mass spectra were obtained using a Fisons VG Quattro mass spectrometer equipped with a triple quadropole analyser. Sample aliquots in 50% acetonitrile were injected directly into the spectrometer, which was operated in negative-ion mode using a capillary voltage of −2.7 kV and a cone voltage of 40 V. In-flight fragmentation was achieved using argon gas collision-induced fragmentation in the quadropole analyser. A collision energy of 13 eV and an argon collision gas pressure of 2×10−3 bar were used.
MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) mass spectra were obtained using a Perseptive Biosystems DE-PRO MALDI mass spectrometer equipped with a TOF analyser. Sample aliquots (1 μl) in 50% acetonitrile were mixed with 1 μl of 2,5 dihydroxybenzoic acid matrix and applied to the gold sample plate. The spectrometer was operated in positive- and negative-ion modes.
NMR spectra were acquired in 2H2O and recorded at 300 K. 1H-NMR spectra were obtained at 400 MHz using a Varian Unity 400 instrument. 13C and two-dimensional NMR spectra were obtained at 300 MHz using a Varian Mercury 300 instrument. The HSQC (heteronuclear single-quantum coherence) spectroscopy experiment was optimized for J=140 Hz. The HMBC (heteronuclear mutliple bond correlation) spectroscopy experiment was optimized for coupling constants of 6, 8 and 12 Hz. Spectra were processed using standard Varian software.
Lipid studies
Liposomes were prepared from phosphatidylcholine and cholesterol (10:1) encapsulating the fluorescent dye calcein as described previously [11]. Differential scanning calorimetry was performed using a PerkinElmer DSC7 calorimeter. Samples (approx. 10 mg) were analysed between 25 and 60 °C at a scanning rate of 5 °C/min as described previously by other authors [12]. Linoleic acid oxidation was performed using AAPH [2,2′-azobis(2-amidino-propane) dihydrochloride] as a free radical source. Oxidation was determined by an increase in absorption at 234 nm, which monitors the production of conjugated diene hydroperoxides (ε=2.8×104) [13].
Transmission electron microscopy
Leaves from five fully hydrated (100% RWC) and five dry (5% RWC) plants were cut into 4 mm2 segments and were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) supplemented with 0.5% caffeine. Dehydration was performed using a graded ethanol series and specimens were infiltrated and then embedded in epoxy resin [14]. Sections were prepared using a Reichert Ultracut-S microtome after which they were stained with uranyl acetate and lead citrate [15]. Sections were viewed and photographed with a Zeiss EM 109 transmission electron microscope.
Quantitative and compositional analysis
Dehydrated and hydrated leaves were dissected longitudinally with a razor blade. One half was used to determine leaf RWC, whereas the other half was freeze-dried to a stable dry weight and then analysed for polyphenol content. The leaf material was homogenized in methanol/water (7:3) using a Polytron Homogeniser (Kinematica PT 2000) for 5 min after which the homogenate was sonicated (Bandelin Sonorex bath sonicator) for 5 min before being centrifuged (13100 g for 5 min). The pellets were re-extracted twice, the supernatants were pooled and the absorbance A280 was determined. Aliquots (20 μl) were also analysed by HPLC. Selected fractions were collected, dried and analysed by MALDI–TOF-MS. Mild acid hydrolysis was performed using 2 M HCl at 80 °C for 30 min.
RESULTS
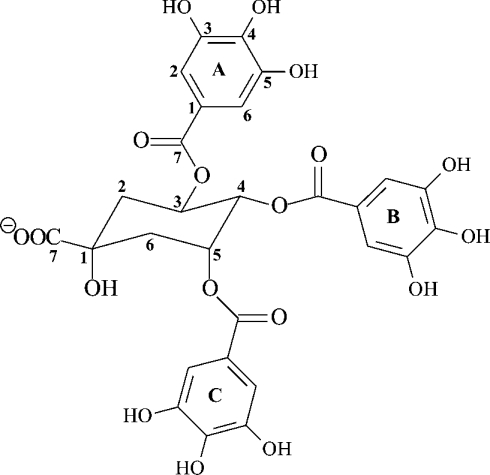
The purified polyphenol compound was assigned to be 3,4,5 tri-O-galloylquinic acid (Figure 2) from the 1H and 13C one- and two-dimensional NMR spectra. The 1H-NMR spectrum displayed three singlets in the aromatic region, characteristic of the ortho protons of galloyl groups; the remaining proton signals were attributed to the methine and methylene protons of the quinic acid moiety (Table 1). The assignment of the quinic acid spin system, H-2–H-6, was followed from the connectivities revealed by the COSY spectrum (Table 1). Although the upfield methylene proton signals at 2.48 and 2.26 ppm may be due to the H-2's or H-6’s, these protons could be assigned unambiguously to H-2ax and H-2eq respectively from chemical shifts and coupling data reported in the literature [16]. Analysis of the coupling patterns of the protons and the determination of coupling constants for the well-resolved H-4 resonance established the relative stereochemistry of the methine protons in the quinic acid ring. Specifically, the large coupling between H-3 and H-4 (3JH4-H3=9.2 Hz) confirm their diaxial orientation, whereas the smaller coupling between H-4 and H-5 (3JH4-H5=3.2 Hz) was characteristic of axial–equatorial splitting [16]. Once proton assignments had been made, identification of the attached carbons followed directly from the HSQC experiment (Table 1). These ring carbon assignments were confirmed by the long-range couplings observed in the HMBC experiments (Table 1). H-2, H-5 and H-6 showed cross-peaks to the quaternary carbon signal at 74.59 ppm, which was assigned to C-1 of the quinic acid ring. The carbon signal at 177.96 ppm, not connected to any of the spin systems through the HMBC experiments, was ascribed to the carboxyl (C-7) of quinic acid in comparison with literature values [16,17].
Figure 2. Structure of 3,4,5 tri-O-galloylquinic acid isolated from M. flabellifolius.
The galloyl rings were annotated A–C to enable proton and carbon atoms to be assigned from the NMR spectrum.
Table 1. 1H- and 13C-NMR spectral data with two-dimensional NMR correlations of 3,4,5 tri-O-galloylquinic acid.
| Carbon number | 13C-NMR (δ, ppm) | Attached proton number | 1H-NMR (δ, ppm; J, Hz) | COSY (1H) | HSQC | HMBC |
|---|---|---|---|---|---|---|
| 1 | 74.59 | |||||
| 2 | 35.32 | 2ax | 2.48 dd | 2ax,eq, 3 | C-2 | C-1, C-3, C-4 |
| 2eq | 2.26 dd | 2ax,eq, 3 | C-2 | C-1, C-3, C-4 | ||
| 3 | 68.84 | 3 | 5.84 m | 2ax,eq, 4 | C-3 | C-4, C=OA |
| 4 | 73.24 | 4 | 5.41 dd | 3, 5 | C-4 | C-5, C=OB |
| (3.2, 9.2) | ||||||
| 5 | 70.03 | 5 | 5.75 m | 4, 6ax,eq | C-5 | C-1, C-5, C=OC |
| 6 | 37.22 | 6ax,eq | 2.36 m | 5, 6ax, 6eq | C-6 | C-1, C-4, C-5 |
| 7 | 177.96 | |||||
| 1A | 120.70 | |||||
| 2A | 110.44 | 2A | 7.12 s | C-2A | C-1A, C-2A, C-3A, C-4A, C-7A | |
| 3A | 144.86 | |||||
| 4A | 138.69 | |||||
| 5A | 144.86 | |||||
| 6A | 110.44 | 6A | 7.12 s | C-6A | C-1A, C-4A, C-5A, C-6A, C-7A | |
| 7A | 167.49 | |||||
| 1B | 119.88 | |||||
| 2B | 110.17 | 2B | 6.93 s | C-2B | C-1B, C-2B, C-3B, C-4B, C-7B | |
| 3B | 144.65 | |||||
| 4B | 138.69 | |||||
| 5B | 144.65 | |||||
| 6B | 110.17 | 6B | 6.93 s | C-6B | C-1B, C-4B, C-5B, C-6B, C-7B | |
| 7B | 167.01 | |||||
| 1C | 120.26 | |||||
| 2C | 110.17 | 2C | 6.77 s | C-2C | C-1C, C-2C, C-3C, C-4C, C-7C | |
| 3C | 144.65 | |||||
| 4C | 138.69 | |||||
| 5C | 144.69 | |||||
| 6C | 110.17 | 6C | 6.77 s | C-6C | C-1C, C-4C, C-5C, C-6C, C-7C | |
| 7C | 167.49 |
The HMBC experiments also contained long-range couplings that linked the quinic acid and galloyl group spin systems. The cross-peaks were between the methine protons (H-3–H-5) of quinic acid and the resonances at 167.49, 167.01 and 167.49 ppm, which correspond to the carbonyl groups of the galloyl groups designated A, B and C respectively (Figure 1, Table 1). Identification of the galloyl rings followed from the long-range couplings that connect the carbonyl groups and their corresponding H-2 and H-6 resonances of the three galloyl groups. Full assignment of the remaining signals of the galloyl A, B and C groups followed from the H-2 and H-6 assignments and use of the HSQC experiment to identify the attached carbons and the HMBC experiments to assign the remainder of the ring carbons (Table 1).
To confirm the identity of the purified polyphenol as 3,4,5 tri-O-galloylquinic acid, the purified polyphenol was subjected to mass spectral analysis using MALDI–TOF-MS to determine the mass of the parent compound. ESI–MS was also performed to show that the fragmentation pattern was consistent with the proposed structure (Figure 2), which would have a molecular mass of 648 Da (all values ±1 Da). The MALDI–TOF positive ion mass spectrum displayed peaks at m/z values of 671, 688 and 694 corresponding to the sodium [M+Na−]+, potassium [M+K−]+ and di-sodium [M−H+2Na−]+ adducts respectively. The MALDI–TOF negative ion spectrum displayed a peak at m/z 647, which was considered to represent the deprotonated parent ion [M–H]−. The ESI–MS spectra of 3,4,5 tri-O-galloylquinic acid gave two peaks [M−2H]2− and [M–H]− at m/z values of 323 and 647 respectively. The most abundant species was the doubly charged m/z 323 ion indicating the tendency of the molecule to readily acquire a second charge during ionization. Collision-induced dissociation fragmentation of this ion resulted in two fragment ions at m/z 247 and 169. Further fragmentation of the m/z 169 ion produced a prominent m/z 125 ion, which was interpreted to cleavage of one of the C-1–C-7 galloyl carbon–carbon bonds releasing a charged pyrogallol ring. The m/z 169 ion was considered to have resulted from cleavage of the C-3, C-4 or C-5 carbon–oxygen bonds releasing charged gallic acid ions (Figure 2). The m/z 247 ion was deduced to represent the doubly charged parent molecule with one of its galloyl groups cleaved at the labile ester between C-7 of a galloyl group and the C-3, C-4 or C-5 oxygen atom of the quinic acid moiety (Figure 2). The presence of these ions supported the prediction of a carboxylic acid group in the structure previously assigned from the signal at 177.96 ppm to the C-7 carboxyl carbon atom. Since the MS data were consistent with the structure assigned from the NMR spectra, we concluded that the proposed structure was indeed 3,4,5 tri-O-galloylquinic acid. Galloylquinic acid derivatives have been reported to be present in other plants. The ethyl ester of 3,4,5 tri-O-galloylquinic acid together with other quinic acid gallates have been reported to be present in Guiera senegalensis [18], tetragalloylquinic acid has been reported to be present in Galphimia glauca [19] and gallotannins have been reported to be present in Lepidoptrys staudtii [20]. These compounds were determined to be galloylquinic acids from their 1H and 13C one-dimensional NMR spectra and the fast atom bombardment mass spectra. However, no complete structure was assigned as two-dimensional NMR spectral analyses were not performed.
The presence of additional polyphenols but in significantly lower abundance were observed during the isolation of 3,4,5 tri-O-galloylquinic acid from the leaves of M. flabellifolius (Figure 1). To investigate these polyphenols, total polyphenol extracts were subjected to HPLC analysis and selected fractions were analysed by MALDI–TOF-MS. This showed that the polyphenols eluted from the HPLC column in the order of increasing molecular mass with masses at m/z 647, 786, 799, 936, 951, 1103, 1255, 1407, 1570 and 1722 (all ±1 Da) being observed (Table 2). Since the cleavage mass of the galloyl ion is m/z 152, this series at approx. 152 Da intervals suggested multiple galloylation of 3,4,5 tri-O-galloylquinic acid with the ions at m/z 647, 799, 951, 1103, 1255 and 1407 deduced to represent the tri- to octa-galloylquinic acids respectively, although the peaks with masses of 1103, 1255 and 1407 Da were present in low abundance. The latter two masses of 1570 and 1722 Da were interpreted to be ellagic acid tannins derived from hexa- and hepta-galloylquinic acids respectively brought about by the oxidative addition of gallic acid moieties [16]. This was confirmed by mild acid hydrolysis of the total polyphenol extract. This resulted in gallic acid together with 3,4,5 tri-O-galloylquinic acid as the major hydrolysis products (results not shown). The depside ester bonds of the gallic acid polyester chains have been reported [17] to be more labile to hydrolysis than the quinic–gallic ester bonds and thus the core molecule would remain largely intact after mild acid hydrolysis. We therefore consider that the chief polyphenol found in M. flabellifolius is 3,4,5 tri-O-galloylquinic acid and that, in addition, a number of higher molecular mass gallic acid polyesters are present, but at markedly lower concentrations. These are due to multiple galloylation of the central 3,4,5 tri-O-galloylquinic acid core.
Table 2. Mass spectral analysis and provisional assignment of polyphenols isolated from M. flabellifolius leaves and purified as described.
Rt is the retention time in minutes from the C18 HPLC column. Only ions present in high abundance have been included (see text for further details). Note that fraction 3 contains a mixture of galloylquinic acids.
| Fraction number | Rt | Galloyl moieties | Quinic acid moieties | Mass | Molecular ion |
|---|---|---|---|---|---|
| 1 | 25.8 | 3 | 1 | 647 | [M−H]− |
| 2 | 26 | 4 | 1 | 799 | [M−H]− |
| 3 | 27 | 4 | 1 | 799 | [M−H]− |
| 5 | 1 | 936 | [M−OH]− | ||
| 5 | 1 | 953 | [M−H]− | ||
| 9 | 1 | 1570 | [M−H]−* | ||
| 10 | 1 | 1722 | [M−H]−* |
* Masses of m/z 1570 and 1722 have been deduced to be oxidative gallic acid condensation products derived from hexa- and hepta-galloylquinic acids and contain one ellagic acid moiety each.
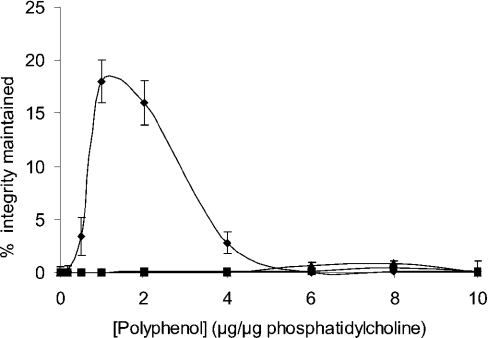
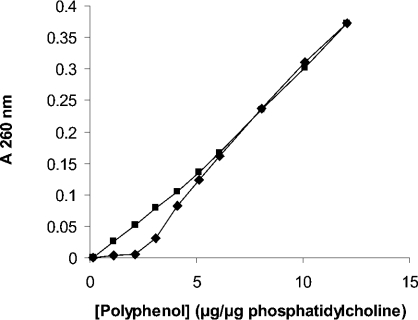
Quantitative analysis of total leaf polyphenols was undertaken to determine whether a difference in polyphenol content existed between fully hydrated (96.4±2.0% RWC) and dehydrated (12.71±5.47% RWC) leaves (Table 3). Hydrated leaves were found to contain 44.3±11.9% (g/g dry wt−1) of 3,4,5 tri-O-galloylquinic acid. This increased to 73.6±9.6% for desiccated leaves suggesting a possible role for these compounds in protection against desiccation stress in M. flabellifolius. Since polyphenols have been shown to protect membranes against desiccation [21,22], we investigated whether 3,4,5 tri-O-galloylquinic acid would protect a model membrane system against desiccation. Various quantities of this compound were therefore added to liposomes made from phosphatidylcholine and cholesterol encapsulating the fluorescent dye calcein, before the liposomes were subjected to desiccation and subsequent rehydration. We found (Figure 3) that the addition of 3,4,5 tri-O-galloylquinic acid indeed protected liposomes against desiccation with a maximum protection observed at a polyphenol/phospholipid ratio of 1 (w/w). Lowering or increasing the polyphenol/phospholipid ratio resulted in decreased protection being observed. In contrast, the addition of equivalent concentrations of gallic acid to the liposome preparation resulted in no protection being observed (Figure 3). Similar results demonstrating maximum protection at low concentrations of the polyphenol with no protection being observed at higher concentrations were reported for arbutin (4-hydroxyphenyl-β-glucopyranoside) binding to liposomes containing monogalactosyldiacylglycerol [22]. It has been reported [23] that arbutin is present in significant quantities in M. flabellifolius, although we were not able to detect any trace of this compound. The addition of arbutin to our liposome preparation resulted in no protection being observed (Figure 3). This result agrees with previous work that demonstrated that the addition of arbutin to liposome preparations only resulted in protection against desiccation when the chloroplast lipid monogalactosyldiacylglycerol was present [22]. We next investigated why maximum protection of liposomes by 3,4,5 tri-O-galloylquinic acid was observed at a polyphenol/phospholipid ratio of 1 (w/w), but that increasing the ratio above 1 resulted in decreased or no protection being observed. This was found not to be due to liposome disruption, since the addition of 3,4,5 tri-O-galloylquinic acid up to a ratio of 6 did not result in calcein leakage (results not shown). The interaction between 3,4,5 tri-O-galloylquinic acid and liposomes was next investigated by the addition of increasing quantities of 3,4,5 tri-O-galloylquinic acid to the liposome preparation, after which the liposomes were pelleted by centrifugation and the 3,4,5 tri-O-galloylquinic acid concentration in the supernatant fraction was determined spectrophotometrically. Buffer without liposomes was used as a control. We found (Figure 4) that the addition of 3,4,5 tri-O-galloylquinic acid to the liposome preparation at low ratios, namely 1 and 2, resulted in no polyphenol being present in the supernatant fraction, suggesting that it was all bound to the liposomes. Increasing the ratio to 3 resulted in some polyphenol to be present in the supernatant fraction, suggesting that the saturation of the liposome preparation had occurred. A further increase to a ratio of 4 resulted in a significant increase in the concentration of 3,4,5 tri-O-galloylquinic acid in the supernatant with the concentration tending towards that present in solution in the absence of liposomes. A further increase to a ratio of 5 resulted in the supernatant concentration being indistinguish-able from that in the absence of liposomes. Interestingly, these results match the data on the protection of liposomes against desiccation by 3,4,5 tri-O-galloylquinic acid (Figure 3). The binding of 3,4,5 tri-O-galloylquinic acid to liposomes was analysed by extrapolation of the data at low (0–2) 3,4,5 tri-O-galloylquinic acid/phosphatidylcholine ratios and comparing this to the data obtained in the absence of liposomes. Assuming that the initial interaction between 3,4,5 tri-O-galloylquinic acid and phosphatidylcholine occurred in the ratio of 2:1, this analysis suggested that 3,4,5 tri-O-galloylquinic acid bound to liposomes with a dissociation constant of approx. 10−8 M.
Table 3. RWC and polyphenol content (g TGAE/g dry wt) of the hydrated and dehydrated leaves of M. flabellifolius from South African and Namibian locations.
TGAE, tri-O-galloylquinic acid equivalents. Polyphenol content is calculated as g TGAE [λ 70% methanol max nm (log ε): 275 (4.3)]/g leaf dry wt. Mean and S.D. values for RWC and polyphenol contents represent n=10 for two independent experiments for South African leaves and n=7 for two independent experiments for Namibian leaves.
| Location | Hydrated RWC* | Polyphenol content | Dehydrated RWC* | Polyphenol content |
|---|---|---|---|---|
| South Africa | 86.03±11.74 | 0.393±0.167 | 9.78±2.34 | 0.569±0.182 |
| Namibia | 86.44±9.70 | 0.443±0.119 | 12.71±5.47 | 0.736±0.096 |
* RWC is expressed as percentage g water/g water at full turgor.
Figure 3. Liposome structural integrity after desiccation and subsequent rehydration determined by calcein leakage as a function of polyphenol addition.
◆, 3,4,5 Tri-O-galloylquinic acid; ■, arbutin; ▲, gallic acid.
Figure 4. Concentration-dependent binding of 3,4,5 tri-O-galloylquinic acid (polyphenol) to liposomes.
3,4,5 Tri-O-galloylquinic acid was added to the liposome preparation. After centrifugation, the concentration in the supernatant was determined spectrophotometrically at 260 nm (■). This was compared with the absorption of an identical concentration of 3,4,5 tri-O-galloylquinic acid in solution (◆).
A possible explanation of these data is that 3,4,5 tri-O-galloylquinic acid binds exclusively to liposomes at low polyphenol/phospholipid ratios, but as the ratio increases, the liposome ‘sites’ become saturated and 3,4,5 tri-O-galloylquinic acid appears in solution. Since 3,4,5 tri-O-galloylquinic acid is amphipathic, micelle formation occurs and the binding of 3,4,5 tri-O-galloylquinic acid molecules to one another to form a micelle is stronger than the interaction between 3,4,5 tri-O-galloylquinic acid and liposomal lipids resulting in the removal of the 3,4,5 tri-O-galloylquinic acid from the liposome preparation. Circumstantial evidence in favour of this model is that an aqueous solution of 3,4,5 tri-O-galloylquinic acid frothed when shaken, a behaviour typical of detergents in solution.
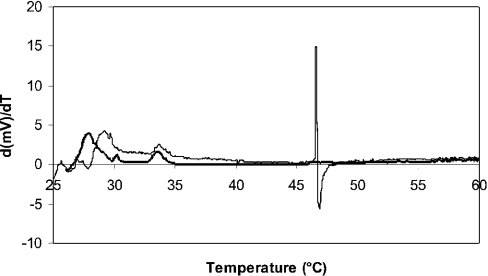
Since 3,4,5 tri-O-galloylquinic acid protected liposomes against desiccation, we next investigated whether the presence of 3,4,5 tri-O-galloylquinic acid affected the temperature of the phase transition between the liquid crystalline and the gel phase. Since differential scanning calorimetry data have been reported to be difficult to interpret for desiccated liposomes [24], only hydrated liposomes were used. The first derivative of the plot of heat flow as a function of temperature for hydrated liposomes (Figure 5) showed an event that occurred at 46 °C. A similar value for liposomes prepared from phosphatidylcholine and cholesterol was reported in a previous study [25]. Addition of 3,4,5 tri-O-galloylquinic acid at a ratio of 1 (w/w) to the liposome preparation resulted in no event being detected between 25 and 60 °C suggesting that 3,4,5 tri-O-galloylquinic acid abolishes the phase transition, an effect observed for liposomes containing high percentages of cholesterol.
Figure 5. Differential scanning calorimetry of a liposome preparation in the absence (thin line) or presence (thick line) of 3,4,5 tri-O-galloylquinic acid.
The first derivative of the milli-watts supplied as a function of time is shown. No transition at 46 °C was observed for liposomes analysed in the presence of 3,4,5 tri-O-galloylquinic acid.
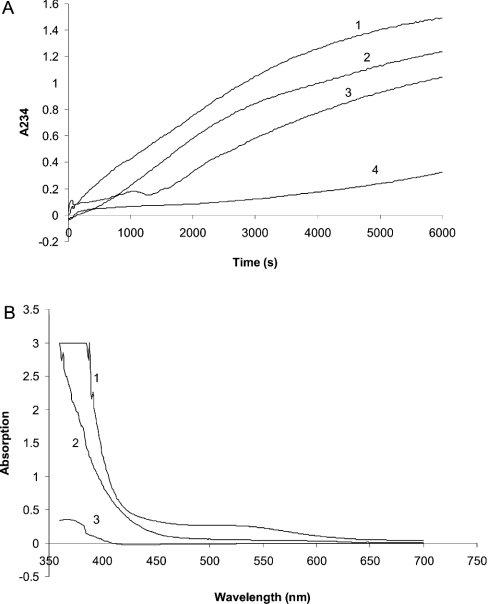
Polyphenols have been proposed to act as antioxidants in plant cells. Our results showing an interaction between 3,4,5 tri-O-galloylquinic acid and liposomes suggested a possible additional role for this compound in protecting membrane lipids against oxidation. Therefore we investigated whether 3,4,5 tri-O-galloylquinic acid would protect linoleic acid (C18:3) against oxidation. The free radical initiator AAPH was therefore added to an aqueous solution of linoleic acid with or without 3,4,5 tri-O-galloylquinic acid, and the oxidation was monitored at 234 nm as a function of time (Figure 6). We found that the addition of increasing concentrations of 3,4,5 tri-O-galloylquinic acid resulted in a decreased rate of linoleic acid oxidation. The addition of 15.4 μM 3,4,5 tri-O-galloylquinic acid, approx. 0.1 × the concentration of the linoleic acid, resulted in a reduction in the rate of linoleic acid oxidation by approx. 95%. Ascorbic acid is a well-known reducing agent in many tissues, and n-propyl gallate is commercially used as an antioxidant. Therefore we compared the inhibition of AAPH-mediated oxidation of linoleic acid brought about by ascorbic acid and n-propyl gallate with that observed for 3,4,5 tri-O-galloylquinic acid. We found that equivalent molar concentrations (13.8 μM) of ascorbic acid and n-propyl gallate were less effective antioxidants when compared with the same molar concentration of 3,4,5 tri-O-galloylquinic acid with the AAPH-mediated oxidation of linoleic inhibited by 58±4 and 40±0.5% respectively compared with 98.3±0.6%. The inhibition mediated by an equivalent molar concentration of gallic acid was also investigated and found to be 29.4±2%. This clearly demonstrated that 3,4,5 tri-O-galloylquinic acid is a far better antioxidant than the other known antioxidants tested and suggested that the redox potential of this compound is markedly more positive than that of linoleic acid. To confirm that the redox state of the 3,4,5 tri-O-galloylquinic acid changed as a result of AAPH-induced oxidation, the spectrum of 3,4,5 tri-O-galloylquinic acid was determined in the presence and absence of AAPH (Figure 6). This showed that the absorbance in the region 450–500 nm was markedly reduced by AAPH, which we interpreted to demonstrate that 3,4,5 tri-O-galloylquinic acid protected linoleic acid against oxidation by itself becoming oxidized.
Figure 6. Prevention of linoleic acid oxidation by 3,4,5 tri-O-galloylquinic acid.
(A) AAPH-induced oxidation of linoleic acid (160 nmol) monitored at 234 nm as a function of time in the absence (1) or in the presence of 3.85 nmol (2), 7.70 nmol (3) and 15.4 nmol (4) 3,4,5 tri-O-galloylquinic acid. (B) Spectra of 3,4,5 tri-O-galloylquinic acid after (1) and before (2) AAPH-induced oxidation of linoleic acid. The spectrum of AAPH is also shown (3).
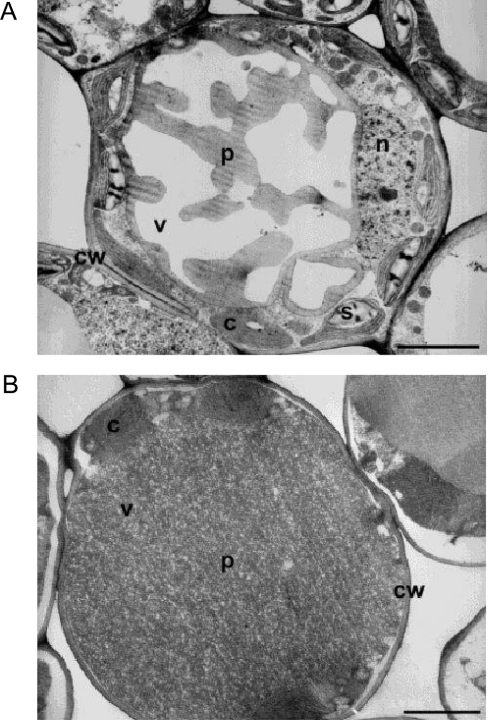
To determine the location of the polyphenols within M. flabellifolius leaves, an ultrastructural investigation was performed using transmission electron microscopy after fixing the tissue with glutaraldehyde and caffeine. Caffeine has been reported to precipitate polyphenols at their cellular location [26,27]. We observed that polyphenol–caffeine complexes occurred within a central vacuole in pallisade and spongy mesophyll cells in both hydrated and dehydrated leaves (Figures 7A and 7B). In hydrated leaves, the polyphenols occupied only a small proportion of the vacuole, whereas in dry leaves they filled the entire vacuolar space. In this capacity they could interact with and stabilize the tonoplast in the desiccated state. The replacement of water in vacuoles with proteins and compatible solutes has been proposed [28,29] as a mechanism for the prevention of mechanical injury [30] during desiccation.
Figure 7. Transmission electron micrographs of a hydrated (A) and a dehydrated (B) leaf mesophyll cell of M. flabellifolius (×3000).
p, Polyphenols; cw, cell wall; v, vacuole; c, chloroplast; s, starch granule; n, nucleus. Scale bar, 3 μm.
DISCUSSION
In the present study, we have identified the main polyphenol constituent of M. flabellifolius leaves to be 3,4,5 tri-O-galloylquinic acid. In addition, smaller quantities of higher molecular mass gallic acid polyesters were also present, the result of multiple galloylation of the central 3,4,5 tri-O-galloylquinic acid core. These compounds are collectively present in very high concentrations; almost half the dry mass of hydrated leaves and approx. three-quarters of the dry mass of desiccated leaves is due to the presence of these compounds.
Electron microscopy demonstrated that the polyphenols are present in the leaf pallisade and spongy mesophyll cell vacuoles in both hydrated and dehydrated leaves. Mechanical stabilization, by replacement of water in vacuoles with compatible solutes during dehydration, has been reported to occur in other resurrection plants [28,29] and it is possible that 3,4,5 tri-O-galloylquinic acid plays a similar role in M. flabellifolius. These compounds absorb UV light with the maximum molar absorption coefficient of 2.5×105 observed at 280 nm (J. P. Moore, G. G. Lindsey and W. F. Brandt, unpublished work). Therefore a role for the absorbing light thereby preventing UV-induced free radical damage may also be inferred.
The results presented here suggest that 3,4,5 tri-O-galloylquinic acid interacts with membranes in two ways. Its one role is to protect membranes against desiccation by presumably intercalating into the lipid bilayer and reducing the Tm of the transition between the liquid crystalline and the gel phases. Such a behaviour, which has been reported to occur for the polyphenol arbutin [31], allows successful desiccation and subsequent rehydration to occur. Whereas arbutin has been shown to have a specific lipid requirement in the target membranes, the liposomes used in this study comprised only egg yolk phosphatidylcholine and cholesterol suggesting a non-specific interaction of 3,4,5 tri-O-galloylquinic acid with membranes. The other suggested role is to protect membranes against free radical-induced damage. This would be an important property since the antioxidant status of M. flabellifolius has been shown to correlate with the duration of viability of the plant in the desiccated state [32]. The results reported in this study agree with that of other authors who have shown gallotannins to be potent antioxidants [27,33]. Our results indicated that using the oxidation of linoleic acid as a model system, 3,4,5 tri-O-galloylquinic acid displays significantly greater antioxidant properties when compared with ascorbic acid and the commercially used n-propyl gallate as well as gallic acid itself.
Since only very low relative concentrations of 3,4,5 tri-O-galloylquinic acid are required for it to act as an antioxidant protecting unsaturated lipids and since the activity observed in protecting liposomes against desiccation only occurred over a limited concentration range, we would postulate that the M. flabellifolius cells must display some mechanism for concentration-dependent release and sequestration of the polyphenol. This might depend on the solubility equilibrium between the soluble and insoluble states, which might be influenced by the metabolic status of the plant affecting parameters such as the intra-vacuolar pH as well as the presence of metal ions. We are currently investigating whether such mechanisms control the effective biological concentration of 3,4,5 tri-O-galloylquinic acid in the cell.
An extract of M. flabellifolius leaves has been reported to be used by indigenous people in Namibia as an aid in wound healing and to treat asthma and general chest ailments [8]. In keeping with this, gallotannins are known for their anti-burn properties [34] and galloylquinic acids have been identified as possessing high activity against bronchial hyper-reactivity and allergic reactions [19,34]. A group of galloylquinic acids has also been shown to display anti-HIV RT (reverse transcriptase) and anti-HIV activity [20,35]. The RT inhibition by galloylquinic acids correlates with the difficulties encountered using Molecular Biology techniques, e.g. RT–PCR, to study this plant [7]. Experiments are currently being performed to investigate this.
Acknowledgments
We express our gratitude to the National Research Foundation and the University of Cape Town Research Fund for financial support. We also thank K. Cooper, M. Jaffer (Electron Microscope Unit) and R. Karreman for their excellent technical assistance.
References
- 1.Gaff D. F. Desiccation tolerant flowering plants in southern Africa. Science. 1971;174:1033–1034. doi: 10.1126/science.174.4013.1033. [DOI] [PubMed] [Google Scholar]
- 2.Child G. F. Brief notes on the ecology of the resurrection plant (Myrothamnus flabellifolius) with mention of its water absorbing abilities. J. S. Afr. Bot. 1960;26:1–8. [Google Scholar]
- 3.Kruger L. A. MSc thesis. Cape Town, South Africa: University of Cape Town; 1998. Towards an understanding of desiccation tolerance in the resurrection plant Myrothamnus flabellifolius Welw. [Google Scholar]
- 4.Schneider H., Thurmer F., Zhu J. J., Wistuba N., Gessner P., Linder K., Herman B., Zimmermann G., Hartung W., Bentrap F.-W., et al. Diurnal changes in xylem pressure of the hydrated resurrection plant Myrothamnus flabellifolius. Evidence for lipid bodies in conducting xylem vessels. New Phytol. 1998;143:471–484. doi: 10.1046/j.1469-8137.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 5.Farrant J. M., Kruger L. A. Longevity of dry Myrothamnus flabellifolius in simulated field conditions. Plant Growth Regul. 2001;35:109–120. [Google Scholar]
- 6.Pizzi A., Cameron F. A. Flavonoid tannins – structural wood components for drought-resistance mechanisms in plants. Wood Sci. Technol. 1986;20:119–124. [Google Scholar]
- 7.Koonjul P. K., Brandt W. F., Farrant J. M., Lindsey G. G. Inclusion of polyvinylpyrrolidone in the polymerase chain reaction reverses the inhibitory effects of polyphenolic contamination of RNA. Nucleic Acids Res. 1999;27:915–916. doi: 10.1093/nar/27.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Wyk B. E., van Oudsthoorn B., Gericke N. Medicinal Plants of Southern Africa. Pretoria: Briza Publications; 1997. [Google Scholar]
- 9.Sherwin H. W., Farrant J. M. Differences in rehydration of three desiccation tolerant angiosperm species. Ann. Bot. 1996;78:703–710. [Google Scholar]
- 10.Cooper K., Farrant J. M. Recovery of the resurrection plant Craterostigma wilmsii from desiccation: protection versus repair. J. Exp. Bot. 2002;53:1805–1813. doi: 10.1093/jxb/erf028. [DOI] [PubMed] [Google Scholar]
- 11.Sales K., Brandt W., Rumbak E., Lindsey G. The LEA-like protein HSP12 in Saccharomyces cerevisiae has a plasma membrane location and protects membranes against desiccation and ethanol-induced stress. Biochim. Biophys. Acta. 2000;1463:267–278. doi: 10.1016/s0005-2736(99)00215-1. [DOI] [PubMed] [Google Scholar]
- 12.Crowe L. M., Crowe J. H., Rudolph A., Womersley C., Appel L. Preservation of freeze-dried liposomes by trehalose. Arch. Biochem. Biophys. 1985;242:240–247. doi: 10.1016/0003-9861(85)90498-9. [DOI] [PubMed] [Google Scholar]
- 13.Liegois C., Lermusieau G., Collin S. Measuring antioxidant efficiency of wort, malt and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J. Agric. Food Chem. 2000;48:1129–1134. doi: 10.1021/jf9911242. [DOI] [PubMed] [Google Scholar]
- 14.Spurr A. R. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds E. S. The use of lead citrate at high pH as an electron opaque stain for electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonaka G. Isolation and structure elucidation of tannins. Pure Appl. Chem. 1989;61:357–360. [Google Scholar]
- 17.Mueller-Harvey I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001;91:3–20. [Google Scholar]
- 18.Bouchet N., Levesque J., Bodo B., Pousset J.-L. 3,4,5-Tri-O-galloylquinic acid ethyl ester from Guiera senegalensi. Pharm. Biol. 1998;36:63–65. [Google Scholar]
- 19.Neszmelyi A., Kreher B., Muller A., Dorsch W., Wagner H. Tetragalloylquinic acid, the major anti-asthmatic principle of Galphimia glauca. Planta Med. 1993;59:164–167. doi: 10.1055/s-2006-959635. [DOI] [PubMed] [Google Scholar]
- 20.Bokesch H. R., McKee T. C., Currens M. J., Gulakowski R. J., McMahon J. B., Cardellina J. H., II, Boyd M. R. HIV-inhibitory gallotannins from Lepidoptrys staudtii. Nat. Prod. Lett. 1996;8:133–136. [Google Scholar]
- 21.Won-Huh N., Porter N. A., McIntosh T. J., Simon S. A. The interaction of polyphenols with bilayers: conditions for increasing bilayer adhesion. Biophys. J. 1996;71:3261–3277. doi: 10.1016/S0006-3495(96)79519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hincha D. K., Oliver A. E., Crowe J. H. Lipid composition determines the effects of arbutin on the stability of membranes. Biophys. J. 1999;77:2024–2034. doi: 10.1016/S0006-3495(99)77043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suau R., Cuevas A., Valpuesta V., Reid M. S. Arbutin and sucrose in the leaves of the resurrection plant Myrothamnus flabellifolius. Phytochemistry. 1991;30:2555–2556. [Google Scholar]
- 24.Chapman D., Dodd G. H. Physicochemical probes of membrane structures. In: Rothfield L. I., editor. Structure and Function of Biological Membranes. New York: Academic Press; 1971. pp. 13–77. [Google Scholar]
- 25.Mabrey S., Mateo P. L., Sturtevant J. M. High sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978;17:2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 26.Hayat M. A. London: Academic Press; 1981. Fixation for Electron Microscopy. [Google Scholar]
- 27.Haslam E. New polyphenols for old tannins. In: Van Sumere C. F., Lea P. J., editors. Annual Proceedings of the Phytochemical Society of Europe, vol. 25. Oxford: Clarendon Press; 1985. pp. 137–196. [Google Scholar]
- 28.Farrant J. M. Comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plants. Plant Ecol. 2000;151:1–11. [Google Scholar]
- 29.Vander Willigen C., Pammenter N. W., Jaffer M. A., Mundree S. G., Farrant J. M. An ultrastructural study using anhydrous fixation of Eragrostis nindensis, a resurrection grass with both desiccation-tolerant and -sensitive tissues. Funct. Plant Biol. 2003;30:281–290. doi: 10.1071/FP02221. [DOI] [PubMed] [Google Scholar]
- 30.Iljin W. S. Drought resistance in plants and physiological processes. Annu. Rev. Plant. Physiol. 1957;3:341–363. [Google Scholar]
- 31.Oliver A. E., Hincha D. K., Crowe L. M., Crowe J. H. Interactions of arbutin with dry and hydrated bilayers. Biochim. Biophys. Acta. 1998;1370:87–97. doi: 10.1016/s0005-2736(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 32.Kranner I., Beckett R. P., Wornik S., Zorn M., Pfeifhofer H. W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002;31:13–24. doi: 10.1046/j.1365-313x.2002.01329.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki H., Sakihama Y., Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onayade O. A., Onayade A. A., Sofowora A. Wound healing with plants: the African perspective. In: Hostettmann K., Chinyanganya F., Maillard M., Wolfender J.-L., editors. Chemistry, Biological and Pharmacological Properties of African Medicinal Plants, vol. 1. Harare: University of Zimbabwe Publications; 1996. pp. 77–120. [Google Scholar]
- 35.Nishizawa M., Yamagashi T., Dutschman G. E., Parker W. B., Bodner A. J., Kilkuskie R. E., Cheng Y.-C., Lee K.-H. Anti-Aids agents, 1. Isolation and characterisation of four new tetragalloylquinic acids as a new class of HIV reverse transcriptase inhibitors from tannic acid. J. Nat. Prod. 1989;52:762–768. doi: 10.1021/np50064a016. [DOI] [PubMed] [Google Scholar]