Abstract
Since 2022, Europe has had 4 cases of extensively drug-resistant Neisseria gonorrhoeae, sequence type 16406, that is resistant to ceftriaxone and highly resistant to azithromycin. We report 2 new cases from France in 2023 involving strains genetically related to the 4 cases from Europe as well as isolates from Cambodia.
Keywords: gonorrhea, antimicrobial resistance, Drug-resistant, Neisseria gonorrhoeae, bacteria, penA-60.001, A2059G mutation, ST16406, sexually transmitted infections, surveillance, Ceftriaxone, Azithromycin, France
Neisseria gonorrhoeae, the causative agent of gonorrhea, remains a persistent global public health concern. N. gonorrhoeae is classified by the World Health Organization as a priority pathogen because of the emergence of multidrug-resistant isolates and rapid increases in antimicrobial drug resistance rates. Because of the frequency of resistance to cefixime, azithromycin, and fluoroquinolones, ceftriaxone is the recommended first-line treatment of N. gonorrhoeae (1). Four cases of extensively drug-resistant (XDR) N. gonorrhoeae, containing the penA-60 allele and the A2059G mutation of 23S rRNA, belonging to sequence type 16406 have been reported in Austria, the United Kingdom, and France since 2022 (2–4). The enhanced gonococcal surveillance program in Cambodia identified 3 similar isolates during 2021–2022 (5). We report 2 new cases of XDR N. gonorrhoeae in France from 2023. Both patients gave consent for the publication of this report.
The Cases
The patient in case 1 is a 40-year-old man living in the Rhône-Alpes region. He sought care in July 2023 for reported urethritis that occurred 15 days after unprotected sexual contact with a person living in Cambodia. The patient did not receive treatment in Cambodia before returning to France, and he had no sexual relations after his return. Laboratory testing of urethral samples was positive for gonococcal DNA and negative for Chlamydia trachomatis. N. gonorrhoeae was identified by using culture and designated as F95. The patient was treated with an intramuscular dose of ceftriaxone (1 g) during the visit. He attended a follow-up consultation with his general practitioner in September, during which control samples from his urethra, anus, and throat were collected. No N. gonorrhoeae DNA was detected from those samples; however, the patient still reported urethritis. Repeat laboratory testing was positive for C. trachomatis, and the patient was treated.
The patient in case 2 is a 60-year-old man living with a partner in the Rhône-Alpes region. The patient sought care for reports of urethritis in August 2023. Symptom onset occurred 1 week after the patient had sexual contact with a sex worker in France. The patient had no documented travel to Asia. Laboratory testing of urine samples by using culture and PCR were positive for N. gonorrhoeae, designated F96, and C. trachomatis. In September, the patient was treated with ceftriaxone (1 g) for the N. gonorrhoeae infection and azithromycin (1 g) for the C. trachomatis infection. At a follow-up consultation a few weeks later, the patient reported no symptoms. Control samples from his urethra, anus, and throat were tested by using PCR and were negative for N. gonorrhoeae and C. trachomatis.
The 2 N. gonorrhoeae isolates, F95 and F96, were sent to the national reference center for sexually transmitted infections in Paris for confirmation and genomic analysis. We conducted phenotypic antimicrobial drug susceptibility testing and found that both isolates were XDR (6). The isolates demonstrated resistance to penicillin G, cefixime, ceftriaxone, ciprofloxacin, azithromycin, tetracycline, and doxycycline; both isolates were susceptible to ertapenem and aminoglycosides (Table). We conducted whole-genome sequencing by using an Illumina MiSeq (Illumina, https://www.illumina.com) system (300 cycles, 2 × 150 bp). We performed bioinformatic analysis as previously described (4). The sequences obtained are available from the European Nucleotide Archive and GenBank (accession no. PRJNA1082518).
Table. MICs and molecular resistance mechanisms of extremely drug-resistant Neisseria gonorrhoeae strains F95 and F96, recovered from 2 patients in France, 2023*.
| Antimicrobial | F95 MIC, mg/L | F96 MIC, mg/L | EUCAST interpretation | Molecular mechanism(s) of resistance |
|---|---|---|---|---|
| Penicillin |
>32 |
>32 |
R |
bla
TEM-135
|
| Cefixime | 1 | 1 | R |
penA 60.001 (mosaic; A311V; T483S); mtrR, A39T; ponA, L421P; porB structure, porB1a |
| Ceftriaxone | 0.25 | 0.25 | R | |
| Cefotaxime |
0.5 |
0.5 |
R |
|
| Ertapenem |
0.032 |
0.016 |
NA |
NA |
| Azithromycin |
>256 |
>256 |
R (high-level) |
23S rRNA, A2059G; mtrR, A39T |
| Tetracycline | 32 | 32 | R (high-level) |
tetM; rpsJ, V57M; mtrR, A39T |
| Doxycycline |
16 |
16 |
R |
|
| Ciprofloxacin |
2 |
4 |
R |
gyrA, S91F and D95A; parC, S87R |
| Spectinomycin |
16 |
16 |
S |
NA |
| Gentamicin |
8 |
8 |
NA |
NA |
| Rifampin |
0.125 |
0.064 |
NA |
NA |
| *MIC testing done using ETEST (bioMérieux, https://www.biomerieux.com). EUCAST, European Committee on Antimicrobial Susceptibility testing; NA, not applicable because of a lack of breakpoints for interpretation; R, resistant; S, susceptible. | ||||
We aligned the genomes of F95 and F96 with ceftriaxone-resistant strains previously isolated in France (WHOY [F89], F90, F91, and F92), Japan (WHOX), and the United States (LRRBGS-1327). We also aligned the F95 and F96 genomes with strains highly resistant to azithromycin and resistant to ceftriaxone recently found in the United Kingdom (WHOQ and H22-494), Austria (AT158), France (F93 and F94), Cambodia (22R655558T, 22R655567T, and 22R655494S), and Australia (A2735 and A2543) (2–5,7–12). We used ParSNP v.1.2 (https://github.com/marbl/parsnp) to correct the alignments for recombination. We then constructed a maximum-likelihood phylogeny for core-genome comparisons. We visualized the comparisons by using iTol (https://itol.embl.de), as previously described (4, 7). The whole-genome sequencing analysis revealed that isolates F95 and F96 belonged to sequence type 16406. The F95 and F96 strains were assigned to sequence type 22862 by using N. gonorrhoeae multiantigen sequence typing (porB 822, tbpB 294) and 5793 by using N. gonorrhoeae sequence typing for antimicrobial resistance (profile no. 60.001_89_13_1_7_3_1).
Our molecular analysis of isolates F95 and F96 revealed several mutations associated with antimicrobial resistance (Table). Both isolates contained the mosaic allele penA-60.001, which encodes a penicillin binding protein 2 with amino-acid substitutions (A311V, T4835) that impair the binding of extended-spectrum cephalosporins (ESCs). This impairment renders the isolates resistant to ESCs. F95 and F96 contain the A39T mutation in the MtrR repressor gene, without promoter deletions, which causes an overexpression of the MtrCDE efflux pump and an increase in the MIC of ESCs. The isolates produced TEM-135 penicillinase with a M182T substitution known to increase the stability of the enzyme, which typically proceeds additional mutations that extend the range of resistance to ESCs. In addition, F95 and F96 carried the A2059G mutation in the 4 alleles of the 23S rRNA-encoding gene, conferring high levels of resistance to azithromycin (6). The quinolone resistance-determining regions of F95 and F96 contained S91F and D95A substitutions in GyrA (a subcomponent of DNA gyrase), and an S87R substitution in ParC (a component of topo-isomerase IV). Those substitutions are responsible for high level resistance to ciprofloxacin. Finally, both strains contained the tet(M) gene and a V57M amino-acid substitution in the rpsJ gene (encoding the S10 ribosomal protein), conferring a high level of tetracycline resistance.
The F95 isolate had no single-nucleotide polymorphism relative to the F96 isolate. Both isolates clustered with other XDR N. gonorrhoeae isolates that contained the penA-60.001 allele and the A2059G mutation in 23S rRNA, indicating a common evolutionary origin (Figure). F95 and F96 were closely related to the recently reported azithromycin-resistant and ceftriaxone-resistant 22R655567T XDR isolates from Cambodia, only differing by 113 single-nucleotide polymorphisms. On the phylogenetic tree, the F95 and F96 isolates belonged to the same lineage as the XDR isolates from Cambodia. This lineage is further divided into multiple sublineages: the A2543 XDR clone, which has been reported to be spreading internationally; the sublineage including AT159, H22-494, and F93; the sublineage F94; the sublineage 22R655558T; and the sublineage including F95, F96, 22R655567T, and 22R655494S (Figure) (2–5,10).
Figure.
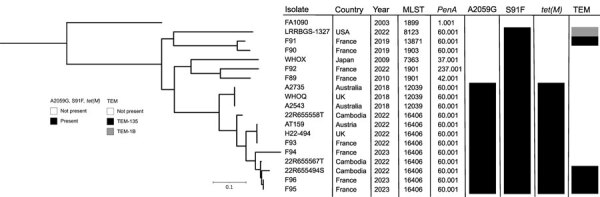
Phylogenetic tree of 19 Neisseria gonorrhoeae isolates, including 2 from patients in France, compared on the basis of their main resistance determinants. The phylogenetic tree was built from 10,907 total core single-nucleotide polymorphism positions. The F95 and F96 isolates from 2 patients in France were compared with ceftriaxone-resistant and extensively drug-resistant N. gonorrhoeae isolates from Europe, Australia, Cambodia, Japan, and the United States. The tree is rooted on N. gonorrheae FA1090, a laboratory reference strain. Scale bar indicates the branch length corresponding to genetic change.
The patients in both cases were successfully treated with 1 g ceftriaxone, which suggests that EUCAST breakpoints (https://www.eucast.org) for ceftriaxone resistance (MIC >0.125 mg/L) might be too low for genital infections (13). In case 2, the patient likely contracted XDR N. gonorrhoeae infection through sexual contact with a sex worker. However, no additional information was obtained about the sex worker, and our investigation did not establish a link to case 1. The XDR N. gonorrhoeae isolates also contained the tetM gene, which confers resistance to tetracycline and doxycycline. Several public health departments in California, USA, recommend the use of doxycycline postexposure prophylaxis in men who have sex with men, and the indirect selection of N. gonorrhoeae containing tet(M) because of the use of doxycycline postexposure prophylaxis is possible (14,15).
Conclusions
We report 2 cases of XDR N. gonorrhoeae strains from France. These strains are highly resistant to azithromycin, resistant to ceftriaxone, and genetically related to isolates from Cambodia. Our findings raise concerns about the spread of XDR N. gonorrhoeae in Southeast Asia. The emergence and spread of XDR N. gonorrhoeae isolates suggest the need to reconsider current recommendations for the first-line treatment of gonococcal infections. Novel and effective therapies against these XDR isolates are required, as is the need for international collaboration to monitor antimicrobial resistance.
Acknowledgments
We thank Monica Lahra and her team for access to the genomes of N. gonorrhoeae strains isolated in Cambodia. We also thank the general practitioner and the patients for agreeing to the publication of this case report.
This study received financial support from the French National Public Health Agency (Saint-Maurice, France) via the French reference center for bacterial sexually transmitted infections.
Biography
Dr. Caméléna is a clinical microbiologist at the French national reference center for bacterial sexually transmitted infections in Paris, France. His main research interests are the epidemiology, genetics, and pathogenicity of N. gonorrhoeae.
Footnotes
Suggested citation for this article: Caméléna F, Mérimèche M, Brousseau J, Mainardis M, Verger P, Le Risbé C, et al. Emergence of extensively drug-resistant Neisseria gonorrhoeae, France, 2023. Emerg Infect Dis. 2024 Sep [date cited]. https://doi.org/10.3201/eid3009.240557
These authors contributed equally to this article.
References
- 1.Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe. 2021;2:e627–36. 10.1016/S2666-5247(21)00171-3 [DOI] [PubMed] [Google Scholar]
- 2.Pleininger S, Indra A, Golparian D, Heger F, Schindler S, Jacobsson S, et al. Extensively drug-resistant (XDR) Neisseria gonorrhoeae causing possible gonorrhoea treatment failure with ceftriaxone plus azithromycin in Austria, April 2022. Euro Surveill. 2022;27:1–5. 10.2807/1560-7917.ES.2022.27.24.2200455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day M, Pitt R, Mody N, Saunders J, Rai R, Nori A, et al. Detection of 10 cases of ceftriaxone-resistant Neisseria gonorrhoeae in the United Kingdom, December 2021 to June 2022. Euro Surveill. 2022;27:2200803. 10.2807/1560-7917.ES.2022.27.46.2200803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maubaret C, Caméléna F, Mrimèche M, Braille A, Liberge M, Mainardis M, et al. Two cases of extensively drug-resistant (XDR) Neisseria gonorrhoeae infection combining ceftriaxone-resistance and high-level azithromycin resistance, France, November 2022 and May 2023. Euro Surveill. 2023;28:1–5. 10.2807/1560-7917.ES.2023.28.37.2300456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouk V, Pham CD, Wi T, van Hal SJ, Lahra MM; EGASP Cambodia working group. EGASP Cambodia working group. The enhanced gonococcal surveillance programme, Cambodia. Lancet Infect Dis. 2023;23:e332–3. 10.1016/S1473-3099(23)00479-6 [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poncin T, Merimeche M, Braille A, Mainardis M, Bebear C, Jacquier H, et al. Two cases of multidrug-resistant Neisseria gonorrhoeae related to travel in south-eastern Asia, France, June 2019. Euro Surveill. 2019;24:1–4. 10.2807/1560-7917.ES.2019.24.36.1900528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poncin T, Fouere S, Braille A, Camelena F, Agsous M, Bebear C, et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill. 2018;23:2–4. 10.2807/1560-7917.ES.2018.23.21.1800264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56:1273–80. 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill. 2019;24:1–4. 10.2807/1560-7917.ES.2019.24.8.1900118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55:3538–45. 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimche JL, Pham CD, Joseph SJ, Hutton S, Cartee JC, Ruan Y, et al. Novel strain of multidrug non-susceptible Neisseria gonorrhoeae in the USA. Lancet Infect Dis. 2024;24:e149–51. 10.1016/S1473-3099(23)00785-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unemo M, Golparian D, Oxelbark J, Kong FYS, Brown D, Louie A, et al. Pharmacodynamic evaluation of ceftriaxone single-dose therapy (0.125–1g) to eradicate ceftriaxone-susceptible and ceftriaxone-resistant Neisseria gonorrhoeae strains in a hollow fibre. J Antimicrob Chemother. 2024;00:1–8. [DOI] [PubMed] [Google Scholar]
- 14.Vanbaelen T, Manoharan-Basil SS, Kenyon C. Doxycycline postexposure prophylaxis could induce cross-resistance to other classes of antimicrobials in Neisseria gonorrhoeae: an in silico analysis. Sex Transm Dis. 2023;50:490–3. 10.1097/OLQ.0000000000001810 [DOI] [PubMed] [Google Scholar]
- 15.Mortimer TD, Grad YH. A genomic perspective on the near-term impact of doxycycline post-exposure prophylaxis on Neisseria gonorrhoeae antimicrobial resistance. Clin Infect Dis. 2023;77:788–91. 10.1093/cid/ciad279 [DOI] [PMC free article] [PubMed] [Google Scholar]


