Abstract
Leucocytes in the bloodstream respond rapidly to inflammatory signals by crossing the blood vessel wall and entering the tissues. This process involves adhesion to, and subsequent transmigration across, the endothelium, mediated by a cascade of interactions between adhesion molecules and stimulation of intracellular signalling pathways in both leucocytes and endothelial cells. This leads to changes in endothelial cell morphology that assist leucocyte extravasation, including endothelial cell contraction, intercellular junction disruption, increased permeability, remodelling of the endothelial apical surface and alterations in vesicle trafficking. Rho GTPases play a central role in many of the endothelial responses to leucocyte interaction. In this review, we discuss recent findings on leucocyte-induced alterations to endothelial cells, and the roles of Rho GTPases in these responses.
Keywords: actin cytoskeleton, intercellular adhesion molecule-1 (ICAM-1), lipid raft, transmigration, vascular cell adhesion molecule-1 (VCAM-1), VE-cadherin
Abbreviations: CHO, Chinese hamster ovary; DRF, diaphanous-related formin; EC, endothelial cell; ERM, ezrin/radixin/moesin; FAK, focal adhesion kinase; GFP, green fluorescent protein; HUVEC, human umbilical-vein endothelial cell; ICAM-1, intercellular adhesion molecule-1; MLC(K), myosin light chain (kinase); MMP, matrix metalloproteinase; PECAM-1, platelet/endothelial cell adhesion molecule-1; PLCγ, phospholipase Cγ; RAGE, receptor for advanced glycation end-products; RhoGDI, Rho guanine-nucleotide dissociation inhibitor; ROCK, Rho-kinase; ROS, reactive oxygen species; TEM, trans-endothelial migration; VCAM-1, vascular cell adhesion molecule-1; VVO, vesiculo-vacuolar organelle
INTRODUCTION
Leucocytes need to cross the endothelium in order to enter tissues during inflammatory and immune responses [1,2]. Leucocyte TEM (trans-endothelial migration; also known as extravasation or diapedesis) is restricted to specific regions of the vasculature, such as capillaries surrounding inflammation or high endothelial venules in secondary lymphoid organs through the localized and regulated expression of receptors. In the current paradigm of leucocyte TEM during inflammation, secretion of cytokines during the initial inflammatory response induces the expression of receptors on the endothelial surface. First, L-selectin on leucocytes and E- and P-selectins, as well as VCAM-1 (vascular cell adhesion molecule-1), on ECs (endothelial cells), slow down leucocytes by establishing intermittent interactions during tethering and rolling (Figure 1). This initial contact allows the leucocyte to recognize chemokines presented on the endothelial apical surface that trigger leucocyte β1-and β2-integrin activation, resulting in leucocyte firm attachment to endothelial adhesion receptors of the immunoglobulin superfamily, such as ICAM-1 (intercellular adhesion molecule-1) and VCAM-1. The arrested leucocyte can then ‘crawl’, searching for a gateway to cross the endothelium [1]. During TEM, the leucocyte engages other transmembrane receptors, including PECAM-1 (platelet/endothelial cell adhesion molecule-1), the JAM ( junction adhesion molecule) family and CD99, which can signal and contribute to TEM [2–4].
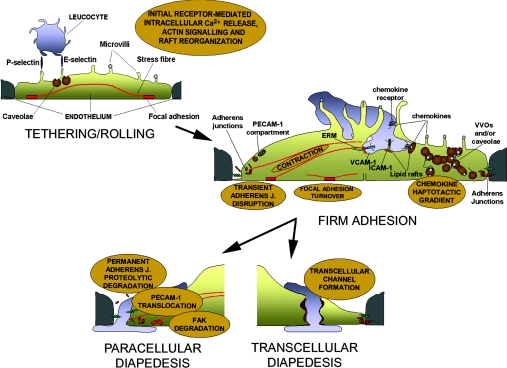
Figure 1. Possible remodelling of the endothelium caused by leucocyte interaction.
Based on signalling induced by ligation (either by leucocytes or by specific antibodies) of endothelial receptors involved in each step of the cascade of transmigration, leucocytes may alter the endothelium in several ways. During the initial tethering and rolling, E-selectin may transiently signal to the actin cytoskeleton and redistribute caveolae. During firm adhesion, the turnover of focal adhesions may be altered and ICAM-1- and VCAM-1-mediated signalling may increase actomyosin contractility. VCAM-1 would also cause disruption of adherens junctions. During TEM, PECAM-1 could be translocated from an internal compartment to the plasma membrane. Simultaneously, a haptotactic gradient of chemokines may guide the leucocyte to the VVOs formed by caveolae. Each of these processes may guide the leucocyte towards either a paracellular or a transcellular route for TEM.
Although ECs were originally believed to play a passive role in leucocyte TEM, merely expressing appropriate cell adhesion receptors, it is now clear that leucocytes induce signalling in ECs to facilitate TEM [5–7]. Leucocytes have been described to migrate either through junctions between ECs, known as paracellular migration, or directly through ECs, known as transcellular migration (Figure 1) [8,9]. These two pathways are likely to involve different signalling mechanisms and morphological changes in the ECs. Here, we will review responses induced by leucocytes in ECs that could facilitate the passage of the leucocytes through either a paracellular or a transcellular route, and, in particular, we discuss the contributions of Rho GTPase signalling in ECs during TEM.
ENDOTHELIAL RECEPTORS INVOLVED IN LEUCOCYTE–ENDOTHELIAL INTERACTIONS
In order to investigate how leucocytes initiate signalling in ECs, it is important to identify which receptors on ECs are engaged during the course of leucocyte TEM. Leucocyte binding to endothelium must be strong enough to overcome the force of blood flow, but transient enough to enable leucocyte motility for further extravasation. Interactions are very dynamic, and leucocytes contact several receptors within seconds.
The identification of receptors involved in the interaction of leucocytes with ECs has involved several complementary strategies. Traditionally, receptor-blocking antibodies have provided the most straightforward tool to determine the role of each receptor, both in vitro and in vivo (Table 1). Microscopy-based approaches have revealed that ICAM-1 and VCAM-1 are localized around leucocytes adhering to ECs (Table 1), and also the dynamic changes in receptor localization following leucocyte adhesion [10–12]. Analysis of leucocyte TEM in vitro allows the role of each receptor to be analysed in detail. For example, combining time-lapse microscopy with receptor-blocking antibodies has recently revealed that ICAM-1 contributes to guiding leucocyte locomotion, as well as to leucocyte adhesion [13].
Table 1. Endothelial receptors involved in leucocyte adhesion and transmigration, and their links with Rho GTPases and/or lipid rafts.
Abbreviations: DN, dominant-negative; ERK, extracellular-signal-regulated kinase; ND, not described; SHP-2, Src homology 2 domain-containing tyrosine phosphatase 2.
| Receptor | Role in vivo* | Role in vitro | Rho signalling | Raft recruitment | Signalling on endothelium |
|---|---|---|---|---|---|
| E-selectin | Rolling [19] | Rolling [120] | DN RhoA or C3 transferase (RhoA inhibitor) reduce monocyte- or antibody-induced clustering [10] | Recruited to rafts and caveolae upon antibody clustering [65,66] | Intracellular Ca2+ release. ERK pathway stimulation [46,66] |
| P-selectin | Rolling [19] | Rolling [121] | C3 transferase or RhoA inhibitor abolish internalization to clathrin-coated pits during rolling [122] | ND | ND |
| VCAM-1 | Rolling [20] and adhesion [123] | Rolling and adhesion [120,124,125] | Rho-dependent Rac1 activation upon VCAM-1 clustering [76,80]. ROCK may contribute to the formation of the ICAM-1- and VCAM-1-containing docking complexes involved in leucocyte TEM [11] | ND | Intracellular Ca2+ release, Rac1 activation, ROS generation, stress fibre induction and adherens junction disruption [46,76,80,82] |
| ICAM-1 | Adhesion [126] | Adhesion and transmigration [120,127,128] | RhoA activation upon ICAM-1 clustering [56,72]. DN RhoA abrogates ICAM-1 clustering induced by monocytes or antibodies [10]. ROCK may contribute to the formation of the ICAM-1- and VCAM-1-containing leucocyte-docking complexes [11] | Recruited to lipid rafts upon clustering [65] | Intracellular Ca2+ release, Rho activation, ROS generation, stress fibre formation, Src and FAK phosphorylation clustering [56,61,62,72,129] |
| RAGE | Adhesion (leucocyte recruitment upon inflammation induction) [87] | Adhesion (counter-receptor of Mac-1) [87] | DN Rac and Cdc42 inhibit RAGE-mediated neurite outgrowth and myoblast differentiation [85] | RAGE has been detected in caveolin-rich fractions in endothelium [130] | Ligation by AGEs induces cell adhesion molecule and cytokine expression via ROS generation [131] |
| PECAM-1 | Diapedesis [132] | Diapedesis [133] | PECAM-1-deficient endothelial cells have impaired Rho GTP loading and motility [88] | Localized in VVOs [134] | Associates with SHP-2, α- and β–catenin, Fer tyrosine kinase. It may modulate endothelial Ca2+ signalling, FAK phosphorylation, apoptosis [135,136] |
* Antibody blockage or genetic manipulation in mice.
The expression level of endothelial receptors varies during the course of inflammation and in different sites in the vasculature, and the levels of cognate receptors on leucocytes differ between leucocyte subtypes [14–16]. The respective contribution of each receptor therefore depends on the site of TEM, and thus the mechanisms of leucocyte TEM differ according to the site in the vasculature, as well as the leucocyte subtype. For example, high ECs in the high endothelial venules of lymph nodes constitutively express receptors involved in leucocyte TEM, including ICAM-1 and VCAM-1, in order to allow continuous entry of naive lymphocytes into the lymph nodes to search for antigen. In culture, ECs rapidly lose many of their location-specific properties, and thus it has been difficult to study differences in TEM with EC subtypes in vitro. However, knockout of receptor genes in mice has provided an important insight into the roles of each adhesion receptor in different parts of the vasculature in vivo. Intravital microscopy has revealed, for instance, the overlapping roles of E- and P-selectin when single and double knockouts are compared [17–19], as well as the contribution of VCAM-1 to neutrophil rolling in bone marrow, independently of selectins [20], or to eosinophil rolling [21]. Similarly, the relative roles of molecules such as ICAM-1 and VCAM-1 in leucocyte–endothelial interaction and in leucocyte homing and recirculation into specific organs have been studied in knockout mice [22–25]. Intravital microscopy combined with electron microscopy has also revealed the importance of other receptors of the immunoglobulin superfamily, such as PECAM-1, during TEM [26] and in the subsequent migration of leucocytes across the basement membrane [27].
RHO GTPases AND THE CYTOSKELETON
Rho GTPases play a central role in leucocyte-induced changes to the endothelium. There are 23 Rho family members in mammals, of which RhoA, Rac1 and Cdc42 are the best characterized [28], and these have been implicated in EC responses during leucocyte TEM [6,29]. Rho GTPases regulate actin and microtubule dynamics, and therefore almost any process that requires motility [30,31]. Most Rho GTPases cycle between an active GTP-bound form and inactive GDP-bound form. Their activity is regulated by GEFs (guanine nucleotide-exchange factors), which promote the exchange of GDP for GTP, and GAPs (GTPase-activating proteins) that enhance the GTPase activity of these proteins. In their active GTP-bound conformation, Rho GTPases bind to and regulate a wide range of downstream effectors [32,33]. In many cultured cell types, RhoA activation promotes the assembly of actin stress fibres and integrin-containing focal adhesions, whereas Rac1 induces lamellipodia and Cdc42 stimulates filopodium extension [34–36]. Rho GTPases normally act on membranes, and are post-translationally modified by prenylation. Their interaction with membranes is regulated in part by RhoGDIs (Rho guanine-nucleotide dissociation inhibitors) in the cytoplasm, which bind Rho proteins and prevent their interaction with membranes and downstream targets.
RhoA induces stress fibres by controlling both actin assembly and actomyosin-based contractility. DRFs (diaphanous-related formins) are Rho targets that stimulate actin polymerization [37]. ROCKs (Rho-kinases) are activated by RhoA, and inactivate MYPT1 (myosin-specific phosphatase-1) by phosphorylating its regulatory subunit [38,39]. This leads to an increase in MLC (myosin light chain) phosphorylation [40], which enhances actin-binding and actin-induced ATPase activity of myosin II, leading to actomyosin contraction and formation of stress fibres (Figure 2).
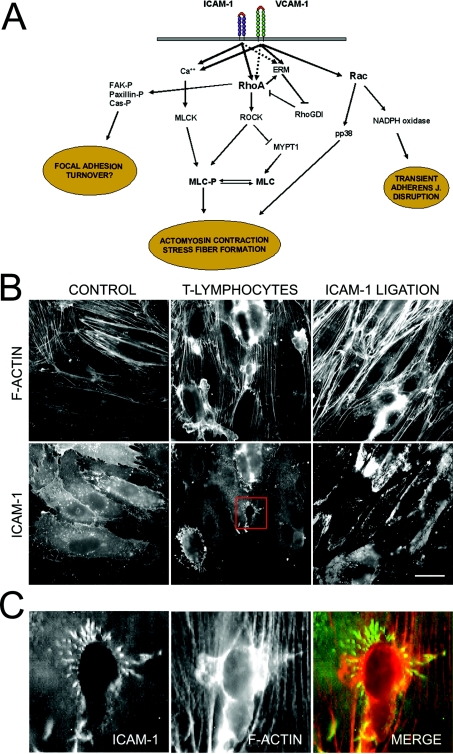
Figure 2. The roles of RhoA and Rac in leucocyte-induced signalling to the endothelium.
(A) ICAM-1, and probably VCAM-1, ligation induces RhoA activation and triggers the formation of stress fibres and actomyosin contractility by increasing the phosphorylation of MLC. In addition, VCAM-1 clustering induces Rac1-mediated generation of ROS via NADPH oxidase, which disrupts adherens junctions. Phosphorylation of FAK, paxillin and Cas proteins induced by ICAM-1 cross-linking may contribute to alter the turnover of endothelial focal adhesions. (B) T-lymphoblasts or ICAM-1 ligation induce RhoA-mediated stress fibre formation. HUVECs were incubated with T-lymphoblasts or clustered with anti-ICAM-1 specific antibodies for 45 min, and ICAM-1 and F-actin were detected with an anti-ICAM-1 antibody or phalloidin respectively. Bar, 20 μm. (C) A 4-fold magnification of the squared area in (B) is shown. Transmigratory T-lymphoblast is surrounded by a microvillus-like docking structure. Despite being enriched in adhesion receptors involved in leucocyte firm adhesion, abrogation of these structures inhibits leucocyte TEM, but not adhesion [12]. ROCK may mediate the formation of these docking complexes [11]. Images in (B) and (C) were taken with a coolview 12-bit integrating cooled CCD camera (Photonic Science, Robertsbridge, East Sussex, U.K.) mounted over an Axiophot microscope (Carl Zeiss Ltd, Welwyn Garden City, Herts., U.K.).
In addition to their effects on the cytoskeleton, Rho GTPases regulate the integrity of intercellular junctions and their adhesion to the underlying cytoskeleton (reviewed in [41,42]). In ECs, Rho and Rac have been shown to affect the integrity of adherens junctions and tight junctions [43,44]. Little is known about how Rho GTPase signalling pathways regulate endothelial junctions, but possible mechanisms for Rac/Rho effects on junctions have been described in epithelial cells. For example, Rac1 has been suggested to stabilize adherens junctions by binding to the protein IQGAP [41]. Downstream of RhoA, the DRF mDia1 and ROCK have been reported to have opposite effects on adherens junctions in epithelial cells [45].
EFFECTS OF LEUCOCYTES ON ENDOTHELIAL STRESS FIBRES AND FOCAL ADHESIONS
Leucocyte interaction has been shown to induce stress fibre formation in various EC types [46,47], and this is often accompanied by an increase in endothelial permeability, which can lead to tissue oedema [48]. Both RhoA/ROCK and Ca2+-dependent phosphorylation of MLC by MLCK (MLC kinase) contribute to leucocyte-induced stress fibre formation, increased permeability and TEM [6,10,49–54]. Stress fibres could facilitate leucocyte TEM by exerting tension on cell–cell junctions. Although stress fibres are normally anchored to the plasma membrane at focal adhesions, under certain conditions they may instead be anchored to intercellular junctions [55], and might pull directly on intercellular junctions.
How do leucocytes stimulate RhoA/ROCK and/or MLCK to induce stress fibres? The contribution of each endothelial receptor involved in leucocyte TEM to endothelial responses has been studied mainly by using specific antibodies to mimic receptor engagement by leucocytes (Figure 2 and Table 1). Antibody-induced clustering of either ICAM-1 or VCAM-1 induces an increase in RhoA activity and in stress fibres [6,10,56]. How endothelial receptors activate RhoA is not clear, but one possible link is via the ERM (ezrin/radixin/moesin) family of actin-filament-binding proteins, which can bind to ICAM-2, ICAM-1 and VCAM-1 cytoplasmic tails [11,57]. RhoGDI binding to ERM proteins dissociates it from RhoA, potentially facilitating RhoA activation [58]. Reciprocally, RhoA regulates membrane localization of ERM proteins in several cell types [59,60], and RhoA activity is required for clustering of ICAM-1 and VCAM-1 with ERM proteins in areas of leucocyte contact (Figure 2) [10,11].
MLCK is activated by Ca2+/calmodulin, and antibody ligation of E-selectin, P-selectin and VCAM-1 can mimic neutrophil-mediated activation and induce an increase in intracellular Ca2+ concentration [46]. ICAM-1 ligation also induces Ca2+-mediated signalling in ECs, probably through activation of PLCγ (phospholipase Cγ), and specific antibodies against ICAM-1 block the leucocyte-mediated increase in intracellular Ca2+ [61,62].
Lipid rafts and/or caveolae could bring together adhesion receptors with specific signalling proteins [63,64]. Both E-selectin and ICAM-1 localize to lipid rafts following antibody clustering [65], and E-selectin clustering induces lipid-raft-dependent phosphorylation of PLCγ. Anti-E-selectin-coated beads bound to HUVECs (human umbilical-vein ECs) appear surrounded by caveolin-1, the structural component of caveolae [66]. RhoA and Rac1 have been reported to localize to lipid rafts and caveolae in some cell types [67–69], although not so far in primary ECs, and this is important for their ability to signal to some of their downstream targets [70,71]. It will therefore be interesting to investigate whether lipid rafts contribute to signalling upstream and/or downstream of Rho and Rac in leucocyte-induced endothelial responses.
As well as activating RhoA, ICAM-1 engagement in brain EC lines promotes phosphorylation of several focal adhesion proteins, including FAK (focal adhesion kinase), paxillin and Src [61,72]. FAK phosphorylation normally accompanies stress fibre and focal adhesion formation, although it usually promotes focal adhesion turnover rather than assembly [73,74]. Consistent with this, Mullaly et al. [75] describe an increase in FAK phosphorylation and a decrease in focal adhesions upon interaction of lymphocytes with ECs. Loosening of endothelial attachment to the extracellular matrix might enhance the migration of leucocytes through the blood vessel wall subsequent to TEM. However, clustering of VCAM-1 was reported to increase focal adhesion formation in HUVECs [76], but whether VCAM-1 affects FAK phosphorylation is not known.
EFFECTS OF LEUCOCYTES ON ENDOTHELIAL CELL–CELL JUNCTIONS
Endothelial cell–cell junctions need to be disrupted when leucocytes use a paracellular pathway to transmigrate across the endothelium. This disruption has been monitored in real time during leucocyte TEM by using fluorescently labelled non-blocking antibodies, or by transfection of constructs coding for junctional components tagged with GFP (green fluorescent protein). For example, expression of VE-cadherin–GFP in HUVECs was used to analyse adherens junction dynamics during TEM of human neutrophils and monocytes [77]. Leucocytes were shown to transmigrate by a paracellular route through transient opening of gaps in the adherens junctions. Leucocytes also transmigrated through pre-existing gaps, or through gaps previously opened by another leucocyte. Importantly, there was no net loss of VE-cadherin at the plasma membrane during TEM, but a reversible reorganization of junctional structures. The average time of gap resealing was measured as 5 min, which seems long enough to account for the increase of endothelial permeability induced by leucocytes [48]. When PECAM-1 and VE-cadherin dynamics were analysed by fluorescence time-lapse microscopy using non-blocking antibodies, a brief gap widening was also observed for both junctional proteins [78].
VCAM-1 clustering has been shown to induce disruption of adherens junctions through Rac1 activation and subsequent generation of ROS (reactive oxygen species) [7,79]. This signalling is dependent on the VCAM-1-induced calcium flux [80]. ROS scavenging inhibits VCAM-1 effects on junctions and monocyte TEM [81,82]. In contrast, ICAM-1 clustering or active RhoA did not alter adherens junctions. As mentioned above, ICAM-1 ligation induces RhoA activation, but its effect on Rac1 has not been reported. Since inhibition of RhoA or Rac1 reduces leucocyte TEM [6,76], these data suggest that activation of RhoA by ICAM-1 and VCAM-1, and subsequent actomyosin contractility, may act co-ordinately with VCAM-1 activation of Rac1 and disruption of endothelial adherens junctions (Figure 2). Together, RhoA and Rac1 would thereby facilitate TEM. This model, however, may be restricted to particular EC types, such as HUVECs, since in human pulmonary microvascular ECs ICAM-1 is necessary not only for stress fibre formation, but also for ROS induction upon monocyte adhesion to the endothelial monolayer [83]. Comparative studies with different EC types have confirmed that ICAM-1-mediated ROS production depends on the cell type [84]. Further investigation is necessary to understand fully the signalling pathways induced by each leucocyte-binding receptor individually and in combination.
Another leucocyte-binding receptor that signals to Rac1 and might thereby affect junctional integrity is RAGE (receptor for advanced glycation end-products) [85]. RAGE is implicated in a variety of inflammatory disorders [86], and has recently been described as a ligand for the β2 integrin Mac-1, αMβ2 [87].
PECAM-1 is present in endothelial cell–cell junctions and may regulate Rho, since lung ECs from PECAM-1-null mice have a decreased motility in wound-healing assays, which correlates with reduced Rho activity [88]. PECAM-1 has been reported to localize in a novel internal parajunctional compartment, and normally cycles continuously between the cell surface and this compartment. Upon interaction with neutrophils, internal PECAM-1 is translocated to the cell surface, and impairment of this trafficking abrogates diapedesis, but not adhesion [89]. It would be interesting to determine whether PECAM-1 engagement by leucocytes stimulates Rho and locally enhances actomyosin contractility, possibly thereby reducing PECAM-1 transport away from the cell surface.
In addition to inducing reversible opening of adherens junctions, there is also some evidence that neutrophils induce proteolytic degradation of endothelial adherens junctions [78]. Fragments of VE-cadherin were found in cell-culture supernatants following adhesion of neutrophils to HUVEC monolayers [90]. Elastase and cathepsin G were identified as proteases on the neutrophil surface that cleave VE-cadherin, and their activity was required for optimal TEM [90]. In addition, it has been proposed that VCAM-1-dependent generation of ROS may activate MMPs (matrix metalloproteinases) that would locally degrade the extracellular matrix at areas of leucocyte binding, promoting EC retraction [79]. However, neutrophils from mice deficient for MMP-9 or neutrophil elastase exhibit normal transmigration through the endothelium, and are able to degrade VE-cadherin [91]. Whether cathepsin G or other proteases are sufficient for degrading VE-cadherin and/or the extracellular matrix will be an important point to address in future studies.
EXTENSION OF APICAL ‘LEUCOCYTE DOCKING STRUCTURES’ BY ECs
Two groups have recently described a dramatic leucocyte-induced remodelling of the apical endothelial plasma membrane mediated by the actin cytoskeleton (Figure 2C) [11,12]. Both groups took advantage of strategies to enhance integrin/cellular-adhesion-molecule-mediated adhesion to perform a detailed microscopical analysis of the interaction zone between leucocytes and ECs. Barreiro et al. [11] primarily used K562 erythroleukaemia cells stably transfected with the VCAM-1 ligand VLA-4 (very late antigen-4)/α4β1, and studied their binding to HUVECs. K562 cells are unable to transmigrate, and remain apically attached via VCAM-1. Carman et al. [12] used CHO (Chinese hamster ovary) cells stably transfected with ICAM-1, co-cultured with K562 cells expressing an LFA-1 (lymphocyte function-associated antigen 1)/αLβ2 trapped in its open conformation. Again, the K562 cells are unable to transmigrate across the CHO cells [12]. Protrusions containing ICAM-1, VCAM-1, F-actin and ERM proteins form on the endothelial or CHO cell apical surfaces and surround the attached K562 cells. Similar docking complexes were found surrounding primary lymphoblasts on HUVECs, indicating that these structures are not restricted to the K562-based model cell systems. It is possible that the docking structure projections are formed by reorganization of endothelial microvilli, since both ICAM-1 and ERM proteins are normally present in microvilli [56,57]. Actin polymerization and microtubule integrity are both required for the formation of these protrusions, and Ca2+ chelation inhibits protrusion formation, although it is not known what the relevant target(s) for Ca2+ are. Whether ROCK contributes to the formation of docking structures is not clear: Barreiro et al. [11] found that ROCK inhibition abrogated the generation of the docking complexes as well as leucocyte rolling, adhesion and TEM, whereas Carman et al. [12] did not detect an appreciable inhibition of endothelial protrusions with ROCK inhibitors. In either case, however, inhibitors that prevent formation of docking structures appear to reduce TEM, but have little effect on adhesion of leucocytes, suggesting that the docking structures contribute to TEM.
RHO GTPases AND LOCALIZATION OF CHEMOKINES AND ADHESION RECEPTORS
All these data together suggest that Rho GTPases contribute to remodelling of EC actin-based structures upon leucocyte binding, and that this facilitates leucocyte TEM. Another endothelium-based mechanism proposed to contribute to TEM is the presentation of chemokines or cell adhesion receptors in a gradient, thereby guiding the leucocyte across the endothelium [92,93]. Chemokines in the tissues are transported across ECs to their apical (luminal) surface via caveolae, which are a non-clathrin-coated vesicle system and are very abundant in ECs [94]. It has been proposed that chemokines form a gradient from the luminal to the abluminal side of blood vessels that would orientate the leucocyte during extravasation [93]. Since Rho and Rac can localize to caveolae [67,69], they might regulate caveolar transport. RhoA, Rac1 and Cdc42 GTPases regulate the transcytosis of the polymeric immunoglobulin receptor (pIgR) across epithelial cells [95–97], but whether they affect transcytosis across ECs is not known. However, dynamin-2 is known to contribute to scission of caveolae from the plasma membrane and endothelial transcytosis [98], and interacts with activators of Cdc42 [99]; thus Cdc42 might contribute to movement of caveolae across ECs by stimulating actin polymerization. Interestingly, caveolae are polarized in ECs under shear stress, and distribute along actin stress fibres in areas upstream of the flow direction [100,101], implying a potential link with Rho.
Recent findings suggest that adhesion molecules may also form a gradient of adhesion to enhance TEM. Blockage of leucocyte β2 integrin interaction in monocytes on HUVECs does not block adhesion (cells remain adhered via β1 integrins), but results in a disorientated crawling of the attached monocytes [13]. Under these conditions, monocytes do not reach intercellular junctions and TEM is impaired. Instead, they move around, ‘blind to the junction’, in what Schenkel et al. [13] call ‘search pirouettes’. The explanation these authors propose is that the β2 ligands ICAM-1 and ICAM-2 form an adhesion gradient that the leucocyte follows to find the best site for extravasation. Taken together, these results suggest a model in which the endothelium is able to guide the leucocyte in order to initiate productive diapedesis.
TRANSCELLULAR TEM OF LEUCOCYTES
Although transcellular TEM has rarely been observed in vitro, there is some evidence that it occurs in vivo. Electron microscopy analysis of neutrophils extravasating in response to fMLP (N-formylmethionyl-leucylphenylalanine) revealed that they preferentially transmigrated through thinner areas of the endothelium far from intercellular junctions [9,102]. Little is known about the mechanistic basis for transcellular TEM of leucocytes, but it has been suggested that a vesicular compartment termed the VVO (vesiculo-vacuolar organelle) could be involved. VVOs result from the fusion of vesicles, which interconnect to form transcellular channels that facilitate the passage of solutes and macromolecules [103]. The nature of these VVOs remains obscure, but they are positive for the caveolar marker caveolin-1 [104], and could therefore be made at least in part from caveolae. It will therefore be important to determine whether Rho GTPases are associated with VVOs. Feng et al. [105] propose that VVOs could transiently form a transcellular pore to allow transcellular diapedesis of a neutrophil. It is possible that a gradient of chemokine could then act across this pore to attract the leucocyte through into the tissues (Figure 1).
In some electron microscopy images, processes extend around neutrophils attached to the endothelium, similar to those observed during phagocytosis [105,106] and in the docking structures described around adherent leucocytes in vitro [11,12]. Interestingly, the endothelium has been shown to possess a remarkable phagocytic capacity [107–109], and Rho GTPases are well known to regulate phagocytosis [110]. Endothelial cells may thus make use of similar machinery to phagocytose cells and to allow transcellular passage of leucocytes.
CONCLUSIONS AND FUTURE PROSPECTS
Leucocytes induce many alterations to the endothelium to facilitate the process of extravasation. Some of these changes, such as endothelial contractility and intercellular junction disruption, are likely to enhance selectively the paracellular pathway of TEM. Other changes, like the formation of docking structures and pores due to VVO fusion, may direct cells preferentially to a transcellular pathway. It is difficult to evaluate the relative contributions of each of these endothelial responses to the process of TEM, because no comparative studies have been performed yet in a single EC type in vivo or in vitro. In addition, the fact that transcellular TEM has rarely been observed in vitro may reflect the decrease in levels and distribution of caveolae in ECs cultured on plastic [104]. For future studies on the mechanistic basis for transcellular TEM, an in vitro system for analysing this process will need to be developed.
The RhoA/ROCK and Rac pathways are key regulators of leucocyte-induced endothelial changes, particularly those relevant to paracellular transmigration (Table 1). Whether Rho GTPases also regulate other leucocyte-induced alterations, such as vesicular reorganization to form transcytotic channels, will be an important topic for future research. In addition, most studies on signal transduction during TEM have concentrated on responses to ICAM-1 and VCAM-1 [3,4]. However, several other receptors have been implicated in leucocyte adhesion and TEM, and it will thus be interesting to know whether they also affect Rho GTPase signalling pathways.
Improvements in technology, such as intravital two-photon microscopy [111], are enabling researchers to address many questions in vivo that before could only be studied in vitro. This, combined with the use of conditional knockout mice, will allow the role of specific signalling molecules in ECs to be addressed during TEM in vivo. In addition, new in vitro systems are being developed to mimic the endothelial environment in blood vessels, such as co-culture of ECs with other vascular cell types [112–114], and systems to study the effects of fluid flow on leucocyte transmigration [115,116]. These systems are suitable for analysis with advanced imaging techniques. For example, fluorescence resonance energy transfer is used to measure local interactions between two proteins [117], and may provide an answer to whether signalling pathways activated by stable cross-linking of different adhesion receptors are also activated at contact areas between the leucocyte and the EC. Fluorescence recovery after photobleaching [118,119] may detect changes in endothelial receptor dynamics during leucocyte engagement. Future studies of endothelial–leucocyte interaction should therefore allow signalling processes during TEM to be analysed without gross perturbations to the endothelium.
Acknowledgments
This work was supported by the Ludwig Institute for Cancer Research. J.M. was supported by a Marie Curie fellowship (no. HPMF-CT-2000-01061) and a British Heart Foundation intermediate fellowship (no. FS/04/006).
References
- 1.Butcher E. C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Muller W. A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 3.Ostermann G., Weber K. S., Zernecke A., Schroder A., Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 4.Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 5.Vicente-Manzanares M., Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 6.Adamson P., Etienne S., Couraud P. O., Calder V., Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J. Immunol. 1999;162:2964–2973. [PubMed] [Google Scholar]
- 7.van Buul J. D., Hordijk P. L. Signaling in leukocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2004;24:824–833. doi: 10.1161/01.ATV.0000122854.76267.5c. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi V. T., Florey H. W. Electron micrographic observations on the emigration of leucocytes. Q. J. Exp. Physiol. Cogn. Med. Sci. 1960;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 9.Feng D., Nagy J. A., Pyne K., Dvorak H. F., Dvorak A. M. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojciak-Stothard B., Williams L., Ridley A. J. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J. Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreiro O., Yanez-Mo M., Serrador J. M., Montoya M. C., Vicente-Manzanares M., Tejedor R., Furthmayr H., Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carman C. V., Jun C. D., Salas A., Springer T. A. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J. Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 13.Schenkel A. R., Mamdouh Z., Muller W. A. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 14.Mebius R. E., Rennert P., Weissman I. L. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 15.McMurray R. W. Adhesion molecules in autoimmune disease. Semin. Arthritis Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 16.Issekutz A. C., Issekutz T. B. The role of E-selectin, P-selectin, and very late activation antigen-4 in T lymphocyte migration to dermal inflammation. J. Immunol. 2002;168:1934–1939. doi: 10.4049/jimmunol.168.4.1934. [DOI] [PubMed] [Google Scholar]
- 17.Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 18.Frenette P. S., Mayadas T. N., Rayburn H., Hynes R. O., Wagner D. D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 19.Labow M. A., Norton C. R., Rumberger J. M., Lombard-Gillooly K. M., Shuster D. J., Hubbard J., Bertko R., Knaack P. A., Terry R. W., Harbison M. L., et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 20.Mazo I. B., Gutierrez-Ramos J. C., Frenette P. S., Hynes R. O., Wagner D. D., von Andrian U. H. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriramarao P., DiScipio R. G., Cobb R. R., Cybulsky M., Stachnick G., Castaneda D., Elices M., Broide D. H. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood. 2000;95:592–601. [PubMed] [Google Scholar]
- 22.Broide D., Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol. Rev. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 23.Dunne J. L., Collins R. G., Beaudet A. L., Ballantyne C. M., Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J. Immunol. 2003;171:6105–6111. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- 24.Hickey M. J., Granger D. N., Kubes P. Molecular mechanisms underlying IL-4-induced leukocyte recruitment in vivo: a critical role for the alpha 4 integrin. J. Immunol. 1999;163:3441–3448. [PubMed] [Google Scholar]
- 25.Bowden R. A., Ding Z. M., Donnachie E. M., Petersen T. K., Michael L. H., Ballantyne C. M., Burns A. R. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ. Res. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 26.Thompson R. D., Wakelin M. W., Larbi K. Y., Dewar A., Asimakopoulos G., Horton M. A., Nakada M. T., Nourshargh S. Divergent effects of platelet–endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J. Immunol. 2000;165:426–434. doi: 10.4049/jimmunol.165.1.426. [DOI] [PubMed] [Google Scholar]
- 27.Wakelin M. W., Sanz M. J., Dewar A., Albelda S. M., Larkin S. W., Boughton-Smith N., Williams T. J., Nourshargh S. An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J. Exp. Med. 1996;184:229–239. doi: 10.1084/jem.184.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood J., Walters C. E., Pryce G., Kanuga N., Beraud E., Baker D., Adamson P. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003;17:905–907. doi: 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 31.Fukata M., Nakagawa M., Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 32.Ridley A. J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 33.Bishop A. L., Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 35.Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 36.Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 37.Wallar B. J., Alberts A. S. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–446. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 38.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 39.Riento K., Ridley A. J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 40.Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukata M., Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 42.Braga V. M. Cell–cell adhesion and signalling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 43.Braga V. M., Machesky L. M., Hall A., Hotchin N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojciak-Stothard B., Potempa S., Eichholtz T., Ridley A. J. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 45.Sahai E., Marshall C. J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzon P., Vecile E., Nardon E., Ferrero E., Harlan J. M., Tedesco F., Dobrina A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J. Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehringer W. D., Edwards M. J., Wintergerst K. A., Cox A., Miller F. N. An increase in endothelial intracellular calcium and F-actin precedes the extravasation of interleukin-2-activated lymphocytes. Microcirculation. 1998;5:71–80. [PubMed] [Google Scholar]
- 48.Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature (London) 1981;289:646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- 49.Huang A. J., Manning J. E., Bandak T. M., Ratau M. C., Hanser K. R., Silverstein S. C. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hixenbaugh E. A., Goeckeler Z. M., Papaiya N. N., Wysolmerski R. B., Silverstein S. C., Huang A. J. Stimulated neutrophils induce myosin light chain phosphorylation and isometric tension in endothelial cells. Am. J. Physiol. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 51.Saito H., Minamiya Y., Kitamura M., Saito S., Enomoto K., Terada K., Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J. Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- 52.Garcia J. G., Verin A. D., Herenyiova M., English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J. Appl. Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 53.Yuan S. Y., Wu M. H., Ustinova E. E., Guo M., Tinsley J. H., De Lanerolle P., Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ. Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 54.Saito H., Minamiya Y., Saito S., Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J. Leukocyte Biol. 2002;72:829–836. [PubMed] [Google Scholar]
- 55.Hordijk P. L., Anthony E., Mul F. P., Rientsma R., Oomen L. C., Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J. Cell Sci. 1999;112:1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 56.Thompson P. W., Randi A. M., Ridley A. J. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J. Immunol. 2002;169:1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 57.Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi K., Sasaki T., Mammoto A., Takaishi K., Kameyama T., Tsukita S., Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 59.Hirao M., Sato N., Kondo T., Yonemura S., Monden M., Sasaki T., Takai Y., Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotani H., Takaishi K., Sasaki T., Takai Y. Rho regulates association of both the ERM family and vinculin with the plasma membrane in MDCK cells. Oncogene. 1997;14:1705–1713. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- 61.Etienne-Manneville S., Manneville J. B., Adamson P., Wilbourn B., Greenwood J., Couraud P. O. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J. Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 62.Pfau S., Leitenberg D., Rinder H., Smith B. R., Pardi R., Bender J. R. Lymphocyte adhesion-dependent calcium signaling in human endothelial cells. J. Cell Biol. 1995;128:969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 64.Parton R. G. Caveolae – from ultrastructure to molecular mechanisms. Nat. Rev. Mol. Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 65.Tilghman R. W., Hoover R. L. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett. 2002;525:83–87. doi: 10.1016/s0014-5793(02)03070-3. [DOI] [PubMed] [Google Scholar]
- 66.Kiely J. M., Hu Y., Garcia-Cardena G., Gimbrone M. A., Jr Lipid raft localization of cell surface E-selectin is required for ligation-induced activation of phospholipase C gamma. J. Immunol. 2003;171:3216–3224. doi: 10.4049/jimmunol.171.6.3216. [DOI] [PubMed] [Google Scholar]
- 67.Michaely P. A., Mineo C., Ying Y. S., Anderson R. G. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J. Biol. Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- 68.Kawamura S., Miyamoto S., Brown J. H. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J. Biol. Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 69.Gingras D., Gauthier F., Lamy S., Desrosiers R. R., Beliveau R. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem. Biophys. Res. Commun. 1998;247:888–893. doi: 10.1006/bbrc.1998.8885. [DOI] [PubMed] [Google Scholar]
- 70.del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H. H., Anderson R. G., Schwartz M. A. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 71.Palazzo A. F., Eng C. H., Schlaepfer D. D., Marcantonio E. E., Gundersen G. G. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 72.Etienne S., Adamson P., Greenwood J., Strosberg A. D., Cazaubon S., Couraud P. O. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J. Immunol. 1998;161:5755–5761. [PubMed] [Google Scholar]
- 73.Ren X. D., Kiosses W. B., Sieg D. J., Otey C. A., Schlaepfer D. D., Schwartz M. A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 74.Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature (London) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 75.Mullaly S. C., Moyse R. J., Nelson R. C., Murray A. G. Stable lymphocyte contact induces remodeling of endothelial cell matrix receptor complexes. Eur. J. Immunol. 2002;32:1493–1501. doi: 10.1002/1521-4141(200205)32:5<1493::AID-IMMU1493>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 76.van Wetering S., van den Berk N., van Buul J. D., Mul F. P., Lommerse I., Mous R., ten Klooster J. P., Zwaginga J. J., Hordijk P. L. VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 77.Shaw S. K., Bamba P. S., Perkins B. N., Luscinskas F. W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 78.Su W. H., Chen H. I., Jen C. J. Differential movements of VE-cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood. 2002;100:3597–3603. doi: 10.1182/blood-2002-01-0303. [DOI] [PubMed] [Google Scholar]
- 79.Cook-Mills J. M. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol. Immunol. 2002;39:499–508. doi: 10.1016/s0161-5890(02)00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cook-Mills J. M., Johnson J. D., Deem T. L., Ochi A., Wang L., Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Buul J. D., Voermans C., van den Berg V., Anthony E. C., Mul F. P., van Wetering S., van der Schoot C. E., Hordijk P. L. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J. Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 82.Matheny H. E., Deem T. L., Cook-Mills J. M. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J. Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q., Doerschuk C. M. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J. Immunol. 2001;166:6877–6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- 84.Wang Q., Pfeiffer G. R., II, Stevens T., Doerschuk C. M. Lung microvascular and arterial endothelial cells differ in their responses to intercellular adhesion molecule-1 ligation. Am. J. Respir. Crit. Care Med. 2002;166:872–877. doi: 10.1164/rccm.2201007. [DOI] [PubMed] [Google Scholar]
- 85.Taguchi A., Blood D. C., del Toro G., Canet A., Lee D. C., Qu W., Tanji N., Lu Y., Lalla E., Fu C., et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature (London) 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 86.Yan S. F., Ramasamy R., Naka Y., Schmidt A. M. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 87.Chavakis T., Bierhaus A., Al-Fakhri N., Schneider D., Witte S., Linn T., Nagashima M., Morser J., Arnold B., Preissner K. T., Nawroth P. P. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gratzinger D., Canosa S., Engelhardt B., Madri J. A. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003;17:1458–1469. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- 89.Mamdouh Z., Chen X., Pierini L. M., Maxfield F. R., Muller W. A. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature (London) 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 90.Hermant B., Bibert S., Concord E., Dublet B., Weidenhaupt M., Vernet T., Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 91.Allport J. R., Lim Y. C., Shipley J. M., Senior R. M., Shapiro S. D., Matsuyoshi N., Vestweber D., Luscinskas F. W. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukocyte Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- 92.Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur. J. Immunol. 1993;23:303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- 93.Middleton J., Patterson A. M., Gardner L., Schmutz C., Ashton B. A. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 94.Gratton J. P., Bernatchez P., Sessa W. C. Caveolae and caveolins in the cardiovascular system. Circ. Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 95.Leung S. M., Rojas R., Maples C., Flynn C., Ruiz W. G., Jou T. S., Apodaca G. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA. Mol. Biol. Cell. 1999;10:4369–4384. doi: 10.1091/mbc.10.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jou T. S., Leung S. M., Fung L. M., Ruiz W. G., Nelson W. J., Apodaca G. Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1. Mol. Biol. Cell. 2000;11:287–304. doi: 10.1091/mbc.11.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rojas R., Ruiz W. G., Leung S. M., Jou T. S., Apodaca G. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 2001;12:2257–2274. doi: 10.1091/mbc.12.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shajahan A. N., Timblin B. K., Sandoval R., Tiruppathi C., Malik A. B., Minshall R. D. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 99.Orth J. D., McNiven M. A. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 2003;15:31–39. doi: 10.1016/s0955-0674(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 100.Isshiki M., Ying Y. S., Fujita T., Anderson R. G. A molecular sensor detects signal transduction from caveolae in living cells. J. Biol. Chem. 2002;277:43389–43398. doi: 10.1074/jbc.M205411200. [DOI] [PubMed] [Google Scholar]
- 101.Isshiki M., Ando J., Yamamoto K., Fujita T., Ying Y., Anderson R. G. Sites of Ca2+ wave initiation move with caveolae to the trailing edge of migrating cells. J. Cell Sci. 2002;115:475–484. doi: 10.1242/jcs.115.3.475. [DOI] [PubMed] [Google Scholar]
- 102.Hoshi O., Ushiki T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP) Arch. Histol. Cytol. 1999;62:253–260. doi: 10.1679/aohc.62.253. [DOI] [PubMed] [Google Scholar]
- 103.Dvorak A. M., Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J. Histochem. Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 104.Vasile E., Qu H., Dvorak H. F., Dvorak A. M. Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J. Histochem. Cytochem. 1999;47:159–167. doi: 10.1177/002215549904700205. [DOI] [PubMed] [Google Scholar]
- 105.Feng D., Nagy J. A., Dvorak H. F., Dvorak A. M. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc. Res. Tech. 2002;57:289–326. doi: 10.1002/jemt.10087. [DOI] [PubMed] [Google Scholar]
- 106.Booth J. W., Trimble W. S., Grinstein S. Membrane dynamics in phagocytosis. Semin. Immunol. 2001;13:357–364. doi: 10.1006/smim.2001.0332. [DOI] [PubMed] [Google Scholar]
- 107.Langeggen H., Namork E., Johnson E., Hetland G. HUVEC take up opsonized zymosan particles and secrete cytokines IL-6 and IL-8 in vitro. FEMS Immunol. Med. Microbiol. 2003;36:55–61. doi: 10.1016/S0928-8244(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 108.Johnson J. D., Hess K. L., Cook-Mills J. M. CD44, alpha(4) integrin, and fucoidin receptor-mediated phagocytosis of apoptotic leukocytes. J. Leukocyte Biol. 2003;74:810–820. doi: 10.1189/jlb.0303092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hess K. L., Tudor K. S., Johnson J. D., Osati-Ashtiani F., Askew D. S., Cook-Mills J. M. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp. Cell Res. 1997;236:404–411. doi: 10.1006/excr.1997.3745. [DOI] [PubMed] [Google Scholar]
- 110.Chimini G., Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- 111.Cahalan M. D., Parker I., Wei S. H., Miller M. J. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat. Rev. Immunol. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kraus J., Ling A. K., Hamm S., Voigt K., Oschmann P., Engelhardt B. Interferon-beta stabilizes barrier characteristics of brain endothelial cells in vitro. Ann. Neurol. 2004;56:192–205. doi: 10.1002/ana.20161. [DOI] [PubMed] [Google Scholar]
- 113.Hermanns M. I., Unger R. E., Kehe K., Peters K., Kirkpatrick C. J. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab. Invest. 2004;84:736–752. doi: 10.1038/labinvest.3700081. [DOI] [PubMed] [Google Scholar]
- 114.Kinard F., Jaworski K., Sergent-Engelen T., Goldstein D., Van Veldhoven P. P., Holvoet P., Trouet A., Schneider Y. J., Remacle C. Smooth muscle cells influence monocyte response to LDL as well as their adhesion and transmigration in a coculture model of the arterial wall. J. Vasc. Res. 2001;38:479–491. doi: 10.1159/000051081. [DOI] [PubMed] [Google Scholar]
- 115.Cuvelier S. L., Patel K. D. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3. J. Exp. Med. 2001;194:1699–1709. doi: 10.1084/jem.194.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cinamon G., Shinder V., Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 117.Jares-Erijman E. A., Jovin T. M. FRET imaging. Nat. Biotechnol. 2003;21:1387–1395. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- 118.McGrath J. L., Hartwig J. H., Tardy Y., Dewey C. F., Jr Measuring actin dynamics in endothelial cells. Microsc. Res. Tech. 1998;43:385–394. doi: 10.1002/(SICI)1097-0029(19981201)43:5<385::AID-JEMT5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 119.Webb D. J., Brown C. M., Horwitz A. F. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr. Opin. Cell Biol. 2003;15:614–620. doi: 10.1016/s0955-0674(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 120.Jones D. A., McIntire L. V., Smith C. W., Picker L. J. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J. Clin. Invest. 1994;94:2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luscinskas F. W., Ding H., Tan P., Cumming D., Tedder T. F., Gerritsen M. E. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J. Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- 122.Setiadi H., McEver R. P. Signal-dependent distribution of cell surface P-selectin in clathrin-coated pits affects leukocyte rolling under flow. J. Cell Biol. 2003;163:1385–1395. doi: 10.1083/jcb.200307178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vajkoczy P., Laschinger M., Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 125.Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J. Exp. Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Argenbright L. W., Letts L. G., Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J. Leukocyte Biol. 1991;49:253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- 127.Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell–cell contact-mediated immune mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lyck R., Reiss Y., Gerwin N., Greenwood J., Adamson P., Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 129.Wang Q., Doerschuk C. M. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J. Immunol. 2000;164:6487–6494. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 130.Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J. Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wautier J. L., Schmidt A. M. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 132.Liao F., Ali J., Greene T., Muller W. A. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J. Exp. Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feng D., Nagy J. A., Pyne K., Dvorak H. F., Dvorak A. M. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J. Histochem. Cytochem. 2004;52:87–101. doi: 10.1177/002215540405200109. [DOI] [PubMed] [Google Scholar]
- 135.Ilan N., Madri J. A. PECAM-1: old friend, new partners. Curr. Opin. Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 136.Kogata N., Masuda M., Kamioka Y., Yamagishi A., Endo A., Okada M., Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell. 2003;14:3553–3564. doi: 10.1091/mbc.E03-02-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]