Abstract
SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) proteins are supposed to mediate the docking and/or fusion of the vesicle with the plasma membrane. However, it is not clearly understood how this process is regulated. In a search for potential SNARE regulators, we recently identified septin 5 (Sept5) as a novel SNARE interacting protein. Septins were first identified as filamentous proteins required for cytokinesis in yeast. Several septins have now been identified in mammals but little is known about their functions. We have previously shown that Sept5 is predominantly expressed in the brain, where it associates with vesicles and membranes through its interaction with the SNARE domain of syntaxin 1A. Furthermore, Sept5 appears to inhibit exocytosis, possibly by regulating vesicle targeting and/or fusion events. To gain insight into the role of Sept5, we have mapped the Sept5 domains important for syntaxin binding. We also investigated the ability of Sept5 to bind to syntaxin when in various protein complexes. Although Sept5 cannot bind an nSec1–syntaxin complex, it can bind syntaxin in a SNARE complex. This interaction is occluded by the binding of α-SNAP, suggesting that Sept5 may regulate the availability of SNARE proteins through its interaction with syntaxin and the 7 S complex.
Keywords: docking, filament, membrane fusion, secretion, septin, SNARE complex
Abbreviations: GST, glutathione S-transferase; NSF, N-ethylmaleimide-sensitive fusion protein; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; SNAP-25, synaptosome-associated protein of 25 kDa; VAMP, vesicle-associated membrane protein
INTRODUCTION
During the exocytosis of synaptic vesicles at the nerve terminal a host of presynaptic proteins ultimately interact to allow release of neurotransmitters into the synaptic cleft. Proteins found both on the vesicle and the presynaptic membrane, called SNAREs (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors), are supposed to be the mediators of vesicle fusion and are the central players in the SNARE hypothesis [1]. The SNARE hypothesis was first formulated in 1993 to describe a general model of vesicle targeting and fusion [2]. The current version of the SNARE hypothesis suggests that a VAMP (vesicle-associated membrane protein) or vesicle (v)-SNARE associates with its cognate target membrane (t)-SNARE, SNAP-25 (synaptosome-associated protein of 25 kDa) and syntaxin, to form a ternary complex with a sedimentation value of 7 S. These SNAREs, localized on opposing membranes, form a tight connection between the membranes in a trans conformation, which is proposed to provide the driving force for membrane fusion. During fusion, the SNARE transmembrane domains will become aligned in parallel in a cis configuration on the same membrane. Once membrane fusion has occurred, this 7 S complex serves as a target for soluble NSF (N-ethylmaleimide-sensitive fusion protein) through its attachment receptor (SNAP), and together they form a 20 S complex. NSF is an ATPase, whose catalytic activity leads to the disassembly of the SNARE complex [3], freeing the SNAREs for subsequent rounds of exocytosis.
Despite the elegance of the SNARE hypothesis, the exact sequence of events underlying SNARE-mediated membrane fusion has not yet been completely elucidated. Recent findings have suggested that the pairing of vesicle and target membrane proteins may not provide sufficient specificity to regulate docking. Significant pools of the target membrane proteins SNAP-25 and syntaxin 1 [4] have also been found on synaptic vesicles, and while these may simply reflect missorted proteins, their abundance suggests that they may also contribute in some way to the specificity or regulation of the fusion process. Interestingly, a recent study has shown that approx. 12% of the syntaxin 1A at boutons is found in intracellular vesicular compartments, yet this pool of syntaxin is present on vesicles that are not capable of evoked release and recycling [5]. Clearly, additional proteins are probably involved in controlling release properties of distinct classes of vesicles in the nerve terminal.
We have recently identified Sept5 (previously called CDCrel-1, see [6] for new nomenclature) in association with components of the membrane fusion apparatus and showed that it may play a role in regulating SNARE function [7]. Sequence analysis of Sept5 reveals that it contains a GTP-binding motif as well as a predicted coiled-coil domain and is homologous with a family of filamentous proteins called septins. First identified as possible components of the 10 nm filaments that surround the mother bud neck in yeast [8], septin proteins have since been found in a wide variety of organisms [9–11]. Near their N-termini, these 40–70 kDa proteins contain a P-loop characteristic of GTP-binding proteins and most of them also contain sequences near their C-termini predicted to form coiled-coils. In budding yeast, mutations in any one of four septins, CDC3, CDC10, CDC11 or CDC12, causes a failure in cell division, presumably through the absence of the septin ring at the mother bud neck [12].
In mammals, the septins Sept2 (Nedd5) and Sept9 (MSF) are concentrated at the cleavage furrow, and the injection of inhibitory antibodies to Sept2 [13] or to Sept9 [14] led to incomplete cytokinesis, implicating a role for both of these septins in this process. Similar to their yeast counterparts, vertebrate septins can also assemble into filaments in vitro [15], although the significance and the regulation of this filamentous organization are not yet understood. As in yeast, the filaments appear to exist as 8 nm wide filaments of variable lengths [8].
In contrast to their yeast counterparts, few proteins interacting with mammalian septins have been discovered. Of these, the exocyst complex was reported to precipitate with a mammalian septin complex [15]. Although a precise role for the mammalian exocyst complex has not yet been elucidated, in yeast this complex is thought to play a role in late stages of post-Golgi transport by either acting as a tether for vesicles or by targeting them to sites of release [16]. Interestingly, the application of dominant-negative forms of exocyst components impaired neurite outgrowth in PC12 cells [17], as did the overexpression of a dominant-negative form of Sept2 [18]. In Drosophila it has recently been found that flies lacking functional copies of Sec5, a component of the exocyst complex, die as first instar larvae [19]. Trafficking assays also demonstrated that there was impairment in the addition of newly synthesized proteins, carried in post-Golgi vesicles, to the plasma membrane, whereas synaptic transmission, which requires the fusion of vesicles to the membrane, was unaltered in the Sec5 mutants. This intriguing observation then suggests that although the exocyst complex may tether or guide vesicles required for cell growth, it does not play a role in transmitter release. Thus, unlike yeast secretion, synaptic vesicle fusions may be mediated by another system of tethers, linking vesicles to the presynaptic membrane.
The only other non-septin interacting partners for mammalian septins that have been identified thus far are the t-SNARE syntaxin [7] and the CDC42p effector protein BORG [20]. We have previously shown, through the purification of synaptic vesicles from rat brain, that a large fraction of Sept5 co-enriches with synaptic vesicles [7]. Septins have also been shown to co-localize with synaptic vesicles by both immunofluorescent microscopy [21] and immunoelectron microscopy [22]. Sept5 is associated with synaptic vesicles through its interaction with syntaxin. Deletion analysis of syntaxin has demonstrated that Sept5 binds predominantly to the H3 coiled-coil region. Interestingly, this is the region of syntaxin that binds to SNAP-25 and VAMP-2 to form the docking–fusion complex, implying a role for Sept5 in exocytosis. Indeed, we found that Sept5 inhibited regulated exocytosis when overexpressed in HIT-T15 cells. Furthermore, platelet secretion assays using platelets from Sept5NULL mice resulted in an enhanced platelet secretion response confirming the hypothesis that Sept5 exerts an inhibitory role in exocytosis [23]. In the present study, we first determine the regions of Sept5 that mediate the binary interaction with syntaxin. We then characterize the ability of Sept5 to bind syntaxin in various protein complexes involving syntaxin and other components of the SNARE cycle. Although Sept5 does not bind to an nSec1–syntaxin complex, Sept5 can bind to syntaxin alone or when it is part of the 7 S complex. However, the addition of α-SNAP to the ternary complex of syntaxin–SNAP-25–VAMP-2 displaces Sept5 suggesting a role for Sept5 before α-SNAP/NSF-mediated SNARE dissociation.
EXPERIMENTAL
Plasmid construction and protein expression
GST–Sept5 (where GST stands for glutathione S-transferase) encompassing residues 1–213 was constructed by digesting the full-length GST–Sept5 [7] with PmlI and EcoRI. The overlapping end created by EcoRI digestion was made blunt through the action of DNA polymerase and the vector was religated. GST–Sept5 (residues 1–60) was digested with AccI and XhoI followed by ligation. The other truncation mutants of GST–Sept5 (residues 110–213 and residues 235–368) were constructed using PCR followed by subcloning into pGEX-KG. Fusion proteins were prepared as described in [24] and affinity purified on glutathione–agarose beads (Sigma).
Isolation of SNARE and SNAP–SNARE complexes
SNARE and SNAP–SNARE complexes were purified essentially as described in [25] on GST–α-SNAP columns except that P2 fractions from five frozen rat brains were used.
Glycerol gradient centrifugation of detergent extracts
Detergent solubilized P2 fractions of frozen rat brains or complexes immunoisolated on GST-α-SNAP as described above were layered on to a 10–35% (w/v) glycerol gradient in buffer A (25 mM Hepes/KOH, pH 7.5, 150 mM NaCl, 0.5% Triton X-100, 1 mM dithiothreitol) plus protease inhibitors (1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 2 mM benzamidine) and subjected to centrifugation at 207000 g in a SW41 centrifuge tube (Beckman Coulter, Mississauga, ON, Canada) for 16 h. Fractions of 1 ml were collected from the top. Catalase (11.3 S) and BSA (4.6 S) were used as sedimentation standards on a parallel gradient.
In vitro protein-binding assay
For syntaxin-binding studies, GST alone or GST-fusion proteins (0.1 nmol) bound to glutathione–agarose beads were incubated at 4 °C for 1.5 h with purified soluble recombinant syntaxin (0.2 nmol) in buffer A but with 0.1% Triton X-100. Beads were then washed three times in buffer A, but with 0.1% Triton X-100. The remaining protein complexes bound to the beads were then electrophoresed on a 10% SDS/PAGE gel and detected by Western blotting using ECL® (Amersham Biosciences).
For GST–Sept5 and either SNAP/SNARE or SNARE-binding studies, the pooled fractions of the 7 S region of glycerol gradient-sedimented SNARE or SNAP–SNARE complexes isolated from a GST–α-SNAP column were incubated with GST alone or GST–Sept5 (0.5 μM) for 2 h. The beads were then washed three times in buffer A, and analysed for the presence of VAMP-2, syntaxin and SNAP-25 by SDS/PAGE and immunoblotting.
For competition studies, GST–Sept5 (0.5 μM) and SNARE complexes were preincubated for 2 h at 4 °C before the addition of recombinant α-SNAP as indicated (0–5 μM). The mixture was further incubated for 1 h and the beads were then washed in buffer A. SNAREs remaining on the GST–Sept5 beads were then resolved by SDS/PAGE followed by Western blotting.
Immunoprecipitations
Anti-syntaxin antibodies (3 μl of HPC-1) were prebound to 15 μl of Protein G–Sepharose beads and incubated with the collected fractions from the glycerol gradient for 1.5 h in buffer A but with 0.1% Triton X-100 and supplemented with 0.1% BSA and 0.1% gelatin (buffer B) plus protease inhibitors. The beads were then washed in buffer B but lacking BSA and gelatin (buffer C) and subjected to SDS/PAGE and Western-blotting analysis. For nSec1 immunoprecipitations, nSec1 antibodies (7.5 μg; StressGen Biotechnologies, Victoria, BC, Canada) were preincubated with 30 μl of Protein A beads in buffer B. Insoluble material from detergent solubilized P2 fractions was first removed by centrifugation at 18500 g for 15 min and the supernatant (0.5 mg) was incubated with the anti-nSec1 beads for 1.5 h at 4 °C with constant rotation. The beads were then washed three times in buffer C and prepared for SDS/PAGE by adding SDS/PAGE loading buffer supplemented with 10 mM N-ethylmaleimide. Proteins bound to the beads were resolved by SDS/PAGE followed by Western blotting. For Sept5 immunoprecipitation, 5 μg of rabbit anti-Sept5 was bound to Protein A beads and mixed with 1 mg of detergent solubilized rat brain P2 fraction. Blots were probed with monoclonal antibodies against syntaxin at 1:7500 (HPC-1), SNAP-25 at 1:1000 and polyclonal rabbit antibody against VAMP-2 (1–500).
RESULTS
Specific association of Sept5 and syntaxin
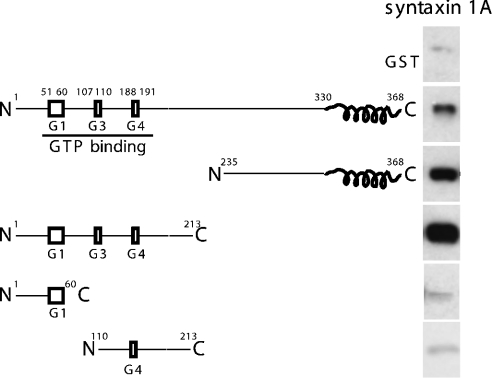
To understand further the molecular interactions that mediate the formation of Sept5–syntaxin complexes, several Sept5 fusion proteins were constructed (Figure 1). N- and C-terminal deletions encompassing various domains of Sept5 were constructed and expressed in bacteria as GST fusion proteins. The GST–Sept5 fusion proteins were immobilized on glutathione beads and the binding of the cytosolic domain of syntaxin to these constructs was analysed. Deletion of the N-terminal 235 amino acids of Sept5 did not abolish its binding to syntaxin suggesting that the C-terminal coiled-coil domains may be involved in these interactions. Surprisingly, proteins lacking the C-terminal 155 amino acids, which included the coiled-coil domain, were also bound to syntaxin. Further deletions within this N-terminal region reduced the binding of syntaxin to levels comparable with the observed background binding to GST, indicating that the intact GTP-binding domain is necessary for syntaxin to bind. Indeed, we had previously shown that the nucleotide-bound state of Sept5 influences binding as point mutations altering the GTP-binding properties of the protein increased binding to syntaxin [24]. The binding of syntaxin to both the N- and C-terminal halves of Sept5 indicates that Sept5 must have at least two syntaxin-binding sites.
Figure 1. Specific interaction of truncation mutants of Sept5 with syntaxin in vitro.
GST alone or truncation mutants of Sept5 (0.1 nmol) were bound to glutathione–agarose and incubated with soluble recombinant syntaxin 1A (0.2 nmol). After washing, syntaxin bound to the various constructs was analysed by SDS/PAGE and immunoblotting with anti-syntaxin antibodies. Whereas GST alone or GST–Sept5 truncations of residues 1–60 and 110–213 did not appreciably bind syntaxin, the binding of the N-terminal region of Sept5 to syntaxin is stronger than that of full-length or C-terminal region of Sept5.
Sept5 is absent from the nSec1–syntaxin complex
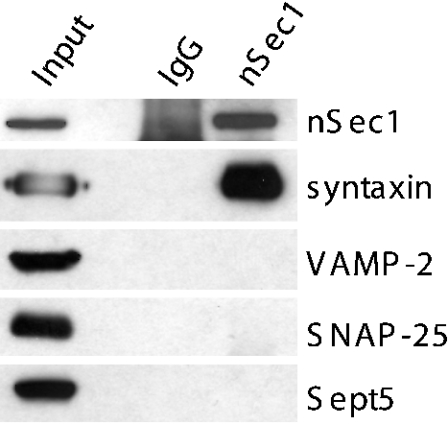
Although these GST-binding assays demonstrate that Sept5 can bind directly to syntaxin in the absence of other proteins, syntaxin in the nerve terminal is known to be associated with many different proteins, forming distinct complexes that probably regulate specific steps in the fusion cycle. We had previously shown that overexpression of Sept5 blocked secretion [7], suggesting an effect on the interaction of syntaxin with components of the core fusion machinery. Structural analyses involving syntaxin 1A fragments have revealed two different structural motifs: a folded three-helix bundle at the N-terminus and a long α-helix at the C-terminus [26]. These two regions of syntaxin can interact intramolecularly to form a closed conformation incapable of binding other SNARE proteins [26]. This closed default conformation of syntaxin, however, is required for its binding to nSec1. The displacement of nSec1 from syntaxin results in the dissociation of the syntaxin C-terminus from the N-terminus, permitting the C-terminal helix to bind SNAP-25 and VAMP-2, thereby forming the 7 S complex. A larger complex of 20 S subsequently forms through the binding of α-SNAP and NSF to the 7 S complex. Since the majority of syntaxin in the brain is present in a binary complex with nSec1 [27], we first asked whether a complex of Sept5, syntaxin and nSec1 could exist. To test this hypothesis detergent solubilized synaptosomal membranes were immunoprecipitated with either anti-nSec1 antibodies or control IgG antibody. As shown in Figure 2, the immunoprecipitates revealed that only syntaxin but not Sept5 co-precipitated with nSec1. Consistent with previous reports [28], neither SNAP-25 nor VAMP-2 was detected in the immunoprecipitates. This indicates that Sept5–nSec1–syntaxin complexes either do not exist in vivo or that they are too transient to be observed by immunoprecipitation. Interestingly, no genetic interaction was seen between a septin yeast mutant cdc12-6 and a mutant of the yeast homologue of nSec1 (sec1-1), providing further evidence that these proteins may not interact [29].
Figure 2. Sept5 does not associate with the nSec1–syntaxin complex.
Detergent extract of rat brain was incubated with anti-nSec1 antibodies (lane 3) or control IgG (lane 2) as described in the Experimental section. The immunoprecipitated samples as well as 20 μg of the starting material were resolved by SDS/PAGE and immunoblotted with various antibodies as indicated.
Sept5 is found in fractions enriched in SNAREs and α-SNAP
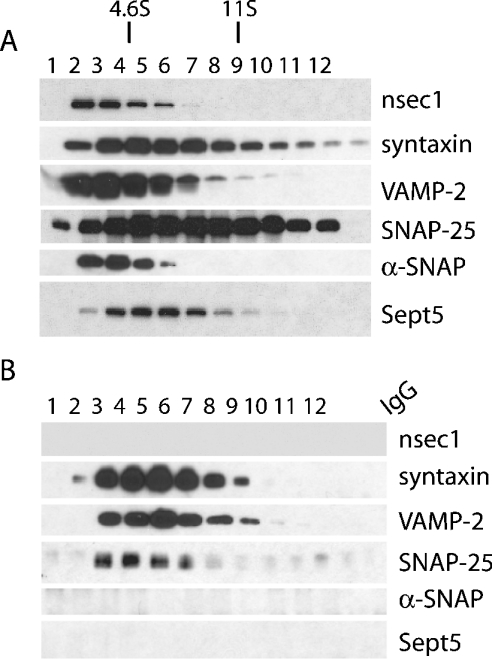
To examine the properties of Sept5 in the brain and determine which syntaxin containing complexes it can associate with, we set out to determine where Sept5 fractionates relative to nSec1 and SNARE components on glycerol gradients from brain. To do this, rat brains were fractionated [7] and LP2 fractions were isolated. These were then solubilized with Triton X-100, spun down to remove insoluble material and the resulting supernatant was layered on a 10–35% glycerol gradient (Figure 3A). The samples were then centrifuged at 207000 g for 16 h and 1 ml fractions were collected and prepared for SDS/PAGE. The distributions of several proteins were then visualized by Western blotting. Syntaxin, VAMP-2 and SNAP-25 were found to predominate in fractions migrating near the 7 S region as reported previously [28]. nSec1 and Sept5 were found to migrate between fractions 2 and 6, in the 4.6–11 S region as did α-SNAP (fractions 2 and 5) [30,31]. To determine whether Sept5 associated with 7 S complexes isolated in this manner, anti-syntaxin antibodies prebound to Protein G beads were incubated with each glycerol gradient fraction. Since these antibodies can immunoprecipitate components of the 7 S complex, immunoprecipitations of the gradient fractions with anti-syntaxin antibodies revealed co-immunoprecipitation of SNAP-25 and VAMP-2, whereas Sept5, like nSec1, was not co-immunoprecipitated (Figure 3B). However, failure to co-immunoprecipitate Sept5 may be a function of the binding site of the HPC-1 antibody since it can recognize syntaxin in 7 S complexes but not when bound to nSec1 or α-SNAP [28].
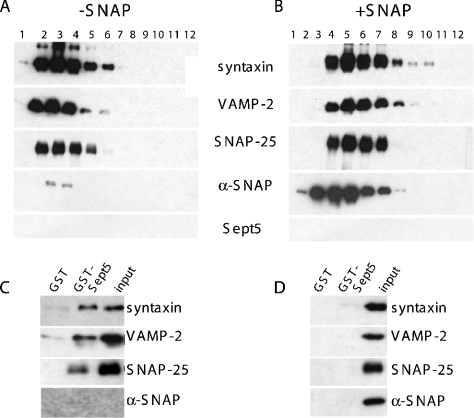
Figure 3. Sedimentation of SNARE proteins.
Rat brain P2 fractions were sedimented on a glycerol gradient (A) and then immunoprecipitated (B) with anti-syntaxin antibodies or control IgG. Although Sept5 fractionated within the 7 S region of the gradient-like syntaxin, SNAP-25 and VAMP-2, no Sept5, α-SNAP or nSec1 was immunoprecipitated with the 7 S complex. The protein markers used were BSA (4.6 S) and catalase (11.3 S).
7 S complexes purified on GST-α-SNAP bind Sept5
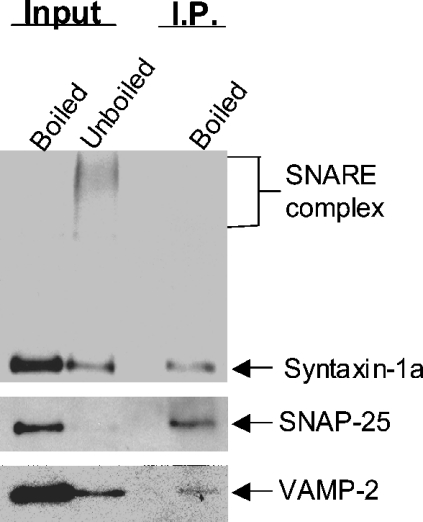
Although the immunoprecipitation of the 7 S complex with anti-syntaxin antibodies suggested that neither nSec1 nor Sept5 was present in the 7 S complex (Figure 3B), the possibility that the immunoprecipitation with anti-syntaxin prevented their visualization, as is the case with α-SNAP, prompted us to search for other evidence that septins interact with the SNARE complex in vivo. We therefore performed immunoprecipitations using anti-Sept5 antibodies. As shown in Figure 4, Triton X-100 extracts from rat brain were immunoprecipitated with anti-Sept5 and probed for VAMP-2, SNAP-25 or syntaxin. The first lane on each blot represents the lysate sample boiled in Laemmli sample buffer to break up the SDS-resistant core SNARE complex and the second lane represents the unboiled sample. The SNARE complex runs as a series of bands between 70 and 150 kDa. After immunoprecipitation with anti-Sept5 antibody, syntaxin 1A, SNAP-25 and VAMP-2 are found in the immunoprecipitates. Since SNAP-25 and VAMP-2 do not bind directly to Sept5 [6], their presence in the immunoprecipitates is due to the binding to syntaxin.
Figure 4. Core SNARE complexes containing SNAP-25 and syntaxin 1A co-immunoprecipitate with Sept5.
Trition X-100 extracts of rat brain were incubated with anti-Sept5 antibodies bound to Protein A beads. Immunoprecipitated material was suspended in Laemmli sample buffer and either loaded directly or boiled (as indicated above each lane). The first two lanes of each blot represent 2% of the initial material (input) used for immunoprecipitation. The probing antibody is indicated to the right of each blot and the bands detected by these antibodies are indicated with arrows.
As further evidence that Sept5 could in fact bind directly to the 7 S SNARE complex, we purified rat brain SNARE complexes based on their α-SNAP-binding properties, since the core complex of syntaxin, SNAP-25 and VAMP-2 [32] serves as a high-affinity α-SNAP receptor. Complexes were isolated from rat brains that were homogenized in high salt buffer to remove endogenous α-SNAP from the SNARE complexes. After extraction of the brain membranes with Triton X-100, GST-α-SNAP fusion proteins were incubated with the salt-washed and detergent-solubilized rat brain extract and then affinity purified on glutathione–agarose beads. Purified SNARE complexes bound to GST–α-SNAP were then eluted from α-SNAP by incubation with n-octylglucoside in high salt concentrations to release SNARE complexes. Alternatively, α-SNAP–SNARE complexes were eluted by incubating the beads with thrombin to cleave the GST–α-SNAP, eluting purified complexes still bound to α-SNAP [25]. These two pools were then sedimented on 10–35% glycerol gradients and resolved by PAGE. Parallel gradients were run using catalase (11.3 S) and BSA (4.6 S) as S standard markers. SNARE complexes (syntaxin, SNAP-25 and VAMP-2) released from the GST–α-SNAP column by detergent and high salt washes migrated between fractions 2 and 6, at the 7 S region (Figure 5A). However, in the presence of α-SNAP, the SNAP–SNARE complex was more dense and sedimented to fractions 3–8 (Figure 5B). Excess free α-SNAP was also eluted from these beads and is predominantly found in fractions 2–5. Of note, Sept5 did not purify by affinity chromatography on a GST–α-SNAP column suggesting (i) that Sept5 does not bind the SNARE complex, (ii) that the initial preparative salt wash to remove endogenous α-SNAP had also extracted Sept5 or (iii) that the SNARE complexes containing Sept5 cannot be purified through a GST–α-SNAP column. To determine if SNARE or SNAP–SNARE complexes can bind Sept5, we first incubated SNARE or SNAP–SNARE fractions from the above glycerol gradients with either GST–Sept5 fusion proteins or GST alone as a control. Interestingly, SNARE complexes lacking α-SNAP (Figure 5C) bound directly to Sept5 but not to GST alone. However, the presence of α-SNAP on these complexes prevented this binding, as shown by the lack of syntaxin, VAMP-2 or SNAP-25 in the GST–Sept5 lane (Figure 5D).
Figure 5. SNAREs but not SNAP–SNAREs associate with Sept5.
GST-α-SNAP immobilized on glutathione–agarose beads were incubated with salt-washed detergent extracts of rat brain and washed to remove unbound material. Precipitated complexes still bound to beads were then either released by incubating with high salt and detergent (A) to yield pure SNARE complexes or thrombin cleaved to yield SNARE complexes bound to α-SNAP (B). These complexes were then sedimented on a glycerol gradient and analysed by SDS/PAGE and immunoblotting with the indicated antibodies. To determine whether Sept5 can bind to 7 S complexes in the absence of α-SNAP, the 7 S region of the gradients containing either pure SNAREs (C) or SNAP–SNAREs (D) were incubated with GST–Sept5 as described in the Experimental section. Whereas SNAREs bound to α-SNAP could not bind GST–Sept5 (D), the absence of α-SNAP allows SNAREs to bind GST–Sept5 (C). The last panel in blot (C) was overexposed to show that no α-SNAP was present. Neither complexes bound GST alone. Results in each panel are representative of three independent experiments.
α-SNAP displaces the 7 S complex from Sept5
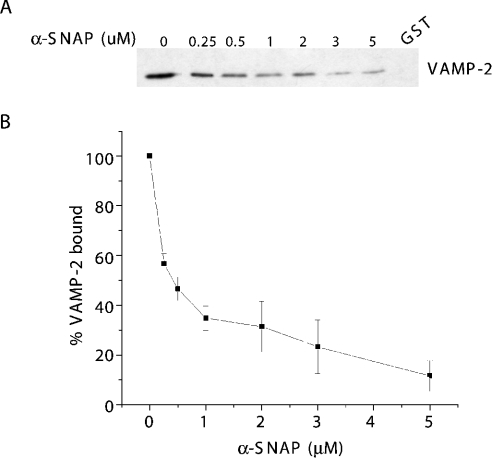
The failure to observe binding of the SNAP–SNARE complex to Sept5 suggested that α-SNAP may occlude the binding site of Sept5 on syntaxin. To examine this further, we measured the ability of α-SNAP to displace SNARE complexes previously bound to GST–Sept5. GST–Sept5–SNARE complexes were mixed with increasing concentrations of recombinant α-SNAP, and the remaining amount of SNARE complexes bound was measured by the presence of VAMP-2 remaining on the bead. As shown in Figure 6, SNARE complexes were readily released from GST–Sept5 as added α-SNAP bound to the SNARE complex. No binding of α-SNAP to GST–Sept5 was detected (results not shown; Figure 5). The results of three independent experiments are shown in graphical form (Figure 6B). Together, these results imply that the Sept5-binding site on syntaxin overlaps with the α-SNAP-binding site but not with those of VAMP-2 or SNAP-25. Since this site is also the one recognized by HPC-1, this probably explains the failure to detect Sept5 in the HPC-1 immunoprecipitates of glycerol gradient fractions (Figure 3B). Furthermore, Sept5 would have been excluded from the α-SNAP purified SNARE complexes (Figure 5) as was the case.
Figure 6. SNARE complexes are released from Sept5 in the presence of α-SNAP.
SNARE complexes immobilized on GST–Sept5 glutathione–agarose beads were incubated with increasing concentrations of recombinant α-SNAP. SNARE complexes remaining bound to GST–Sept5 were then detected by resolving the material on SDS/PAGE followed by immunoblotting with anti-VAMP-2 antibodies to detect the presence of the SNARE complex (A). The values expressed as percentages in (B) are quantifications of the blots from three independent experiments, where 100% VAMP-2 bound represents the amount bound in the absence of α-SNAP.
DISCUSSION
In mammals, Sept5 is predominantly expressed in the brain in post-mitotic neurons [33], suggesting that it is involved in mechanisms other than cytokinesis. In the mouse brain, Sept5 is expressed in presynaptic axon terminals where it is densely distributed near synaptic vesicles [22]. Indeed, we have previously shown that Sept5 co-purifies with synaptic vesicles and is associated with them because of its direct interaction with the t-SNARE syntaxin. In the present study, we have used a combination of biochemical approaches to characterize this interaction in detail since syntaxin exists, in vivo, as part of a larger complex involved in exocytosis. Our results show that monomeric syntaxin is not the only binding partner for Sept5, it also binds to the ternary SNARE complex composed of syntaxin, SNAP-25 and VAMP-2. Moreover, Sept5 is capable of discriminating between different SNARE complexes: the addition of α-SNAP to the 7 S complex causes the displacement of the ternary complex from Sept5. Interestingly, the binding sites for Sept5 as well as those of VAMP-2, SNAP-25 and α-SNAP are all located within the H3 coiled-coil region of syntaxin. Deletion mutagenesis of Sept5 revealed that, unlike SNAP-25 and VAMP-2, the coiled-coil region is not solely responsible for its binding to syntaxin. The interaction of αSNAP with the SNARE complex is largely through electrostatic interactions [34] and Sept5 may bind by similar means. The fact that syntaxin bound to both ends of Sept5, including the N-terminal portion encompassing the GTP-binding region, correlates with our previous observation that syntaxin binding was affected by the nucleotide-bound state of Sept5 [24]. Interestingly, yeast two-hybrid analysis of SPR28p interactions, a sporulation-specific septin in yeast, showed that the binding of this protein with other septins (Spr3p and Ccd11p) appeared to be independent of the C-terminal coiled-coil regions [35], whereas yeast two-hybrid analyses of the Drosophila septins using truncated constructs of Pnut, Sep1 and Sep2 revealed that interactions between specific septin pairs could be mediated by either N-terminal interactions (Sep1–Sep2) and C-terminal interactions (Sep2–Sep2) or a combination of N- and C-terminal-binding interactions (Sep2–Pnut) [36].
The observation that both ends of Sept5 can bind syntaxin suggests that this septin may play an important role in vesicle dynamics through its interaction with syntaxin. We have previously shown that Sept5 inhibits secretion [7] and although Sept5 binds to SNAREs it is unlikely that it is involved in the assembly of the ternary complex. However, Sept5 may recruit other late acting proteins necessary for exocytosis to the fusion site. This role for septins as local concentrating factors has been suggested in yeast, where septins localize factors required for morphogenesis (Spa2p) and exocytosis (Sec3p and Sec5p), maintain actin patches [37] and serve as a template for a contractile ring of F-actin and myosin II [38].
In view of the fact that septin proteins are capable of forming filaments in vitro [15,39,40], an alternative possibility is that mammalian septins may play a negative role in secretion in one of two ways. In one way, septin filaments could tether vesicles away from the plasma membrane, holding vesicles in reserve by physically restricting their movements and preventing the fusion of the vesicle lipid bilayer to the presynaptic membrane (Figure 7A). It is noteworthy that Sept5 co-localizes with the t-SNARE syntaxin in PC12 cells [7] and immunogold labelling [22] of mice brain terminals reveals the presence of Sept5 around synaptic vesicles near the presynaptic terminals. Additionally, quick-freeze deep-etch electron microscopy studies of the presynaptic terminal has revealed filament-like strands linking vesicles to each other as well as strands between synaptic vesicles and plasma membrane [41] that remain unidentified. Septins are attractive candidates for the protein composition of these filaments. In studies involving the giant synaptic terminals of goldfish retinal bipolar neurons, newly arrived vesicles from the reserve pools stopped approx. 20 nm away from the cell membrane [42] suggesting that these vesicles were physically restricted from the plasma membrane. Interestingly, septin filaments isolated from rat brain have a subunit size of 8.25 nm and filament lengths in multiples of 25 nm [15]. Since ternary complexes can be found both in the presynaptic membrane and in the membranes of synaptic vesicles [43], Sept5 could bridge the space between vesicle and plasma membrane, by forming filaments and binding to 7 S complexes, thereby confining the movement of the vesicle until the proper releasing signal occurs. Interestingly, two SNARE family members have recently been identified in the mid-body of mammalian cells during cytokinesis: syntaxin 2 and VAMP-8 [44]. Since septins have also been identified in the mid-body region during cell division [14,24] and since Sept5 preferentially binds to syntaxins 1 and 2 but not to 3 or 4 [7], it is conceivable that the septin–syntaxin interaction during cytokinesis regulates the delivery, or fusion, of vesicles at the membrane, necessary for eventual cell separation.
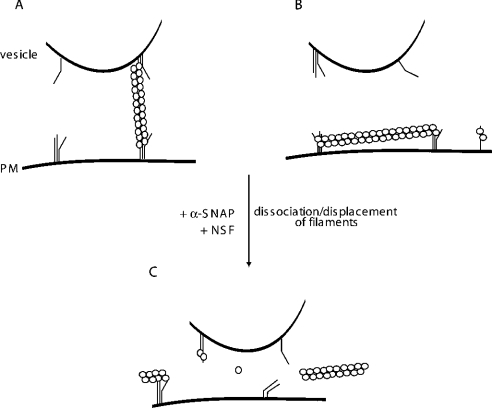
Figure 7. Models for Sept5 organization and role in secretion.
(A) Septins may act as molecular tethers. By binding to 7 S complexes both on the vesicle and the plasma membrane, Sept5 and associated septins (○○) can physically restrict the movement of vesicles towards the membrane. (B) Septins may create a physical barrier to the membrane. Alternatively, septins may form networks parallel to the membrane demarcating inactive zones where SNAREs are incompetent for fusion. (C) α-SNAP displaces the septins. In either scenario, α-SNAP can then displace Sept5 from the 7 S complex, priming the SNAREs for fusion. The formation of trans complexes would then lead to exocytosis.
An alternative version would describe Sept5 as a protein that could physically restrict the fusion of lipid bilayers by creating a grid-like barrier along the plasma membrane, preventing the access of vesicles to specific domains on the plasmalemma (Figure 7B). In a recent study using chromaffin cells, the movement of granules towards and away from the plasma membrane was analysed [45]. As granules approached the membrane, they moved more slowly. This restriction in movement was neither due to the actin cytoskeleton nor to VAMP-2 or SNAP-25, since treatment of the cells with Latrunculin A, tetanus toxin or botulinum toxin respectively caused little change in the restricted motion of granules approaching the plasma membrane. Such septin filaments may also play a role in determining the ‘non-active’ zones of the plasmalemma.
Given the recent results of Mitchell and Ryan [5], who showed that intracellular syntaxin is not localized in actively recycling vesicles, this latter possibility seems more probable, although the septin filaments could serve to exclude vesicles containing syntaxin from the recycling pool. In either case, since α-SNAP can displace Sept5, this displacement could be linked to the ‘priming’ step that may precede vesicle docking. Displacement of Sept5 by α-SNAP would then allow binding of NSF, and the subsequent dissociation of the cis 7 S complexes on the vesicle and membrane (Figure 7C). The dissociated and thus primed SNAREs would then be capable of forming trans SNARE complexes between the vesicle and the membrane that would result in membrane fusion.
In summary, these results provide new insight into the role of Sept5 in exocytosis. Given the diversity in the pattern of expression of eukaryotic septins, these proteins probably regulate events of vesicle exocytosis not only in neuronal cells but also in various cell types. Recently, it has been suggested that animal cytokinesis, very much similar to plant cell division, may occur through the transport of vesicles to the intercellular canal where they could fuse and thus separate mother and daughter cells [46,47]. The presence of both septins [13,48,49] and syntaxins [44] at areas of cleavage furrow formation suggest that these two proteins could co-operate to regulate the membrane fusion events required for cytokinesis.
Acknowledgments
We thank Dr G. Boulianne, M. Surka and Dr H. Xu for helpful comments and critical reading of the manuscript. We are grateful to Dr J.E. Rothman (Sloan Kettering Cancer Center, NY, U.S.A.) for providing us with the α-SNAP cDNA, Dr W.G. Honer (University of British Columbia, Vancouver, Canada) for the Sept5 cDNA and Dr M. Mohtashami (Sunnybrook Hospital Research Institute, Toronto, Canada) for antibodies to α-SNAP. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR). W.S.T. is a recipient of an Investigator Award and C.L.B. is a recipient of a CIHR Doctoral award.
References
- 1.Jahn R., Lang T., Südhof T. C. Membrane fusion. Cell (Cambridge, Mass.) 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 3.Haas A. NSF–fusion and beyond. Trends Cell Biol. 1998;8:471–473. doi: 10.1016/s0962-8924(98)01388-9. [DOI] [PubMed] [Google Scholar]
- 4.Walch-Solimena C., Blasi J., Edelmann L., Chapman E., von Mollard G., Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J. Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell S. J., Ryan T. A. Syntaxin-1A is excluded from recycling synaptic vesicles at nerve terminals. J. Neurosci. 2004;24:4884–4888. doi: 10.1523/JNEUROSCI.0174-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macara I. G., Baldarelli R., Field C. M., Glotzer M., Hayashi Y., Hsu S. C., Kennedy M. B., Kinoshita M., Longtine M., Low C., et al. Mammalian septins nomenclature. Mol. Biol. Cell. 2002;13:4111–4113. doi: 10.1091/mbc.E02-07-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beites C. L., Xie H., Bowser R., Trimble W. S. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- 8.Byers B., Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J. Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimble W. S. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J. Membr. Biol. 1999;169:75–81. doi: 10.1007/s002329900519. [DOI] [PubMed] [Google Scholar]
- 10.Kartmann B., Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J. Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M. The septins. Genome Biol. 2003;4:236. doi: 10.1186/gb-2003-4-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field C. M., Kellogg D. Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita M., Kumar S., Mizoguchi A., Ide C., Kinoshita A., Haraguchi T., Hiraoka Y., Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 14.Surka M. C., Tsang C. W., Trimble W. S. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol. Biol. Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu S. C., Hazuka C. D., Roth R., Foletti D. L., Heuser J., Scheller R. H. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 16.Roth D., Guo W., Novick P. Dominant negative alleles of SEC10 reveal distinct domains involved in secretion and morphogenesis in yeast. Mol. Biol. Cell. 1998;9:1725–1739. doi: 10.1091/mbc.9.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega I. E., Hsu S. C. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega I. E., Hsu S. C. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport. 2003;14:31–37. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Murthy M., Garza D., Scheller R. H., Schwarz T. L. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 20.Joberty G., Perlungher R. R., Sheffield P. J., Kinoshita M., Noda M., Haystead T., Macara I. G. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- 21.Peng X. R., Jia Z., Zhang Y., Ware J., Trimble W. S. The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol. Cell. Biol. 2002;22:378–387. doi: 10.1128/MCB.22.1.378-387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita A., Noda M., Kinoshita M. Differential localization of septins in the mouse brain. J. Comp. Neurol. 2000;428:223–239. doi: 10.1002/1096-9861(20001211)428:2<223::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Dent J., Kato K., Peng X. R., Martinez C., Cattaneo M., Poujol C., Nurden P., Nurden A., Trimble W. S., Ware J. A prototypic platelet septin and its participation in secretion. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beites C. L., Peng X. R., Trimble W. S. Expression and analysis of properties of septin CDCrel-1 in exocytosis. Methods Enzymol. 2001;329:499–510. doi: 10.1016/s0076-6879(01)29111-3. [DOI] [PubMed] [Google Scholar]
- 25.Hohl T. M., Parlati F., Wimmer C., Rothman J. E., Söllner T. H., Engelhardt H. Arrangement of subunits in 20 S particles consisting of NSF, SNAPs, and SNARE complexes. Mol. Cell. 1998;2:539–548. doi: 10.1016/s1097-2765(00)80153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes L. P., Morgan A., Burgoyne R. D. nSec-1 (munc-18) interacts with both primed and unprimed syntaxin 1A and associates in a dimeric complex on adrenal chromaffin granules. Biochem. J. 1999;342:707–714. [PMC free article] [PubMed] [Google Scholar]
- 28.Pevsner J., Hsu S., Braun J., Calakos N., Ting A., Bennett M., Scheller R. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 29.Finger F. P., Novick P. Synthetic interactions of the post-Golgi sec mutations of Saccharomyces cerevisiae. Genetics. 2000;156:943–951. doi: 10.1093/genetics/156.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita Y., Shirataki H., Sakisaka T., Asakura T., Ohya T., Kotani H., Yokoyama S., Nishioka H., Matsuura Y., Mizoguchi A., et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 31.McBride H. M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell (Cambridge, Mass.) 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 32.McMahon H., Südhof T. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J. Biol. Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 33.Honer W., Hu L., Davies P. Human synaptic proteins with a heterogeneous distribution in cerebellum and visual cortex. Brain Res. 1993;609:9–20. doi: 10.1016/0006-8993(93)90848-h. [DOI] [PubMed] [Google Scholar]
- 34.Marz K. E., Lauer J. M., Hanson P. I. Defining the SNARE complex binding surface of alpha-SNAP: implications for SNARE complex disassembly. J. Biol. Chem. 2003;278:27000–27008. doi: 10.1074/jbc.M302003200. [DOI] [PubMed] [Google Scholar]
- 35.De Virgilio C., DeMarini D. J., Pringle J. R. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology. 1996;142:2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- 36.Al-Awar O. S., Pringle J. R. Ph.D. thesis. Chapel Hill: Department of Biology, University of North Carolina at Chapel Hill; 1996. The role of septins in the morphogenesis of Schizosaccharomyces pombe and Drosophila melanogaster; pp. 60–103. [Google Scholar]
- 37.Barral Y., Mermall V., Mooseker M. S., Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- 38.Lippincott J., Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazier J. A., Wong M. L., Longtine M. S., Pringle J. R., Mann M., Mitchison T. J., Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field C. M., Al-Awar O., Rosenblatt J., Wong M. L., Alberts B., Mitchison T. J. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J. Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirokawa N., Sobue K., Kanda K., Harada A., Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J. Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zenisek D., Steyer J. A., Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature (London) 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- 43.Otto H., Hanson P., Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low S. H., Li X., Miura M., Kudo N., Quinones B., Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev. Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 45.Johns L. M., Levitan E. S., Shelden E. A., Holz R. W., Axelrod D. Restriction of secretory granule motion near the plasma membrane of chromaffin cells. J. Cell Biol. 2001;153:177–190. doi: 10.1083/jcb.153.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skop A. R., Bergmann D., Mohler W. A., White J. G. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr. Biol. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu H., Brill J. A., Hsien J., McBride R., Boulianne G. L., Trimble W. S. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev. Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- 48.Adam J. C., Pringle J. R., Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol. Biol. Cell. 2000;11:3123–3135. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fares H., Peifer M., Pringle J. R. Localization and possible functions of Drosophila septins. Mol. Biol. Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]