Abstract
In response to UV irradiation, mammalian cells elicit a gene expression programme designed to repair damage and control cell proliferation and apoptosis. Important members of this stress response include the NF-κB (nuclear factor-κB) family. However, the mechanisms by which UV irradiation activates NF-κB are not well understood. In eukaryotes, a variety of environmental stresses are recognized and remediated by a family of protein kinases that phosphorylate the α subunit of eIF2 (eukaryotic initiation factor-2). In the present study we show that NF-κB in MEF (murine embryo fibroblast) cells is activated by UV-C and UV-B irradiation through a mechanism requiring eIF2α phosphorylation. The primary eIF2α kinase in response to UV is GCN2 (general control non-derepressible-2), with PEK/PERK (pancreatic eIF2α kinase/RNA-dependent-protein-kinase-like endoplasmic-reticulum kinase) carrying out a secondary function. Our studies indicate that lowered protein synthesis accompanying eIF2α phosphorylation, combined with eIF2α kinase-independent turnover of IκBα (inhibitor of κBα), reduces the levels of IκBα in response to UV irradiation. Release of NF-κB from the inhibitory IκBα would facilitate NF-κB entry into the nucleus and targeted transcriptional control. We also find that loss of GCN2 in MEF cells significantly enhances apoptosis in response to UV exposure similar to that measured in cells deleted for the RelA/p65 subunit of NF-κB. These results demonstrate that GCN2 is central to recognition of UV stress, and that eIF2α phosphorylation provides resistance to apoptosis in response to this environmental insult.
Keywords: eukaryotic initiation factor-2 (eIF2), general control non-derepressible-2 (GCN2), nuclear factor κB (NF-κB), stress response, translational control, UV irradiation
Abbreviations: ATF4, activating transcription factor 4; bZIP, basic leucine zipper; CHOP, C/EBP (CCAAT-enhancer-binding protein)-homologous protein; CK2, casein kinase II; CRE, cAMP response element; CREB, cAMP-response-element-binding protein; CreP, constitutive repressor of eIF2α (eukaryotic initiation factor-2α) phosphorylation; DMEM, Dulbecco's modified Eagle's medium; EMSA, electrophoretic-mobility-shift assay; ER, endoplasmic reticulum; GADD153 and GADD34, growth arrest and DNA damage inducible gene 153 and 34; GCN2, general control non-derepressible-2; HRI kinase, haem-regulated inhibitory kinase; IκB, inhibitory κB; IKK, IκB kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEF, mouse embryo fibroblast; MG132, N-Cbz (benzyloxycarbonyl)-Leu-Leu-Leu-al; NF-κB, nuclear factor κB; OCT1, Octamer-1; PEK, pancreatic eIF2α kinase; PERK, RNA-dependent-protein-kinase-like ER kinase; PEST, Pro-Glu-Ser-Thr; PKR, protein kinase regulated by RNA; PPP1R15B, phosphorylation/protein phosphatase 1, regulatory (inhibitor) subunit 15b; RSB, reticulocyte standard buffer; SAPK, stress-activated protein kinase; TNFα, tumour necrosis factor α; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin in situ nick end labelling; URE, upstream regulatory element
INTRODUCTION
UV irradiation from the sun is the main causative agent in the formation of non-melanoma skin cancer, and the risk for exposure to harmful UV irradiation is rising with the continued destruction of the protective atmospheric ozone layer. Exposure of mammalian cells to UV irradiation rapidly stimulates many well known transcription factors, such as p53 and NF-κB (nuclear factor κB) [1–4]. Whereas activation of p53 is thought to be a direct consequence of nuclear signals triggered by damaged DNA, the molecular mechanisms by which exposure to UV-C and UV-B activate NF-κB rely on cytoplasmic signals that are generated independently of DNA damage [4–7].
The NF-κB/Rel family of transcription factors regulate the expression of genes critical for multiple biological processes, including inflammatory reactions, immune responses, cell proliferation and apoptosis [8–11]. The cellular localization of NF-κB is tightly controlled by a protein family designated IκBs (inhibitory κB), the most prominent and well-studied being IκBα. Nuclear uptake of NF-κB is blocked by its tight association with IκBα. Exposure of cells to a variety of extracellular signals, such as TNFα (tumour necrosis factor α), interleukin-1 or bacterial lipopolysaccharide leads to the rapid phosphorylation of IκBα at two amino acid residues, namely Ser32 and Ser36, by IKK (IκB kinase). This targeted IκBα phosphorylation contributes to its ubiquitination and, ultimately, proteasome-mediated degradation. Decreased levels of IκBα lead to the release of NF-κB, facilitating translocation of this transcription factor into the nucleus to regulate expression of its target genes.
The molecular mechanisms regulating NF-κB in response to UV exposure are less well understood. Activation of NF-κB by UV irradiation is slower than cytokine stimulation, taking between 1 and 3 h after UV treatment [12,13]. Whereas ubiquitin-mediated degradation of IκBα is proposed to be required for full induction of NF-κB by UV exposure, such degradation appears to occur independently of IKK phosphorylation of IκBα [13,14]. Recently, Kato et al. [15] suggested that the UV-mediated reduction in IκBα levels occurs via a mechanism involving CK2 (casein kinase II) phosphorylation of one or more serine residues in the PEST (Pro-Glu-Ser-Thr) domain of IκBα. CK2 activity is proposed to be UV inducible through a mechanism involving activation of the p38 MAPK (mitogen-activated protein kinase) pathway.
We are interested in the mechanisms by which eukaryotic cells process diverse stress signals to regulate programmes of gene expression designed to prevent or remedy cellular damage. Central to this stress response is a family of protein kinases that phosphorylate the α subunit of eIF2 (eukaryotic initiation factor-2) [16,17]. The eIF2α kinases includes GCN2 (general control non-derepressible-2), which is activated by amino acid limitation [18–22], PEK/PERK [pancreatic eIF2α kinase/RNA-dependent-protein-kinase-like ER (endoplasmic recticulum) kinase], which is important for remedying protein malfolding in the ER [23], HRI kinase (haem-regulated inhibitory kinase), which couples protein synthesis to haem availability in erythroid cells [24] and PKR (protein kinase regulated by RNA), which participates in an antiviral defence pathway induced by interferon [25]. Phosphorylation of eIF2α during cellular stress impedes the ability of eIF2 to deliver initiator Met-tRNAiMet to the translation machinery [26]. The resulting reduction in eIF2 activity lowers global protein synthesis concomitant with induced translation of selected mRNAs including ATF4 (activating transcription factor 4), a transcriptional activator of stress remedy genes [18,27,28]. Furthermore, we recently reported that eIF2α phosphorylation is linked to activation of NF-κB in response to ER stress and nutritional deprivation by mechanisms independent of IKK phosphorylation of IκBα [29].
In addition to the stress conditions highlighted above, there have been several reports coupling UV irradiation to eIF2α phosphorylation or its target transcription activator. Deng et al. [30] reported that UV-C irradiation elicits phosphorylation of eIF2α by GCN2 via a mechanism that does not require SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) or p38 MAP kinases. Wu et al. [31] suggested that UV-C treatment induced the eIF2α kinase PEK. Phosphorylation of eIF2α by PEK may play a role in cell-cycle arrest after UV irradiation. Exposure of yeast to UV irradiation leads to activation of Gcn4, a bZIP (basic leucine zipper) transcriptional activator related to ATF4, whose expression is induced by GCN2 phosphorylation of eIF2α during amino acid limitation [32]. Such activation of Gcn4 was proposed to be required for induction of target genes involved in nucleotide biosynthesis, contributing to DNA repair.
Given the important role of eIF2α phosphorylation as an upstream sensor and regulator in stress-response pathways, and the linkage between eIF2α kinases and UV exposure, we addressed the question as to whether phosphorylation of eIF2α facilitates activation of NF-κB by UV irradiation. We find that mouse embryo fibroblasts (MEFs) deleted for GCN2 or containing a homozygous mutation at the eIF2α phosphorylation site (Ser51→Ala) block activation of NF-κB. The basis for this reduction is that eIF2α phosphorylation by GCN2 contributes to significantly lower levels of general translation, including decreased synthesis of IκBα. Decreased IκBα translation, along with eIF2α kinase-independent turnover of IκBα, contributes to the lowered levels of IκBα and activation of NF-κB. These results indicate that, in addition to induced translational expression of regulatory proteins such as ATF4, eIF2α phosphorylation and the accompanying reduced general translation can regulate stress-gene expression by lowering the levels of key regulatory proteins that are subject to rapid turnover.
EXPERIMENTAL
Cell culture and stress conditions
GCN2−/− and PEK−/−, and their wild-type counterparts, were immortalized by simian-virus-40 large T antigen and have been described previously [29,33,34]. Immortalized S/S MEF cells expressing wild-type eIF2α, and A/A MEF cells (containing eIF2α with alanine replacing serine at the position-51 phosphorylation site), were previously described [33]. The RelA/p65−/− MEF cells [35,36] and the immortalized cells were cultured in DMEM (Dulbecco's modified Eagle's medium; BioWhittaker), supplemented with 2 mM glutamine, 1 mM non-essential amino acids, 10% (v/v) fetal-bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. A/A MEF cells require additional amino acid supplements for growth viability, and therefore the A/A and the wild-type counterpart S/S cells were maintained under these enriched-media conditions. MEF cells were grown to 50–70% confluency and were irradiated at the indicated UV-C dose (254 nm; UV Stratalinker 1800, Stratagene). Alternatively, UV-B irradiation of cells was carried out using a Philips FS-20 UVB lamp that was filtered to eliminate residual UV-C. The intensity of the UV-B exposure was measured for each experiment using an IL1700 radiometer and an SED240 UV-B detection instrument (International Light, Newburyport, MA, U.S.A.) at a distance of 8 cm from the UV-B source to the monolayer of cells. As a control for IκBα phosphorylation and subsequent degradation, 10 ng/ml TNFα was added to MEF cells in DMEM for 30 min.
EMSAs (electrophoretic-mobility-shift assays)
Nuclear extracts were prepared from MEF cells as described previously [37]. MEF cells were subjected 40 J/m2 UV-C irradiation, 1 μM thapsigargin or 10 ng/ml TNFα, as indicated. To determine the role of ubiquitin-mediated degradation in the activation of NF-κB, one set of experiments involved the addition of 1 μM MG132 [N-Cbz (benzyloxycarbonyl)-Leu-Leu-Leu-al, a proteasome inhibitor] 30 min prior to exposure to these stress agents. Following the stress treatment, cells were resuspended in 1 ml of cold hypo-osmotic RSB (reticulocyte standard buffer) [10 mM Tris (pH 7.4)/10 mM NaCl/3 mM MgCl2] supplemented with 0.5% Nonidet P40 and protease inhibitors (100 μM of PMSF/0.15 μM aprotinin/1 μM leupeptin/1 μM pepstatin). Cell lysis was carried out using a Dounce homogenizer and, following centrifugation (13000 g, 10 min), the nuclei pellets were resuspended in 2 packed nuclear vol. of extraction buffer C [420 mM KCl/20 mM Hepes (pH 7.9)/1.5 mM MgCl2/0.2 mM EDTA/20% (v/v) glycerol] supplemented with protease inhibitors. Protein concentrations were determined using the Bradford assay. The sequence of the DNA fragment containing the URE (upstream regulatory element) NF-κB binding element derived from c-myc was 5′-GATCCAAGTCCGGGTTTTCCCCAACC-3′ [29] and the Octamer-1 (OCT1) binding element was 5′-GATCTGTCGAATGCAAATCACTAGAA-3′ [37]. Binding reaction mixtures contained 32P-labelled DNA fragments (20000–25000 c.p.m.), 5 μg of nuclear extract, and 2.5 μg of poly(dI-dC) as non-specific competitor were added to a solution of 10 mM Hepes, pH 7.9, 4 mM dithiothreitol, 0.5% Triton X-100, 100 mM KCl and 2.5% (v/v) glycerol in a final assay volume of 25 μl. Binding mixtures were incubated at room temperature for 30 min. DNA/protein complexes were separated by gel electrophoresis and visualized by autoradiography. To address NF-κB binding specificity, unlabelled competitor URE or CREB (cAMP-response-element-binding protein) DNA fragments were added at the indicated stoichiometry to the binding mixture. The CREB competitor DNA included a previously reported ATF consensus binding sequence, 5′-TGACGTCA-3′ [38]. Supershift studies were carried out by including polyclonal antibody specific to p65 (Upstate Biotechnology), p50 (Santa Cruz) or c-Rel (Santa Cruz), as indicated, in the binding mixture. Mixtures were analysed by EMSA and autoradiography.
Preparation of protein lysates and immunoblot analyses
MEF cells that were exposed to UV irradiation, or to no stress, were washed twice with ice-cold PBS and lysed using Breaking Solution [50 mM Tris/HCl (pH 7.9)/150 mM NaCl/1% Nonidet P40/0.1% SDS/100 mM NaF/17.5 mM β-glycerol phosphate/10% glycerol, supplemented with protease inhibitors]. Lysates were subjected to sonication for 30 s and clarified by centrifugation (13000 g, 10 min). Protein content was measured by using the Bio-Rad protein quantification kit for detergent lysis, and equal amounts of each sample were subjected to SDS/PAGE. Proteins were transferred to nitrocellulose filters, and the filters were incubated in TBS-T solution [20 mM Tris/HCl (pH 7.9)/150 mM NaCl/0.2% Tween-20, supplemented with 4% (w/v) non-fat dried milk) and antibodies that specifically recognize ATF4, ATF3, CHOP [C/EBP (CCAAT-enhancer-binding protein)-homologous protein], p65, IκBα, actin or eIF2α. Phosphorylation of eIF2α and IκBα were measured by using a polyclonal antibody that recognizes eIF2α phosphorylated at Ser51 (Invitrogen, Carlsbad, CA, U.S.A.), or IκBα phosphorylated at serine-32 (Cell Signaling Technology, Inc., Beverly, MA, U.S.A.). Following three washes in TBS-T, filters were incubated with TBS-T containing secondary antibody conjugated to horseradish peroxidase (Bio-Rad) and spots were visualized using a chemiluminescent substrate. Low- and high-molecular-mass ranges of polypeptide markers (Bio-Rad) were used in the SDS/PAGE analysis to determine the sizes of proteins detected in the immunoblots.
Radiolabelling assays
Translation inhibition was measured as previously described [18]. Briefly, GCN2+/+ and GCN2−/− MEF cells were seeded at 2×105 cells per 60 mm-diameter dish and grown to 50% confluency in DMEM as described above. Cells were washed twice with PBS and incubated in 4 ml of trans-labelling medium without methionine or cysteine supplemented with 10% (w/v) dialysed foetal bovine serum. The MEF cells were then labelled with 500 μCi of [35S]Met/Cys Express Labeling Mix (ICN) for 30 min, and washed twice with ice-cold PBS containing non-radiolabelled methionine and cysteine. Cell lysates were prepared using Breaking Solution and clarified by centrifugation. Alternatively, the MEF cells were then subjected to 40 J/m2 UV-C, or no stress, and incubated for between 0 and 2 h before transferring to the trans-labelling medium for 30 min and the [35S]Met/Cys Labeling Mix for 30 min. Equal amounts of total protein were separated by SDS/PAGE, and radiolabelled protein was visualized by autoradiography. Half-life measurements were carried out by radiolabelling cells under nonstress conditions for 30 min, washing with non-radiolabelled medium, and incubation in medium containing non-radiolabelled methionine and cysteine. MEF cells were then treated with 40 J/m2 UV-C, followed by incubation in non-radiolabelled medium for the indicated length of time. Lysates were prepared from the radiolabelled cells, and IκBα and p65 were immunoprecipitated using protein-specific antibodies. Immunoprecipitated proteins were analysed by SDS/PAGE and visualized by autoradiography. Immunoblots measuring total levels of actin were carried out in parallel to be certain that similar levels of total protein were analysed in each lysate preparation.
Apoptosis assays
GCN2−/− and RelA/p65−/− MEF cells, and their wild-type counterparts, were exposed to UV-B or UV-C irradiation and the number of apoptotic cells were visually counted by using the In Situ Cell Death Detection Kit (Roche) that uses the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin in situ nick end labelling) reaction to label fragmented DNA with free 3-ends. Cells were visualized by microscopy, and 100 cells in each field were scored for apoptosis. Mean values±S.D. were derived from three independent experiments. ANOVA and a Bonferroni post test (post hoc test) of the apoptotic measurements were carried out by using GraphPad Prism (San Diego, CA, U.S.A.) software. Significant differences were defined as P<0.05 for all tests. Activation of caspases 3 and 8 were measured by immunoblot analysis using polyclonal antibodies specific to each caspase.
RESULTS
Phosphorylation of eIF2α by GCN2 is induced in response to UV-C and UV-B irradiation
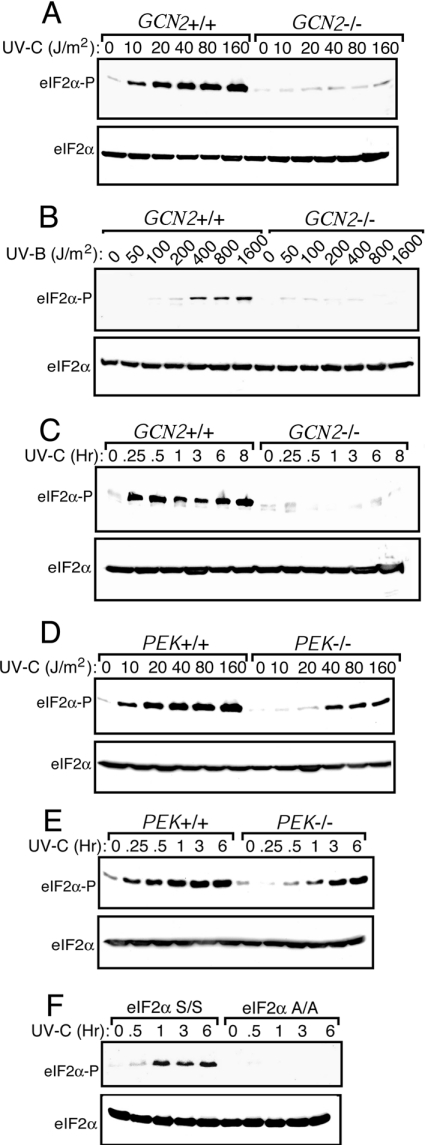
eIF2α kinases GCN2 and PEK have been suggested to elicit translational control in response to UV irradiation [30,31]. To address this model, we measured eIF2α phosphorylation in GCN2−/− and PEK−/− MEF cells and their wild-type counterparts in response to increasing amounts of UV-C and UV-B irradiation. Phosphorylation of eIF2α, as judged by immunoblot using polyclonal antibody specific to eIF2α phosphorylated at the regulatory residue Ser51, occurs with as little as 10 J/m2 of UV-C, with a maximum at 40 J/m2 (Figure 1A). Similar levels of total eIF2α were observed between the stressed and non-stressed lysates using antibody that recognizes both phosphorylated and non-phosphorylated forms of eIF2α. In the case of UV-B exposure, significant eIF2α phosphorylation was detected between 200 and 400 J/m2 (Figure 1B). Induction of eIF2α phosphorylation was rapid, within 15 min of UV-C exposure of MEF cells, and was sustained following 8 h of UV stress (Figure 1C). Minimal eIF2α phosphorylation was detected in MEF cells devoid of GCN2 function in response to either UV-C or UV-B irradiation (Figures 1A, B, and C).
Figure 1. Phosphorylation of eIF2α by GCN2 is enhanced in response to UV irradiation.
GCN2+/+ and GCN2−/− MEF cells were subjected to increasing dosages of UV-C (A) or UV-B irradiation (B). After UV treatment, cells were incubated in the culture medium for 3 h and the levels of phosphorylation of eIF2α were measured by immunoblot by using a polyclonal antibody specific to eIF2α phosphorylated at Ser51. Equal amounts of proteins were analysed in each lane. Levels of total eIF2α were assayed by using an antibody that recognizes both phosphorylated and non-phosphorylated versions of the translation initiation factor. GCN2+/+ and GCN2−/− (C) or PEK+/+ and PEK−/− (E) MEF cells were subjected to 30 J/m2 UV-C irradiation, incubated in the culture medium for between 0.25 and 8 h as indicated, and eIF2α phosphorylation was measured by immunoblot. (D) PEK+/+ and PEK−/− MEF cells were subjected to increasing dosages of UV-C, cultured for 3 h, and the amounts of eIF2α phosphorylation were measured by immunoblot. (F) S/S and A/A MEF cells were exposed to 30 J/m2 UV-C irradiation, and after between 0.5 and 6 h incubation as indicated, phosphorylated eIF2α and total eIF2α levels were measured by immunoblot. Immunoblots in each panel are representative of three independent experiments.
Deletion of PEK led to some reduction in eIF2α phosphorylation in MEF cells exposed to UV-C. Analysis of increasing dosages of UV-C indicated that there was significantly lowered eIF2α phosphorylation in PEK−/− MEF cells treated with 10 or 20 J/m2, whereas cells exposed to higher amounts of UV-C (40–160 J/m2) showed induced levels of phosphorylation that were partially decreased compared with wild-type cells (Figure 1D). Phosphorylation of eIF2α in response to 30 J/m2 was modestly delayed with the loss of PEK activity, with eIF2α phosphorylation in PEK+/+ being maximally induced between 0.5 and 1 h, and upwards of 3 h in PEK−/− cells (Figure 1). No UV induction of eIF2α phosphorylation was detected using A/A MEF cells, demonstrating the specificity of our immunoblot assay (Figure 1F). We conclude that UV-C or UV-B exposure enhances GCN2 phosphorylation of eIF2α at Ser51 in MEF cells, and that PEK functions as a secondary eIF2α kinase during UV stress.
eIF2α kinase-targeted genes, ATF4 and CHOP are not induced in response to UV stress
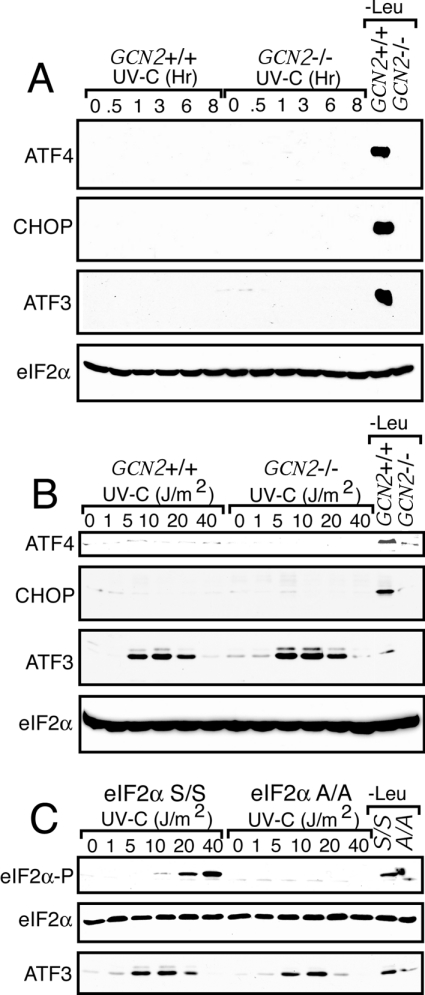
Phosphorylation of eIF2α induces stress-gene expression through activation of transcription factors ATF4 and NF-κB [18,27,29]. In the example of ATF4, eIF2α phosphorylation in response to ER or nutritional stress stimulates ATF4 translation via a mechanism involving translation reinitiation and two open reading frames in the 5′-leader of the ATF4 mRNA [18,28]. Increased levels of ATF4 mRNA are also associated with cellular stress [39]. ATF4 contributes to induction of additional transcription factors, including ATF3 and CHOP/GADD153 (growth arrest and DNA damage inducible gene 153), which together direct a programme of stress-related gene expression [18,33,40]. We measured induction of ATF4 and its target genes in GCN2+/+ and GCN2−/− MEF cells by immunoblot analysis after 40 J/m2 UV-C irradiation, an exposure that gave high levels of eIF2α phosphorylation (Figure 2). No increase in ATF4, ATF3 or CHOP protein levels were detected in either GCN2+/+ or GCN2−/− MEF cells after 8 h of 40 J/m2 UV irradiation (Figure 2A). Additionally, there was no increase in the levels of ATF4, ATF3 or CHOP mRNA after 40 J/m2 UV-C irradiation in either GCN2+/+ or GCN2−/− MEF cells (results not shown). Consistent with previous reports, expression of each of these transcription factors was significantly increased in GCN2+/+ MEF cells subjected to leucine starvation, and this induction was blocked in GCN2−/− cells (Figure 2A) [18,33]. We also measured expression of these eIF2α kinase stress-response genes after exposure from between 1 and 40 J/m2 UV irradiation. No expression of ATF4 or CHOP were detected after treatment with these UV-C dosages (Figure 2B). Interestingly, ATF3 expression was significantly increased between 5 and 20 J/m2, with minimal expression at 40 J/m2 UV-C. This induction was detected in both GCN2+/+ and GCN2−/− MEF cells, as well as in S/S (wild-type eIF2α) and A/A ([Ser51→Ala]eIFα) MEF cells, indicating that there are regulatory mechanisms in response to certain stress conditions that can lead to enhanced expression of ATF3 independent of eIF2α phosphorylation (Figures 2B and 2C).
Figure 2. Phosphorylation of eIF2α in response to UV irradiation does not induce expression of ATF4 and its target genes.
(A) GCN2+/+ and GCN2−/− MEF cells were exposed to 40 J/m2 UV-C or no stress (0 h), and incubated in culture medium for between 0.5 and 8 h, as indicated. Levels of ATF4, CHOP, ATF3 and eIF2α were measured by immunoblot using antibodies specific to each of these proteins. (B) GCN2+/+ and GCN2−/− MEF cells were exposed to increasing dosages of UV-C, as indicated, and then cultured for 6 h prior to immunoblot measurements of ATF4, CHOP, ATF3 and eIF2α. (C) S/S and A/A MEF cells were exposed to increasing dosages of UV-C, cultured for 6 h, and ATF3, phosphorylated eIF2α, and total eIF2α levels were measured by immunoblot analysis. Results shown are representative of three independent experiments.
Phosphorylation of eIF2α activates NF-κB during UV irradiation
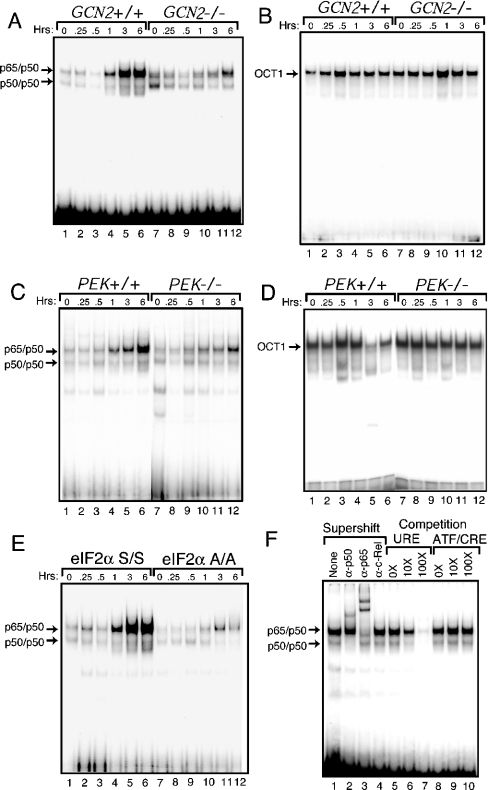
To address whether phosphorylation of eIF2α contributes to NF-κB activation in MEF cells in response to UV irradiation, we performed an EMSA using radiolabelled DNA containing a NF-κB binding site. Nuclear lysates were prepared from GCN2+/+ and GCN2−/− MEF cells exposed to 40 J/m2 UV-C or no stress. Significant DNA binding was detected in the GCN2+/+ cells 1 h after the UV exposure, with further increases following 3 and 6 h of UV irradiation (Figure 3A). By contrast, there were only low levels of NF-κB activity in GCN2−/− cells after 6 h of the UV insult. No differences were found in the EMSA using radiolabelled DNA containing OCT1 binding site (Figure 3B). A similar EMSA analysis of PEK−/− MEF cells revealed a similar time course for activation of NF-κB compared with PEK+/+ cells, although the levels of NF-κB binding were partially decreased (Figure 3C).
Figure 3. GCN2 is required for activation of NF-κB in response to UV exposure.
GCN2+/+ and GCN2−/− (A and B) or PEK+/+ and PEK−/− (C and D) MEF cells were exposed to 40 J/m2 UV-C or no stress (0 h). After UV stress, the MEF cells were cultured for the indicated time, and nuclear lysates were utilized in an EMSA with radiolabelled DNA containing a NF-κB (A and C) or control OCT1 (B and D) binding site. (E) A similar EMSA study was carried out using nuclear lysates prepared from UV-stressed MEF cells containing eIF2α with wild-type Ser51 (S/S) or with alanine replacing serine in the eIF2α phosphorylation site at position 51 (A/A). (F) In lanes 2–4, supershift indicates that polyclonal antibodies that specifically recognize NF-κB subunits, p50, p65, or Rel-C were added to the EMSA binding mixture. None indicates that no antibody was used in the assay (lane 1). To determine the specificity for the NF-κB binding site in the EMSA, non-radiolabelled DNA containing the NF-κB site (lanes 5–7) or ATF/CRE (8–10) were added to the EMSA binding mixtures containing nuclear lysates prepared from S/S MEF cells. Competitor DNA was added at 10- or 100-fold molar excess. Arrows indicate DNA complexed with p65/p50 or p50/p50. In each experiment excess free radiolabelled probe was present at the bottom of the panel. Portions of the radiolabelled free probes in (B) and (E) migrated off the bottom of the polyacrylamide gel during the EMSA. EMSA results shown are representative of three independent experiments.
The role of phosphorylation of eIF2α in the activation of NF-κB was directly addressed by using MEF cells containing wild-type (S/S) or the mutant form of eIF2α (A/A). Activation of NF-κB occurred in the S/S cells exposed to UV, while minimal NF-κB binding was detected in the A/A cells (Figure 3E). The enhanced DNA binding was specific for NF-κB, as confirmed by using non-radiolabelled competitor DNA containing an NF-κB binding site (Figure 3F). By contrast, no competition was found using a non-radiolabelled DNA containing an ATF/CRE (cAMP response element) DNA binding element. The NF-κB binding was attributed to NF-κB dimers p65/p50 and p50/p50, as judged by antibody supershift experiments [14] (Figure 3F). These results indicate that eIF2α phosphorylation by GCN2 is central to activation of NF-κB in response to UV irradiation.
Decreased levels of IκB contribute to activation of NF-κB during UV stress
One mechanism facilitating nuclear localization of NF-κB involves phosphorylation and proteolysis of IκBα. We wished to measure IκBα phosphorylation at Ser32 and IκBα protein levels in the GCN2+/+ and GCN2−/− MEF cells in response to UV irradiation. As a control, we treated the MEF cells with TNFα, a cytokine that induces IKK phosphorylation of IκB at Ser32 and Ser36, leading to ubiquitin-mediated proteolysis of IκB [41,42]. No phosphorylation of eIF2α was detected after exposure to TNFα (results not shown) [29]. While TNFα induced phosphorylation of IκBα in both the GCN2+/+ and GCN2−/− cells, as judged by immunoblot using a polyclonal antibody specific to IκBα phosphorylated at Ser32, no IκBα phosphorylation was detected in either MEF cell line exposed to UV irradiation (Figure 4A). Total levels of IκBα were also measured by immunoblot analysis. Consistent with the TNFα-mediated phosphorylation of IκB, there was a decrease in IκBα levels in both the GCN2+/+ and GCN2−/− MEF cells after treatment with this cytokine. Interestingly, the IκBα levels were also rapidly reduced in the GCN2+/+ MEF cells in response to UV irradiation, with a significant decrease in the amounts of IκBα within 1 h following UV stress (Figure 4A). By comparison, an appreciable decrease in IκBα levels was detected in the GCN2−/− cells only after 8 h of UV insult. Levels of p65, as well as actin, were similar in GCN2+/+ and GCN2−/− MEF cells during the time course after UV irradiation. A similar reduction in IκBα levels was measured in UV-treated S/S MEF cells, which was alleviated in A/A cells, indicating that the regulatory function of GCN2 is via phosphorylation of eIF2α (results not shown).
Figure 4. Phosphorylation of eIF2α facilitates lowered levels of IκBα in response to UV irradiation.
(A) GCN2+/+ and GCN2−/− MEF cells were exposed to 40 J/m2 UV-C irradiation or no stress (0), and incubated in the culture medium for up to 8 h as indicated. Alternatively, the MEF cells were incubated with TNFα for 30 min. Equal amounts of proteins were analysed in each lane. Levels of phosphorylated IκBα, total IκBα, p65 and actin were measured by immunoblot analysis using polyclonal antibody specific to each protein. (B) Levels of protein synthesis were measured in GCN2+/+ and GCN2−/− MEF cells subjected to 40 J/m2 UV-C irradiation or no stress (0). Following the UV stress, cells were incubated in the culture medium for the indicated time. Thirty minutes prior to harvesting, cells were radiolabelled with [35S]Met/Cys. Cells were then washed, and radiolabelled proteins were separated by SDS/PAGE, and visualized by autoradiography. IκBα and p65 were immunoprecipitated using protein-specific antibodies, subjected to SDS/PAGE, and imaged by autoradiography. To show that equal amounts of proteins were analysed in the electrophoretic analysis, actin was measured by immunoblot analysis using the radioactive lysate preparation. Levels of general protein synthesis, IκBα and p65 in the GCN2+/+ and GCN2−/− MEF cells are listed relative to 100% synthesis in the non-stressed cells. (C) The turnover of IκBα was measured by radiolabelling GCN2+/+ or GCN2−/− MEF cells with [35S]methionine/[35S]cysteine for 30 min. Radiolabelled MEF cells were washed, incubated in DMEM supplemented with non-radiolabelled amino acids, and then exposed to 40 J/m2 UV-C or no stress (0 min). Following incubation for between 20 and 160 min, IκBα was immunoprecipitated, separated by SDS/PAGE and visualized by autoradiography. Total levels of actin were measured by immunoblot to insure that equal amounts of lysates were analysed in each experimental lane. Results shown in (A)–(C) are representative of three experiments.
Phosphorylated eIF2α prevents the exchange of eIF2-GDP for eIF2-GTP that is required for general translation initiation [16,26]. To determine the effects of phosphorylation of eIF2α during UV irradiation, we measured [35S]methionine/[35S]cysteine incorporation after exposure of GCN2+/+ and GCN2−/− MEF cells exposed to 40 J/m2 of UV-C. There was a significant decrease in protein synthesis in GCN2+/+ cells 1–3 h following UV-C irradiation (Figure 4B). By contrast, GCN2−/− MEF cells devoid of phosphorylated eIF2α showed minimal reductions in translation following UV stress. These results are similar to that reported by Deng et al. [30], who found that loss of GCN2 activity alleviated the translation repression associated with UV exposure. We extended our analysis to the synthesis of IκBα and p65 by using immunoprecipitation followed by SDS/PAGE and autoradiography to visualize the radiolabelled proteins. We found that translation of IκBα was significantly decreased upon eIF2α phosphorylation in the GCN2+/+ MEF cells as compared with minimal change in the UV-stressed GCN2−/− cells (Figure 4B). Interestingly, synthesis of p65 was more resistant to the decrease in translation initiation, with significant p65 expression in the GCN2+/+ cells after 1.5 h of the UV treatment. In the GCN2−/− MEF cells subjected to UV, p65 synthesis was decreased to about half the levels measured for non-stressed cells (Figure 4B).
We next compared the turnover of IκBα in GCN2+/+ and GCN2−/− MEF cells after exposure to UV. Using pulse–chase experiments we found that there was a rapid decay of [35S]methionine/[35S]cysteine-labelled IκBα in both GCN2+/+ and GCN2−/− MEF cells after UV stress (Figure 4C). The UV-triggered turnover of IκBα had been reported previously and is disrupted by proteasome inhibitors, suggesting that ubiquitinassisted degradation was taking place [13]. These results suggest that turnover in response to UV irradiation occurs independently of GCN2 phosphorylation of eIF2α. We conclude that decreased synthesis of the repressing IκBα is the central contribution of eIF2α phosphorylation in the activation of NF-κB in response to UV irradiation. Decreased translation of IκBα, combined with turnover of IκBα, results in detachment from NF-κB. Release of NF-κB from IκBα would lead to translocation of the transcription factor into the nucleus and expression of NF-κB-targeted genes.
Different mechanisms activate NF-κB in response to UV and ER stress
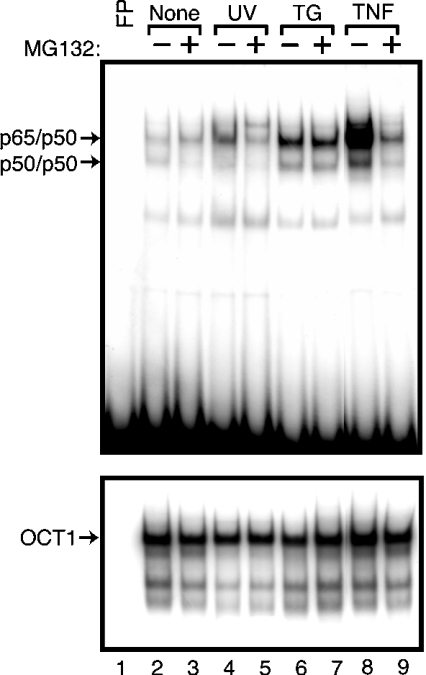
We had shown previously that activation of NF-κB in response to ER stress involved the release of IκBα from NF-κB without measurable reductions in IκBα levels [29]. Thus it appears that the mechanisms by which eIF2α phosphorylation modulates NF-κB activity can vary depending on the cellular stress condition and the participation of additional stress-response pathways. We wished to formally delineate the role of ubiquitin-mediated degradation of IκBα in the activation of NF-κB between the UV and ER stress conditions. Wild-type MEF cells were pretreated with MG132, followed by exposure to UV-C, thapsigargin (a standard ER stress agent), TNFα or no stress. Inhibition of ubiquitin-mediated degradation significantly decreased activation of NF-κB in response to UV or TNFα exposure, but did not diminish NF-κB induction in response to ER stress (Figure 5). These results support the notion that there are differences between the mechanisms activating NF-κB during ER stress and in response to UV or TNFα treatments. Ubiquitin-mediated turnover of IκBα, which would contribute to lowered levels of this inhibitory protein, does not appear to be central to modulation of NF-κB activity in response to ER stress.
Figure 5. Phosphorylation of eIF2α in response to UV or ER stress activates NF-κB by different mechanisms.
Wild-type MEF cells were pretreated with MG132 as indicated, followed by exposure to UV-C (lanes 4 and 5), the ER stress agent thapsigargin (TG, lanes 6 and 7), TNFα (lanes 8 and 9), or no stress agent (None, lanes 2 and 3). Nuclear lysates were prepared from these MEF cells and included in an EMSA with radiolabelled DNA containing a binding site for NF-κB (top panel) or OCT1 (bottom panel). Free probe (FP) indicates that only radiolabelled DNA was included in the EMSA. Arrows indicate the protein–DNA complexes. EMSA results shown are representative of three independent experiments.
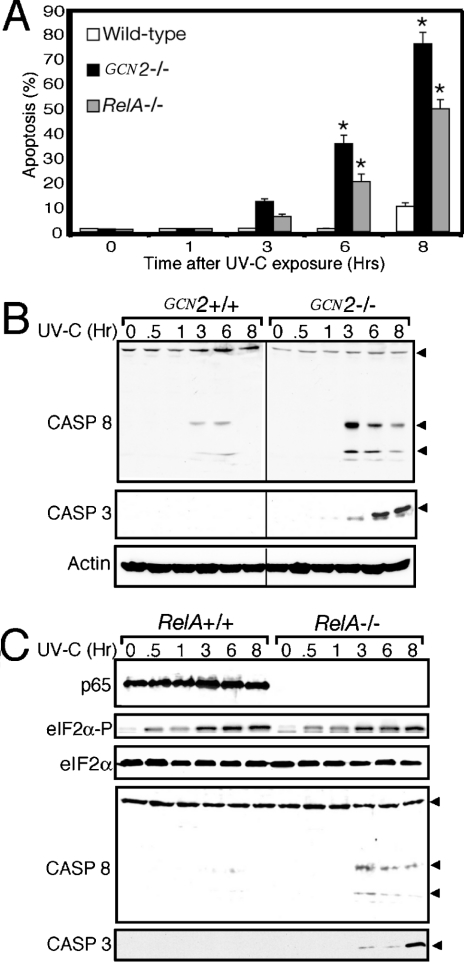
Loss of GCN2 eIF2α kinase enhances apoptosis in response to UV irradiation
NF-κB regulates the transcription of genes involved in stress remediation and apoptosis. We wished to determine whether impaired activation of NF-κB in GCN2−/− MEF cells affected their viability upon treatment with UV irradiation. GCN2+/+ and GCN2−/− MEF cells were exposed to UV-C irradiation and the number of apoptotic cells was counted by using the TUNEL assay. While less than 10% of GCN2+/+ MEF cells elicited apoptosis after 8 h of the UV insult, almost 80% of cells devoid of GCN2 eIF2α kinase activity underwent programmed cell death (P<0.0001) (Figure 6A). UV-triggered apoptosis has been shown to be facilitated by cleavage and activation of a cascade of caspase proteases [43]. Cleavage of caspase 3 and 8 were measured by immunoblot and found to be significantly enhanced in the GCN2−/− MEF cells compared with the wild-type cells (Figure 6B). Cleavage and activation of caspase 8, an early event in the caspase cascade, was at a maximum 3 h after UV irradiation. By comparison, cleavage of caspase 3 was detected at low levels 3 h after the UV insult, with maximum levels of cleaved protein following 6 and 8 h of the stress. These results indicate that phosphorylation of eIF2α by GCN2 facilitates activation of caspases in response to UV irradiation, contributing to enhanced apoptosis.
Figure 6. Loss of GCN2 activity enhances apoptosis in response to UV exposure.
GCN2−/− and RelA/p65−/− MEF cells, and their wild-type counterparts, were exposed to 30 J/m2 UV-C irradiation or no stress (0), and incubated in the culture medium for up to 8 h as indicated. (A) Apoptosis was monitored by using the TUNEL assay to detect fragmented DNA. Wild-type GCN2+/+ cells and RelA/p65+/+ gave similar results, and the GCN2+/+ apoptotic measurements are presented. Means values±S.D. were derived from three independent experiments. Two-way ANOVA and post hoc analyses indicated that there were significant differences in apoptosis between genotypes (P<0.001) and with time (P<0.001) as indicated by the asterisks. (B) Cleavage and activation of caspase (CASP) 3 and 8 were monitored by immunoblot analysis using polyclonal antibody specific to each caspase. Arrowheads indicate the non-cleaved caspase 8 (upper band), and cleaved/activated capase 8 (two lower bands). The polyclonal antibody used in the capase 3 immunoblot recognizes only the cleaved, activated caspase 3 polypeptide highlighted by the arrowhead. (C) RelA/p65+/+ and RelA/p65−/− MEF cells were subjected to 30 J/m2 UV-C irradiation or no stress (0), and cultured for between 0.5 and 8 h as indicated. Levels of phosphorylated eIF2α, total eIF2α, actin and cleaved and activated caspase 3 and 8 were measured by immunoblot analysis. Arrowheads indicate the uncleaved and cleaved/activated versions of caspase 8, and activated caspase 3, as described for (B). Immunoblot experiments shown in (B) and (C) are representative of three independent experiments.
Loss of RelA/p65 was reported to enhance apoptosis in response to UV irradiation [44,45]. We found that whereas the pattern of induced eIF2α phosphorylation was similar in RelA/p65+/+ and RelA/p65−/− MEF cells in response to UV exposure, the RelA/p65−/− cells preferentially elicited cleavage of caspase 3 and 8 (Figure 6C). Furthermore, about 50% of the RelA/p65−/− MEF cells underwent apoptosis 8 h after the UV treatment, while only 10% of the wild-type cells were apoptotic. These results are consistent with the idea that activation of NF-κB and its target genes is one important reason for GCN2 protection against UV-induced apoptosis.
DISCUSSION
Translation repression by eIF2α phosphorylation activates NF-κB and reduces apoptosis in response to UV irradiation
Lowered levels of the inhibitory protein IκBα are central to mechanisms activating NF-κB in response to UV irradiation. We show that reduced general protein synthesis in response to UV irradiation is the result of eIF2α phosphorylation by GCN2, and this lowered translation facilitates decreased levels of IκBα (Figures 1 and 4). Translation of IκBα mRNA appears to be very sensitive to eIF2α phosphorylation, with a notable reduction in IκBα synthesis as compared with RelA/p65 translation (Figure 4B). Lowered synthesis of IκBα, coupled with the rapid turnover of this inhibitory protein by eIF2α kinase-independent mechanisms, is thought to trigger the nuclear translocation of NF-κB and activation of its target genes (Figures 3 and 4). Loss of GCN2 or RelA/p65 increases activation of capases 3 and 8 and enhances apoptosis in response to UV irradiation, suggesting that GCN2 modulation of NF-κB activity is important for signalling programmed cell death (Figure 6).
The basic features of this model are supported by a recent report by Wu et al. [46] that appeared after the completion of the present study. Wu and colleagues found that eIF2α phosphorylation contributed to decreased IκBα levels in response to UV-C exposure, and that PEK/PERK was the eIF2α kinase facilitating this regulation, as judged by analysis of MC-7 cells transfected with a dominant-negative version of PEK. We analysed PEK−/− MEF cells for eIF2α phosphorylation in response to UV stress, and found a partial reduction compared with wild-type cells (Figure 1). These results suggest that GCN2 is the primary eIF2α kinase, with a near elimination of eIF2α phosphorylation over a range of UV-C exposures, and PEK may carry out a secondary role. The results obtained in the present study is also consistent with previous reports that conclude that IκBα turnover in response to UV stress is independent of IKK phosphorylation of IκBα N-terminal residue Ser32 (Figure 4) [13,14].
In addition to NF-κB, eIF2α phosphosphorylation can enhance expression of ATF4 and its target genes. However, there was minimal expression of the ATF4 response pathway after UV exposure (Figure 2), suggesting that the ATF4/CHOP pathway is not required for the anti-apoptotic function of GCN2 in response to UV irradiation. MAP kinase cascades anchored by JNK and p38 are also induced by UV exposure and contribute to expression of genes regulating cellular stress responses, including cell-cycle control and apoptosis [47]. In the example of p38, induction of this MAP kinase by UV irradiation is proposed to be critical for CK2-directed ubiquitination and degradation of IκBα via the proteasome [15]. Deng et al. [30] found that GCN2 is not required for the phosphorylation of JNK and p38 MAP kinases. These results suggest that GCN2 is not a sensor of UV stress for multiple stress pathways. Furthermore, eIF2α phosphorylation appears to be effectively induced upon UV exposure in the absence of either JNK or p38 MAP kinase activities, further supporting a delineation between these stress pathways [30]. Combined with the earlier reports, our study suggests that while GCN2-deficient MEF cells are prevented from inducing NF-κB, and its protective function against UV-directed apoptosis, these cells retain the pro-apoptotic signals of the JNK MAP kinase pathway.
Phosphorylation of eIF2α triggers changes in the levels of regulatory proteins
Our results indicate that there are at least two fundamental processes by which eIF2α phosphorylation can regulate stress response pathways. GCN2 activation of NF-κB in response to UV irradiation illustrates the model whereby a decrease in general translation can preferentially lower the levels of regulatory proteins subject to rapid turnover. In the example of the inhibitory IκBα protein, this reduction would lead to activation of the NF-κB transcriptional regulator in response to UV irradiation. Another example is CReP/PPP1R15B [constitutive repressor of eIF2α phosphorylation/protein phosphatase 1, regulatory (inhibitor) subunit 15b], which functions in the dephosphorylation of eIF2α under basal conditions in the absence of stress. In response to translation inhibition, there is reduced synthesis of the labile CReP that contributes to elevated levels of eIF2α phosphorylation [48]. Such a mechanism could also be adapted to the lowered synthesis of an activator protein subject to a short half-life. In this case, such protein turnover would lead to repression of a stress-responsive pathway.
The second fundamental process by which eIF2α phosphorylation can control stress response pathways involves preferential translation of specific mRNAs. There are several examples of such gene-specific translation control, including ATF4, CAT-1 (cationic amino acid transporter 1) and C/EBPα [16,18,28,49,50]. Phosphorylation of eIF2α leads to preferential translation of ATF4, triggering expression of a large number of genes involved in metabolism, antioxidation and apoptosis [27]. It is noteworthy that exposure to UV irradiation does not enhance ATF4 levels, despite significant induction of eIF2α phosphorylation (Figure 2). Furthermore, expression of CHOP was not induced in response to UV-C, consistent with an earlier study [51]. This result would suggest that eIF2α phosphorylation alone is not sufficient for increased ATF4 expression under certain stress conditions. The underlying difference between ER and nutritional stresses, which induce ATF4 translation, and UV irradiation, may involve lowered ATF4 mRNA that accompanies this UV treatment of MEF cells. In addition to UV exposure, we previously reported that ER stress in PEK−/− MEF cells does not elicit expression of ATF4, or its target genes ATF3 and CHOP [33]. Initially this may not appear to be significant, given that PEK is the primary eIF2α kinase that is activated when malfolded proteins accumulate in the ER. However, it was found that PEK−/− MEF cells treated for extended periods of time with the ER stress agent thapsigargin display robust eIF2α phosphorylation that is comparable with that shown by PEK+/+ cells. This delayed eIF2α phosphorylation was nearly absent in MEF cells with a combined deletion of PEK and GCN2, suggesting that the secondary eIF2α kinase activated by ER stress is GCN2. These two examples, whereby significant eIF2α phosphorylation is not linked to induced levels of ATF4 protein, suggest that there are one or more mechanisms that can thwart ATF4 expression. Such mechanisms would provide for versatility in the regulatory proteins induced by eIF2α phosphorylation. Therefore, the programme of gene expression can be tailored to a given stress condition.
The transcriptional regulator ATF3 is induced in response to diverse environmental stress conditions [52,53]. Previously, we found that eIF2α phosphorylation, and the subsequent expression of ATF4, was required for increased ATF3 levels in response to amino acid limitation or ER stress [33]. ATF3 was found to be important for expression of CHOP during amino acid limitation, and for induction of GADD34, an eIF2α protein phosphatase regulatory subunit involved in feedback control, in response to either nutrient or ER stress. However, upon exposure of MEF cells to between 5 and 20 J/m2 UV-C, significant ATF3 expression was measured independently of GCN2 activity (Figure 2). This observation indicates that there are multiple mechanisms that enhance ATF3 expression in response to different stresses, and that ATF4 induced by eIF2α phosphorylation is required for only a subset of these stress conditions.
eIF2α kinases activate NF-κB in response to diverse stress conditions
Previously we reported that eIF2α phosphorylation is required for the activation of NF-κB in MEF cells exposed to ER stress or leucine starvation [29]. Characterization of the mechanisms regulating NF-κB during ER stress indicated that whereas IκBα was released from NF-κB, there was no significant reduction in the steady-state levels of IκBα. Supporting this conclusion, the addition of the proteasome inhibitor MG132 along with ER stress did not block activation of NF-κB in MEF cells (Figure 5) [29]. This is a distinguishing difference compared with UV stress, whereby proteasome inhibitors block activation of NF-κB (Figure 5) [13].
An explanation for how eIF2α phosphorylation can contribute to different mechanisms of NF-κB regulation involves the synergism between eIF2 kinases and other stress-response pathways. In the example of UV irradiation, decreased translation by GCN2 phosphorylation of eIF2α is proposed to combine with p38-MAP-kinase-directed turnover of IκBα to significantly lower the steady-state levels of this repressor protein, facilitating NF-κB translocation into the nucleus and direct gene expression. Disruption of one stress pathway, loss of GCN2 or deletion of the p38α gene, blocks activation of NF-κB in response to UV irradiation (Figure 3) [15]. Decreased translation accompanying PEK phosphorylation of eIF2α is suggested to prevent further synthesis of secreted proteins that would further overload the folding capacity of the ER [23,54]. Using pulse–chase style experiments, we have also found lowered IκBα synthesis during ER stress, but no measurable lowering of the steady-state levels of IκBα ([29]; H. Y. Jiang, unpublished work). There does not appear to be a directed turnover of IκBα during ER stress as has been described for UV irradiation. We do not yet understand the mechanism by which eIF2α phosphorylation contributes to release of IκBα from NF-κB in response to ER stress. Elaborating on our discussion above, in the case of activation of NF-κB by ER stress, lowered translation may combine with preferential synthesis of a regulatory protein, such as an NF-κB subunit, or alternatively targeted degradation of a regulator distinct from IκBα.
Acknowledgments
We thank Hong Chang for technical assistance, Dan Spandau and Jana Narasimhan for helpful discussions, and Douglas Cavener (Pennsylvania State University, University Park, PA, U.S.A.), Barbara McGrath (Pennsylvania State University, University Park, PA, U.S.A.), Gail Sonenshein (Boston University, Boston, MA, U.S.A.), Randal Kaufman (University of Michigan, Ann Arbor, MI, U.S.A.), and Harikrishna Nakshatri for sharing cell lines and reagents (where no address is given, the people cited are in our Department). We also acknowledge the Biochemistry Biotechnology Facility at Indiana University for technical support. This study was supported in part by grants RO1GM49164 and R01GM64350 (to R.C.W.) from the National Institutes of Health, and by a Biomedicine Research Grant from Indiana University School of Medicine (to H.-Y.J.).
References
- 1.Bender K., Blattner C., Knebel A., Iordanov M., Herrlich P., Rahmsdorf H. J. UV-induced signal transduction. J. Photochem. Photobiol. B Biol. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 2.Dent P., Yacoub A., Fisher P. B., Hagan M. P., Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 3.Herrlich P., Blattner C., Knebel A., Bender K., Rahmsdorf H. J. Nuclear and non-nuclear targets of genotoxic agents in the induction of gene expression. Shared principles in yeast, rodents, man and plants. Biol. Chem. 1997;378:1217–1229. doi: 10.1515/bchm.1997.378.11.1217. [DOI] [PubMed] [Google Scholar]
- 4.Vousden K. H. Activation of p53 tumor suppressor protein. Biochim. Biophys. Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 5.Devary Y., Rosette C., DiDonato J. A., Karin M. NF-κB activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 6.Jayaraman J., Prives C. Activation of p53 sequence-specific DNA binding by short single strands of DNA requires the p53 C-terminus. Cell. 1995;81:1021–1029. doi: 10.1016/s0092-8674(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z.-G., Baskaran R., Lea-Chou E. T., Wood L. D., Chen Y., Karin M., Wang J. Y. J. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature (London) 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 8.Barkett M., Gilmore T. D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 9.Baud V., Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen F. E., Ghosh G. Regulation of DNA binding by Rel/NF-κB transcription factors: structural views. Oncogene. 1999;18:6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- 11.Li Q., Verma I. M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 12.Huang T. T., Feinsberg S. L., Suryanarayanan S., Miyamoto S. The zinc finger domain of NEMO is selectively required for NF-kappaB activation by UV radiation and topoisomerase inhibitors. Mol. Cell. Biol. 2002;22:1513–1525. doi: 10.1128/MCB.22.16.5813-5825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N., Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender K., Gottlicher M., Whiteside S., Rahmsdor H. J., Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-κB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T., Delhase M., Hoffmann A., Karin M. CK2 is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol. Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 16.Dever T. E. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 17.Wek R. C. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem. Sci. 1994;19:491–496. doi: 10.1016/0968-0004(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 18.Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 19.Hinnebusch A. G. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 20.Wek S. A., Zhu S., Wek R. C. The histidyl-tRNA synthetase-related sequence in eIF2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P., McGrath B. C., Reinert J., Olsen D. S., Lei L., Gill S., Wek S. A., Vattem K. M., Wek R. C., Kimball S. R., et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthony T. G., McDaniel B. J., McGrath B. C., Cavener D. R., McNurlan M. A., Wek R. C. Preservation of liver mass during dietary leucine starvation occurs at the expense of skeletal muscle mass in GCN2 knockout mice. J. Biol. Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 23.Harding H. P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 24.Chen J.-J. Heme-regulated eIF2α kinase. In: Sonenberg N., Hershey J. W. B., Mathews M., editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 529–546. [Google Scholar]
- 25.Kaufman R. J. Double-stranded RNA-activated protein kinase. In: Sonenberg N., Hershey J. W. B., Mathews M., editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 503–528. [Google Scholar]
- 26.Hershey J. W. B., Merrick W. C. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N., Hershey J. W. B., Mathews M., editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 33–88. [Google Scholar]
- 27.Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 28.Vattem K. M., Wek R. C. Reinitiation involving upstream open reading frames regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H. Y., Wek S. A., McGrath B. C., Scheuner D., Kaufmann R. J., Cavener D. R., Wek R. C. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stress. Mol. Cell. Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng J., Harding H., Raught B., Gingras A., Berlanga J., Scheuner D., Kaufman R., Ron D., Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr. Biol. 2002;12:1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- 31.Wu S., Hu Y., Wang J. L., Chatterjee M., Shi Y., Kaufman R. J. Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2002;277:18077–18083. doi: 10.1074/jbc.M110164200. [DOI] [PubMed] [Google Scholar]
- 32.Engelberg D., Klein C., Marinetto H., Struhl K., Karin M. The UV response involving the RAS signalling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H. Y., Wek S. A., McGrath B. C., Lu D., Hai T., Harding H. P., Wang X., Ron D., Cavener D. R., Wek R. C. Activating transcription factor 3 (ATF3) is integral to the eIF2 kinase stress response. Mol. Cell. Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 35.Beg A. A., Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 36.Nozaki S., Sledge G. W., Nakshatri H. Repression of GADD153/CHOP by NF-κB: A possible cellular defense against endoplasmic reticulum stress-induced cell death. Oncogene. 2001;20:2178–2185. doi: 10.1038/sj.onc.1204292. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H. Y., Petrovas C., Sonenshein G. E. RelB-p50 NF-κB complexes are selectively induced by cytomegalovirus immediate-early protein 1: differential regulation of Bcl-x(L) promoter activity by NF-κB family members. J. Virol. 2002;76:5737–5747. doi: 10.1128/JVI.76.11.5737-5747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinson C. R., Hai T., Boyd S. M. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev. 1993;7:1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- 39.Siu F., Blain P. J., LeBlanc-Chaffin R., Chen H., Kilberg M. S. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J. Biol. Chem. 2002;277:24120–24127. doi: 10.1074/jbc.M201959200. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y., Brewer J. W., Diehl J. A., Hendershot L. M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S., May M. J., Kopp E. B. NF-κB and rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 42.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 43.Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol. 2003;66:1547–1554. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 44.Karin M., Lin A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 45.Wang C. Y., Mayo M. W., Baldwin A. S. TNF- and cancer-therapy-induced apoptosis: Potentiation by inhibiton by NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 46.Wu S., Tan M., Hu Y., Wang J.-L., Scheuner D., Kaufman R. J. Ultraviolet light activates NFκB through translation inhibition of IκBα synthesis. J. Biol. Chem. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 47.Karin M., Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr. Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 48.Jousse C., Oyadomari S., Novoa I., Lu P. D., Zhang H., Harding H. P., Ron D. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calkhoven F. C., Muller C., Leutz A. Translational control of C/EBPα and C/EBPβ isoform expression. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 50.Yaman I., Fernandez J., Liu H., Caprara M., Komar A. A., Koromilas A. E., Zhou L., Snider M. D., Scheuner D., Kaufman R. J., Hatzoglou M. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell. 2003;113:519–531. doi: 10.1016/s0092-8674(03)00345-3. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt-Ney M., Habener J. F. CHOP/GADD153 gene expression response to cellular stresses inhibited by prior exposure to ultraviolet light wavelength band c (UVC) J. Biol. Chem. 2000;275:40839–40845. doi: 10.1074/jbc.M007440200. [DOI] [PubMed] [Google Scholar]
- 52.Hai T., Hartman M. G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 53.Hai T., Wolfgang C. D., Marsee D. K., Allen A. E., Sivaprasad U. ATF3 and stress responses. Gene Expression. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 54.Harding H. P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature (London) 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]