Abstract
The C-terminal region of EC-SOD (extracellular superoxide dismutase) mediates the binding to both heparin/heparan sulphate and type I collagen. A mutation (Arg213→Gly; R213G) within this extracellular matrix-binding region has recently been implicated in the development of heart disease. This relatively common mutation affects the heparin affinity, and the concentration of EC-SOD in the plasma of R213G homozygous individuals is increased 10- to 30-fold. In the present study we confirm, using R213G EC-SOD purified from a homozygous individual, that the heparin affinity is reduced. Significantly, the collagen affinity of the R213G EC-SOD variant was similarly affected and both the heparin and collagen affinities were reduced by 12-fold. Structural analysis of synthetic extracellular matrix-binding regions suggests that the mutation alters the secondary structure. We conclude that the increased concentration of EC-SOD in the plasma of R213G carriers is caused by a reduction in both heparin and collagen affinities.
Keywords: collagen, extracellular superoxide dismutase (EC-SOD), oxidative damage, reduced affinity, Arg213→Gly (R213G), structure
Abbreviations: ECM, extracellular matrix; EC-SOD, EC superoxide dismutase; HRP, horseradish peroxidase; TFA, trifluoroacetic acid; TFE, trifluoroethanol; R213G (etc.), Arg213→Gly (etc.)
INTRODUCTION
Aerobic organisms have developed several defence mechanisms to protect cellular components and tissues against oxidative damage. Central to these defence mechanisms are the antioxidant enzymes of which EC-SOD (extracellular superoxide dismutase or SOD3) is an important component [1]. EC-SOD was initially purified from human lung and is a 135 kDa homotetrameric glycoprotein composed of two disulphide-linked dimers [2,3]. Recently, we have shown that the human EC-SOD subunit exists in two folding variants with distinct disulphide bridge patterns; one of the variants is enzymically active (aEC-SOD) and the other one is inactive (iEC-SOD) [4].
The mature EC-SOD subunit (Trp1–Ala222) encompasses a C-terminal ECM (extracellular matrix)-binding region (Arg210–Ala222). This region binds heparin, other sulphated proteoglycans [5–8] and collagen [9,10]. Immediately before secretion, the EC-SOD subunit can be processed intracellularly to produce a truncated variant lacking the ECM-binding region (Trp1–Glu209) [11]. The process is a two-step proteolytic event involving an initial cut by a member of the proprotein convertase family of processing proteinases and a subsequent trimming by an unknown carboxypeptidase [11–13]. Therefore native EC-SOD presents variable affinity for heparin and collagen depending on the ratio of intact and processed subunits in the tetramer: type A without affinity (cleaved subunits only), type B with intermediate affinity (composed of both intact and cleaved subunits) and type C with high affinity (intact subunits only) [2,8,9]. The ECM-binding region contains an unusually high content of charged amino acid residues (seven Arg/Lys and two Glu) [14]. Six of the basic amino acid residues are located in an Arg/Lys-cluster: Arg210-Lys-Lys-Arg-Arg-Arg. Biophysical studies and secondary structure predictions indicate that the ECM-binding region adopts an α-helical structure [15]. This structure is probably important for heparin binding [16]. However, a series of single amino acid substitutions within the ECM-binding region of a recombinant EC-SOD homologue suggested that the affinity for heparin is minimally affected by structural perturbations of the ECM-binding region and is more dependent on the charge density [17]. Interestingly, a naturally occurring Arg213→Gly (R213G) mutation within the ECM-binding region exists [18–20]. The concentration of EC-SOD in the plasma of homozygous individuals is increased 10- to 30-fold and it has been suggested that this phenotype is caused by a reduction of the heparin affinity of the R213G variant [18,20–23].
In the present study, we show that the collagen affinity of R213G EC-SOD is reduced and that the relative reduction in ECM affinity is caused by a combination of reduced collagen and heparin affinity. Structural analyses suggested that the perturbation of the ECM-binding region imposed by Gly213 is responsible for the reduced affinity for collagen. This is in contrast with the heparin affinity that depends more on the density of positively charged amino acid residues [17]. We conclude that the increased concentration of EC-SOD in the plasma of R213G carriers may be explained not only by a reduced heparin affinity but also by the disruption of the EC-SOD–collagen interaction. This may increase the risk of oxidative damage to type I collagen of R213G carriers and possibly affect the integrity of the vascular wall.
MATERIALS AND METHODS
Proteins
Wild-type human EC-SOD was purified from human aorta as described previously, except that the cation exchange chromatography step was omitted [3]. The R213G variant of EC-SOD was purified from plasma obtained from a homozygous R213G individual using heparin- and immunoaffinity chromatography as described previously [13]. BSA conjugated with heparin (heparin–BSA) and HRP (horseradish peroxidase)-conjugated goat anti-rabbit Ig were purchased from Sigma. HRP-conjugated rabbit anti-mouse IgG was obtained from Dako (Glostrup, Denmark). A monoclonal antibody developed against native EC-SOD used for ELISA was a gift from Dr R. P. Bowler (National Jewish Medical and Research Center, Denver, CO, U.S.A.).
PAGE and Western blotting
Proteins were separated by SDS/PAGE in 5–15% polyacrylamide gels [24]. Before electrophoresis, samples were boiled for 5 min in the presence of 30 mM dithiothreitol and 1% SDS. For Western blotting, proteins were electrophoretically transferred on to a PVDF membrane in 10 mM 3-(cyclohexylamino)-1-propane sulphonic acid/10% methanol (pH 11) [25]. The membranes were blocked with 5% (w/v) skimmed milk in 20 mM Tris/HCl and 150 mM NaCl (pH 7.4). EC-SOD was subsequently detected by enhanced chemiluminescence (Amersham Biosciences) using a rabbit anti-human EC-SOD antiserum and HRP-conjugated goat anti-rabbit Ig.
Analyses of the relative heparin affinities of wild-type and R213G EC-SOD
Heparin–Sepharose chromatography
Heparin affinities were initially analysed by affinity chromatography using a 1 ml heparin–Sepharose column (HiTrap; Amersham Biosciences) connected to an FPLC system. Human plasma from a wild-type individual (25 ml) and from a homozygous R213G individual (5 ml) was diluted 5-fold in 20 mM Tris/HCl (pH 7.4) containing 5 mM EDTA (buffer A) and applied to the column. The column was washed in buffer A and bound proteins were subsequently eluted using a 1 ml/min flow rate and a linear gradient (1%/min) from buffer A to buffer A containing 1 M NaCl. Fractions of 1 ml were collected and aliquots were analysed by ELISA using heparin–BSA-coated microtitre plates (see below) and by SDS/PAGE followed by Western blotting using a polyclonal rabbit anti-EC-SOD antiserum. For these analyses, we used 40 μl from the R213G fractions and 600 μl from the wild-type fractions. The 600 μl aliquots were precipitated by using trichloroacetic acid before SDS/PAGE analysis.
Heparin-ELISA
To assess further the heparin binding properties of wild-type and R213G EC-SOD, we used an ELISA assay. Maxisorb microtitre wells (Nunc, Roskilde, Denmark) were coated with 0.1 μg of heparin–BSA in 100 μl of 50 mM NaHCO3 (pH 9.6) overnight at 23 °C. The wells were emptied and residual binding sites were blocked by the addition of 200 μl of 0.1% (w/v) BSA in 20 mM Tris/HCl and 135 mM NaCl, pH 7.4 (TBS) for 1 h at 23 °C. Two-fold dilution series of wild-type and R213G EC-SOD were made with concentration ranging from 2560 to 2.5 ng/ml in TBS containing 0.05% (v/v) Tween 20 (TBS-T). The dilutions were assayed for SOD activity to verify that comparable concentrations were used. Wells were incubated with 100 μl of the samples in doublet for 90 min at 23 °C. After washing the wells three times in TBS-T, 0.1 μg of monoclonal anti-EC-SOD antibody in 100 μl of TBS-T was added and allowed to bind for 90 min at 23 °C. Wells were washed in TBS-T and the bound primary antibody was subsequently detected by the addition of HRP-conjugated rabbit anti-mouse IgG antibody. Wells were developed using the o-phenylenediamine dihydrochloride-system according to the manufacturer's instructions. Colour development was assessed by absorption at 450 nm. The relative binding capacities of the wild-type and R213G EC-SOD were estimated in the linear area with an approximately parallel increase in absorbance. The influence of ionic strength on heparin binding was also investigated by using heparin–BSA-coated microtitre wells. Wild-type and R213G EC-SOD were diluted to 100 ng/ml in 20 mM Tris/HCl containing 50–500 mM NaCl. To the heparin–BSA-coated wells, 100 μl of the samples in doublet were added and EC-SOD was allowed to bind for 90 min at 23 °C. Bound EC-SOD was detected as described above, except that 20 mM Tris/HCl and 50 mM NaCl (pH 7.4) containing 0.05% Tween 20 were used for both the washing steps and for dilution of primary and secondary antibodies.
ELISA analysis of the relative collagen affinities of wild-type and R213G EC-SOD
The binding of EC-SOD to immobilized type I collagen was performed essentially as described previously [9]. Briefly, maxisorb microtitre wells (Nunc) were coated with 1 μg of collagen in 100 μl of 30 mM NaH2PO4/30 mM Tes/135 mM NaCl (pH 7.4) overnight at 23 °C. The wells were emptied and blocked by the addition of 20 mM NaH2PO4/50 mM NaCl/0.05% Tween 20, pH 7.4 (blocking buffer) for 1 h at 23 °C. Wild-type and R213G EC-SOD were diluted in blocking buffer at the indicated concentrations. Doublet samples (100 μl) were added to the wells and EC-SOD was allowed to bind for 90 min at 23 °C. Bound EC-SOD was detected as described in the analyses of the relative heparin affinities of wild-type and R213G EC-SOD subsection using 20 mM NaH2PO4/50 mM NaCl/0.05% Tween 20 (pH 7.4) for all dilutions and washings.
Production of synthetic ECM-binding regions
To facilitate structural analyses of the ECM-binding region and assess supposed differences between the wild-type and R213G EC-SOD, we produced synthetic ECM-binding regions. Synthetic peptides (residues Glu201-Ala222) representing the ECM-binding regions of wild-type and R213G EC-SOD were assembled in a 433A peptide synthesizer (Applied Biosystems, Foster city, CA, U.S.A.) using Fmoc [N-(9-fluorenyl)methoxycarbonyl] chemistry (FastMoc™; Applied Biosystems). The wild-type and R213G peptides were N-ERQAREHSERKKRRRESECKAA-C and N-ERQAREHSERKKGRRESECKAA-C respectively. The peptides were synthesized using Fmoc-Ala resin as solid support, de-blocked on ice for 3 h in 88% TFA (trifluoroacetic acid), 2% tri-isopropylsilane, 5% dithiothreitol, 5% water, extracted using diethyl ether and recovered by centrifugation. The peptides were then rehydrated in 10 mM NH4HCO3, desalted into 10 mM NH4HCO3 using Sephadex G-10, and purified by reversed-phase HPLC (Nova-Pak C18, 150 mm×3.9 mm; Waters, Milford, MA, U.S.A.). Peptides were applied in solvent A (0.1% TFA), eluted by a linear gradient of 2%/min of solvent B (90% acetonitrile containing 0.08% TFA) and detected by absorption at 220 nm. Fractions were collected manually and the structures of the peptides were verified by matrix-assisted-laser desorption ionization mass spectrometry in a quadrupole–time-of-flight Ultima Global mass spectrometer (Micromass, Wythenshaw, Manchester, U.K.) as described previously [4]. The collected fractions were aliquoted and freeze-dried. To generate homodimers, Cys219 was oxidized by atmospheric oxygen in 10 mM NaH2PO4 (pH 8.0). The peptide solutions were continuously stirred for up to 5 days at 5 °C until more than 50% of the peptide consisted of dimers. The progression of disulphide bridge formation was monitored by reversed-phase HPLC analysis using a C18 column (2.1 mm×210 mm; Vydac, Hesperia, CA, U.S.A.). Homodimers were subsequently purified by reversed-phase HPLC using a C18 column (Nova-Pak) as described above. Collected fractions were aliquoted, freeze-dried and stored at −20 °C until further use. All chromatographic separations were performed at 23 °C. The concentration of peptides was determined by amino acid composition analysis [26].
Structural analysis by CD
Far-UV CD spectra of the synthetic peptides (wild-type and R213G homodimers) were recorded at 20 °C in a closed 0.5 mm Suprasil quartz cell (Helma) using the UV1 photo-biology beamline with synchroton radiation provided by the ASTRID storage ring (Institute for Storage Ring Facilities, University of Aarhus; www.isa.au.dk). The peptides were dissolved at 0.2 mg/ml in 3 mM NaPO4 (pH 7.4) containing 0–40% (v/v) TFE (trifluoroethanol). Three consecutive scans with 1 nm intervals between 185 and 270 nm were collected and corrected for background signal by subtracting the average of three scans of solvent only. The CD spectra were normalized to give mean residue ellipticity.
RESULTS
R213G EC-SOD elutes earlier from heparin–Sepharose than wild-type EC-SOD
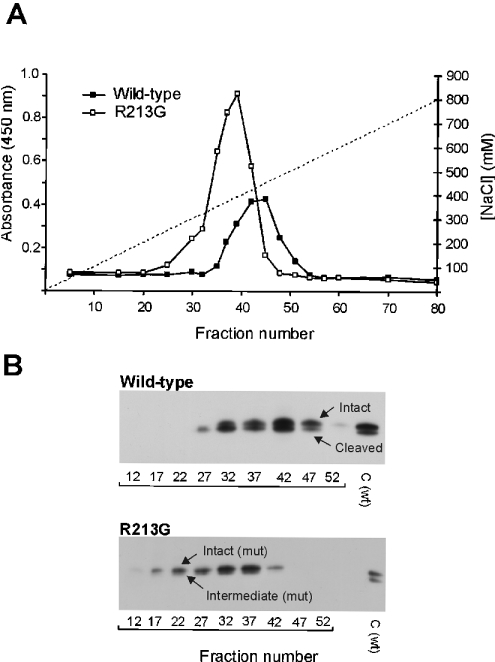
Plasma from wild-type (25 ml) or homozygous R213G (5 ml) donors was subjected to heparin–Sepharose affinity chromatography to evaluate the relative heparin affinity of EC-SOD. EC-SOD from wild-type individuals eluted from approx. 320 to 500 mM NaCl, as detected by ELISA (Figure 1A). EC-SOD from a homozygous R213G individual eluted in a slightly broader peak between 200 and 470 mM NaCl (Figure 1A). This indicates that the heparin affinity of the R213G variant is reduced relative to wild-type EC-SOD. In addition, the increased concentration of EC-SOD in plasma of homozygous R213G individuals was evident from this analysis according to the relative absorbance (Figure 1A). Both the intact and cleaved subunits of wild-type EC-SOD were detected by Western blotting (Figure 1B). Similarly, the intact and the intermediate subunits of R213G EC-SOD were detected (Figure 1B). The latter subunit is only partially processed, producing a mature C-terminus at Gly213 and not at Glu209, the mature C-terminus of the cleaved wild-type subunit [13]. The ratio between the intact and processed subunits in plasma-derived EC-SOD from wild-type and R213G homozygous individuals was not significantly different, as estimated by Western blotting (Figure 1B). The difference in heparin affinity observed is therefore not likely to reside in a different distribution of intact and processed subunits.
Figure 1. Affinity purification of EC-SOD.
(A) Plasma from a wild-type individual (25 ml) and a homozygous R213G individual (5 ml) was applied to a heparin–Sepharose column and bound proteins were subsequently eluted with NaCl (indicated by broken line). Collected fractions were analysed for the presence of EC-SOD by using a heparin-based ELISA assay. The amount of EC-SOD is given as the absolute absorbance. (B) Collected fractions were analysed by SDS/PAGE using 600 and 40 μl of fractions representing wild-type and R213G plasma respectively. EC-SOD was subsequently detected by using Western blotting. The intact, intermediate and cleaved forms of EC-SOD are indicated. C, wild-type EC-SOD purified from human aorta used as control.
The binding of R213G EC-SOD to heparin is reduced under physiological conditions
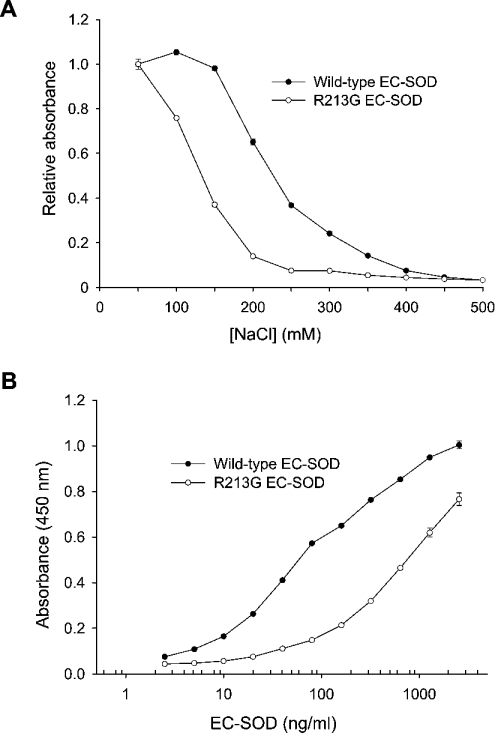
The influence of ionic strength on the heparin–EC-SOD interaction was analysed by using heparin–BSA-coated ELISA plates. Samples were applied in a buffer containing increasing concentrations of NaCl and the level of binding was evaluated relative to the binding in the presence of 50 mM NaCl (Figure 2A). The binding of both wild-type and R213G EC-SOD was affected by increasing the ionic strength. The absorbance produced by wild-type and R213G EC-SOD was reduced by 50% in the presence of 230 and 130 mM NaCl respectively (Figure 2A). At physiological ionic strength, corresponding to approx. 137 mM NaCl, no significant change was observed for the binding of wild-type EC-SOD. However, the absorbance obtained from R213G EC-SOD was reduced significantly at 137 mM NaCl (Figure 2A). This suggests that the binding of R213G EC-SOD to heparin is impaired under physiological conditions as opposed to the wild-type protein. Both wild-type and R213G EC-SOD bound in a dose-dependent manner at physiological ionic strength, suggesting the presence of a saturable binding process (Figure 2B). The binding curves were used to evaluate the relative affinities of wild-type and R213G EC-SOD. The concentration of R213G EC-SOD required to obtain the same level of bound wild-type EC-SOD (based on the absorbance) was approx. 12-fold higher in the parallel segment of the curves. Taken together, these results show that the heparin affinity of R213G EC-SOD is reduced approx. 12-fold when compared with the wild-type EC-SOD.
Figure 2. Heparin-binding analysis of EC-SOD.
(A) Approximately 100 ng/ml of wild-type and R213G EC-SOD were allowed to bind a heparin–BSA-coated surface in the presence of increasing concentrations of NaCl. For comparison, the binding of EC-SOD is depicted as the absorbance relative to the binding in the presence of 50 mM NaCl. (B) Wild-type and R213G EC-SOD were diluted to the indicated concentrations in a buffer containing 135 mM NaCl. The qualitative amount of bound material is given as absorbance at 450 nm. The experiment was repeated three times producing similar results. The data points represent the mean for doublet estimations and error bars indicate S.D.
The collagen-binding capacity of R213G EC-SOD is significantly reduced
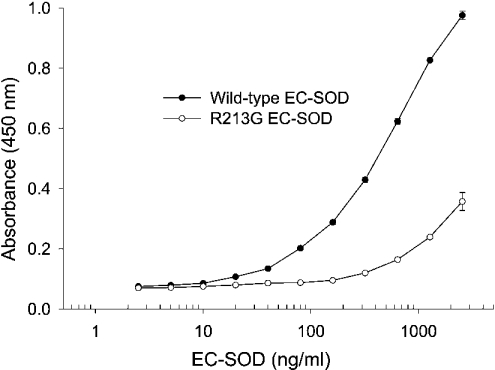
We have recently shown that the wild-type human and mouse EC-SOD binds to type I collagen [9,10] and that this binding is mediated by the ECM-binding region [9]. To evaluate the effect of the R213G mutation, we examined the binding of purified wild-type and R213G EC-SOD to collagen-coated ELISA plates. As previously shown, wild-type EC-SOD bound in a dose-dependent manner suggesting a specific interaction [9] (Figure 3). Significantly, the binding of R213G EC-SOD was reduced (Figure 3). Approximately 12-fold more R213G EC-SOD (2560 ng/ml) was required to produce the same response as wild-type EC-SOD (200 ng/ml) in this assay. This difference indicates that the collagen affinity of R213G EC-SOD relative to the wild-type protein is reduced by approximately the same degree, as is the heparin affinity. This implies that the amino acid residue at position 213 is essential for maintaining the collagen binding capacity.
Figure 3. Collagen-binding capacity of native EC-SOD.
Microtitre wells were coated with type I collagen and subsequently incubated with wild-type and R213G EC-SOD at the concentrations indicated. The R213G variant was purified from a homozygous individual. The amount of bound material is given as absorbance at 450 nm. The experiment was repeated three times producing similar results. The data points represent the mean for doublet estimations and error bars indicate S.D.
Gly213 disrupts the structure of the ECM-binding region
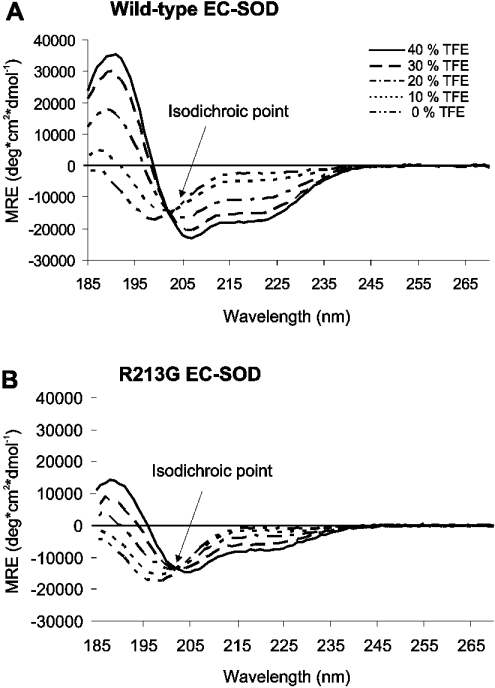
To study the structural impact of the R213G substitution, we analysed the synthetic homodimeric peptides representing the ECM-binding region of both wild-type and R213G EC-SOD by CD spectroscopy. Secondary structure predictions and experimental evidence suggest that the ECM-binding region adopts an α-helical structure in native EC-SOD [6,15]. The CD spectra of both homodimers in a purely aqueous solution (0% TFE) exhibited a singular negative peak at approx. 198 nm indicating that they predominantly have random coil (unfolded) structures (Figures 4A and 4B). To study the ability of the peptides to form α-helical structures, we added increasing amounts of the organic solvent TFE. TFE will induce the formation of an α-helix in peptides that have the propensity to form this secondary structure [27]. The CD spectra of the wild-type homodimer displayed an increasing positive peak at 190 nm, and negative peaks at 208 and 222 nm (typical of α-helical structure) concomitant with increasing TFE concentration (Figure 4A). This suggests that the homodimer has the capacity to form an α-helix. The increase in mean residue ellipticity at the wavelengths indicative of α-helix formation was significantly reduced for the R213G homodimer (Figure 4B). The presence of an isodichroic point in both sets of spectra indicates that the conversion from a predominantly random coil into a predominantly α-helical structure is a two-state process. These results show that the peptide variants have a random-coiled structure in the absence of TFE. However, in the presence of TFE the wild-type homodimer exhibited a substantially higher propensity to form an α-helical structure when compared with the R213G homodimer. Therefore it is probable that the R213G mutation within the ECM-binding region of native EC-SOD disrupts the α-helical structure.
Figure 4. Structural analysis of homodimeric peptides.
Far-UV CD spectra of (A) wild-type and (B) R213G homodimeric peptides were recorded at 20 °C. Peptides were analysed at 0.2 mg/ml in 3 mM NaPO4 (pH 7.4) in the presence of increasing amounts of TFE, as indicated. Local peaks at 190, 208 and 222 nm indicate the presence of α-helical structures. The isodichroic point at 203 nm indicates a two-state process.
DISCUSSION
The 10- to 30-fold increase in the EC-SOD concentration in the plasma of R213G carriers has been suggested to reside in reduced affinity for heparin and heparan sulphate [18,19,28]. We find, as reported by others, that the heparin affinity of plasma-derived R213G EC-SOD is lower than that of the wild-type form as assessed by affinity chromatography [18,28,29]. The interactions were further evaluated using purified components in an ELISA assay. These analyses showed that the binding of R213G EC-SOD was impaired at physiological ionic strength, whereas the binding of wild-type EC-SOD was maintained (see Figure 2A). This confirms that the ionic interaction between heparin and the ECM-binding region of EC-SOD is perturbed by the R213G substitution. Qualitative analysis of the heparin-binding capacity of wild-type and R213G EC-SOD indicated that the heparin affinity of R213G EC-SOD was reduced 12-fold relative to wild-type EC-SOD (see Figure 2B). This difference in heparin affinity is comparable with the 6-fold reduction determined by surface plasmon resonance analysis using a recombinant protein variant with the ECM-binding region [17].
The collagen binding capacity was also investigated by using ELISA. A qualitative estimation indicated that the affinity of R213G EC-SOD was reduced 12-fold relative to the wild-type form. EC-SOD is found at high concentrations in systemic vessel walls, especially the arteries where it is located throughout the adventitia below the endothelium [30,31], but also in the arterial intima surrounding the endothelial cells [32]. As the adventitia contains high amounts of type I collagen, we speculated that EC-SOD is bound to collagen in this area. Consequently, these areas are probably less protected against oxidative stress in individuals carrying the R213G EC-SOD variant. Significantly, a recent study has shown that heterozygous R213G carriers have a higher risk of ischaemic heart disease [23]. In human lung, EC-SOD was found to be associated with type I collagen fibres and not diffusely dispersed on cell surfaces [30]. The disruption of collagen binding will probably reduce the antioxidant level in these areas. We have recently illustrated the ability of EC-SOD to protect collagen from oxidative fragmentation both in vitro and in vivo using a mouse model of bleomycin-induced pulmonary fibrosis [9,10]. Therefore it is plausible that R213G carriers are more susceptible to pulmonary diseases such as asthma, idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease where reactive oxygen species are supposed to play an important role [33,34].
The R213G substitution probably destabilizes an α-helical structure because Arg213, in contrast with Gly213, has the capacity to interact with Glu209 [17]. Analysis by CD spectroscopy in the presence of increasing amounts of TFE confirmed that the wild-type dimer is more likely to form an α-helical structure compared with the R213G dimer. Therefore it is probable that the tertiary structure of the ECM-binding region of R213G EC-SOD is altered when compared with wild-type EC-SOD. When Stenlund et al. [17] analysed the heparin affinities of artificial EC-SOD homologues encompassing single Arg/Lys→Ala substitutions in the Arg/Lys-cluster they found that the affinity decreased by 2- to 6-fold. In addition, they showed that a series of mutations disrupting the α-helical structure of the ECM-binding region (E206P, K212P and R215P) had similar effects on the heparin affinity as the corresponding Ala variants. As discussed by these authors, this indicates that the ECM-binding region has sufficient flexibility to accept several disruptive amino acid substitutions and sustain some heparin-binding activity. This hypothesis implies that the heparin–heparan sulphate binding is less affected by structural changes and more dependent on the charge density of the ECM-binding region. The structural organization of the rod-shaped collagen fibril will probably impose structural constraints on the EC-SOD–collagen interaction. Owing to the size and heterogeneity of complexes between collagen fibrils and collagen-binding proteins, there is only limited structural information available [35,36]. However, it is probable that a more rigid molecule such as collagen will impose higher structural demands on EC-SOD compared with a more flexible molecule like heparin. The structural disruption imposed by the R213G substitution may thus account for the reduced collagen-binding capacity.
We have shown that R213G EC-SOD has a significantly reduced affinity for collagen in addition to a reduced heparin-binding capacity. The relative contribution of the reduced affinities to the increased concentration of EC-SOD in the plasma of individuals carrying the R213G mutation is difficult to assess. Nonetheless, the increased concentration of EC-SOD in plasma of affected individuals may correspondingly reflect a reduced antioxidant level in tissue and consequently a concomitant increase in radical-mediated disease susceptibility. Indeed, it has recently been shown that heterozygous individuals have an increased risk of acquiring ischaemic heart disease [23]. Similarly, a prospective study in a selected population of haemodialysis patients showed that heterozygotes had an increased risk of death from ischaemic heart disease and cerebrovasular disease [37]. In addition, mice lacking EC-SOD develop normally; however, when stressed by hyperoxia they display an increased mortality when compared with wild-type animals [38]. It may thus be hypothesized that an R213G phenotype will be observed primarily in combination with a disease creating increased oxidative stress. Our finding that the R213G mutation disrupts the interaction with collagen may indicate that the affected individuals will be more susceptible to diseases where collagenous tissue is destroyed.
Acknowledgments
We thank S. V. Hoffmann for his assistance in CD analysis and the Institute for Storage Ring Facilities (ISA), for access to the UV1 photobiology beamline at ASTRID. This work was supported by the Carlsberg Foundation (S.V.P.), the Danish Natural Science Research Council (J.J.E.), and the National Institutes of Health grants RO1 HL63700 (T.D.O.), P01 HL31992E (J.D.C.) and RO1 EY12712 (J.J.E.).
References
- 1.Fattman C. L., Schaefer L. M., Oury T. D. Extracellular superoxide dismutase in biology and medicine. Free Radical Biol. Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 2.Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. U.S.A. 1982;79:7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oury T. D., Crapo J. D., Valnickova Z., Enghild J. J. Human extracellular superoxide dismutase is a tetramer composed of two disulphide-linked dimers: a simplified, high-yield purification of extracellular superoxide dismutase. Biochem. J. 1996;317:51–57. doi: 10.1042/bj3170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen S. V., Oury T. D., Valnickova Z., Thogersen I. B., Hojrup P., Crapo J. D., Enghild J. J. The dual nature of human extracellular superoxide dismutase: one sequence and two structures. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13875–13880. doi: 10.1073/pnas.2436143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem. J. 1988;256:29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi T., Marklund S. L. Interactions between human extracellular superoxide dismutase C and sulfated polysaccharides. J. Biol. Chem. 1989;264:8537–8541. [PubMed] [Google Scholar]
- 7.Adachi T., Kodera T., Ohta H., Hayashi K., Hirano K. The heparin binding site of human extracellular-superoxide dismutase. Arch. Biochem. Biophys. 1992;297:155–161. doi: 10.1016/0003-9861(92)90654-f. [DOI] [PubMed] [Google Scholar]
- 8.Sandstrom J., Carlsson L., Marklund S. L., Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J. Biol. Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- 9.Petersen S. V., Oury T., Oestergaard L., Valnickova Z., Wegrzyn J., Thogersen I. B., Jacobsen C., Bowler R. P., Fattman C. L., Crapo J. D., et al. Extracellular superoxide dismutase (EC-SOD) binds to type I collagen and protects against oxidative fragmentation. J. Biol. Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 10.Fattman C. L., Chang L. Y., Termin T. A., Petersen L., Enghild J. J., Oury T. D. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radical Biol. Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 11.Enghild J. J., Thogersen I. B., Oury T. D., Valnickova Z., Hojrup P., Crapo J. D. The heparin-binding domain of extracellular superoxide dismutase is proteolytically processed intracellularly during biosynthesis. J. Biol. Chem. 1999;274:14818–14822. doi: 10.1074/jbc.274.21.14818. [DOI] [PubMed] [Google Scholar]
- 12.Bowler R. P., Nicks M., Olsen D. A., Thogersen I. B., Valnickova Z., Hojrup P., Franzusoff A., Enghild J. J., Crapo J. D. Furin proteolytically processes the heparin-binding region of extracellular superoxide dismutase. J. Biol. Chem. 2002;277:16505–16511. doi: 10.1074/jbc.M105409200. [DOI] [PubMed] [Google Scholar]
- 13.Olsen D. A., Petersen S. V., Oury T. D., Valnickova Z., Thogersen I. B., Kristensen T., Bowler R. P., Crapo J. D., Enghild J. J. The intracellular proteolytic processing of extracellular superoxide dismutase (EC-SOD) is a two step event. J. Biol. Chem. 2004;279:22152–22157. doi: 10.1074/jbc.M401180200. [DOI] [PubMed] [Google Scholar]
- 14.Hjalmarsson K., Marklund S. L., Engstrom A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibell L. A., Sethson I., Buevich A. V. Characterization of the heparin-binding domain of human extracellular superoxide dismutase. Biochim. Biophys. Acta. 1997;1340:21–32. doi: 10.1016/s0167-4838(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 16.Lookene A., Stenlund P., Tibell L. A. Characterization of heparin binding of human extracellular superoxide dismutase. Biochemistry. 2000;39:230–236. doi: 10.1021/bi991512x. [DOI] [PubMed] [Google Scholar]
- 17.Stenlund P., Lindberg M. J., Tibell L. A. Structural requirements for high-affinity heparin binding: alanine scanning analysis of charged residues in the C-terminal domain of human extracellular superoxide dismutase. Biochemistry. 2002;41:3168–3175. doi: 10.1021/bi011454r. [DOI] [PubMed] [Google Scholar]
- 18.Sandstrom J., Nilsson P., Karlsson K., Marklund S. L. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J. Biol. Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 19.Folz R. J., Peno-Green L., Crapo J. D. Identification of a homozygous missense mutation (Arg to Gly) in the critical binding region of the human EC-SOD gene (SOD3) and its association with dramatically increased serum enzyme levels. Hum. Mol. Genet. 1994;3:2251–2254. doi: 10.1093/hmg/3.12.2251. [DOI] [PubMed] [Google Scholar]
- 20.Yamada H., Yamada Y., Adachi T., Goto H., Ogasawara N., Futenma A., Kitano M., Hirano K., Kato K. Molecular analysis of extracellular-superoxide dismutase gene associated with high level in serum. Jpn. J. Hum. Genet. 1995;40:177–184. doi: 10.1007/BF01883574. [DOI] [PubMed] [Google Scholar]
- 21.Marklund S. L., Nilsson P., Israelsson K., Schampi I., Peltonen M., Asplund K. Two variants of extracellular-superoxide dismutase: relationship to cardiovascular risk factors in an unselected middle-aged population. J. Intern. Med. 1997;242:5–14. doi: 10.1046/j.1365-2796.1997.00160.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X. L., Adachi T., Sim A. S., Wilcken D. E. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1998;18:1915–1921. doi: 10.1161/01.atv.18.12.1915. [DOI] [PubMed] [Google Scholar]
- 23.Juul K., Tybjaerg-Hansen A., Marklund S., Heegaard N. H., Steffensen R., Sillesen H., Jensen G., Nordestgaard B. G. Genetically reduced antioxidative protection and increased ischaemic heart disease risk: the Copenhagen City Heart Study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 24.Bury A. F. Analysis of protein and peptide mixtures. Evaluation of three sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer systems. J. Chromatogr. 1981;213:491–500. [Google Scholar]
- 25.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 26.Meltzer N. M., Tous G. I., Gruber S., Stein S. Gas-phase hydrolysis of proteins and peptides. Anal. Biochem. 1987;160:356–361. doi: 10.1016/0003-2697(87)90060-1. [DOI] [PubMed] [Google Scholar]
- 27.Luo P., Baldwin R. L. Mechanism of helix induction by trifluoroethanol: a framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry. 1997;36:8413–8421. doi: 10.1021/bi9707133. [DOI] [PubMed] [Google Scholar]
- 28.Adachi T., Yamada H., Yamada Y., Morihara N., Yamazaki N., Murakami T., Futenma A., Kato K., Hirano K. Substitution of glycine for arginine-213 in extracellular-superoxide dismutase impairs affinity for heparin and endothelial cell surface. Biochem. J. 1996;313:235–239. doi: 10.1042/bj3130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi T., Morihara N., Yamazaki N., Yamada H., Futenma A., Kato K., Hirano K. An arginine-213 to glycine mutation in human extracellular-superoxide dismutase reduces susceptibility to trypsin-like proteinases. J. Biochem. (Tokyo) 1996;120:184–188. doi: 10.1093/oxfordjournals.jbchem.a021383. [DOI] [PubMed] [Google Scholar]
- 30.Oury T. D., Chang L. Y., Marklund S. L., Day B. J., Crapo J. D. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab. Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 31.Oury T. D., Day B. J., Crapo J. D. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radical Biol. Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 32.Stralin P., Karlsson K., Johansson B. O., Marklund S. L. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 33.Oury T. D., Thakker K., Menache M., Chang L. Y., Crapo J. D., Day B. J. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am. J. Respir. Cell Mol. Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 34.Rahman I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targets. Curr. Drug Targets Inflamm. Allergy. 2002;1:291–315. doi: 10.2174/1568010023344607. [DOI] [PubMed] [Google Scholar]
- 35.Emsley J., Knight C. G., Farndale R. W., Barnes M. J., Liddington R. C. Structural basis of collagen recognition by integrin alpha2beta1. Cell (Cambridge, Mass.) 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 36.Nishida N., Sumikawa H., Sakakura M., Shimba N., Takahashi H., Terasawa H., Suzuki E. I., Shimada I. Collagen-binding mode of vWF-A3 domain determined by a transferred cross-saturation experiment. Nat. Struct. Biol. 2003;10:53–58. doi: 10.1038/nsb876. [DOI] [PubMed] [Google Scholar]
- 37.Yamada H., Yamada Y., Adachi T., Fukatsu A., Sakuma M., Futenma A., Kakumu S. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron. 2000;84:218–223. doi: 10.1159/000045580. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson L. M., Jonsson J., Edlund T., Marklund S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]