Abstract
Renal tubular epithelial cells in all nephron segments express a distinct member of the metalloprotease-disintegrin family, ADAM9 (a disintegrin and metalloprotease 9), in a punctate basolateral distribution co-localized to the β1 integrin chain [Mahimkar, Baricos, Visaya, Pollock and Lovett (2000) J. Am. Soc. Nephrol. 11, 595–603]. Discrete segments of the nephron express several defined β1 integrins, suggesting that ADAM9 interacts with multiple renal integrins and thereby regulates epithelial cell–matrix interactions. Intact ADAM9 and a series of deletion constructs sequentially lacking the metalloprotease domain and the disintegrin domain were assembled as chimaeras with a C-terminal GFP (green fluorescent protein) tag. Stable expression of the ADAM9/GFP protein on the surface of HEK-293 cells (human embryonic kidney 293 cells) significantly decreased adhesion to types I and IV collagen, vitronectin and laminin, but had little effect on adhesion to fibronectin. Expression of the disintegrin/cysteine-rich/GFP construct yielded a similar, but more marked pattern of decreased adhesion. Expression of the cysteine-rich/GFP construct had no effect on adhesion, indicating that the disintegrin domain was responsible for the competitive inhibition of cell–matrix binding. To define the specific renal tubular β1 integrins interacting with the ADAM9 disintegrin domain, a recombinant GST (glutathione S-transferase)-disintegrin protein was used as a substrate in adhesion assays in the presence or absence of specific integrin-blocking antibodies. Inclusion of antibodies to α1, α3, α6, αv and β1 blocked adhesion of HEK-293 cells to GST-disintegrin protein. Immobilized GST-disintegrin domain perfused with renal cortical lysates specifically recovered the α3, α6, αv and β1 integrin chains by Western analysis. It is concluded that ADAM9 is a polyvalent ligand, through its disintegrin domain, for multiple renal integrins of the β1 class.
Keywords: adisintegrin and metalloprotease 9 (ADAM9), cell–matrix interaction, disintegrin, human embryonic kidney 293 cell (HEK-293 cell), β1 integrin, renal tubular epithelial cell
Abbreviations: ADAM, adisintegrin and metalloprotease; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; GFP, green fluorescent protein; GST, glutathione S-transferase; HEK-293 cells, human embryonic kidney 293 cells; PDL, poly(D-lysine)
INTRODUCTION
ADAM (a disintegrin and metalloprotease) represents a large family of integral membrane proteins with diverse tissue distributions and functions, including sperm– egg interactions, neurogenesis and ectodomain proteolysis of structural proteins and growth factors [1–3]. We previously reported the identification of ADAM9 as the dominant form of the metalloprotease-disintegrin family present in the kidney [4]. Histochemical analysis indicated that the ADAM9 protein was present in all components of the nephron, including the glomerulus and tubular epithelial cells of both proximal and distal origin. Tubular epithelial staining was concentrated in a punctate pattern on the baso-lateral surfaces of the cells, consistent with an interaction of the ADAM9 protein with specific renal integrins. As the α1–α6 and αv components of the β1 integrins in the nephron exhibit defined patterns of expression, the pan-nephron distribution of the ADAM9 protein suggested an interaction with multiple renal integrin proteins of the β1 class. Confocal microscopy of dual histochemical stains for the β1 and ADAM9 proteins confirmed the co-localization of these proteins in the nephron [4], raising the issue of the precise identities of the members of the β1 integrin family that interact with the ADAM9 protein. Also, we had observed that expression of the disintegrin domain of ADAM9 in glomerular epithelial cells led to major changes in cellular morphology, with rounding and detachment from the extracellular matrix. These observations prompted the present study, which focused on the identification of renal β1 integrins that potentially interact with the ADAM9 protein and determination of the specific interactions of defined ADAM9 structural domains with individual components of the extracellular matrix. These studies demonstrate that ADAM9 is a unique member of the ADAM protein family capable of interaction with multiple renal β1 integrins and that this interaction, through the disintegrin domain, regulates cellular attachment to critical components of the extracellular matrix.
EXPERIMENTAL
Cell culture
HEK-293 cells (human embryonic kidney 293 cells; A.T.C.C.) were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 2 mM penicillin/streptomycin, 1% glutamine and 10% (v/v) fetal calf serum. A375 melanoma cells (A.T.C.C.) were maintained in DMEM supplemented with 4.5 g/l glucose, 2 mM penicillin/streptomycin and 10% (v/v) fetal bovine serum.
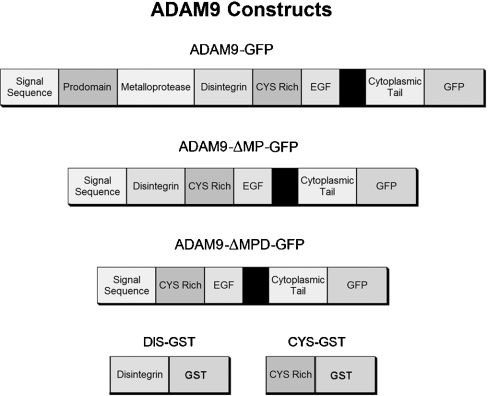
Generation of chimaeric ADAM9-GFP (green fluorescent protein) and N-terminal deletion mutants
A 2548 bp nucleotide fragment consisting of the entire open reading frame of murine ADAM9 [4] was amplified by PCR using a 5′-primer (5′-ACTGGTCGACCACCATGGGGCCGCGCGCGCTCTCG-3′) and a 3′-primer (5′-CAGTGGATCCCGGGTGAGGGAGCTATATAAAGG-3′), to permit cloning into the SalI and BamHI sites of the EGFP-N1 (ClonTech, Palo Alto, CA, U.S.A.) expression vector. The resulting chimaera was designated ADAM9-GFP and encoded the GFP component in-frame at the C-terminus of ADAM9 (Figure 1).
Figure 1. Design of ADAM-GFP expression constructs.
The cDNA for murine ADAM9 was cloned into the EGFP-N1 (ClonTech) expression vector. The two ADAM9 deletion constructs were prepared by serial deletion of the metalloprotease and disintegrin domains, while retaining the native ADAM9 signal sequence. The completed chimaeric constructs were subsequently inserted into an episomal expression vector, pCEP4 (Invitrogen) as described in the Experimental section.
The two ADAM9 deletion constructs were prepared by the sequential N-terminal deletion of the metalloprotease and the disintegrin domains (Figure 1). Both of these deletion mutants retained the intrinsic N-terminal ADAM9 signal sequence. The signal sequence was amplified by PCR using a 5′-primer containing a SalI site (5′-ACTGGTCGACCACCATGGGGCCGCGCGCGCTCTCG-3′) and a 3′-primer containing a MluI site (5′-ACGTACGCGTCCCGGCCTCGAGGACTGG-3′).
To generate the ADAM9 mutant with the deleted metalloprotease domain, the cDNA encoding residues 447–845 was amplified by PCR from the full-length ADAM9 cDNA using a 5′-primer containing a MluI site (5′-GATCACGCGTCCTTGCCAGAACAGCAAAG-3′) and a 3′-primer containing a BamHI site (5′-CAGTGGATCCCGGGTGAGGGAGCTATATAAAGG-3′). This PCR product and the signal sequence PCR product were digested with MluI, followed by SalI and BamHI. In a three-way ligation, the PCR fragments were cloned into the SalI and BamHI cloning sites of EGP-N1 (chimaera designated ADAM9-ΔMP-GFP).
To generate the ADAM9 mutant with deleted metalloprotease and disintegrin domains, the cDNA encoding residues 512–845 was amplified by PCR from the full-length ADAM9 cDNA using a 5′-primer containing a MluI site (5′-GATCACGCGTCCTTGCCAGAACAGCAAAG-3′) and a 3′-primer containing a BamHI site (5′-CAGTGGATCCCGGGTGAGGGAGCTATATAAAGG-3′). This PCR product and the signal sequence product were digested with MluI, followed by SalI and BamHI. In a three-way ligation the PCR products were cloned into the SalI and BamHI cloning sites of EGP-N1 (chimaera designated ADAM9-ΔMPD-GFP).
To achieve controlled and equivalent expression of the original ADAM9 protein and the deletion constructs, the above described constructs were amplified by PCR and directionally subcloned into the KpnI and NotI cloning sites of the episomal expression vector, pCEP4 (Invitrogen). pCEP4 contains the cytomegalovirus enhancer and a hygromycin B resistance cassette. Stable transfectants for each construct were selected initially after transfection of HEK-293 cells with hygromycin B (200 μg/ml). To avoid selection bias based on varying extracellular matrix substrate-binding affinities, the cells were grown on dishes coated with PDL [poly(D-lysine)]. After initial selection, resistant colonies were pooled and sorted by flow cytometry using the GFP signal intensity as a marker of chimaeric protein expression. Peaks corresponding to equivalent amounts of GFP intensity for each construct were collected and maintained in 50 μg/ml hygromycin B to sustain a constant level of ADAM9 and deletion mutant protein expression.
Microsomal protein extracts
Crude microsomal fractions of HEK-293 clones expressing chimaeric ADAM9-GFP and deletion mutants were made as described previously [4]. Confluent cells were suspended in lysis buffer, consisting of 20 mM sodium borate (pH 8.0), 1 mM EDTA, 1 mM PMSF, 1 mM leupeptin, 1 mM pepstatin and 1 mg/ml aprotonin, for 20 min at 4 °C. Subsequently, cells were disrupted by Dounce homogenization and NaCl was added to a final concentration of 150 mM. Cellular debris and nuclei were removed by centrifugation at 500 g for 10 min. The supernatant was sonicated briefly, re-centrifuged at 15000 g for 30 min and the pellet resuspended in PBS containing 1% Triton X-100, 1 mM EDTA, 1 mM PMSF, 1 mM leupeptin, 1 mM pepstatin and 1 mg/ml aprotonin. Crude microsomal protein extracts were stored at −70 °C until use. Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce) with BSA as a standard.
Western blots
Crude microsomal extracts (5 μg) were resolved by denaturing SDS/PAGE under reducing conditions and transferred on to nitrocellulose membranes for Western analysis. The membranes were blocked in PBS containing 0.5% casein and 0.5% Tween 20, for 4 h at 4 °C.
For the detection of ADAM9, the membranes were incubated overnight at 4 °C in blocking buffer containing either 5 μg/ml affinity-purified rabbit anti-ADAM9 metalloprotease domain IgG or preimmune IgG [4]. The following day, the membranes were washed in blocking buffer and incubated for 2 h at 4 °C with 2 μg/ml biotinylated F(ab′)2 goat anti-rabbit IgG. For detection of the GFP component of the chimaeric proteins, the membranes were incubated overnight at 4 °C in blocking buffer containing 5 μg/ml rabbit polyclonal anti-GFP IgG (Clontech). The following day, the membranes were washed in blocking buffer and incubated for 2 h at 4 °C with 1 μg/ml biotinylated F(ab′)2 goat anti-rabbit IgG. Finally, the membranes were incubated with avidin/biotin-conjugated alkaline phosphatase (Vector, Burlingame, CA, U.S.A.) for 1 h at 4 °C, developed using CDP-Star substrate (Tropix, Applied Biosystems, Foster City, CA, U.S.A.) according to the manufacturer's instructions and exposed to an X-ray film.
Cell–matrix adhesion assays
Individual wells of 96-well Immulon-2 microtitre plates (Dynatech Laboratories, Chantilly, VA, U.S.A.) were coated overnight at 4 °C with 150 μl of different culture matrices (Sigma) at the following concentrations: 3 μg/ml type I collagen, 3 μg/ml type IV collagen, 3 μg/ml fibronectin, 3 μg/ml vitronectin, 3 μg/ml laminin, 50 μg/ml PDL or 1% BSA. On the following day, the wells were washed with PBS and non-specific binding sites were blocked by incubating with 200 μl of 1% BSA at 37 °C for 1 h.
For cell–matrix adhesion assays, stable HEK-293 clones expressing GFP alone or the discrete chimaeric ADAM9-GFP proteins were detached from their culture surfaces using PBS containing 1 mM EDTA. Detached cells were centrifuged at 500 g, washed thrice with PBS and resuspended in DMEM supplemented with 0.1% fetal calf serum. To individual wells coated with different culture matrices, 30000 cells/150 μl were added and allowed to adhere at 37 °C for 2 h. Non-adherent cells were aspirated off and further removed by gently rinsing the wells with PBS. Adherent cells were fixed on to the culture surface using 4% formaldehyde and stained with 0.5% Crystal Violet dye (Sigma). The excess dye was removed by washing with PBS and cells were solubilized using 10% acetic acid. The staining intensity, an indicator of the number of adherent cells, was determined by measuring absorbance at 595 nm on a microplate reader (Bio-Rad). For each stable clone, the values were determined in quadruplicate for each culture matrix. The values reported are the means for at least three separate determinations.
ADAM9-GST (glutathione S-transferase) fusion proteins
The cDNA encoding the ADAM9-disintegrin domain (residues 423–514) was amplified by PCR from murine ADAM9 cDNA template using a 5′-primer (5′-ACTGGGATCCAGCGCGCCCTCC-3′) and a 3′-primer (5′-ACTGGAATTCTTAATATCCATTCTGAATGAAGAC-3′). The resulting PCR fragment was subcloned into the BamHI and EcoRI cloning sites of the pGEX6P1 bacterial expression vector (Amersham Biosciences). The expressed chimaeric protein was designated DIS-GST, with the GST component in-frame, C-terminally, with the open reading frame of the ADAM9-disintegrin domain.
The cysteine-rich domain of ADAM9 (residues 513–649) was amplified by PCR using a 5′-primer (5′-ACTGGGATCCCCTTGCCAGAACAGC-3′) and a 3′-primer (5′-ACTGGAATTCTTAGTCATAATTCAGGACAGA-3′). The resulting PCR fragment was subcloned into the BamHI and EcoRI cloning sites of pGEX6P1. The resulting chimaeric protein was designated CYS-GST, with the GST component placed C-terminally to the ADAM9-cysteine rich domain.
Plasmids encoding DIS-GST or CYS-GST were transformed into BL21(DE3) competent cells (Stratagene). Expression of GST fusion proteins was induced by the inclusion of 1 mM isopropyl β-D-thiogalactoside in culture medium for 4 h. Thereafter, the BL21(DE3) cells were centrifuged at 12000 g for 20 min, washed twice with PBS and disrupted by French-press extrusion. Cellular debris was removed by centrifugation at 15000 g and the GST fusion proteins were purified from the supernatant using a glutathione-coupled Sepharose 4B matrix (Amersham Biosciences), according to the manufacturer's instructions. The purified fusion proteins were dialysed against 1000 vol. of PBS and concentrated using an Amicon concentrator with a molecular mass cut-off of 10 kDa. Protein concentrations were determined by the bicinchoninic acid protein assay (Pierce) using BSA as a standard. The purity of the expressed GST chimaeric proteins was determined by analytical SDS/PAGE (results not shown).
Cell adhesion assay using immobilized ADAM9-GST fusion proteins
Individual wells of 96-well Immulon-2 microtitre plates were coated overnight at 4 °C with 150 μl of different culture matrices (Sigma) or GST fusion proteins at the following concentrations: 3 μg/ml type I collagen, 3 μg/ml type IV collagen, 20 μg/ml DIS-GST, 20 μg/ml CYS-GST, 20 μg/ml GST, 50 μg/ml PDL or 1% BSA. The following day, the wells were washed with PBS and the non-specific binding sites were blocked by incubation with 200 μl of 1% BSA at 37 °C for 1 h. Quantification of the adhesion of HEK-293 cells or A375 cells was performed as detailed above. The values reported are the means for quadruplicates and at least three separate determinations.
Inhibition of cell adhesion to DIS-GST using anti-integrin antibodies
Individual wells of 96-well Immulon-2 microtitre plates were coated overnight at 4 °C with 150 μl of 20 μg/ml DIS-GST and, the following day, were blocked with 1% BSA/PBS at 37 °C for 1 h.
HEK-293 cells were detached from the culture surface using 1 mM EDTA in PBS/CMF (where CMF stands for calcium-, magnesium-free). Detached cells were centrifuged at 500 g, washed three times in PBS/CMF and resuspended in DMEM supplemented with 0.1% fetal calf serum alone or with one of the integrin blocking antibodies listed in Table 1. After incubation at 37 °C for 30 min, the cells were centrifuged at 500 g, washed once with DMEM and finally resuspended in DMEM with 0.1% fetal calf serum. To each well, 30000 cells were added per 150 μl and allowed to adhere at 37 °C for 2 h. The number of adherent cells was determined as detailed above. The values reported are the means±S.D. for quadruplicate determinations and the studies were repeated three times.
Table 1. Blocking antibodies for adhesion assay.
| Antigen | Antibody (5 μg/ml) | Clone | Manufacturer |
|---|---|---|---|
| α1, human | Mouse IgG1 | FB12 | Chemicon (Temecula, CA, U.S.A.) |
| α2, human | Mouse IgG1, κ | P1E6 | Dako (Carpinteria, CA, U.S.A.) |
| α3, human | Mouse IgG1 | P25 | Gibco BRL |
| α4, human | Mouse IgG3, κ | P4G9 | Dako |
| α5, human | Mouse IgG3, κ | P1D6 | Dako |
| α6, human | Mouse IgG2b | 4F10 | Research Diagnostics (Flanders, NJ, U.S.A.) |
| αv, human | Mouse IgG1 | 13C2 | Chemicon |
| β1, human | Mouse IgG1 | P4C10 | Gibco BRL |
Enrichment of renal integrins using immobilized DIS-GST fusion protein
Sprague–Dawley rat kidney cortices were separated by standard procedures and resuspended in PBS containing 1% Triton X-100, 1 mM EDTA, 1 mM PMSF, 1 mM leupeptin, 1 mM pepstatin and 1 mg/ml aprotonin. The renal cortices were homogenized using a Polytron tissue homogenizer, tissue debris was removed by centrifugation at 250 g for 30 min at 4 °C, and the cortical extract was then frozen overnight at –70 °C. The following day, the cortical extract was centrifuged at 12000 g and the supernatant was used for enrichment of renal integrins. Rat cortical protein (200 μg≈100 μl) was added to 10 μg of DIS-GST, CYS-GST or GST alone (≈10 μl) in a final volume of 500 μl and incubated on ice for 4 h. This mixture was incubated with the glutathione-coupled Sepharose 4B matrix (Amersham Biosciences) overnight on a shaker at 4 °C. The following day, the beads were separated by centrifugation at 1000 g, washed four times with PBS containing 1% Triton X-100 and eluted with reducing SDS sample buffer. The eluates were resolved by denaturing SDS/PAGE on 10% gels and transferred on to nitrocellulose membranes for Western analysis. The membranes were blocked in PBS containing 0.5% casein and 0.5% Tween for 4 h at 4 °C and washed with blocking buffer. The membranes were cut into individual strips and incubated overnight at 4 °C with one of the primary antibodies listed in Table 2. The next day, individual strips were washed with blocking buffer and incubated with the appropriate biotinylated F(ab′)2 secondary antibody (5 μg/ml, goat anti-rabbit IgG, goat anti-mouse IgG or rabbit anti-goat IgG; Zymed, San Francisco, CA, U.S.A.) in blocking buffer for 2 h at room temperature (25 °C). Finally, the membrane strips were washed with blocking buffer and developed as described above.
Table 2. Antibodies for Western blots of renal eluates.
| Antigen | Antibody isotype | Clone | Manufacturer | Concentration |
|---|---|---|---|---|
| α1, human | Mouse IgG1 | FB12 | Chemicon | 2 μg/ml |
| α2, human | Mouse IgG1, κ | P1E6 | Dako | 1 μg/ml |
| α3, human | Mouse IgG1 | P25 | Gibco BRL | 10 μg/ml |
| α4, human | Mouse IgG3, κ | P4G9 | Dako | 5 μg/ml |
| α5, human | Mouse IgG3, κ | P1D6 | Dako | 1 μg/ml |
| α6, human | Goat IgG | Polyclonal | Santa Cruz Biotechnologies | 2 μg/ml |
| αv, human, cytoplasmic domain | Rabbit IgG | Polyclonal | Chemicon | 5 μg/ml |
Statistical analysis
Statistical significance was determined by Student's t test for paired comparisons or by ANOVA for multiple comparisons where appropriate.
RESULTS
Establishment of ADAM9 expressing cell lines
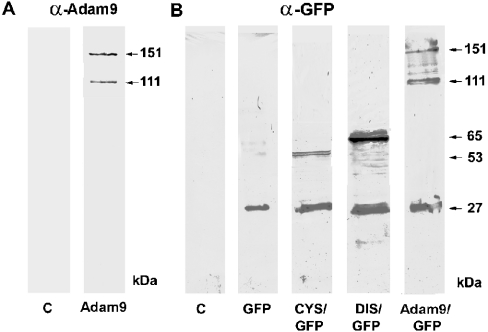
Previous studies from our laboratory had suggested that the ADAM9 protein is capable of interaction with more than one individual renal β1 integrin and that this interaction, through the disintegrin domain, affects cellular binding affinities to the extracellular matrix [4]. To evaluate these issues, a series of ADAM9 deletion constructs were assembled to investigate the role of individual functional domains in extracellular matrix adhesion and integrin binding (Figure 1). To facilitate localization and measurement of levels of expression, the constructs were expressed as fusion proteins with the enhanced GFP at the C-termini. To achieve controlled and equivalent expression of the intact ADAM9 protein and the sequential deletion constructs, an episomal expression vector, pCEP4, was used. Stable transfectants for each construct were selected initially in hygromycin during growth on the non-selective adherent PDL. Resistant colonies were pooled and sorted by FACS using the GFP intensity as a marker of chimaeric protein expression. Peaks corresponding to equivalent amounts of ADAM9 chimaeric protein expression were collected and maintained in selection medium to sustain a constant level of chimaeric protein expression. Microsomal protein extracts were prepared from HEK-293 cells expressing each of the ADAM9-GFP fusion proteins and analysed by Western blotting (Figure 2). When probed with a specific affinity-purified anti-ADAM9 antibody directed against residues 288–308 in the metalloprotease domain, two proteins with apparent molecular masses of 151 and 111 kDa were detected in cells expressing the full-length ADAM9-GFP construct. The presence of the two forms was reported previously [4], with the higher molecular mass protein corresponding to precursor ADAM9-GFP protein and the small molecular mass protein corresponding to the mature form of ADAM9-GFP subsequent to the removal of the prodomain. Similar results were also obtained when the same panel of extracts was probed with a specific monoclonal anti-GFP antibody (Figure 2). Two proteins with molecular masses of 151 and 111 kDa were detected in cells expressing the full-length ADAM9-GFP construct. In addition, a 27 kDa band was also present. This band corresponds to the molecular mass of the GFP protein and is presumably generated by autocatalysis during protein extract preparation. We note that free ADAM9 protein (i.e. catalytically separated from GFP) was not detected in blots probed with the anti-ADAM9 antibody, which presumably represents loss of the single antigenic epitope used to detect this protein, whereas the GFP protein was detected with a polyclonal antibody raised against the entire molecule. In extracts from HEK-293 cells expressing the ADAM9-ΔMP-GFP construct, a 65 kDa band corresponding to the chimaeric disintegrin-like, cysteine-rich, EGF (epidermal growth factor)-domain-GFP chimaera was detected, whereas in cells expressing the ADAM9-ΔMPD-GFP construct, the expected 53 kDa protein corresponding to the chimaeric cysteine-rich, EGF-domain-GFP protein was detected. Incubation of protein extracts from control HEK-293 cells with the anti-ADAM9 antibody did not demonstrate any specifically reactive bands, indicating that endogenous ADAM9 levels were lower than the detection limits of the Western analysis.
Figure 2. Western-blot analysis of stably transfected HEK-293 cells expressing ADAM9-GFP constructs.
(A) Lysates from control HEK-293 cells (C) and HEK-293 cells expressing full-length ADAM9-GFP probed with an affinity purified antibody to ADAM9 metalloprotease domain (α-Adam9). Cells expressing the ADAM9-GFP reveal protein bands of 151 and 111 kDa molecular mass, consistent with precursor ADAM9-GFP and processed ADAM9-GFP after the removal of the prodomain. (B) Western blots probed with anti-GFP antibody from control HEK-293 cells (C), and cells expressing GFP alone, the ADAM9-ΔMPD-GFP (CYS/GFP), the ADAM9-ΔMP-GFP (DIS/GFP) or the full-length Adam9-GFP (ADAM9/GFP) constructs respectively. GFP is detected at the expected 27 kDa, cysteine-rich GFP at 53 kDa, disintegrin-GFP at 65 kDa and full-length ADAM9-GFP at 151 and 111 kDa.
Role of individual ADAM9 domains in cell–matrix adhesion
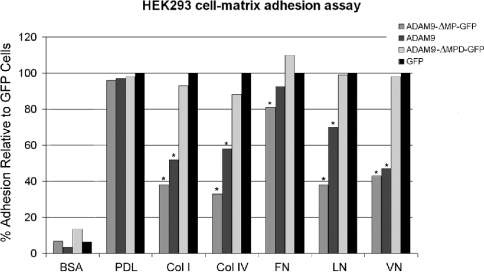
A quantitative assay to assess cell adhesion to defined extracellular matrix substrates was established as described in the Experimental section. The results of these studies are summarized in Figure 3. All ADAM9 cell lines exhibited equivalent degrees of adherence to the non-specific substrates, BSA and PDL. HEK-293 cells expressing GFP alone showed unaltered adhesion irrespective of the matrix protein used, indicating an absence of the effect of GFP. Major quantitative differences in cellular adhesion to specific matrix proteins were observed with the cells expressing the ADAM9-GFP or ADAM9-Δ-MP-GFP constructs. When compared with HEK-293 cells expressing GFP alone, the ADAM9-GFP cells showed a 50% decrease in adhesion when plated on collagens I and IV, a 30% decrease on binding to laminin, a 60% decrease in adhesion to vitronectin, but only a marginal decrease when plated on fibronectin. Cells expressing ADAM9-ΔMP-GFP showed a similar, but more augmented, decrease in adhesion, namely a 70% decrease in adhesion on collagens I and IV, a 60% decrease in adhesion to laminin and vitronectin, but only a minor decrease in adhesion to fibronectin. In contrast with the decreased adhesion to specific matrix proteins observed with the ADAM9-GFP and ADAM9-ΔMP-GFP constructs, cells expressing the ADAM9-ΔMPD-GFP construct, which lacks both the metalloproteinase and disintegrin domains, demonstrated adhesion profiles identical with the control GFP cell population. These results demonstrate that the major determinant of ADAM9 binding to a specific profile of extracellular matrix proteins resides exclusively within the disintegrin domain under these conditions.
Figure 3. Quantitative HEK-293 cell–matrix adhesion assay on defined extracellular matrix substrates.
HEK-293 cells expressing the respective ADAM9-GFP chimaeric constructs or GFP alone were allowed to adhere to the defined substrates, followed by quantification as given in the Experimental section. The data are expressed as percentage adhesion relative to the GFP control HEK-293 cell population and are given as the means for quadruplicate to pentuplicate determinations. To maintain visual clarity, the S.D. bars are not shown, but were <10% (*P<0.05). Col I, type I collagen; Col IV, type IV collagen; FN, fibronectin; LN, laminin; VN, vitronectin.
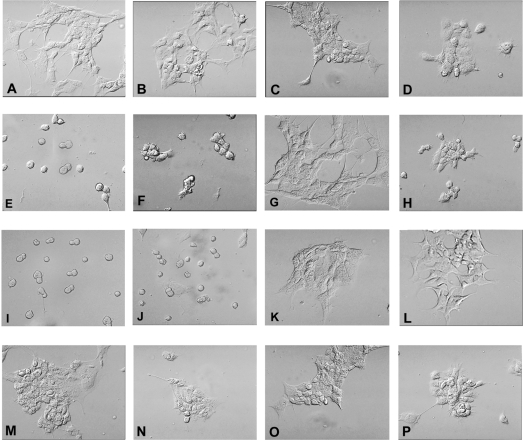
The morphological features of cells expressing discrete ADAM9 constructs when plated on defined substrates confirmed the impressions obtained with the formal adhesion assay. These studies are summarized in Figure 4. Control cells expressing GFP alone show a typical flattened or spread morphology with local extensions on vitronectin, type IV collagen, fibronectin and PDL. In contrast, cells expressing the ADAM9-GFP or ADAM9-ΔMP-GFP show markedly reduced adhesion to vitronectin and type IV collagen, with a rounded morphology and minimal cytoplasmic extension. Consistent with the results of the formal quantitative adhesion assay, cells expressing ADAM9-GFP or ADAM9-ΔMP-GFP adhered well to fibronectin substrates with normal patterns of spreading. Cells expressing the deletion construct lacking the metalloproteinase and disintegrin domains, ADAM9-ΔMPD-GFP, exhibited normal patterns of adhesion and spreading to all the tested matrix substrates. Taken together, the morphological patterns of adhesion to defined matrix substrates closely reflect the quantitative results detailed above in the adhesion assay and localize the site affecting matrix interaction specifically to the disintegrin domain of the ADAM9 protein.
Figure 4. Phase-contrast morphological features of HEK-293 cells expressing ADAM9-GFP chimaeric constructs on defined extracellular matrices.
(A–D) Control HEK-293 cells expressing GFP alone plated on vitronectin (A), type IV collagen (B), fibronectin (C) and PDL (D). (E–H) HEK-293 cells expressing ADAM9-GFP; (I–L) HEK-293 cells expressing ADAM9-ΔMP-GFP; (M–P) HEK-293 cells expressing ADAM9-ΔMPD-GFP. The order of substrates is the same as (A–D). Magnification ×350.
Confirmation of the role of the ADAM9 disintegrin domain in cell–matrix adhesion
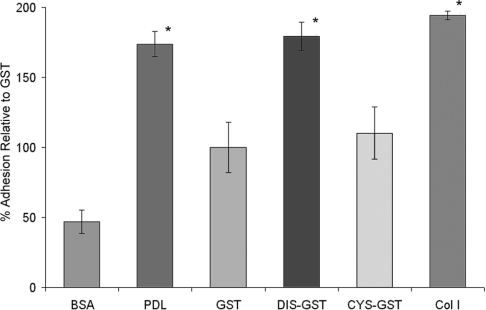
Cell–matrix adhesion studies using stable expression of defined ADAM9 deletion constructs suggested that cell–matrix interaction was specifically modulated by the disintegrin domain alone. To confirm this observation, we evaluated whether recombinant ADAM9 disintegrin domain protein expressed as a chimaera with GST (designated DIS-GST) or cysteine-rich domain expressed with GST (designated CYS-GST) could support adhesion of HEK-293 cells. Recombinant GST protein was used as a recombinant protein control and collagen I and PDL were used as positive binding controls. These studies are summarized in Figure 5. Binding of the HEK-293 cells to the recombinant DIS-GST protein was significantly greater than that observed with control GST protein, whereas binding to the CYS-GST protein was not significantly increased. The levels of HEK-293 cell binding observed with the DIS-GST protein were similar to those obtained with collagen I and PDL positive controls. In contrast, the binding of HEK-293 cells to recombinant CYS-GST protein was not significantly different from binding to GST protein alone. These results confirm the impression obtained in the cell–matrix-binding assays and localize adhesion activity specifically to the disintegrin domain.
Figure 5. Adhesion assay of HEK-293 cells to recombinant ADAM9 disintegrin and cysteine-rich domains.
Recombinant GST, disintegrin-GST (DIS-GST) and cysteine-rich GST (CYS-GST) fusion proteins were prepared as described in the Experimental section and were used as substrates for quantitative HEK-293 cell binding assays. PDL and type I collagen were used as positive controls; BSA was used as a negative control. Results are expressed as the means±S.D. for percentage adhesion relative to GST (*P<0.05).
Identification of the integrin binding specificity of the ADAM9-disintegrin domain
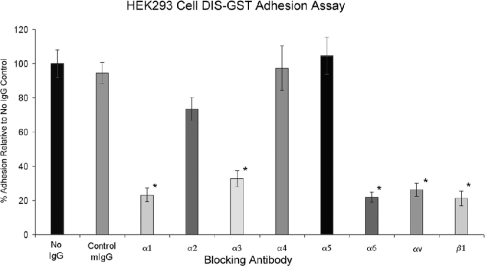
The adhesion assays detailed above pinpoint the site of matrix protein adhesion interaction to the disintegrin domain of ADAM9. These observations were supplemented by a further series of studies designed to identify the specific renal β1 integrins that interact with the disintegrin domain. Two complementary approaches were utilized. In the first approach, culture wells were coated with the recombinant DIS-GST protein and a quantitative adhesion assay was performed using HEK-293 cells preincubated with specific anti-integrin subunit monoclonal antibodies or with control murine IgG. The results of these experiments are depicted in Figure 6. Incubation with control murine IgG had no quantitative effect on HEK-293 cell adhesion to the DIS-GST protein, whereas preincubation with monoclonal antibodies to the integrin subunits α1, α3, α6, αv and β1 significantly decreased HEK-293 cell adhesion to the recombinant DIS-GST protein. Monoclonal antibodies to integrin subunits α2, α4 and α5 (or β3, results not shown) had no significant effect on cellular adhesion.
Figure 6. Definition of ADAM9 disintegrin–integrin interactions.
HEK-293 cells were preincubated with saline (no IgG), control murine IgG (mIgG) or specific anti-integrin antibodies as described in the Experimental section, followed by a quantitative adhesion assay on the recombinant disintegrin-GST fusion peptide. Results are expressed as the means±S.D. for percentage adhesion relative to the saline control (P<0.05).
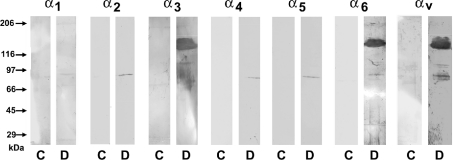
In the second approach, immobilized DIS-GST protein was perfused with renal cortical lysates to confirm the pattern of integrin subunit binding to the disintegrin domain. Controls consisted of immobilized GST protein alone. After elution, the material was subjected to SDS/PAGE and Western-blot analysis for individual integrin subunit proteins. These studies are summarized in Figure 7 and indicate that immobilized DIS-CYS specifically recovered from renal cortical lysates the α3, α6 and αv subunits. In addition, the β1, but not the β3 subunit was detected (results not shown). The α1 integrin subunit was not detected by this method, but this is probably due to the fact that the expression of this integrin in the kidney is limited to glomerular mesangial cells, [5,6], which represent a very minor component of the cortical lysate preparation (<1% of total protein).
Figure 7. Western-blot analysis of renal cortical integrins recovered from immobilized recombinant ADAM9 disintegrin-GST fusion peptide.
Rat renal cortical lysates were prepared as described in the Experimental section, followed by perfusion over immobilized recombinant disintegrin-GST peptide (D) or control GST (C). Western blots of eluted proteins were performed using specific anti-integrin antibodies. Reactive bands of approx. 120 kDa were detected for α3, α6 and αv integrins.
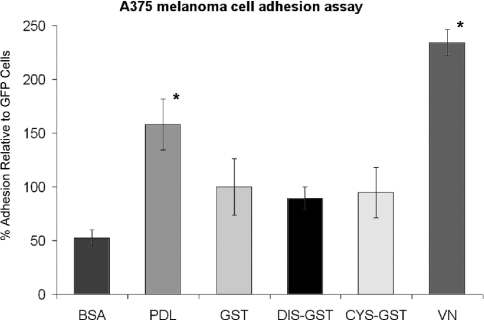
To confirm further the specificity of DIS–GST interaction with αvβ1 as opposed to αvβ3 integrin, both of which act as vitronectin receptors, an adhesion assay using A375 melanoma cells, which only express the αvβ3 integrin [7,8], was performed. These results are summarized in Figure 8 and indicate that this cell type does not manifest specific adhesion to the ADAM9 DIS-GST peptide, whereas clear adhesion to vitronectin was observed.
Figure 8. A375 melanoma cell adhesion to recombinant ADAM9 disintegrin and cysteine-rich domains.
Recombinant GST, disintegrin-GST (DIS-GST) and cysteine-rich GST (CYS-GST) fusion peptides were prepared as described in the Experimental section and were used as substrates for quantitative A375 cell binding assays. PDL and type I collagen were used as positive controls; BSA was used as a negative control. Results are expressed as the means±S.D. for percentage adhesion relative to GST (*P<0.05).
DISCUSSION
The present study demonstrates that the ADAM9 protein interacts, through the disintegrin domain, with multiple renal integrins of the β1 class, thereby affecting adherence of HEK-293 cells to defined extracellular matrix proteins. The HEK-293 cell line is derived from HEK and displays multiple renal integrins of the β1 family, including α1–6 and αv [9]. Controlled expression of equivalent amounts of defined regions of the ADAM9 protein permitted a quantitative analysis of the adhesion properties of individual structural domains and localized the site of interaction affecting cellular adhesion to the disintegrin domain. ADAM9 was shown to interact with multiple β1 integrins present in the kidney, including α1, α3, α6 and αv. These specific β1 integrin–ADAM9 disintegrin interactions correlate well with the disruption of cell binding to collagens I and IV (α1β1), laminin (α3β1 and α6β1) and vitronectin (αvβ1), whereas no effect of the ADAM9 protein or disintegrin domain was seen on cellular binding to fibronectin (α5β1) or the non-specific substrates BSA and PDL.
Recently, there has been considerable attention given to the protease properties of the ADAM family, where several members have been shown to act as membrane sheddases [2,3]. The extensively studied tumour necrosis factor α converting enzyme (TACE, ADAM17) is a membrane sheddase with multiple substrates in addition to tumour necrosis factor α [10–13]. Other members of the ADAM family, including ADAM10 and ADAM9, may affect cellular development or differentiation through the proteolytic shedding of growth factors or receptors [14,15].
The disintegrin domains of the ADAM protein family were originally postulated to play a role in cell–matrix interactions based on the structural similarity with the RGD-containing snake venom disintegrins. Significantly, only one member of the ADAM gene family, metargidin, ADAM15, includes an RGD sequence within the disintegrin domain [16,17]. The disintegrin domain of metargidin interacts, through the RGD sequence, with αvβ3 integrin [17] and α5β1 [18]. Interactions with integrins do not absolutely require the presence of an RGD consensus sequence; ADAM23, which lacks an RGD sequence in the disintegrin domain, exhibits highly specific interaction with the αvβ3 integrin [19]. The lymphocyte MDC-L (ADAM28) also lacks an RGD sequence, yet specifically interacts with the α4β1 integrin [20]. Eto et al. [21,22] demonstrated that a conserved RX6DLPEF motif flanking the RGD sequence in ADAM15 directs specific binding to the α9β1 integrin. These studies also demonstrated that the RX6DLPEF motif is conserved within all members of the ADAM family with the exception of ADAM10 and ADAM17. Thus ADAM 1, ADAM2, ADAM3, ADAM9, ADAM12 and ADAM15 bind the α9β1 integrin.
There have been two additional reports concerning specific ADAM9 interactions with integrins. Nath et al. [23] demonstrated that ADAM9 mediated fibroblast adhesion and motility through a specific interaction with α6β1 integrin. Zhou et al. [24] found that ADAM9 functions as an adhesion molecule by binding the disintegrin domain to integrin αvβ5 expressed by human myeloma cells. The present study expands the list of ADAM9 integrin ligands to a total of six, making ADAM9 the most polyvalent member of the ADAM family investigated to date. Two of these integrin species, α9β1 and αvβ5, are not expressed in the adult kidney, whereas the other four integrins determined in the present study have distinct distributions within the nephron. Integrin α1β1 is expressed in the glomerular mesangium, whereas integrin α3β1 is expressed in the glomerulus and distal nephron [5,6,25]. Integrins α6β1 and αvβ1 are present in both proximal and distal tubular segments. Thus the distributions of the four β1 integrins identified as ADAM9 interactors matches precisely the distribution of ADAM9 protein identified by histochemical analysis.
The biological roles of the ADAM9 protein in terms of renal structure and function remain conjectural at this point. The intimate relationship of the ADAM9 protein on the basolateral surfaces of the tubular epithelial cells in close association with four discretely distributed β1 integrins suggests a role in the modulation of integrin-dependent cell–cell or cell–matrix interactions. Such modulation may be important during development or after a renal tubular injury, in which major changes in cell–cell and cell–matrix interactions occur. The identification of the ADAM9 interacting renal β1 integrins will provide the basis for future analysis of these important questions.
Acknowledgments
This study was supported by National Institutes of Health grants DK 39776 (to D.H.L.) and DK 31398 (to A.S.P.).
References
- 1.Wolfsberg T. G., Primakoff P., Myles D. G., White J. M. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J. Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Primakoff P., Myles D. G. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 3.Kheradmand F., Werb Z. Shedding light on sheddases: role in growth and development. Bioessays. 2002;24:8–12. doi: 10.1002/bies.10037. [DOI] [PubMed] [Google Scholar]
- 4.Mahimkar R., Baricos W. H., Visaya O., Pollock A. S., Lovett D. H. Identification, cellular distribution and potential function of the metalloprotease-disintegrin MDC9 in the kidney. J. Am. Soc. Nephrol. 2000;11:595–603. doi: 10.1681/ASN.V114595. [DOI] [PubMed] [Google Scholar]
- 5.Korhonen M., Ylanne J., Laitinen L., Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J. Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy-Chaudhury P., Hillis G., McDonald S., Simpson J. G., Power D. A. Importance of the tubulointerstitium in human glomerulonephritis. II. Distribution of integrin chains beta 1, alpha 1 to 6 and alpha V. Kidney Int. 1997;52:103–110. doi: 10.1038/ki.1997.309. [DOI] [PubMed] [Google Scholar]
- 7.Gehlsen K. R., Davis G. E., Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin. Exp. Metastasis. 1992;10:111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- 8.Iba K., Albrechtsen R., Gilpin B. J., Loechel F., Wewer U. M. Cysteine-rich domain of human ADAM 12 (meltrin alpha) supports tumor cell adhesion. Am. J. Pathol. 1999;154:1489–1501. doi: 10.1016/s0002-9440(10)65403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J. Biol. Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 10.Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature (London) 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 11.Schlondorff J., Blobel C. P. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 12.Black R. A. Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 2001;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 13.Sunnarborg S. W., Hinkle C. L., Stevenson M., Russell W. E., Raska C. S., Peschon J. J., Castner B. J., Gerhart M. J., Paxton R. J., Black R. A., et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 14.Izumi Y., Hirata M., Hasuwa H., Iwamoto R., Umata T., Miyado K., Tamai Y., Kurisaki T., Sehara-Fujisawa A., Ohno S., et al. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi H., Rand M. D., Wu X., Sestan N., Wang W., Rakic P., Xu T., Artavanis-Tsakonas S. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283:91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- 16.Kratzschmar J., Lum L., Blobel C. P. Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J. Biol. Chem. 1996;271:4593–4596. doi: 10.1074/jbc.271.9.4593. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X.-P., Kamata T., Yokoyama K., Puzon-McLaughlin W., Takada Y. Specific interaction of the recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15) with integrin alphavbeta3. J. Biol. Chem. 1998;273:7345–7350. doi: 10.1074/jbc.273.13.7345. [DOI] [PubMed] [Google Scholar]
- 18.Nath D., Slocombe P. M., Stephens P. E., Warn A., Hutchinson G. R., Yamada K. M., Docherty A. J. P., Murphy G. Interaction of metargidin (ADAM-15) with alphavbeta3 and alpha5beta1 integrins on different haemopoietic cells. J. Cell Sci. 1999;112:579–587. doi: 10.1242/jcs.112.4.579. [DOI] [PubMed] [Google Scholar]
- 19.Cal S., Freije J. M. P., Lopez J. M., Takada Y., Lopez-Otin C. ADAM 23/MDC3, a human disintegrin that promotes cell adhesion via interaction with the alphavbeta3 integrin through an RGD-independent mechanism. Mol. Biol. Cell. 2000;11:1457–1469. doi: 10.1091/mbc.11.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges L. C., Tani P. H., Hanson K. R., Roberts C. M., Judkins M. B., Bowditch R. D. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4beta1. J. Biol. Chem. 2002;277:3784–3792. doi: 10.1074/jbc.M109538200. [DOI] [PubMed] [Google Scholar]
- 21.Eto K., Puzon-McLaughlin W., Sheppard D., Sehara-Fujisawa A., Zhang X.-P., Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J. Biol. Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- 22.Eto K., Huet C., Tarui T., Kupriyanov S., Liu H.-Z., Puzon-McLaughlin W., Zhang X.-P., Sheppard D., Engvall E., Takada Y. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 2002;277:17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- 23.Nath D., Slocombe P. M., Webster A., Stephens P. E., Docherty A. J., Murphy G. Meltrin gamma (ADAM-9) mediates cellular adhesion through alpha(6)beta(1) integrin, leading to a marked induction of fibroblast cell motility. J. Cell Sci. 2000;111:2319–2328. doi: 10.1242/jcs.113.12.2319. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M., Graham R., Russell G. G., Croucher P. I. MDC-9 (ADAM-9/meltrin gamma) functions as an adhesion molecule by binding the alpha(v)beta(5) integrin. Biochem. Biophys. Res. Commun. 2001;280:574–580. doi: 10.1006/bbrc.2000.4155. [DOI] [PubMed] [Google Scholar]
- 25.Kreidberg J. A., Symons J. M. Integrins in kidney development, function, and disease. Am. J. Physiol. Renal Physiol. 2000;279:F233–F242. doi: 10.1152/ajprenal.2000.279.2.F233. [DOI] [PubMed] [Google Scholar]