Abstract
Natural cellulose exists as a composite of cellulose forms, which can be broadly characterized as crystalline or non-crystalline. The recognition of both of these forms of cellulose by the CBMs (carbohydrate-binding modules) of microbial glycoside hydrolases is important for the efficient natural and biotechnological conversion of cellulosic biomass. The category of CBM that binds insoluble non-crystalline cellulose does so with an affinity approx. 10–20-fold greater than their affinity for cello-oligosaccharides and/or soluble polysaccharides. This phenomenon has been assumed to originate from the effects of changes in configurational entropy upon binding. The loss of configurational entropy is thought to be less profound upon binding to conformationally restrained insoluble non-crystalline cellulose, resulting in larger free energies of binding. However, using isothermal titration calorimetry, it is shown that this is not the case for the high-affinity interactions of CcCBM17 (the family 17 CBM from EngF of Clostridium cellulovorans) and BspCBM28 (the family 28 CBM from Cel5A of Bacillus species 1139) with regenerated cellulose, an insoluble preparation of primarily non-crystalline cellulose. The enhanced free energy of binding of non-crystalline cellulose relative to cello-oligosaccharides is by virtue of improved enthalpy, not entropy.
Keywords: adsorption, carbohydrate-binding module, cellulose, cellulose-binding domain, non-crystalline, thermodynamics
Abbreviations: CBM, carbohydrate-binding module; ΔCp, change in heat capacity; ΔG, free energy of binding; ΔH, change in enthalpy; ITC, isothermal titration calorimetry; [N]o, binding capacity or density of binding sites; RC, regenerated cellulose; ΔS, change in entropy
INTRODUCTION
CBMs (carbohydrate-binding modules) are discrete modules found within numerous carbohydrate-active enzymes. Their near ubiquity in plant cell wall polysaccharide-degrading enzymes is testament to their importance in the recycling of this abundant carbon source. CBMs that are specific for insoluble cellulose, arguably the most predominant CBMs, can be grouped into two general categories: those that interact with crystalline cellulose and those that interact with non-crystalline cellulose. The former, which fall into the Type A CBMs [1], make up a unique class of carbohydrate-binding proteins that have been the subject of a number of studies over the last 15 years. The latter category of CBMs is included in Type B [1], and has only recently been gaining more attention.
Type B CBMs have binding sites that are ‘valley’-shaped and well suited to accommodating individual glycan chains of three sugars or longer [1]. Those Type B CBMs that are specific for cellulose (e.g. CfCBM4-1 [2], CfCBM4-2 [3], CcCBM17 [4,5] and BspCBM28 [6]) bind soluble cello-oligosaccharides in addition to insoluble cellulose. In general, the association constants of these CBMs for their optimal cello-oligosaccharide ligands and/or soluble polysaccharides are ∼2×104 M−1. The thermodynamics of these interactions are typified by large favourable ΔH (change in enthalpy) values and partially offsetting unfavourable TΔS values, like those of most other protein–carbohydrate interactions. Interestingly, the association constants (Ka values) of these CBMs for non-crystalline insoluble cellulose are invariably greater than 105 M−1. Recent studies of the adsorption of CcCBM17 (the family 17 CBM from EngF of Clostridium cellulovorans) and BspCBM28 (the family 28 CBM from Cel5A of Bacillus species 1139) showed that a preparation of RC (regenerated cellulose), a primarily non-crystalline cellulose in an insoluble form, presented at least two classes of binding sites to each of these CBMs [7]. The high-affinity sites were bound with Kas upwards of ∼106 M−1, and the low-affinity sites with Kas of ∼104 M−1. An important question is how these Type B CBMs interact with insoluble non-crystalline cellulose 10–100-fold more tightly than with sugars free in solution. This question is particularly salient considering that natural cellulose, the true ligand for these CBMs, is insoluble.
ITC (isothermal titration calorimetry) is a powerful method for studying molecular interactions. A single experiment can be used to determine the Ka, ΔH and stoichiometry (n) of an interaction. From these values, ΔG (free energy of binding) and ΔS (change in entropy) can be derived. This method has been applied twice to the challenging problem of studying the interaction of CBMs with insoluble cellulose. The binding of the family 9 CBM from Thermotoga maritima to RC revealed an enthalpically favourable binding mechanism. However, this CBM is the only one to be shown to interact with the reducing termini of polysaccharides, making this a somewhat unique situation [8]. Creagh et al. [9] used ITC to quantify the interaction of CfCBM2a, a family 2 CBM from Cellulomonas fimi, with a highly crystalline form of cellulose, BMCC (bacterial microcrystalline cellulose). This seminal study showed that, in contrast with most protein–carbohydrate interactions, this process was driven by a favourable ΔS. This thermodynamic signature was rationalized to result from the release of ordered water from the relatively apolar CBM binding site and cellulose surface. In many systems, favourable contributions to ΔS from such dehydration may be wiped out by compensating losses in ΔS from changes in the configurational entropy of the reactants. However, in the case of this CBM, the ligand (i.e. cellulose) has a rigid and fixed structure. Thus it was argued that the favourable ΔS of the CBM2a–BMCC interaction results from the lack of the loss of configurational entropy in BMCC upon binding. This assumption has been applied to the interaction of Type B CBMs with non-crystalline cellulose, such as RC or acid-swollen cellulose, to rationalize the differences in affinities between non-crystalline cellulose and cello-oligosaccharides. Due to its insoluble nature and somewhat restrained structure, non-crystalline cellulose is thought to lose less configurational entropy than a cello-oligosaccharide upon binding, resulting in a smaller entropic penalty and, thus, better overall free energy of binding. This supposition is logical, but currently not supported by experimental data. In order to examine this, ITC was used to measure the thermodynamics of the binding of CcCBM17 and BspCBM28 to RC. The results confirm the presence of two classes of binding sites for each CBM on RC. The argument of less net loss of configurational entropy may apply to the recognition of the low-affinity site, but this is offset by a less favourable ΔH of binding. Recognition of the high-affinity site at the temperatures used (15–35 °C) is a distinctly enthalpically driven process whereby the enhanced ΔG of binding relative to cello-oligosaccharides comes by virtue of improved enthalpy, not entropy.
MATERIALS AND METHODS
Materials
Microcrystalline cellulose (Avicel™ PH101) was obtained from FMC International (Little Island, County Cork, Ireland). RC was prepared by phosphoric acid treatment of Avicel™ as reported previously [8]. The batch of RC used in this study was the same as that used for previous studies of CcCBM17 and BspCBM28 [7].
Purification of CBMs
Gene fragments encoding CBMs were cloned, expressed and the products purified as described elsewhere [5,6]. The CBMs used in this study were CcCBM17, the C-terminal module from Clostridium cellulovorans Cel5A, and BspCBM28, the C-terminal module from Bacillus sp. 1139 Cel5A. The concentrations of purified proteins were determined by UV absorbance (280 nm) using calculated molar absorption coefficients [10] of 31010 M−1·cm−1 and 32290 M−1·cm−1 for CcCBM17 and BspCBM28 respectively.
ITC analysis
All ITC experiments were carried out with a VP-ITC isothermal titration calorimeter (MicroCal Inc., Northampton, MA, U.S.A.). CcCBM17, BspCBM28 and RC were dialysed together and extensively (3 weeks with buffer changes every 2–3 days; last change was left for 4 days) against 50 mM potassium phosphate buffer, pH 7.0. The concentrations of CcCBM17 and BspCBM28, filtered previously through 0.2 μm filters, were determined to be 1.202 mM and 1.14 mM respectively, by UV absorbance. Appropriate dilutions of the proteins for the UV readings were prepared by mass on an analytical balance, assuming a density of 1 g/ml for the protein solution. The concentration of RC was determined by dry weight and corrected for the mass of buffer components, which were also measured by the dry weight of residue in the dialysis buffer. The stock of RC was diluted to 5 mg/ml in the dialysis buffer and the concentration reconfirmed by dry weight. Protein and cellulose samples were degassed extensively with stirring prior to use. Titrations were performed by titrating 26 samples of 10 μl of protein into the ITC cell, which contained RC at 5 mg/ml. The sample was stirred at 550 rev./min during the titration. Triplicate titrations were performed at each of the temperatures 15, 25 and 35 °C. Titration of buffer into buffer and buffer into RC did not produce significant heats. However, titration of CBM into buffer did yield significant heats, so a heat of dilution experiment of protein titrated into buffer was performed for each temperature, and the value obtained was subtracted from that from the experimental titration to correct for this effect.
The binding isotherms corrected for heat of dilution were analysed using the MicroCal ORIGIN software (version 7.0) supplied with the instrument. Based on previous depletion isotherm results with CcCBM17 and BspCBM28 [7], the total concentrations of binding sites on the RC were fixed at 122.5 μM (5 mg/ml at 24.5 μmol of binding sites per g of RC) and 52.5 μM (5 mg/ml at 10.5 μmol of binding sites per g of RC) for CcCBM17 and BspCBM28 respectively during the fitting process. The software provided with the instruments includes very commonly used binding models treating the acceptor (i.e. cellulose in this case) as having one class of independent binding sites or two classes of independent binding sites. In the first case, the stoichiometry (n value) represented the fraction of the total expected binding sites that acted as acceptors. In the latter case, two n values are regressed, one for each class of binding site, which represent the fractions of the total expected binding sites that are of one class or the other. C-values [C=Ka×A×n, where Ka is the association constant, A is the acceptor concentration (i.e. binding sites on the cellulose) and n is the number of binding sites on the acceptor] for the high-affinity sites were always greater than 6, allowing accurate deconvolution of the binding parameters (Ka, ΔH and n). The n value for the high-affinity site was determined during the regression and represented the fraction of the total binding sites that were high-affinity sites. [N]o, an expression of the stoichiometry representing the concentration of binding sites on the cellulose, was determined by multiplying n by the total initial concentration of binding sites (i.e. 122.5 μM or 52.5 μM) and dividing by the cellulose concentration (i.e. 5 mg/ml) to give a value in μmol/g of RC. C-values for the low-affinity binding sites were to low to allow accurate deconvolution of both ΔH and n. Thus the n values were fixed at values such that the concentrations of low-affinity binding sites were equal to the values determined previously by depletion isotherms [7]. Thus ΔH and Ka could be determined for the low-affinity sites.
RESULTS AND DISCUSSION
CcCBM17 and BspCBM28 recognize two classes of binding sites in RC
The cellulose depletion isotherm is a method adapted to direct measurement of the partitioning of CBM between the bound and free states when using cellulose as an insoluble sorbent. We previously studied the binding of CcCBM17 and BspCBM28 to RC by this method. Binding models accounting for multiple independent binding sites on the cellulose and one-dimensional overlapping lattices of binding sites, with and without co-operativity, were discriminated using Scatchard plots and statistical evaluation of non-linear fits to the models. Based on this, it appeared that these two CBMs each recognized two independent classes of binding sites on non-crystalline cellulose [7]. Both CBMs bound completely reversibly, and there did not appear to be any co-operativity to the binding.
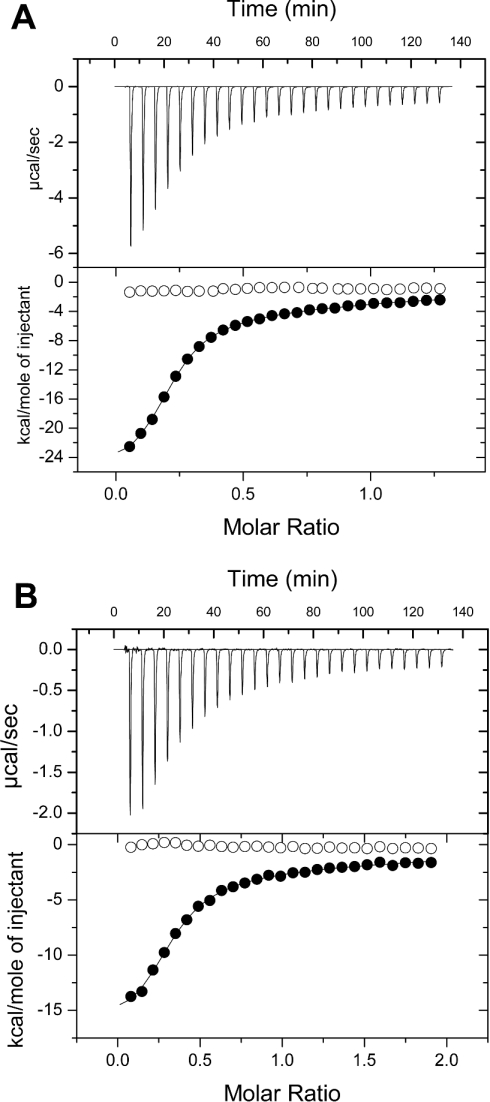
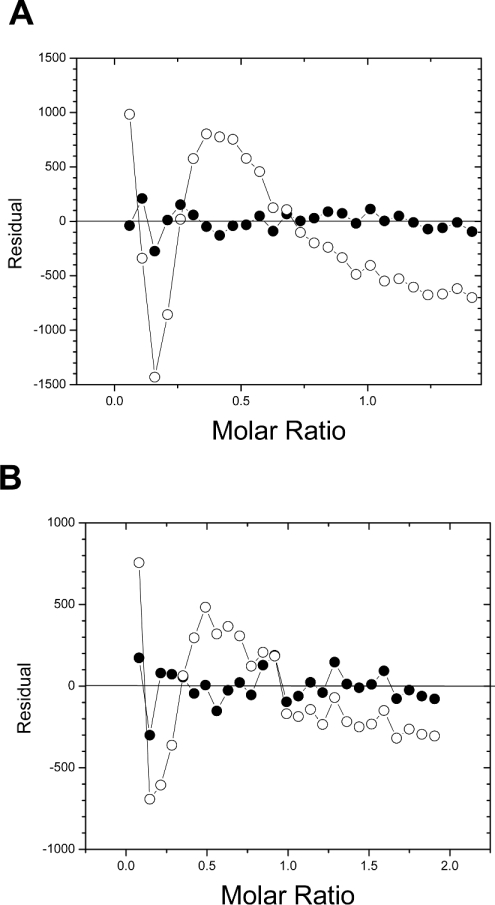
Titrations of the CBMs into RC yielded negative power deflections, indicating exothermic binding, and integrated isotherms of sigmoidal shape (Figure 1). These calorimetric isotherms also appeared to show two classes of binding site on RC for both CBMs. Visual inspection of the residuals from fits of a one-site binding model to the data (differences between the fit and experimental Y values for each X value) clearly indicated systematic deviations in the one-site fit, whereas a model involving two independent binding sites gave apparently random scatter about the X-axis (Figure 2). Runs-test analysis of the residuals from the fits of a one-site binding model to the isotherms gave P-values of 0.0004 for both CBMs. Using a standard P-value threshold of 0.05, this confirmed a strong systematic deviation from the one-site model and, thus, its rejection. In contrast, a similar analysis with a two independent binding site model gave P-values of 0.212 and 0.115 (i.e. significantly greater than 0.05) for CcCBM17 and BspCBM28 respectively, indicating that the two-site model was statistically appropriate.
Figure 1. Representative calorimetric isotherms of binding of CcCBM17 (A) and BspCBM28 (B) to RC at 25 °C and 15 °C respectively in 50 mM potassium phosphate, pH 7.0.
The upper panels show the raw data, and the lower panels the integrated data. The CcCBM17 and BspCBM28 titrations into RC are shown as solid circles. The lines show the fits to a two independent binding site model. The corresponding heat of dilution data (CBM titrated into buffer) are shown for reference as open circles. 1 kcal=4.184 kJ.
Figure 2. Residual plots of the fits of a one-site binding model (○) and a two independent binding site model (●) to the calorimetric isotherms of binding of CcCBM17 (A) and BspCBM28 (B) to RC at 25 °C and 15 °C respectively.
As was acknowledged previously, there may indeed be more than two classes of binding sites on RC. However, Scatchard analysis of our previous depletion isotherms showed two distinct binding phases [7]. Furthermore, fitting both the cumulative ITC isotherms (results not shown) and depletion isotherms [7] with binding models accounting for more than two independent binding sites failed to reduce the sum-of-squares of the fits and, therefore, were not statistically better. Thus, although there may be more than two distinct classes of binding sites for each of CcCBM17 and BspCBM28 in RC, it is concluded that there are two dominant classes of binding sites, the general properties of which are revealed in this analysis. On this basis, all of the ITC isotherms were analysed using a two independent binding site model.
Interaction of CcCBM17 with RC
The association constants for binding of CcCBM17 to high- and low-affinity sites on RC were approx. 6×105 M−1 and 1×104 M−1 respectively, and agreed reasonably well with values determined previously by depletion isotherms (Table 1). The high-affinity binding site density ([N]o value) was also in acceptable agreement with that determined previously (Table 1).
Table 1. Thermodynamic parameters of the interactions of CcCBM17 and BspCBM28 with RC in 50 mM potassium phosphate, pH 7.0.
Values are means±S.D. of triplicate experiments. The stoichiometry of the interaction (n) was determined as described in the Materials and methods section. [N]o indicates the concentration of binding sites per g of cellulose, and was determined as described in the Materials and methods section. N/A, not applicable. 1 cal=4.184 J.
| CBM/method | Temp (°C) | No. of sites | 10−4×Ka (M−1) | ΔG (kcal/mol) | ΔH (kcal/mol) | ΔS (cal/mol per K) | n | [N]o (μmol/g) |
|---|---|---|---|---|---|---|---|---|
| CcCBM17 | ||||||||
| ITC | 15 | 1 | 60.7±14.0 | −7.6±0.1 | −21.3±0.6 | −47.5±2.6 | 0.25±0.01 | 6.2±0.2 |
| 2 | 1.2±0.2 | −5.3±0.1 | −8.8±0.3 | −11.8±1.3 | 0.68 | 15.9 | ||
| ITC | 25 | 1 | 54.0±18.0 | −7.8±0.2 | −24.2±0.7 | −55.0±3.1 | 0.23±0.00 | 5.5±0.0 |
| 2 | 1.2±0.4 | −5.5±0.2 | −10.3±1.1 | −15.9±4.5 | 0.68 | 15.9 | ||
| ITC | 35 | 1 | 59.6±0.4 | −8.1±0.0 | −21.5±0.5 | −43.4±1.7 | 0.25±0.01 | 6.2±0.2 |
| 2 | 1.3±0.3 | −5.8±0.2 | −10.5±0.9 | −15.5±3.5 | 0.68 | 15.9 | ||
| Depletion* | 25 | 1 | 113.0±0.1 | −8.4±0.3 | N/A | N/A | N/A | 8.6±1.4 |
| 2 | 1.8±0.5 | −5.9±0.4 | N/A | N/A | N/A | 15.9±1.3 | ||
| ITC – G6† | 25 | − | 5.9±0.1 | −6.6±0.0 | −14.5±0.0 | −27.5±0.7 | 1 | N/A |
| BspCBM28 | ||||||||
| ITC | 15 | 1 | 44.6±2.4 | −7.3±0.3 | −19.3±2.7 | −41.7±10.4 | 0.26±0.02 | 2.7±0.3 |
| 2 | 2.0±0.3 | −5.7±0.1 | −7.3±2.3 | −5.6±7.6 | 0.65 | 6.8 | ||
| Depletion* | 25 | 1 | 99.0±0.2 | −8.3±0.4 | N/A | N/A | N/A | 3.7±0.4 |
| 2 | 2.1±0.1 | −5.9±0.4 | N/A | N/A | N/A | 6.8±0.6 | ||
| ITC – G6‡ | 25 | − | 4.0±0.1 | −6.4±0.0 | −14.9±0.0 | −28.6±0.5 | 1 | N/A |
At 25 °C, CcCBM17 bound to cellohexaose with an affinity of 5.8×104 M−1 [ΔG=−27.2 kJ/mol (−6.5 kcal/mol)], a ΔH of −60.7 kJ/mol (−14.5 kcal/mol) and a ΔS of −113 J/mol per K (−27 cal/mol per K) [5] (Table 1). Thus at this temperature the recognition of the high-affinity site in RC by CcCBM17 is less entropically favourable by approx. −117 J/mol per K (−28 cal/mol per K) [or TΔΔS of approx. −35.1 kJ/mol (−8.4 kcal/mol)] than the recognition of cellohexaose (Table 1). The improvement in ΔG [approx. −5.4 kJ/mol (−1.3 kcal/mol)] comes by virtue of an interaction that is enthalpically more favourable by approx. −40.6 kJ/mol (−9.7 kcal/mol). Thus, if the loss of configurational entropy upon binding RC is less than for cellohexaose, this effect is eclipsed by other less favourable contributions to entropy and more favourable contributions to enthalpy.
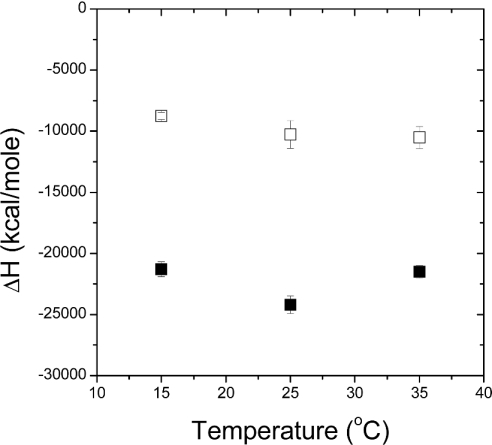
The dependence of ΔH on experimental temperature for the recognition of the high-affinity sites on RC by CcCBM17 was not linear (Figure 3). There was a decrease in ΔH when going from 15 °C to 25 °C, giving a ΔCp (change in heat capacity) of −1213±377 J/mol per K (−290±90 cal/mol per K). The increase in ΔH when going from 25 °C to 35 °C gave a ΔCp of 1130±377 J/mol per K (270±90 cal/mol per K). Such non-linear behaviour of ΔCp has been documented for an antibody–carbohydrate interaction [11] and was suggested to arise from changes in the antibody conformation [12]. The conformation of CcCBM17 is identical in its liganded and unliganded states [5]; furthermore, CcCBM17 showed a linear relationship between ΔH and temperature for binding to cello-oligosaccharides [5]. Therefore it is unlikely to be a result of structural changes in the CBMs, but it is possible that the behaviour of ΔCp, in this case, represents a structural change in the RC.
Figure 3. Enthalpy against temperature data for binding of CcCBM17 to RC in 50 mM potassium phosphate, pH 7.0.
■, High-affinity binding sites; □, low-affinity sites. Error bars represent the S.D. of triplicate experiments. 1 kcal=4.184 kJ.
The concept of structural rearrangements in cellulose upon CBM binding is not a new one: CfCBM2a was observed to release cellulose fragments non-catalytically from crystalline cellulose [13]. The mechanism by which this occurs is unknown, but it is assumed to be via mechanical disruption of the cellulose fibres [14]. In the case of CcCBM17 binding, a structural rearrangement in the cellulose may occur as changes in inter- or intra-molecular hydrogen bonding while the cellulose chain(s) are induced to adopt a different conformation. Such a possibility implies that the recognition of the high-affinity sites may occur via a multi-step equilibrium. It is not possible to determine conclusively if this is in fact true and, in this event, the parameters reported for this binding site would omit details of the individual steps required to form the bound species. However, the values reported would, to a first approximation, give a general representation of energetics involved in the overall equilibrium and, thus, the interpretation would remain the same.
The recognition of the low-affinity site in RC by CcCBM17 is more entropically favourable by ∼46.0 J/mol per K (∼11 cal/mol per K) [or TΔΔS of ∼13.8 kJ/mol (∼3.3 kcal/mol)] and ∼17.6 kJ/mol (∼4.2 kcal/mol) less enthalpically favourable than the recognition of cellohexaose, to give a differential in ΔG of approx. 4.2 kJ/mol (1 kcal/mol) (Table 1). ΔH for this site appeared to vary approximately linearly (R2=0.87) with temperature (Figure 3), yielding a ΔCp,RC of −368±146 J/mol per K (−88±35 cal/mol per K), compared with a ΔCp,G6 of −611±46 J/mol per K (−146±11 cal/mol per K) for the recognition of cellohexaose. The magnitude of ΔCp is thought to be the best indicator of ΔSsolv, the contribution of solvent rearrangement to entropy, where a large negative ΔCp generally indicates a more favourable ΔSsolv [15]. Based on this generalization, because ΔCp,RC is greater than ΔCp,G6, then ΔSsolv for RC must be less than ΔSsolv for cellohexoase, meaning the contribution of ΔSsolv to binding at the low-affinity RC site is less favourable than for cellohexaose binding. Overall, ΔS can be generally be considered the sum of ΔSsolv and ΔSconfig/rot/trans, where ΔSconfig/rot/trans is change in entropy resulting from alterations in the configurational, rotational and translational freedom of the system components [15]. Because the difference between the ΔSsolv values for RC and cellohexaose is negative, but the overall difference between ΔS values is positive [+46 J/mol per K (+11 cal/mol per K)], the difference between ΔSconfig/rot/trans values for binding to RC compared with cellohexaose must be substantially positive to overcome the deficit of ΔSsolv. Thus the change in entropy due to the changes in the configurational, rotational and translational freedom of the system components is more favourable for binding RC than for the recognition of cellulose. Although this analysis is somewhat rough due to the limited precision of the experimental measurements, this does suggest consistency with the assumption of less loss of configurational entropy when binding to the low-affinity site in insoluble cellulose. However, this appears to be at the cost of an overcompensating change in ΔH which results in a poorer ΔG of binding.
Interaction of BspCBM28 with RC
Due to the low density of binding sites for BspCBM28 on RC, the inability to use RC in the calorimeter at concentrations greater than those used, and the resulting difficulty in maintaining adequate C-values, ITC using this CBM was less tractable. However, the appropriate conditions were met when performing the experiments at a sufficiently low temperature, i.e. 15 °C. As with CcCBM17, the calorimetric values of Ka and [N]o for binding of BspCBM28 to the high-affinity site were similar to those determined by depletion isotherms at 25 °C (Table 1).
At 25 °C, BspCBM28 bound to cellohexaose with an affinity of 4.0×104 M−1 [ΔG=−26.4 kJ/mol (−6.3 kcal/mol)], a ΔH of −61.1 kJ/mol (−14.6 kcal/mol) and a ΔS of −117 J/mol per K (−28 cal/mol per K) [6]. Although the parameters for binding of BspCBM28 to RC were determined at 15 °C, it is clear that the ΔH for the high-affinity site on RC was substantially more favourable and the ΔS substantially less favourable in comparison with binding to cellohexaose (Table 1), a pattern identical with that observed for CcCBM17. Likewise, the pattern for binding at the low-affinity site was the same, i.e. a less favourable ΔH and more favourable ΔS in comparison with cellohexaose. The thermodynamic signatures of cello-oligosaccharide binding by CcCBM17 and BspCBM28 are extremely similar; it would also appear that the thermodynamic signatures for RC recognition by the two CBMs are very similar.
Conclusions
CcCBM17 and BspCBM28 are CBMs that each recognize two distinct classes of binding sites on RC: one of high affinity and one of low affinity. Both classes are recognized via enthalpically driven processes at the temperatures tested. Contrary to assumption, the high-affinity interactions with RC are 10–100-fold stronger relative to those for cello-oligosaccharide binding due to gains in ΔH, not gains in configurational ΔS. The individual contributors to the thermodynamic parameters remain unknown, as structural rearrangements in the cellulose are a possibility, leaving us with the question of how the gain in ΔH is generated. Is this a result of differences in more favourable direct CBM–cellulose interactions, such as hydrogen bonding? Or does it somehow result indirectly from a thermodynamically favourable structural rearrangement in the cellulose? We currently have insufficient evidence to comment. In contrast with binding at the high-affinity site, the low-affinity interaction does appear to have gains in configurational entropy, but with a compensating enthalpic loss.
Although this study does suggest a thermodynamic basis for the tight binding of CBMs to RC, it also highlights that the structure of non-crystalline cellulose is largely unknown, making it difficult to associate these energetic observations with structural features. Indeed, even the aetiology of the multiple classes of binding sites on RC remains unclear. The presence of particular ‘micro-structures’ in non-crystalline cellulose and their solvation may have effects on binding of which we are currently poorly informed. In short, although we have made strides in the last 10 years, there is much to learn about how CBMs interact with both non-crystalline and crystalline cellulose.
Acknowledgments
I thank Alicia Lammerts van Bueren for preparing some of the protein used in this work. This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. A. B. B. is a Canada Research Chair in Molecular Interactions.
References
- 1.Boraston A. B., Bolam D. N., Gilbert H. J., Davies G. J. Carbohydrate-binding modules: fine tuning polysaccharide recognition. Biochem. J. 2004;382:769–782. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomme P., Creagh A. L., Kilburn D. G., Haynes C. A. Interaction of polysaccharides with the N-terminal cellulose-binding domain of Cellulomonas fimi CenC. 1. Binding specificity and calorimetric analysis. Biochemistry. 1996;35:13885–13894. doi: 10.1021/bi961185i. [DOI] [PubMed] [Google Scholar]
- 3.Brun E., Johnson P. E., Creagh A. L., Tomme P., Webster P., Haynes C. A., McIntosh L. P. Structure and binding specificity of the second N-terminal cellulose-binding domain from Cellulomonas fimi endoglucanase C. Biochemistry. 2000;39:2445–2458. doi: 10.1021/bi992079u. [DOI] [PubMed] [Google Scholar]
- 4.Boraston A. B., Chiu P., Warren R. A. J., Kilburn D. G. Specificity and affinity of substrate binding by a family 17 carbohydrate-binding module from Clostridium cellulovorans cellulase 5A. Biochemistry. 2000;39:11129–11136. doi: 10.1021/bi0007728. [DOI] [PubMed] [Google Scholar]
- 5.Notenboom V., Boraston A. B., Chiu P., Freelove A. C., Kilburn D. G., Rose D. R. Recognition of cello-oligosaccharides by a family 17 carbohydrate-binding module: an X-ray crystallographic, thermodynamic and mutagenic study. J. Mol. Biol. 2001;314:797–806. doi: 10.1006/jmbi.2001.5153. [DOI] [PubMed] [Google Scholar]
- 6.Boraston A. B., Ghaffari M., Warren R. A., Kilburn D. G. Identification and glucan-binding properties of a new carbohydrate-binding module family. Biochem. J. 2002;361:35–40. doi: 10.1042/0264-6021:3610035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boraston A. B., Kwan E., Chiu P., Warren R. A., Kilburn D. G. Recognition and hydrolysis of noncrystalline cellulose. J. Biol. Chem. 2003;278:6120–6127. doi: 10.1074/jbc.M209554200. [DOI] [PubMed] [Google Scholar]
- 8.Boraston A. B., Creagh A. L., Alam M. M., Kormos J. M., Tomme P., Haynes C. A., Warren R. A., Kilburn D. G. Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry. 2001;40:6240–6247. doi: 10.1021/bi0101695. [DOI] [PubMed] [Google Scholar]
- 9.Creagh A. L., Ong E., Jervis E., Kilburn D. G., Haynes C. A. Binding of the cellulose-binding domain of exoglucanase Cex from Cellulomonas fimi to insoluble microcrystalline cellulose is entropically driven. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12229–12234. doi: 10.1073/pnas.93.22.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach H., Middaugh C. R., Lewis R. V. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 1992;200:74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- 11.Sigurskjold B. W., Bundle D. R. Thermodynamics of oligosaccharide binding to a monoclonal antibody specific for a Salmonella O-antigen point to hydrophobic interactions in the binding site. J. Biol. Chem. 1992;267:8371–8376. [PubMed] [Google Scholar]
- 12.Williams B. A., Chervenak M. C., Toone E. J. Energetics of lectin-carbohydrate binding. A microcalorimetric investigation of concanavalin A-oligomannoside complexation. J. Biol. Chem. 1992;267:22907–22911. [PubMed] [Google Scholar]
- 13.Din N., Gilkes N. R., Tekant B., Miller R. C., Warren R. A., Kilburn D. G. Non-hydrolytic disruption of cellulose fibres by the binding domain of a bacterial cellulase. Bio/Technology. 1991;9:1096–1099. [Google Scholar]
- 14.Din N., Damude H. G., Gilkes N. R., Miller R. C., Jr, Warren R. A., Kilburn D. G. C1-Cx revisited: intramolecular synergism in a cellulase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11383–11387. doi: 10.1073/pnas.91.24.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chervenak M. C., Toone E. J. Calorimetric analysis of the binding of lectins with overlapping carbohydrate-binding ligand specificities. Biochemistry. 1995;34:5685–5695. doi: 10.1021/bi00016a045. [DOI] [PubMed] [Google Scholar]