Abstract
The four members of the AP (adaptor protein) family are heterotetrameric cytosolic complexes that are involved in the intracellular trafficking of cargo proteins between different organelles. They interact with motifs present in the cytoplasmic tails of their specific cargo proteins at different intracellular locations. While AP-1, AP-2 and AP-3 have been investigated extensively, very few studies have focused on the fourth member, AP-4. In the present study, we report on the intracellular localization of AP-4 in the MDCK (Madin–Darby canine kidney) and MelJuSo cell lines after immunogold labelling of ultrathin cryosections. We find that AP-4 is localized mainly in the Golgi complex, as well as on endosomes and transport vesicles. Interestingly, we show for the first time that AP-4 is localized with the clathrin coat machinery in the Golgi complex and in the endocytic pathway. Furthermore, we find that AP-4 is localized with the CI-MPR (cation-independent mannose 6-phosphate receptor), but not with the transferrin receptor, LAMP-2 (lysosomal-associated membrane protein-2) or invariant chain. The difference in morphology between CI-MPR/AP-4-positive vesicles and CI-MPR/AP-1-positive vesicles raises the possibility that AP-4 acts at a location different from that of AP-1 in the intracellular trafficking pathway of CI-MPR.
Keywords: adaptor protein-4 (AP-4), cation-independent mannose 6-phosphate receptor, clathrin coat, immuno-electron microscopy, intracellular trafficking
Abbreviations: AP, adaptor protein; CD-MPR, cation-dependent mannose 6-phosphate receptor; CI-MPR, cation-independent mannose 6-phosphate receptor; HRP, horseradish peroxidase; Ii, invariant chain; LAMP, lysosomal-associated membrane protein; LDLR, low-density lipoprotein receptor; MDCK, Madin–Darby canine kidney; MPR, mannose 6-phosphate receptor; PAG, Protein A-coated colloidal gold particles; TfR, transferrin receptor; TGN, trans-Golgi network
INTRODUCTION
The heterotetrameric AP (adaptor protein) complexes are involved in the sorting of cargo proteins into transport vesicles that traffick between the different organelles of the cell. Four members, AP-1 to AP-4, and their isoforms have been characterized in this family of cytosolic complexes [1,2]. Each AP consists of two heavy subunits of ∼100 kDa (γ/β1, α/β2, δ/β3 and ε/β4), one medium subunit of ∼50 kDa (μ1–μ4), and one small subunit of ∼20 kDa (σ1–σ4). APs mediate the recruitment of cargo proteins into the nascent transport vesicle by interacting with their cytoplasmic tails. In addition, APs recruit cytosolic components of the transport machinery, which assemble into protein coats and form the budding vesicle on the membrane of a donor organelle [3]. The coated vesicle is then separated from the donor organelle and subsequently transported to the acceptor organelle. Meanwhile, the coat is removed to permit membrane fusion between the transport vesicle and the acceptor organelle.
The specificity of cargo protein sorting in donor organelles is arbitrated by the differential locations of APs inside the cell or on organelles, and by their specific interactions with different cargo proteins through sorting motifs in the cytoplasmic domain [4]. APs interact with tyrosine sorting signals (YxxΦ) via their μ subunit [5–7], as well as with di-leucine sorting signals (D/ExxxLL) via their β and/or μ subunits [8,9]. AP-1 sorts cargo proteins at the TGN (trans-Golgi network) and endosomes. Two major isoforms of AP-1 are known: the ubiquitous AP-1A, which contains the μ1A subunit, and the cell type-specific AP-1B, which contains the μ1B subunit. AP-1A is critical for embryonic development [10], and has been shown to be involved in the bi-directional transport of the two MPRs (mannose 6-phosphate receptors) between the TGN and endosomes [10–12]. AP-1B is found only in polarized epithelial cells, and mediates the sorting of numerous cargo proteins to the basolateral membrane of polarized cells [13]. It has been shown that AP-1B mediates recycling from endosomes to the basolateral membrane [14], and that the intracellular distribution of AP-1B differs from that of AP-1A [14,15]. AP-2 acts exclusively at the plasma membrane in the clathrin-mediated internalization mechanism [16]. AP-3 is found at the TGN and endosomes. It is believed to play a role in the sorting of cargo proteins containing a tyrosine [LAMP-1 (lysosomal-associated membrane protein-1)] or a di-leucine [LIMP II (lysosomal integral membrane protein II)] motif to lysosomes [17–19]. Finally, AP-4 has been suggested to be involved in two different sorting processes. In HeLa cells, the μ4 subunit interacts with the cytoplasmic tyrosine motif of lysosomal cargo proteins such as LAMP-2, and mediates their direct transport to lysosomes without passing via the plasma membrane [6]. In MDCK (Madin–Darby canine kidney) cells, the μ4 subunit interacts with different cargo proteins destined for the basolateral membrane [e.g. the LDLR (low-density lipoprotein receptor), CD-MPR (cation-dependent MPR) and furin] [7].
The main coat protein in the exocytic and endocytic pathways is clathrin, which is found at the Golgi complex, the plasma membrane and the early endosomes [20–22]. It is well established that AP-1 and AP-2 interact with clathrin at the TGN and plasma membrane respectively [23]. Clathrin binding is mediated mainly by the hinge and appendage of the β subunit [24,25]. AP-3 also contains clathrin-binding sequences, but its interaction with clathrin is still controversial. While AP-3 was found to interact with clathrin [20,26], it was not detected on purified clathrin-coated vesicles [27], and nor was AP-4 [28]. Notably, AP-4 is phylogenetically different from the other three family members [29]. A direct comparison revealed that AP-4 lacks the appendage of the β subunit [30]. In addition to being a site for clathrin binding, this appendage also contains the binding site for cytosolic factors such as microtubule-associated protein 1A, γ synergin, rabaptin-5, auxilin 2, Eps15, epsin, clathrin assembly lymphoid myeloid leukaemia protein and sorting nexin 9. So far, none of these factors has been found to interact with AP-4.
We have used immuno-electron microscopy of ultrathin sections of canine epithelial cells and human melanoma cells to study the intracellular distribution of AP-4. We show here that AP-4 is distributed on tubulo-vesicular structures of the Golgi complex, on transport vesicles and on endosomes. For the first time, AP-4 has been found on clathrin-coated vesicles as well as the clathrin-coated membrane of early endosomes. We also show that AP-4 is partly localized with the CI-MPR (cation-independent MPR) in vesicles that are morphologically distinct from AP-1-positive vesicles, suggesting that AP-4 interacts with the CI-MPR at a different location in the intracellular trafficking pathway from that used by AP-1.
EXPERIMENTAL
Cell culture
The epithelial MDCK cell line and the human melanoma MelJuSo cell line were grown in Dulbecco's modified Eagle's medium (MedProbe, Oslo, Norway) containing 10% (v/v) foetal bovine serum (Saveen, Oslo, Norway), 2 mM L-glutamine, 25 units/ml penicillin, and 25 μg/ml streptomycin (all from MedProbe) at 37 °C in a 6% CO2 incubator. For experiments, cells were seeded in 10 cm-diameter culture dishes and grown until confluence, and then fixed for immuno-electron microscopy. For internalization experiments, MelJuSo cells were incubated with HRP (horseradish peroxidase) coupled to 5 nm colloidal gold particles (final absorbance=5) for 1 h at 37 °C without chase, and then washed and fixed as described below in order to label the different types of endocytic compartments (early and late endosomes, and lysosomes).
Antibodies
Rabbit polyclonal antibodies against the ε subunit and the μ4 subunit of human AP-4 were obtained from Margaret Robinson (University of Cambridge, U.K.) and Stefan Höning (University of Göttingen, Germany) respectively, and have been described previously [7,28]. Rabbit polyclonal antibody against human LAMP-2 was a gift from Sven Carlsson (University of Umeå, Sweden) [31]. Rabbit polyclonal antibody against bovine CI-MPR was obtained from Bernard Hoflack (University of Dresden, Germany) and has been characterized previously [32]. Mouse monoclonal antibody VicY1 directed against the cytoplasmic tail of human Ii (invariant chain) was a gift from Walter Knapp (University of Vienna, Austria) [33]. Mouse monoclonal antibodies against the γ subunit of AP-1 (clone 100/3), the cytoplasmic tail of TfR (transferrin receptor) (clone H68.4) and the clathrin heavy chain (clone 23) were purchased from Sigma (Seelze, Germany), Zymed (South San Francisco, CA, U.S.A.) and BD Biosciences (Heidelberg, Germany) respectively. For immunogold labelling of mouse antibodies, we used a second step with a rabbit polyclonal antibody directed against mouse IgG+M (DAKO, Glostrup, Denmark). The labelling was detected using PAG (Protein A-coated colloidal gold particles) of varying sizes (purchased from George Posthuma, Utrecht, the Netherlands).
Immuno-electron microscopy and quantification
Cells were prepared for ultrathin cryosectioning and immunogold labelling essentially as described in [34]. Briefly, cells were fixed for 3 h at room temperature in 0.1 M phosphate buffer containing 0.1% glutaraldehyde alone or 0.1% glutaraldehyde plus 0.5% paraformaldehyde. After washing in 1×PBS, cells were scraped off and spun down. Cell pellets were embedded in 1×PBS/12% (w/v) gelatine and, after infiltration with 2.3 M sucrose overnight at 4 °C, were cut into small blocks, which were mounted on pins and frozen in liquid nitrogen. Ultrathin cryosections of about 60–70 nm thickness were obtained by cutting at −120 °C with a Reichert Ultracut S ultracryomicrotome from Leica (Heidelberg, Germany). Cryosections were picked up in a 1:1 (v/v) mixture of 2% (w/v) methylcellulose and 2.3 M (w/v) sucrose.
Ultrathin cryosections were then single- or double-labelled. Primary antibodies, secondary antibody (in the case of a mouse primary antibody) and PAG diluted in 1×PBS/1% BSA were incubated sequentially with the cryosections for 30 min at room temperature, then washed extensively. In the case of double labelling, cryosections were post-fixed with 1% (v/v) glutaraldehyde between the two labelling sequences. Finally, cryosections were contrasted with a 1:9 (v/v) mixture of 3% (w/v) uranyl acetate and 2% (w/v) methylcellulose. Sections were examined using a Philips CM100 transmission electron microscope (FEI, Acht, the Netherlands).
For determination of the distribution of AP-4 in the Golgi complex, on various endocytic compartments and on transport vesicles, quantification was performed under the microscope by screening the sections and scoring each gold particle associated with the compartments. Because we observed that labelling on vesicles surrounding early endosomes was lower in MelJuSo cells than in MDCK cells, the vesicular compartment was divided into three categories: vesicles spread in the cytoplasm, those close to early endosomes, and those close to late endosomes. Positive vesicles were scored as close to an endocytic compartment when found within a distance of approx. 500 nm around the compartment.
For double labelling, quantification was performed using randomly photographed cell areas at a magnification of ×21000 or ×39000 of the various double-labelled preparations by counting gold particles assigned only to transport vesicles. A minimum of 10 pictures per labelling were taken for analysis. A vesicle was considered as positive for a marker when a minimum of one gold particle was found for this marker.
For all quantifications, a gold particle was assigned to a membrane when the label was observed within a distance from that membrane of twice the particle size. The different types of endocytic compartments were identified by their morphology as described in [35].
RESULTS
AP-4 is localized on clathrin-coated vesicles and clathrin-coated membrane of endosomes
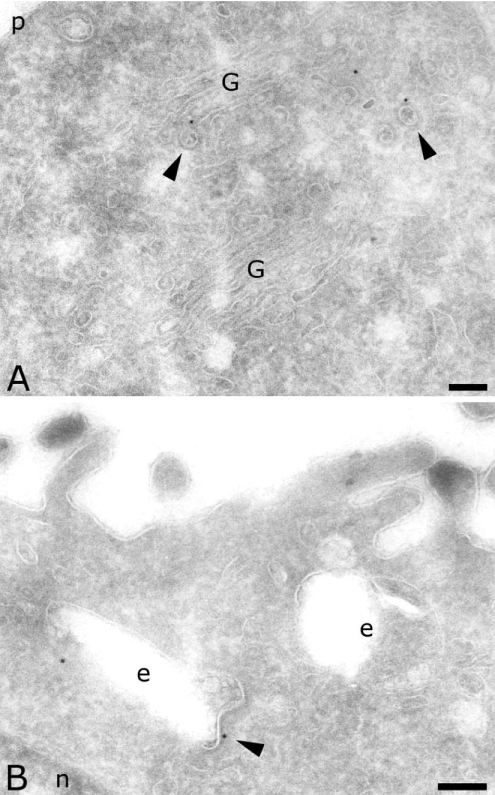
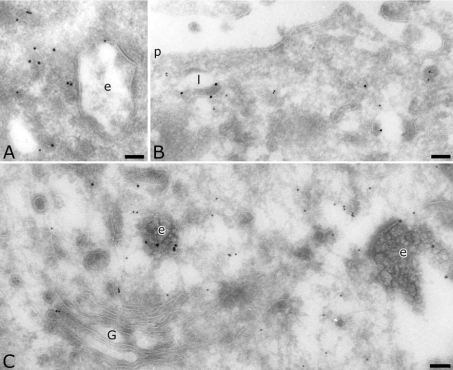
To determine the precise intracellular location of AP-4, we used immunogold labelling of ultrathin cryosections of two different cell lines. In MDCK cells, we detected endogenous AP-4 with a polyclonal antibody directed against the ε subunit [28]. Despite a low labelling density, AP-4 was clearly present in the Golgi complex (Figure 1A), on endosomal compartments (Figure 1B) as well as on transport vesicles. Quantification showed that a majority of the AP-4 was localized on transport vesicles spread in the cytoplasm (Table 1). Whereas a previous study did not observe AP-4 on coated vesicles by electron microscopy nor on purified clathrin-coated vesicles [28], the present labelling of the ε subunit revealed the presence of AP-4 on coated vesicles as well as on coated membranes of endosomal compartments (arrowheads in Figure 1).
Figure 1. Localization of AP-4 ε subunit in MDCK cells.
Sections were labelled for the ε subunit of AP-4 (10 nm). Arrowheads indicate coated vesicles and membranes labelled for AP-4. Scale bar, 100 nm. G, Golgi complex; e, endosomes; n, nucleus; p, plasma membrane.
Table 1. Subcellular distribution of AP-4.
Immunogold particles were quantified on single-labelled cryosections from MDCK and MelJuSo cells. Gold particles were scored under the microscope and assigned to Golgi complex, transport vesicles and endocytic compartments. The total numbers of gold particles were: anti-ε in MDCK cells, 240; anti-μ4 in MDCK cells, 427; anti-μ4 in MelJuSo cells, 311.
| Immunogold labelling (% of total particles) | |||
|---|---|---|---|
| Compartment | Anti-ε in MDCK | Anti-μ4 in MDCK | Anti-μ4 in MelJuSo |
| Golgi complex | 33 | 39 | 38 |
| Vesicles | 56 | 45 | 47 |
| Close to early endosomes | 16 | 20 | 10 |
| Close to late endosomes | 11 | 8 | 17 |
| Others | 29 | 17 | 20 |
| Endosomes | 11 | 16 | 15 |
| Early | 9 | 13 | 6 |
| Late | 2 | 3 | 9 |
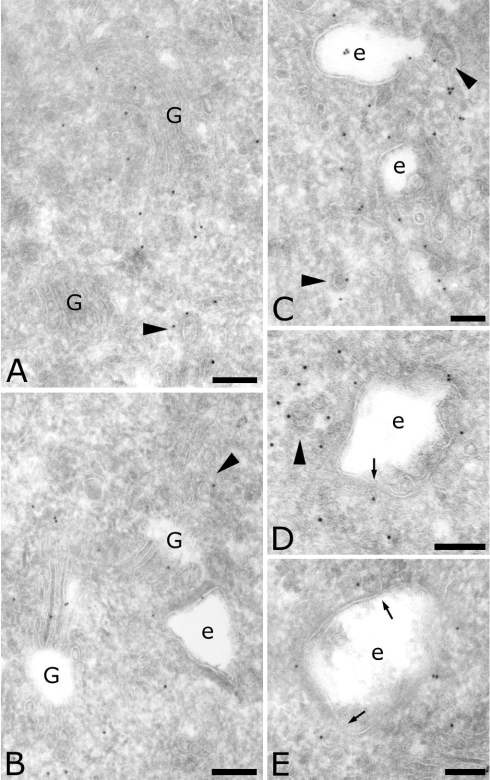
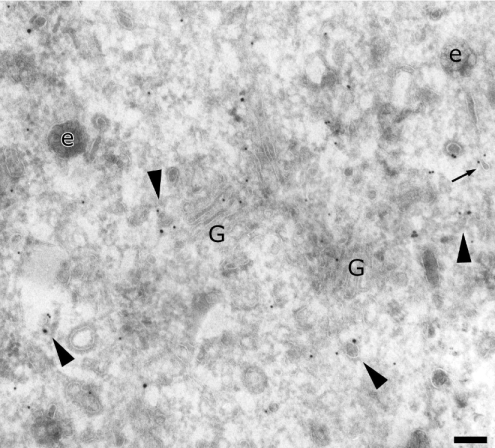
We next used a polyclonal antibody directed against the μ4 subunit [7]. Its higher labelling density confirmed the presence of AP-4 in the Golgi complex (Figures 2A, 2B and 2E) and on endosomal compartments (Figures 2B–2E). Two different μ4-positive endosomal compartments have been defined by their morphology [35]: the recycling endosomes, composed of vesicles and tubules close to early endosomes (as shown in Figure 2C), and the early endosomes, characterized by their lucent contents, including internal vesicles (Figures 2B–2E). In addition, the anti-μ4 antibody labelled numerous vesicles surrounding early endosomes (Figures 2D and 2E), indicating that AP-4 may play a role in the sorting of cargo proteins from early endosomes (arrows in Figures 2D and 2E). Quantification showed that more label was found on early endosomes than on late endosomes, and that the majority of μ4-positive vesicles were close to early endosomes (Table 1). Finally, some of the μ4-positive vesicles displayed a coat (arrowheads in Figure 2). All of these observations indicate that AP-4 may be involved in the sorting processes from the TGN as well as from early endosomes.
Figure 2. Localization of AP-4 μ subunit in MDCK cells.
Sections were labelled for the μ subunit of AP-4 (10 nm). Arrowheads indicate coated vesicles labelled for AP-4. Arrows indicate vesicles bound to endosomes and labelled for AP-4. Scale bar, 100 nm. G, Golgi complex; e, endosomes.
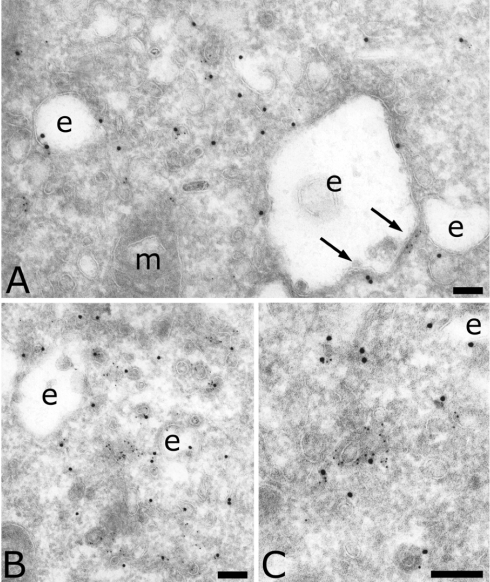
To determine the nature of the vesicle coat, we double-labelled MDCK cells for the μ4 subunit and the clathrin heavy chain. Clathrin is the main coat used in the endocytic and exocytic pathways. It is known to interact with AP-1 and AP-2, but its interaction with AP-3 is still controversial. Figure 3(A) shows that the coat associated with the endosomal membrane contained clathrin as well as μ4 (arrows in Figure 3A). Figures 3(B) and 3(C) show that μ4 was also found on clathrin-coated vesicles. Quantification indicated that 23% of μ4-positive transport vesicles were also positive for clathrin (Table 2). In conclusion, the presence of AP-4 in the clathrin coat of early endosomes and vesicles indicates that AP-4 may also interact with clathrin.
Figure 3. Localization of AP-4 and clathrin in MDCK cells.
Sections were double-labelled for the clathrin heavy chain (5 nm) and the μ subunit of AP-4 (15 nm). Arrows indicate the presence of AP-4 on the clathrin-coated membrane of endosomes. Scale bar, 100 nm. e, endosomes; m, mitochondria.
Table 2. Distribution of clathrin and μ4.
Immunogold particles were quantified on clathrin- and μ4-double-labelled cryosections from MDCK cells. Only gold particles found on transport vesicles were scored in random micrographs at ×39000. A vesicle was considered positive when a minimum of one gold particle was found.
| Immunogold-labelled proteins | Number of positive vesicles |
|---|---|
| Clathrin | 161 |
| μ4 | 158 |
| Clathrin plus μ4 | 37 |
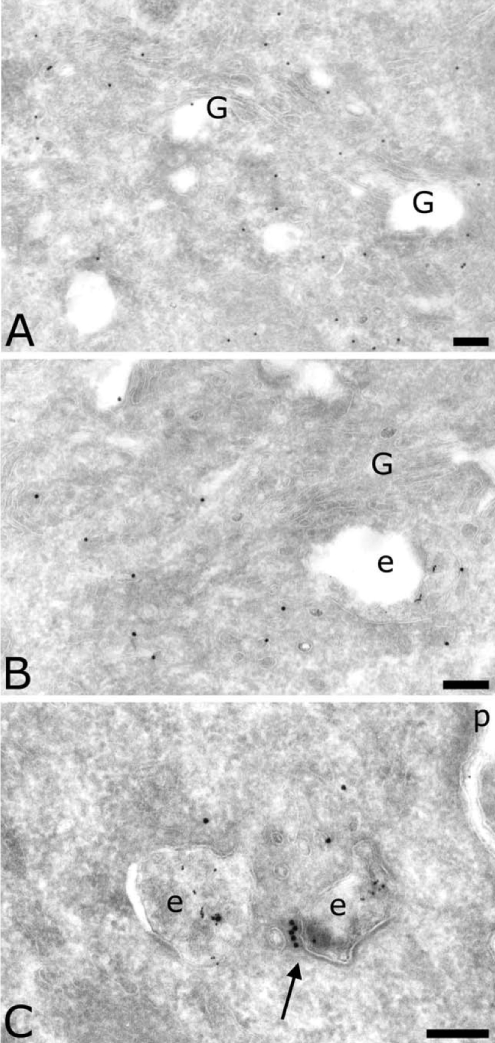
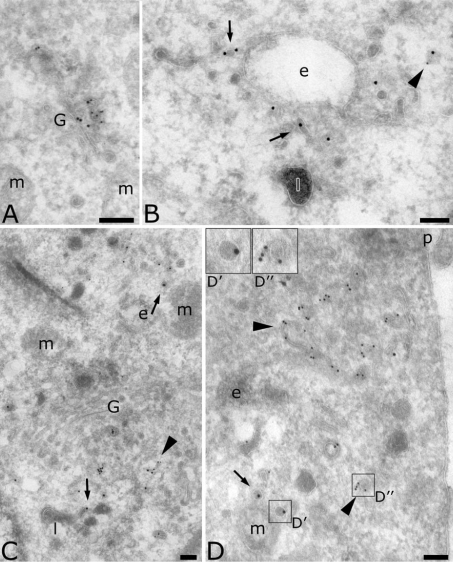
MelJuSo cells showed a distribution of AP-4 similar to that seen in MDCK cells. μ4 was also found in the Golgi complex (Figures 4A and 4B), on endosomes containing internalized HRP (Figures 4B and 4C) as well as on transport vesicles. Figure 4(C) also shows that the coated membrane on the early endosome contained AP-4 (arrow). Quantification indicated that MDCK and MelJuSo cells have the same overall AP-4 distribution in the Golgi complex, in the endocytic pathway and on transport vesicles (Table 1). However, detailed observation and quantification showed that in MelJuSo cells more labelling was found on late endosomes than on early endosomes, and that the majority of μ4-positive vesicles were close to late endosomes (compare Figure 4 with Figures 2 and 3). This discrepancy between MDCK and MelJuSo cells suggests that AP-4 plays a more important role in sorting from early endosomes in polarized cells such as MDCK cells [7], whereas in non-polarized cells such as MelJuSo cells, AP-4 has a more important role in sorting from late endosomes.
Figure 4. Localization of AP-4 in MelJuSo cells.
Sections were labelled for the μ subunit of AP-4 (10 nm). In (B) and (C), endosomes contain internalized HRP (5 nm). The arrow indicates the coated membrane of an early endosome labelled for the μ4 subunit. Scale bar, 100 nm. G, Golgi complex; e, endosomes; p, plasma membrane.
AP-4 is not localized with TfR, LAMP-2 or Ii
Two previous studies have shown that AP-4 is involved in the sorting processes of cargo proteins that contain a tyrosine motif in their cytoplasmic tail [6,7]. In the first study, the AP-4 μ chain was shown by two-hybrid analysis to interact with the cytoplasmic tail of LAMP-2, while in the second study it was found by surface plasmon resonance binding experiments to interact with the cytoplasmic tail of various cargo proteins, such as LDLR, CD-MPR, TfR and furin, but not with LAMP-2. In the present study, we investigated whether or not AP-4 localizes with different cargo proteins in MelJuSo cells. First we double-labelled cells for the μ4 subunit and TfR (Figure 5A). Both molecules were present in vesicles surrounding early endosomes, but no strong co-localization was observed. Quantification showed that only 9% of TfR-positive transport vesicles were also labelled for the μ4 subunit (Table 3). In contrast, we found TfR in vesicles positive for AP-1 (results not shown), in accordance with AP-1 being involved both in transport of the receptor from apical to basolateral membranes and in its recycling to the basolateral membrane [14,21]. We next analysed the location of AP-4 in the trafficking pathway of lysosomal proteins, and LAMP-2 was used as a lysosomal marker [36,37]. Labelling in MelJuSo cells showed that LAMP-2 was present in small vesicles, but was located mainly in electron-dense endocytic compartments corresponding to lysosomes (Figure 5B). No strong co-localization of LAMP-2 with the μ4 subunit was observed. Quantification showed that only 5.5% of LAMP-2-positive transport vesicles were also labelled for the μ4 subunit (Table 3), indicating that AP-4 is not directly involved in the trafficking of LAMP-2 to lysosomes.
Figure 5. Localization of AP-4 and different markers of the endocytic pathway in MelJuSo cells.
Sections were double-labelled for the μ subunit of AP-4 (10 nm) plus (A) TfR (15 nm), (B) LAMP-2 (15 nm) or (C) Ii (15 nm). Scale bar, 100 nm. G, Golgi complex; e, endosomes; l, lysosomes; p, plasma membrane.
Table 3. Co-distribution of different cargo proteins with AP-4 and AP-1.
Immunogold particles were quantified on double-labelled cryosections from MelJuSo cells. Only gold particles found on transport vesicles were scored on random micrographs at ×21000. A vesicle was considered positive when a minimum of one gold particle was found. The percentage of double-positive vesicles is indicated in parentheses.
| Immunogold-labelled proteins | Number of positive vesicles |
|---|---|
| TfR | 87 |
| TfR plus μ4 | 8 (9%) |
| LAMP-2 | 53 |
| LAMP-2 plus μ4 | 3 (5.5%) |
| Ii | 80 |
| Ii plus μ4 | 6 (7.5%) |
| CI-MPR | 121 |
| CI-MPR plus μ4 | 29 (24%) |
| CI-MPR | 143 |
| CI-MPR plus γ1 | 41 (29%) |
The MHC-associated Ii contains two leucine-based sorting signals [38], and in vitro studies have shown that it interacts with both AP-1 and AP-2 [8]. Whether or not AP-4 is involved in Ii trafficking has not been studied, and we therefore double-labelled MelJuSo cells for both molecules. Ii was found in late endocytic compartments containing internal vesicles (Figure 5C), but not in the Golgi complex or on transport vesicles positive for μ4. Quantification showed that only 7.5% of Ii-positive transport vesicles were labelled for the μ4 subunit (Table 3). Thus AP-4 is not involved in the intracellular trafficking of Ii.
AP-4 is localized with CI-MPR
CI-MPR transports soluble lysosomal proteins from the TGN to the endocytic pathway, and also plays a role at the plasma membrane [39]. It cycles several times between the TGN and the endocytic pathway, as well as between the plasma membrane and the endocytic pathway. Its cytoplasmic tail, which contains both a tyrosine and a di-leucine sorting motif, interacts with numerous cytosolic molecules, such as AP-1, AP-2, GGAs (Golgi-associated γ-adaptin homologous, ADP-ribosylation factor-interacting proteins) and TIP47 (47 kDa tail-interacting protein) [11,12,40]. While transport between the TGN and early endosomes is well characterized, the transport of CI-MPR from the endocytic pathway to the cell surface and from early to late endosomes is less well documented. In MelJuSo cells, CI-MPR labelling was detected at the TGN, early and late endosomes and plasma membrane as expected (results not shown). As shown in Figure 6, we could observe electron-dense vesicles labelled both for CI-MPR and for the μ4 subunit (arrowheads). Quantification indicated that 24% of CI-MPR-positive transport vesicles were also positive for the μ4 subunit (Table 3). AP-4 may thus in principle interact with CI-MPR and regulate its intracellular traffic, as reported by Simmen and co-workers [7].
Figure 6. Localization of AP-4 and CI-MPR in MelJuSo cells.
Sections were double-labelled for the μ subunit of AP-4 (10 nm) and CI-MPR (15 nm). Arrowheads indicate vesicles positive for AP-4 and CI-MPR. The arrow indicates the presence of AP-4 on the tip of a tubule emerging from an endosome. Scale bar, 100 nm. G, Golgi complex; e, endosomes.
Since AP-1 is known to interact and localize with the CD- and CI-MPRs at the TGN for sorting to endosomes [11,12], we hypothesized that both AP-1 and AP-4 are involved in the transport of CI-MPR, but at different locations. Thus we double-labelled for AP-1 and CI-MPR. Both molecules were localized at the TGN (Figure 7A) and on vesicles (arrowheads in Figures 7B and 7C). Quantification indicated that 29% of CI-MPR-positive vesicles were also positive for the γ1 subunit (Table 3). AP-4- and AP-1-positive vesicles seemed to be morphologically different. Vesicles labelled for CI-MPR only (arrows in Figures 7B–7D) or vesicles labelled for CI-MPR and AP-4 (arrowheads in Figure 6) had more electron-dense contents than vesicles labelled for CI-MPR and AP-1 or for AP-1 alone (arrowheads in Figure 7B–7D). This indicates the presence of two morphologically distinct populations of vesicles (insets 7D′ and 7D″), which possibly reflects their different location in the trafficking pathway of CI-MPR.
Figure 7. Localization of AP-1 and CI-MPR in MelJuSo cells.
Sections were double-labelled for the γ subunit of AP-1 (10 nm) and CI-MPR (15 nm). Arrowheads indicate vesicles positive for AP-1 and CI-MPR or AP-1 alone. Arrows indicate electron-dense vesicles labelled for CI-MPR only. Inset D′ shows an electron-dense vesicle positive for CI-MPR at 2× magnification; insert D″ shows a coated vesicle positive for AP-1 at 2× magnification. Scale bar, 100 nm. G, Golgi complex; e, endosomes; l, lysosomes; m, mitochondria; p, plasma membrane.
DISCUSSION
In the present study, the intracellular distribution of AP-4 was studied by immunogold labelling of ultrathin cryosections. AP-4 is located mainly in the Golgi complex, in the endocytic pathway and on transport vesicles in both the MDCK and MelJuSo cell lines. Interestingly, we found AP-4 on clathrin-coated vesicles and the membrane of early endosomes, indicating an association with the clathrin coat machinery. Our results also showed that AP-4 is localized with CI-MPR.
AP-4 has been shown to be involved in the transport of lysosomal membrane proteins without cycling by the plasma membrane [6]. AP-4 has also been shown to interact with the cytoplasmic tails of LDLR, CD-MPR, TfR and furin [7]. Our attempt to identify cargo that may use AP-4 as part of the trafficking machinery revealed that AP-4 showed low co-localization with TfR, LAMP-2 or Ii. In contrast, AP-4 was localized with 24% of CI-MPR-positive transport vesicles. As control, AP-1, which is well known to localize with MPRs, was found on 29% of CI-MPR-positive transport vesicles. Electron microscopy showed that the vesicles that contained CI-MPR and AP-4 were morphologically different from those containing CI-MPR and AP-1, with the first population being more electron-dense than the second. These observations raise the possibility than CI-MPR uses both APs, but at different stages in its intracellular pathway.
Until the present investigation, the precise intracellular location of AP-4 had been poorly documented. While AP-4 is expressed ubiquitously in all cell types [28,41], immunofluorescence labelling in fibroblasts has shown that AP-4 is expressed at a lower level than the other family members [28]. Immunofluorescence labelling has also shown that AP-4 is located in the Golgi region, and not with the TfR and lysosomal proteins [28,41]. Immuno-electron microscopy has confirmed that AP-4 is localized on tubulo-vesicular structures of the Golgi complex [28]. More recently, AP-4 has also been detected in endocytic compartments, positive for internalized BSA, by immuno-electron microscopy in MDCK cells [7]. However, conclusions regarding the intracellular localization of AP-4 were based on electron microscopy experiments with a low labelling efficiency. In the present study, using immunogold labelling of ultrathin cryosections, we have analysed the distribution of AP-4 in two different cell lines, MDCK and MelJuSo. Our results show AP-4 located on the Golgi complex stacks and surrounding tubulo-vesicular structures, suggesting that AP-4 is involved in sorting processes at the Golgi complex. AP-4 was also distributed in endocytic compartments, mainly on early endosomes in MDCK cells and on late endosomes in MelJuSo cells. The same discrepancy is observed with transport vesicles that surround endocytic compartments. Thus, in MDCK cells, AP-4-positive transport vesicles are localized more often around early endosomes, while in MelJuSo cells they are localized more around late endosomes. This discrepancy in labelling may represent different expression levels of AP-4 in the two cell lines, but the overall AP-4 distribution in both cells is identical. This may also point to a special role of AP-4 in intracellular trafficking in polarized cells. Indeed, AP-4 has been suggested to play a role in the sorting of cargo proteins to the basolateral plasma membrane in MDCK cells [7]. Vesicles that we find bound to the membrane of early endosomes could correspond to this sorting event. In non-polarized cells, AP-4 may have a more important role in sorting events from late endosomes. The presence of AP-4 on the tip of a tubule emerging from a late endosome suggests such a role in the sorting from late endosomes (arrow in Figure 6). In conclusion, the intracellular distribution of AP-4 indicates that it may be involved in sorting processes at the Golgi complex and/or endocytic compartments.
In the present study, we observed that AP-4 may be associated with the clathrin coat machinery, as it is detected on clathrincoated vesicles and clathrin-coated membranes of early endosomes. How may we explain the discrepancy between our observation and the lack of a clathrin-binding consensus sequence, as well as the reported absence of AP-4 from purified clathrin-coated vesicles? First, the ε subunit of AP-4 may also bind clathrin, as has been shown for the γ and α subunits of AP-1 and AP-2 respectively [42,43]. Secondly, AP-4 may interact indirectly with clathrin via a third component present in the coat. A direct or indirect interaction could also be weaker than an interaction with the clathrin-binding consensus sequence, and thus be lost during vesicle purification. Thirdly, association with a coat may not be essential for AP-4 function, since only 23% of AP-4-positive vesicles were also labelled with clathrin, compared with 82% for AP-1 [12] and 65% for AP-3 [20]. A sorting mechanism from early endosomes to lysosomes has been characterized [22]. In that study, the growth hormone receptor, which is internalized for degradation in lysosomes, was found on clathrin-coated membranes of early endosomes that did not contain AP-1, AP-2 or AP-3; AP-4 was not tested for. With our observation of the presence of AP-4 in clathrin coats, it is tempting to speculate that AP-4 has a role in the mechanisms of sorting of cargo proteins from early endosomes to lysosomes in a clathrin-dependent manner.
Acknowledgments
We thank Inger Sandlie and John Ormerod for critical reading of the manuscript. We also thank the EM department at Oslo University for the use of equipment. This research was supported by a Marie Curie Fellowship of the European Community programme Improving Human Research Potential and the Socio-economic Knowledge Base under contract number HPMF-CT-2001-01414.
References
- 1.Boehm M., Bonifacino J. S. Genetic analyses of adaptin function from yeast to mammals. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- 2.Boehm M., Bonifacino J. S. Adaptins. The final recount. Mol. Biol. Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhausen T. Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 5.Ohno H., Aguilar R. C., Yeh D., Taura D., Saito T., Bonifacino J. S. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar R. C., Boehm M., Gorshkova I., Crouch R. J., Tomita K., Saito T., Ohno H., Bonifacino J. S. Signal-binding specificity of the μ4 subunit of the adaptor protein complex, AP-4. J. Biol. Chem. 2001;276:13145–13152. doi: 10.1074/jbc.M010591200. [DOI] [PubMed] [Google Scholar]
- 7.Simmen T., Höning S., Icking A., Tikkanen R., Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann M. W., Höning S., Rodionov D., Dobberstein B., von Figura K., Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J. Biol. Chem. 1999;274:36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport I., Chen Y. C., Cupers P., Shoelson S. E., Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer C., Zizioli D., Lausmann S., Eskelinen E. L., Hamann J., Saftig P., von Figura K., Schu P. μ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Borgne R., Schmidt A., Mauxion F., Griffiths G., Hoflack B. Binding of AP-1 Golgi adaptors to membranes requires phosphorylated cytoplasmic domains of the mannose 6-phosphate/insulin-like growth factor II receptor. J. Biol. Chem. 1993;268:22552–22556. [PubMed] [Google Scholar]
- 12.Doray B., Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- 13.Fölsch H., Ohno H., Bonifacino J. S., Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 14.Gan Y., McGraw T. E., Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 15.Folsch H., Pypaert M., Maday S., Pelletier L., Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson M. S. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J. Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Borgne R., Alconada A., Bauer U., Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- 18.Feng L., Seymour A. B., Jiang S., To A., Peden A. A., Novak E. K., Zhen L., Rusiniak M. E., Eicher E. M., Robinson M. S., et al. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum. Mol. Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 19.Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 20.Dell'Angelica E. C., Klumperman J., Stoorvogel W., Bonifacino J. S. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- 21.Futter C. E., Gibson A., Allchin E. H., Maxwell S., Ruddock L. J., Odorizzi G., Domingo D., Trowbridge I. S., Hopkins C. R. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachse M., Urbe S., Oorschot V., Strous G. J., Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 24.Gallusser A., Kirchhausen T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen D. J., Vallis Y., Pearse B. M., McMahon H. T., Evans P. R. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drake M. T., Zhu Y., Kornfeld S. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell. 2000;11:3723–3736. doi: 10.1091/mbc.11.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson F., Peden A. A., Christopoulou L., Robinson M. S. Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirst J., Bright N. A., Rous B., Robinson M. S. Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson M. S., Bonifacino J. S. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 30.Lundmark R., Carlsson S. R. The beta-appendages of the four adaptor-protein (AP) complexes: structure and binding properties, and identification of sorting nexin 9 as an accessory protein to AP-2. Biochem. J. 2002;362:597–607. doi: 10.1042/0264-6021:3620597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahlgren C., Carlsson S. R., Karlsson A., Lundqvist H., Sjolin C. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem. J. 1995;311:667–674. doi: 10.1042/bj3110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Méresse S., Ludwig T., Frank R., Hoflack B. Phosphorylation of the cytoplasmic domain of the bovine cation-independent mannose 6-phosphate receptor. Serines 2421 and 2492 are the targets of a casein kinase II associated to the Golgi-derived HAI adaptor complex. J. Biol. Chem. 1990;265:18833–18842. [PubMed] [Google Scholar]
- 33.Quaranta V., Majdic O., Stingl G., Liszka K., Honigsmann H., Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3) J. Immunol. 1984;132:1900–1905. [PubMed] [Google Scholar]
- 34.Raposo G., Kleijmeer M. J., Posthuma G., Slot J. W., Geuze H. J. Immunogold labelling of ultrathin cryosections: application in immunology. In: Herzenberg L. A., Weir D. M., Herzenberg L. A., Blackwell C., editors. Weir's Handbook of Experimental Immunology. Malden, MA: Blackwell Science Inc.; 1997. pp. 208–201. [Google Scholar]
- 35.Kleijmeer M. J., Morkowski S., Griffith J. M., Rudensky A. Y., Geuze H. J. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J. Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Höning S., Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J. Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarnieri F. G., Arterburn L. M., Penno M. B., Cha Y., August J. T. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J. Biol. Chem. 1993;268:1941–1946. [PubMed] [Google Scholar]
- 38.Pieters J., Bakke O., Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J. Cell Sci. 1993;106:831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh P., Dahms N. M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 2003;4:202–213. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 40.Orsel J. G., Sincock P. M., Krise J. P., Pfeffer S. R. Recognition of the 300-kDa mannose 6-phosphate receptor cytoplasmic domain by 47-kDa tail-interacting protein. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9047–9051. doi: 10.1073/pnas.160251397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dell'Angelica E. C., Mullins C., Bonifacino J. S. AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- 42.Goodman O. B., Jr, Keen J. H. The alpha chain of the AP-2 adaptor is a clathrin binding subunit. J. Biol. Chem. 1995;270:23768–23773. doi: 10.1074/jbc.270.40.23768. [DOI] [PubMed] [Google Scholar]
- 43.Morgan J. R., Prasad K., Hao W., Augustine G. J., Lafer E. M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 2000;20:8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]