Abstract
Vasopressin and other phospholipase-C-coupled hormones induce oscillations (waves) of [Ca2+]cyt (cytoplasmic Ca2+ concentration) in liver cells. Maintenance of these oscillations requires replenishment of Ca2+ in intracellular stores through Ca2+ inflow across the plasma membrane. While this may be achieved by SOCs (store-operated Ca2+ channels), some studies in other cell types indicate that it is dependent on AA (arachidonic acid)-activated Ca2+ channels. We studied the effects of AA on membrane conductance of rat liver cells using whole-cell patch clamping. We found no evidence that concentrations of AA in the physiological range could activate Ca2+-permeable channels in either H4IIE liver cells or rat hepatocytes. However, AA (1–10 μM) did inhibit (IC50=2.4±0.1 μM) Ca2+ inflow through SOCs (ISOC) initiated by intracellular application of Ins(1,4,5)P3 in H4IIE cells. Pre-incubation with AA did not inhibit ISOC development, but decreased maximal amplitude of the current. Iso-tetrandrine, widely used to inhibit receptor-activation of phospholipase A2, and therefore AA release, inhibited ISOC directly in H4IIE cells. It is concluded that (i) in rat liver cells, AA does not activate an AA-regulated Ca2+-permeable channel, but does inhibit SOCs, and (ii) iso-tetrandrine and tetrandrine are effective blockers of CRAC (Ca2+-release-activated Ca2+) channel-like SOCs. These results indicate that AA-activated Ca2+-permeable channels do not contribute to hormone-induced increases or oscillations in [Ca2+]cyt in liver cells. However, AA may be a physiological modulator of Ca2+ inflow in these cells.
Keywords: arachidonic acid, Ca2+-release-activated Ca2+ channel (CRAC channel), H4IIE liver cell, iso-tetrandrine, patch-clamp recording, store-operated Ca2+ channel
Abbreviations: AA, arachidonic acid; [Ca2+]cyt, Ca2+ concentration in cytoplasmic space; CRAC, Ca2+-release-activated Ca2+; DMEM, Dulbecco's modified Eagle's medium; m3HEK293 cells, HEK-293 cells stably transfected with human M3 muscarinic receptor; IARC, current mediated by AA-regulated channels; ICRAC, current mediated by CRAC channels; ISOC, current mediated by store-operated Ca2+ channels; SOC, store-operated Ca2+ channel
INTRODUCTION
In non-excitable cells, agonists acting at phospholipase-C-coupled receptors generate repetitive rises in free [Ca2+]cyt (Ca2+ concentration in the cytoplasmic space) by releasing Ca2+ from intracellular Ca2+ stores and activating Ca2+ influx through plasma membrane Ca2+ channels. It has been generally accepted that the major component of the receptor-activated Ca2+ entry into the cell is produced by a store-operated Ca2+ entry mechanism, which involves activation of SOCs (store-operated Ca2+ channels) in response to emptying intracellular Ca2+ stores by Ins(1,4,5)P3 [1]. At the same time, a significant body of evidence suggests that a non-store-operated Ca2+ entry may also be activated by phospholipase-C-coupled receptors [2–4]. In A7r5 vascular smooth muscle cells, avian nasal gland cells and m3HEK293 cells (HEK-293 cells stably transfected with human M3 muscarinic receptor), non-store-operated Ca2+ influx stimulated by AA (arachidonic acid) has been suggested to be a major pathway for Ca2+ entry activated by physiological concentrations of specific agonists that induce intracellular Ca2+ waves [5–7].
AA, a cis-polyunsaturated fatty acid, is a constituent of membrane phospholipids that can be released by cellular phospholipases, particularly by phospholipase A2 and diacylglycerol lipase [8]. Free AA has been shown to modulate the activity of a number of ion channels, including a range of Ca2+-permeable channels [9–11]. There is no single mechanism of action by which AA modulates ion channels. Thus there is evidence that it binds directly to some channel proteins, but, for other channels, it can also have an indirect effect through its metabolites, free radicals and AA-sensitive protein kinases and phosphatases [12–14]. In some cells in which AA has been shown to inhibit SOCs, it has also been shown to activate a specific Ca2+ conductance [15]. Patch-clamping of m3HEK293 cells revealed that, in these cells, AA activates a Ca2+ current (IARC) that is distinctively different from that activated by store depletion (ISOC) [16]. This current was implicated in mediating Ca2+ oscillations activated by low concentrations of carbachol in these cells [17].
Hepatocytes are polarized epithelial cells in which Ca2+ oscillations can be activated by a variety of Ca2+-mobilizing hormones. They exhibit a Ca2+-selective current mediated by SOCs (ISOC) that has been characterized by patch-clamp recording [18,19]. The hepatocyte ISOC exhibits many of the characteristics, including high Ca2+ selectivity, of ICRAC studied in lymphocytes and mast cells [18,19]. Indirect evidence indicates that hormone-induced Ca2+ oscillations in hepatocytes are maintained by a store-operated Ca2+ entry mechanism, as they are inhibited by known blockers of SOCs [19]. On the other hand, there is evidence that Ca2+-mobilizing hormones induce AA release [20].
The present study was designed to elucidate the effects of AA on SOCs in liver cells, and to establish whether AA activates a specific Ca2+ conductance in these cells. The results indicate that AA, at concentrations within the estimated physiological range, inhibits ISOC in rat liver H4IIE cells, but does not itself activate any type of membrane conductance in either H4IIE cells or rat hepatocytes. We also show that iso-tetrandrine, commonly used to inhibit activation of phospholipase A2 by receptors and therefore AA release [21], is a potent blocker of the ISOC in H4IIE cells.
EXPERIMENTAL
Cell culture
H4IIE cells (A.T.C.C. CRL 1548) were cultured at 37 °C in 5% (v/v) CO2 in air in DMEM (Dulbecco's modified Eagle's medium; Gibco) supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), 10 mM Hepes (pH 7.4) and 10% (v/v) foetal bovine serum (complete DMEM) [22]. The cells were subcultured for a maximum of 15 passages. Hepatocytes were isolated from male Hooded Wistar rats by liver perfusion with collagenase, plated on glass coverslips in complete DMEM (as above) and used for patch clamping on the following day [23].
Electrophysiology
Whole-cell patch clamping was performed at room temperature (24 °C) using a computer-based patch-clamp amplifier (EPC-9, HEKA Electronics, Lambrecht/Pfalz, Germany) and PULSE software (HEKA Electronics). The usual bath solution contained 140 mM NaCl, 4 mM CsCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM glucose and 10 mM Hepes, adjusted to pH 7.4 with NaOH. The internal solution 1 contained 120 mM caesium glutamate, 5 mM CaCl2, 5 mM MgCl2, 1 mM MgATP, 10 mM EGTA and 10 mM Hepes, adjusted to pH 7.2 with NaOH. The internal solution 2 contained 130 mM caesium glutamate, 10 mM CsCl, 0.5 mM CaCl2, 6 mM MgATP, 1 mM EGTA and 10 mM Hepes, adjusted to pH 7.2 with NaOH. The calculated internal free Ca2+ concentration for each internal solution was approx. 100 nM (EQCAL; Biosoft, Cambridge, U.K.). Depletion of the intracellular Ca2+ stores was achieved by addition of 20 μM Ins(1,4,5)P3 (D-myo-inositol 1,4,5-trisphosphate hexapotassium salt; Sigma) to internal solution 1 or 1 μM thapsigargin (Sigma) to the bath solution. Patch pipettes were pulled from borosilicate glass, coated with Sylgard and fire-polished; pipette resistance ranged between 2 and 4 MΩ. In order to monitor the development of ISOC, voltage ramps between −138 and +102 mV were applied every 2 s, starting immediately after achieving the whole-cell configuration. Acquired currents were filtered at 2.7 kHz and sampled at 10 kHz. Traces presented in the Figures were further digitally filtered at 1.5 kHz. All voltages shown have been corrected for the liquid junction potential of −18 mV between the bath and electrode solutions (estimated by JPCalc [24]). The holding potential was −18 mV throughout. Cell capacitance was compensated automatically by the EPC9 amplifier. AA and tetrandrine were purchased from Sigma; iso-tetrandrine was purchased from Calbiochem.
RESULTS
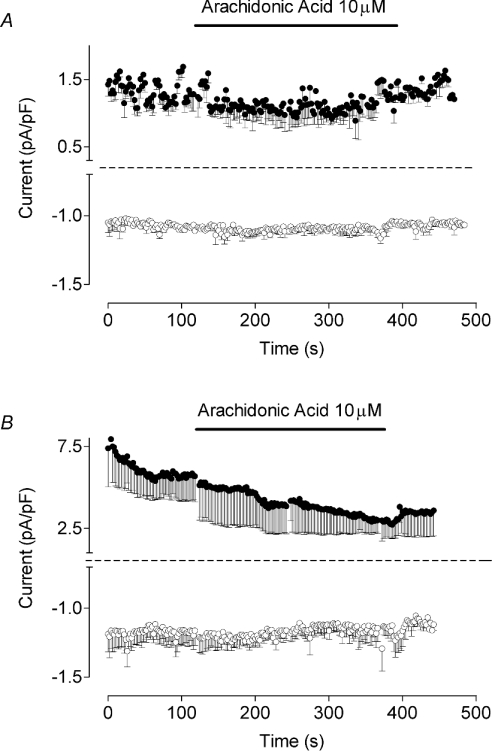
First, the effect of AA on the membrane conductance of H4IIE rat liver cells was investigated to establish if AA activates a specific Ca2+ current. As it has been suggested that pre-activation of capacitative Ca2+ entry may inhibit AA-induced Ca2+ entry [15], in these experiments, we used an intracellular solution containing 6 mM Mg ATP and Ca2+ buffered to 100 nM with 1 mM EGTA. This would prevent the spontaneous development of ISOC, and would also minimize the contribution of Mg2+-regulated non-selective cation current attributed to TRPM7 (transient receptor potential melastatin-7 cation channel) [25]. Addition of 10 μM AA to the bath solution under these conditions failed to activate any current within 10 min of recording (Figure 1A).
Figure 1. AA fails to activate any current in either H4IIE cells or in rat hepatocytes.
Time courses of the inward and outward membrane currents measured at −118 mV (bottom trace) and 82 mV (top trace) in H4IIE cells (n=5) (A) and in rat hepatocytes (n=5) (B). Application of 10 μM AA in the bath is indicated by a horizontal bar. Internal solution 2 (see the Experimental section) was used in the pipette in order to prevent development of ISOC.
H4IIE cells are likely to have lost receptors that are normally present in rat hepatocytes, as they do not respond to ATP (<100 μM) or vasopressin (G. Rychkov and G. Barritt, unpublished work). It might be argued that AA-activated Ca2+ entry requires the presence of functional G-protein-coupled receptors on the plasma membrane, or that AA-activated Ca2+ channels themselves have been lost in this particular cell line. Therefore similar experiments were performed on rat hepatocytes in primary culture that are known to generate cytoplasmic Ca2+ waves in response to a variety of hormones [19,26,27]. AA (<20 μM) in the bath solution failed to activate any current in rat hepatocytes within 10 min of recording (Figure 1B), indicating that the failure to observe any AA-activated current in H4IIE cells is unlikely to be due to the loss of expression of G-protein-coupled receptors, and that the absence of AA-activated channels is not unique to H4IIE cells.
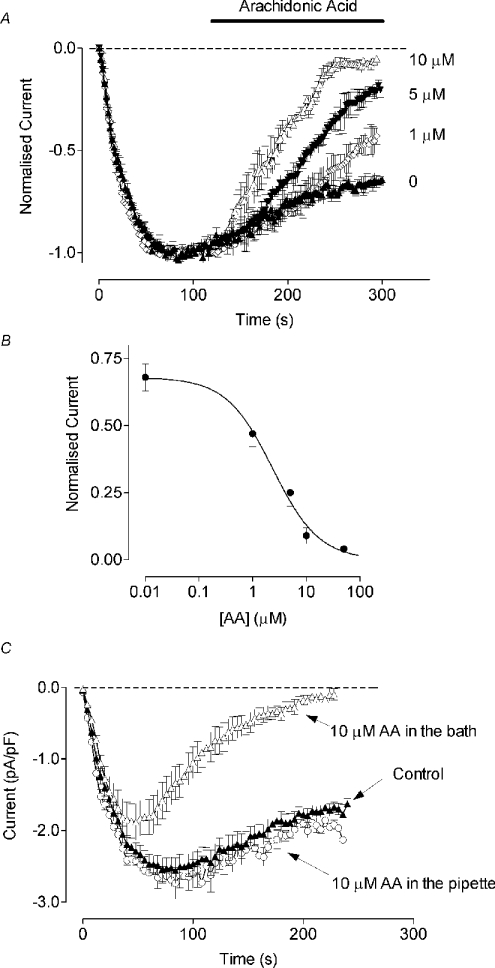
The fact that AA does not activate Ca2+ conductance in liver cells does not imply that it has no role in Ca2+ signalling in these cells. AA has been shown to inhibit store-operated Ca2+ entry in various cell types [15,28–30]. However, its effects on SOCs in liver cells and on similar CRAC (Ca2+-release-activated Ca2+)-type SOCs in other cell lines are not known. Therefore, in the next set of experiments, we investigated the effect of AA on ISOC in H4IIE cells. We have characterized ISOC in H4IIE cells previously and have shown that it has many of the properties of ICRAC in lymphocytes and mast cells [18]. In the absence of AA, depletion of intracellular Ca2+ stores in H4IIE cells activated an inward current (ISOC) with a magnitude of approx. −2.5 pA/pF, measured at −118 mV, as described previously [18] (Figure 2A). The current reached its maximum within 60 s after achieving the whole-cell configuration with the pipette containing 20 μM Ins(1,4,5)P3, and then slowly decayed to a level of 60–70% of the maximal amplitude. Addition of 10 μM AA to the bath solution after full development of the current produced an almost complete block of ISOC within 150 s. At lower concentrations, the block was slower in onset and less complete. The lowest concentration of AA that produced a significant effect within 200 s of application was 1 μM (Figure 2A). Higher concentrations of AA (20–50 μM) blocked ISOC faster; however, they frequently caused development of a non-specific leakage and electrical breakdown of the membrane during steps to negative potentials (results not shown). The apparent IC50 for AA measured 200 s after AA application was 2.4±0.1 μM (n=4) (Figure 2B).
Figure 2. Effects of AA on ISOC in H4IIE cells.
(A) Time course of ISOC inhibition by AA. Amplitude of ISOC measured at −118 mV is plotted against time. Application of AA is indicated by a horizontal bar (n=4–9). (B) Dose-dependent inhibition of ISOC by AA. Each point on the graph is the normalized average amplitude of ISOC at −118 mV measured after 200 s of application of the corresponding concentration of AA in the bath (n=4–9). (C) Effect of AA on ISOC development. Cells were either pre-incubated with AA in the bath for at least 2 min before achieving the whole-cell configuration or AA was added to the pipette solution (n=6).
Pre-incubation of H4IIE cells with 10 μM AA in the bath for at least 2 min before achieving whole-cell configuration did not prevent development of ISOC (Figure 2C). However, the maximal amplitude of the current was significantly smaller and it was completely inactivated within 200 s of recording. The time constants of ISOC development were 24±2 s (n=6) for the control cells and 20±3 s (n=6) for the cells pre-incubated with AA. These results indicate that it is unlikely that AA interferes with the mechanism of ISOC activation, or that it inhibits ISOC by binding to the closed channel. It is more likely that AA either inhibits the open channel directly or modulates the mechanism by which ISOC is slowly inactivated [31]. Addition of 10 μM AA to the internal solution containing 20 μM Ins(1,4,5)P3 had no effect on the development or amplitude of ISOC (Figure 2C).
Under conditions used in the experiments with H4IIE cells described above (Figure 1), AA did not seem to activate any specific conductance. However, in other cell types, AA has been shown to activate highly selective Ca2+ channels with properties distinct from that of the SOCs in those cells [16]. One of the main differences between ISOC and the current activated by AA (IARC) in HEK-293 cells is the lack of the fast inactivation in the latter [16]. ISOC in H4IIE cells also shows a significant fast Ca2+-dependent inactivation at negative potentials [32], and therefore can be distinguished easily from a current that shows no fast inactivation. While there was no evidence that AA activated any current in H4IIE cells, the possibility of a transient activation of such a current when ISOC is blocked could not be excluded. Therefore we compared current traces obtained in response to −138 mV voltage steps before, and 60 s after, application of AA. The kinetics of the ISOC inactivation and the relative amplitude of the non-inactivating component at negative potentials remained unaffected in the presence of AA (Figures 3A and 3B). This argues against the presence of any non-inactivating Ca2+ current additional to ISOC. Moreover, the I–V plot remained inwardly rectifying in the presence of AA, with no evidence for the development of any outward current or a shift in the reversal potential (which would be expected if any other conductance had developed) (Figure 3C).
Figure 3. AA does not alter the kinetics of fast inactivation or the inward rectification of ISOC in H4IIE cells.
(A) Current traces were obtained in response to voltage steps to −138 mV before and after application of 10 μM AA in the bath for 60 s. (B) Current traces shown on (A) were normalized to the peak value and superimposed. (C) Current–voltage plots in the absence and presence of AA in the bath.
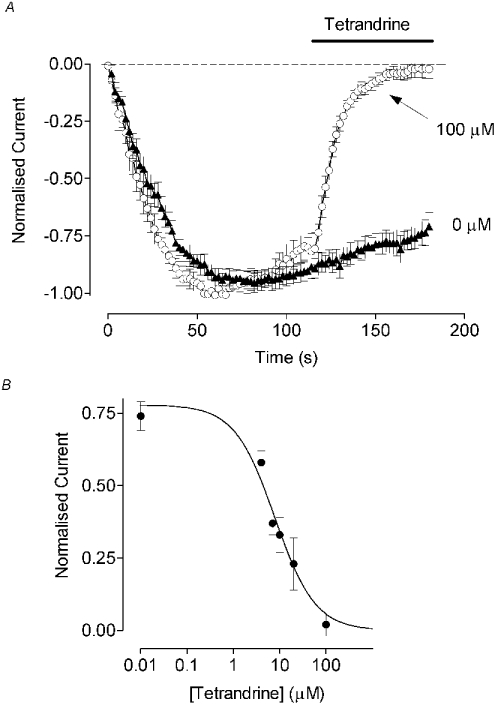
One of the methods of distinguishing between Ca2+ influxes carried by ISOC and IARC in m3HEK293 cells, where IARC was first described, was to use iso-tetrandrine. Iso-tetrandrine is known to inhibit the activation of phospholipase A2 by receptors [21], and has therefore been used to inhibit agonist-induced AA release [6,15]. Iso-tetrandrine is also one of the stereoisomers of tetrandrine that blocks L-type and T-type Ca2+ channels [33]. The effects of either compound on store-operated Ca2+ entry have not been investigated previously in most cell types. However, in avian nasal gland cells, iso-tetrandrine had no effect on thapsigargin-activated Ca2+ inflow [6]. In H4IIE cells, both tetrandrine and iso-tetrandrine caused a dose-dependent inhibition of ISOC (Figure 4, only tetrandrine is shown). The apparent IC50 for tetrandrine was 8.6±0.3 μM (n=4–12) (Figure 4B). At concentrations of 10 and 100 μM, iso-tetrandrine showed potency similar to that of tetrandrine in inhibiting ISOC (results not shown). The onset of the block induced by tetrandrine and iso-tetrandrine was as rapid as that observed with La3+ (T. Litjens and G. Rychkov, unpublished work) and was easily reversible upon washout (results not shown). Both tetrandrine and iso-tetrandrine had the same effect when ISOC was activated by either thapsigargin or Ins(1,4,5)P3.
Figure 4. Inhibition of ISOC by tetrandrine in H4IIE cells.
(A) Time course of ISOC inhibition by 100 μM tetrandrine (application is indicated by the horizontal bar). (B) Dose-dependent inhibition of ISOC by tetrandrine (n=4–12).
DISCUSSION
The existence of AA-activated Ca2+ entry has been shown in a variety of cell types [5,6,34,35]. In some of these cell types, AA-activated Ca2+ channels provide a major pathway for Ca2+ entry during Ca2+ oscillations triggered by receptor stimulation [5,17,29]. In contrast, in astrocytes, AA inhibits Ca2+ oscillation activated by ATP and activates sustained Ca2+ influx [30]. The results of the present study demonstrate that AA, in the predicted physiological range of concentrations [36], does not activate Ca2+-permeable channels in H4IIE liver cells, but strongly inhibits Ca2+ entry through SOCs (cf. [15,28–30]). The observation that AA also did not activate Ca2+-permeable channels in primary rat hepatocytes indicates that the failure to observe an AA-activated current in H4IIE liver cells is unlikely to be due to the absence of receptors for agonists in this immortalized cell line.
These results imply that, unlike some other cell types, including vascular smooth muscle cells and m3HEK293 cells, liver cells do not rely on AA-activated Ca2+ entry to maintain Ca2+ oscillations generated in response to phospholipase-C-coupled hormones. Moreover, the idea that SOCs are responsible for Ca2+ inflow that maintains Ca2+ oscillations in liver cells [19] is consistent with the present observations of the absence of AA-activated Ca2+-permeable channels in liver cells.
The observation that the intracellular application of AA had no effect on ISOC development and amplitude suggests an extracellular or membrane-delimited site of action for AA. However, an intracellular site cannot be unequivocally ruled out, since application of a membrane-permeable substance, such as AA, through a patch pipette may not be as effective in maintaining the exogenous AA concentration, since the AA will diffuse into the infinitely large (compared with the cell volume) bath faster than it diffuses through a patch pipette. AA may also be metabolized faster than it is incorporated into the plasma membrane, if the site of action is membrane-delimited. This appears to be the case in studies of the regulation of ICRAC by sphingosine, another membrane-permeable compound. Sphingosine that accumulates in the membrane, as assessed by the changes in the membrane capacitance, and inhibits ICRAC in RBL (rat basophil leukaemia) cells when applied in the bath has no effect on either capacitance or ICRAC when applied through the pipette [37]. Regardless of the site of action in liver cells, AA may be an important modulator of Ca2+ entry through SOCs when these are activated by physiological concentrations of hormones that induce the formation of AA. Under physiological conditions, AA is produced in the membrane [8], and, in vivo, when cells are packed close to each other in an intact organ, the concentration of AA is likely to rise both inside and outside the cells.
In m3HEK293 cells, Ca2+ currents activated by AA and depletion of Ca2+ stores were additive [16]. Thus addition of AA after ISOC development in m3HEK293 cells increased membrane current further. In contrast, in the present study with H4IIE cells, ISOC was inhibited completely by similar concentrations of AA. This suggests that ISOC in HEK-293 cells and ISOC in H4IIE cells are likely to be mediated by different types of SOC. Moreover, these results emphasize that conclusions about the nature of Ca2+-permeable pathways in the plasma membrane for one cell type cannot necessarily be applied to another cell type.
Another important observation of the present study is that iso-tetrandrine and tetrandrine are potent inhibitors of ISOC in liver cells. The mechanism of inhibition is unlikely to involve an effect of these agents on phospholipase A2, as the block is very rapid and readily reversible. A direct effect of tetrandrine and iso-tetrandrine on the SOC is consistent with these observations. In addition, if iso-tetrandrine and tetrandrine were acting via phospholipase A2, they would be expected to enhance ISOC, not to inhibit it, since the present results show that AA, a product of phospholipase A2 activity, inhibits SOCs in liver cells. In avian nasal gland cells, iso-tetrandrine has been shown to inhibit Ca2+ oscillations activated by a low concentration (0.5 μM) of carbachol, but was found to be ineffective in inhibiting Ca2+ entry activated by a high concentration (10 μM) of carbachol or by thapsigargin [6]. It was concluded from these experiments that, in avian nasal gland cells, iso-tetrandrine does not affect SOCs, that SOCs are not activated by low concentrations of carbachol, and therefore that the major physiological pathway for Ca2+ entry in these cells is through Ca2+ channels activated by AA. In view of the present study, caution should be exercised in interpretation of the results obtained using these compounds as inhibitors of receptor-activation of phospholipase A2 in studies involving the measurement of SOCs, especially when employing Ca2+ fluorescent dyes to measure Ca2+ inflow, and when the nature of the channels underlying that inflow is not entirely certain.
The observations made by several research groups with different cell types on the effects of AA on plasma membrane Ca2+-permeable channels can be summarized as follows: in different cell types, AA has been shown either to induce Ca2+ oscillations [6], or to inhibit them [30]; to inhibit SOC-mediated Ca2+ entry [15,28–30], or to have no effect on SOCs [16]; to activate Ca2+ channels with a high selectivity for Ca2+ [16], or to activate Ca2+-permeable non-selective cation channels [38]; and to activate Ca2+-permeable channels which are very sensitive to inhibition by Gd3+ [34], or channels which are insensitive to Gd3+ [5,39,40]. On the basis of all these results, it can be suggested that AA plays different physiological roles in regulating Ca2+ entry, and may activate or inhibit different types of Ca2+-permeable channels in different cell types. The results also indicate that SOCs are likely to be different in different cell types, and no single model of the mechanism of Ca2+ oscillations can be applied to all cell types.
Acknowledgments
We thank Rachael Hughes for excellent technical assistance. This work was supported by the Australian Research Council and NHMRC (National Health and Medical Research Council), Australia.
References
- 1.Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 2.Bird G. S., Aziz O., Lievremont J. P., Wedel B. J., Trebak M., Vazquez G., Putney J. W., Jr Mechanisms of phospholipase C-regulated calcium entry. Curr. Mol. Med. 2004;4:291–301. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- 3.Shuttleworth T. J. Intracellular Ca2+ signalling in secretory cells. J. Exp. Biol. 1997;200:303–314. doi: 10.1242/jeb.200.2.303. [DOI] [PubMed] [Google Scholar]
- 4.Taylor C. W. Controlling calcium entry. Cell. 2002;111:767–769. doi: 10.1016/s0092-8674(02)01197-2. [DOI] [PubMed] [Google Scholar]
- 5.Broad L. M., Cannon T. R., Taylor C. W. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J. Physiol. 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuttleworth T. J. Arachidonic acid activates the non-capacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem. 1996;271:21720–21725. doi: 10.1074/jbc.271.36.21720. [DOI] [PubMed] [Google Scholar]
- 7.Shuttleworth T. J., Thompson J. L. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J. Biol. Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- 8.Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem. J. 1984;220:345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke A. L., Petrou S., Walsh J. V., Jr, Singer J. J. Modulation of BKCa channel activity by fatty acids: structural requirements and mechanism of action. Am. J. Physiol. Cell Physiol. 2002;283:C1441–C1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature (London) 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 11.Barrett C. F., Liu L., Rittenhouse A. R. Arachidonic acid reversibly enhances N-type calcium current at an extracellular site. Am. J. Physiol. Cell. Physiol. 2001;280:C1306–C1318. doi: 10.1152/ajpcell.2001.280.5.C1306. [DOI] [PubMed] [Google Scholar]
- 12.Katsuki H., Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- 13.Meves H. Modulation of ion channels by arachidonic acid. Prog. Neurobiol. 1994;43:175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Skinner J., Sinclair C., Romeo C., Armstrong D., Charbonneau H., Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J. Biol. Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- 15.Luo D., Broad L. M., Bird G. S., Putney J. W., Jr Mutual antagonism of calcium entry by capacitative and arachidonic acid-mediated calcium entry pathways. J. Biol. Chem. 2001;276:20186–20189. doi: 10.1074/jbc.M100327200. [DOI] [PubMed] [Google Scholar]
- 16.Mignen O., Shuttleworth T. J. IARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J. Biol. Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 17.Mignen O., Thompson J. L., Shuttleworth T. J. Reciprocal regulation of capacitative and arachidonate-regulated non-capacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 18.Rychkov G., Brereton H. M., Harland M. L., Barritt G. J. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938–947. doi: 10.1053/jhep.2001.23051. [DOI] [PubMed] [Google Scholar]
- 19.Gregory R. B., Barritt G. J. Evidence that Ca2+-release-activated Ca2+ channels in rat hepatocytes are required for the maintenance of hormone-induced Ca2+ oscillations. Biochem. J. 2003;370:695–702. doi: 10.1042/BJ20021671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes B. P., Polverino A. J., Lim F., Barritt G. J. Vasopressin decreases total free fatty-acids but enhances release of radioactivity from isolated hepatocytes labeled with [3H]arachidonic acid. Horm. Metab. Res. 1987;19:15–20. doi: 10.1055/s-2007-1011725. [DOI] [PubMed] [Google Scholar]
- 21.Akiba S., Kato E., Sato T., Fujii T. Biscoclaurine alkaloids inhibit receptor-mediated phospholipase A2 activation probably through uncoupling of a GTP-binding protein from the enzyme in rat peritoneal mast cells. Biochem. Pharmacol. 1992;44:45–50. doi: 10.1016/0006-2952(92)90036-i. [DOI] [PubMed] [Google Scholar]
- 22.Brereton H. M., Chen J. L., Rychkov G., Harland M. L., Barritt G. J. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim. Biophys. Acta. 2001;1540:107–126. doi: 10.1016/s0167-4889(01)00124-0. [DOI] [PubMed] [Google Scholar]
- 23.Rychkov G. Y., Litjens T., Roberts M. L., Barritt G. J. ATP and vasopressin activate a single type of store-operated Ca2+ channel, identified by patch clamp recording, in rat hepatocytes. Cell Calcium. 2005 doi: 10.1016/j.ceca.2004.09.001. in the press. [DOI] [PubMed] [Google Scholar]
- 24.Barry P. H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 25.Hermosura M. C., Monteilh-Zoller M. K., Scharenberg A. M., Penner R., Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J. Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J. Biol. Chem. 1989;264:17131–17141. [PubMed] [Google Scholar]
- 27.Dixon C. J., Cobbold P. H., Green A. K. Oscillations in cytosolic-free Ca2+ induced by ADP and ATP in single-rat hepatocytes display differential sensitivity to application of phorbol ester. Biochem. J. 1995;309:145–149. doi: 10.1042/bj3090145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso-Torre S. R., Garcia-Sancho J. Arachidonic acid inhibits capacitative calcium entry in rat thymocytes and human neutrophils. Biochim. Biophys. Acta. 1997;1328:207–213. doi: 10.1016/s0005-2736(97)00094-1. [DOI] [PubMed] [Google Scholar]
- 29.Moneer Z., Taylor C. W. Reciprocal regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells: only the latter operates during receptor activation. Biochem. J. 2002;362:13–21. doi: 10.1042/0264-6021:3620013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sergeeva M., Strokin M., Wang H., Ubl J. J., Reiser G. Arachidonic acid in astrocytes blocks Ca2+ oscillations by inhibiting store-operated Ca2+ entry, and causes delayed Ca2+ influx. Cell Calcium. 2003;33:283–292. doi: 10.1016/s0143-4160(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 31.Zweifach A., Lewis R. S. Slow calcium-dependent inactivation of depletion-activated calcium current: store-dependent and -independent mechanisms. J. Biol. Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 32.Litjens T., Harland M. L., Roberts M. L., Barritt G. J., Rychkov G. Y. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J. Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q. Y., Karpinski E., Pang P. K. Tetrandrine inhibits both T and L calcium channel currents in ventricular cells. J. Cardiovasc. Pharmacol. 1992;20:513–519. doi: 10.1097/00005344-199210000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Mignen O., Thompson J. L., Shuttleworth T. J. Ca2+ selectivity and fatty acid specificity of the non-capacitative, arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 35.Peppiatt C. M., Holmes A. M., Seo J. T., Bootman M. D., Collins T. J., McDonald F., Roderick H. L. Calmidazolium and arachidonate activate a calcium entry pathway that is distinct from store-operated calcium influx in HeLa cells. Biochem. J. 2004;382:929–939. doi: 10.1042/BJ20040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brash A. R. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathes C., Fleig A., Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J. Biol. Chem. 1998;273:25020–25030. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 38.Fiorio Pla A., Munaron L. Calcium influx, arachidonic acid, and control of endothelial cell proliferation. Cell Calcium. 2001;30:235–244. doi: 10.1054/ceca.2001.0234. [DOI] [PubMed] [Google Scholar]
- 39.Watson E. L., Jacobson K. L., Singh J. C., DiJulio D. H. Arachidonic acid regulates two Ca2+ entry pathways via nitric oxide. Cell. Signalling. 2004;16:157–165. doi: 10.1016/s0898-6568(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 40.Luo D., Broad L. M., Bird G. S., Putney J. W., Jr Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J. Biol. Chem. 2001;276:5613–5621. doi: 10.1074/jbc.M007524200. [DOI] [PubMed] [Google Scholar]