Abstract
The gene for NAT (arylamine N-acetyltransferase) from Pseudomonas aeruginosa (panat) has been cloned from genomic DNA, and the gene product (PANAT) expressed as an N-terminal histidine-tagged protein in Escherichia coli and purified via nickel ion affinity chromatography. The specific activities of PANAT against a broad range of substrates have been investigated and compared with those of other prokaryotic NAT enzymes. For most arylamine substrates identified, PANAT exhibits in vitro specific activities typically one order of magnitude greater than those of recombinant NAT enzymes from Mycobacterium smegmatis or Salmonella typhimurium. Among the substrates of PANAT so far identified are the anti-tubercular drug isoniazid, 5-aminosalicylate (a drug used in the treatment of inflammatory bowel disease), as well as important environmental pollutants such as 3,4-dichloroaniline and 2-aminofluorene. As well as acetylating common NAT substrates, PANAT is unique among the prokaryotic NATs so far studied in acetylating the folate precursor 4-aminobenzoic acid and the folate catabolite 4-aminobenzoylglutamate. The recombinant protein has been expressed in sufficient quantity to allow protein crystallization, and we have subsequently determined the 1.95 Å structure of PANAT by X-ray crystallography.
Keywords: 5-aminosalicylic acid, arylamine N-acetyltransferase (NAT), Pseudomonas aeruginosa, xenobiotic metabolism, X-ray crystallography
Abbreviations: AcCoA, acetyl-CoA; 5-AS, 5-aminosalicylate; LB, Luria–Bertani; MALDI-TOF, matrix-assisted laser desorption ionization–time-of-flight; MSNAT, NAT from Mycobacterium smegmatis; NAT, arylamine N-acetyltransferase; pABA, 4-aminobenzoic acid; pABA-Glu, 4-aminobenzoylglutamate; PANAT, NAT from Pseudomonas aeruginosa; RMSD, root mean square deviation; RT-PCR, reverse-transcription–PCR; STNAT, NAT from Salmonella typhimurium; TBNAT, NAT from Mycobacterium tuberculosis
INTRODUCTION
NATs (arylamine N-acetyltransferases) constitute a family of drug-metabolizing enzymes of molecular mass 30–34 kDa that are found in a range of eukaryotes and prokaryotes [1]. NATs catalyse the transfer of an acetyl group from AcCoA (acetyl-CoA) to arylamines, arylhydrazines and hydroxyarylamines [2–5]. The NAT enzyme was first identified in humans in the 1960s due to its ability to inactivate the anti-tubercular drug isoniazid (INH) [6]. NATs have been identified more recently in a range of prokaryotes, including Salmonella typhimurium, Mycobacterium smegmatis, Pseudomonas aeruginosa and Mycobacterium tuberculosis [3,7–9].
According to current World Health Organization reports, approximately one-third of the world's population carry the M. tuberculosis bacterium, the causative agent of tuberculosis (www.who.int/gtb). The recent emergence of multidrug-resistant strains of M. tuberculosis has reduced the efficacy of the traditional three-drug anti-tubercular therapy of isoniazid, rifampicin and pyrazinamide [10,11], and so there is an urgent need to increase our understanding of tuberculosis biology.
Previous attempts to study TBNAT (NAT from M. tuberculosis; NAT104*1) have been unsuccessful due to low yields of recombinant protein [8]. Escherichia coli-based expression levels of recombinant NAT enzymes from both M. smegmatis (NAT103*1; MSNAT) and S. typhimurium (NAT101*1; STNAT) are significantly higher [8,12], and hence much of our biochemical and structural knowledge of NATs is based on these proteins. The X-ray crystal structures of MSNAT and STNAT have revealed a conserved three-domain fold whose active site contains a triad of residues (Cys-His-Asp) that is proposed to catalyse substrate acetylation [13,14]. In vitro, both MSNAT and STNAT have been shown to acetylate a range of known NAT substrates, including 5-AS (5-aminosalicylate) and isoniazid [8,12,14–17]. Furthermore, it has been shown that overexpression of TBNAT in M. smegmatis decreases the susceptibility of the organism to isoniazid, while a genetic nat knockout in M. smegmatis exhibited heightened susceptibility to the drug [8,18]. More recently, characterization of a genetic nat deletion mutant of the M. tuberculosis model organism, Mycobacterium bovis BCG, has indicated that NAT is essential for mycolic acid biosynthesis in this organism [19].
Using the TBNAT sequence as a search template, we have identified a single putative NAT-encoding DNA sequence (panat) within the P. aeruginosa genome. Despite sharing only low sequence identity at the amino acid level (35%), the PANAT amino acid sequence contains many of the sequence motifs associated with NATs, for example the catalytic triad of Cys-His-Asp residues and the Phe-Glu-Asn-Leu (FENL) motif [20]. Interestingly, whereas STNAT and TBNAT share 43% sequence identity over the first and second domains, but only 16% identity in the third domain, the pattern of sequence identity between PANAT and TBNAT is distributed fairly homogeneously throughout the protein (≈35%). The third domain of NATs confers substrate specificity [15], and hence in order to improve and complement our current enzymic and structural models of TBNAT in drug screening, we have recombinantly expressed and purified the NAT from P. aeruginosa (PANAT).
5-AS is commonly used in the treatment of inflammatory bowel diseases, such as Crohn's disease and ulcerative colitis [21]; however, its efficacy is reduced as a result of acetylation. Cultures containing P. aeruginosa have been demonstrated to acetylate and hence inactivate 5-AS [3,22]. P. aeruginosa is found in the gut, and so it is important to determine whether PANAT contributes to the acetylation of 5-AS.
A detailed characterization of PANAT is therefore pertinent to inflammatory bowel disease treatment and as a model system for the design of anti-tubercular agents. As a contribution to both of these research areas, in this paper we present the 1.95 Å structure of PANAT, determined by X-ray crystallography, and a detailed profile of PANAT activity against a broad range of substrates.
EXPERIMENTAL
Materials
All chemicals and reagents were purchased from Sigma Aldrich (Poole, Dorset, U.K.).
Protein and RNA isolation, and RT-PCR (reverse transcription–PCR)
Total RNA was isolated from a 4 ml culture of Pseudomonas aeruginosa (PA01 strain) using TRIzol (Gibco BRL, Cergy-Pontoise, France). Protein, produced as a by-product, was used for gel electrophoresis and Western blotting. cDNA was synthesized from 1 μg of total RNA template using the C. therm. Polymerase RT-PCR kit (Roche, Meylan, France). The reaction was performed in a total volume of 20 μl at 70 °C using the antisense primer 5′-GGAATTCAGGCGGAGATCAGTCCCGCCA-3′.
For PCR analysis, 0.25 μg of cDNA was used as a template in a 50 μl amplification mixture containing 200 μM of each deoxynucleotide triphosphate, 10 mM Tris/HCl, pH 8.3, 1.5 mM MgCl2, 50 mM KCl, 5% (v/v) DMSO, 0.3 μM of each primer and 1.5 units of DyNAzyme EXT DNA polymerase (Ozyme, Saint-Quentin en Yvelines, France). The primers used in the PCR were 5′-CGAGTGCCATATGACCCCGCTGACGCCCGA-3′ (sense) and 5′-GGAATTCAGGCGGAGATCAGTCCCGCCA-3′ (antisense). The PCR conditions were as follows: denaturation (94 °C, 1 min), followed by 35 cycles of combined annealing and polymerization (72 °C, 4 min) and denaturation (94 °C, 1 min), followed by a final extension step (72 °C, 10 min). To assess whether genomic DNA had contaminated the RNA samples, an additional PCR reaction was carried out under the same conditions with purified total RNA (1 μg). The PCR products were separated by gel electrophoresis in ethidium bromide-stained agarose (1.8%, w/v).
Cloning
The panat open reading frame was amplified from P. aeruginosa strain PA01 genomic DNA using PCR with the primers 5′-CGAGTGCCATATGACCCCGCTGACGCCCGA-3′ (sense) and 5′-GGAATTCAGGCGGAGATCAGTCCCGCCA-3′ (antisense). The start and stop codons for the nat gene are shown in bold type, and NdeI and EcoRI restriction sites are shown in italics. The PCR used Pfu DNA polymerase (Promega) and consisted of a denaturation step (95 °C, 2 min) followed by 30 cycles of annealing (60 °C, 30 s), extension (72 °C, 2 min) and denaturation (95 °C, 1 min), and a final extension step (72 °C, 5 min). The PCR product was subcloned into the pET28b(+) vector (Novagen) using the NdeI and EcoRI restriction sites. The pET28b(+) vector containing the panat insert was transformed initially into Escherichia coli strain JM109, which was grown in LB (Luria–Bertani) medium containing kanamycin (30 μg/ml). The identity of the insert was confirmed by DNA sequencing (University of Oxford Biochemistry Department Sequencing Service).
Expression and purification
The pET28b(+)-panat plasmid was transformed into the expression host E. coli BL21(DE3)pLysS as described previously [12]. An overnight culture was diluted into 100 vol. of LB broth containing kanamycin (30 μg/ml). Bacterial cultures of 1 litre were grown in a shaking incubator at 37 °C to a D600 of 0.4– 0.6. Expression of PANAT was induced by addition of isopropyl β-D-thiogalactopyranoside to a final concentration of 0.25 mM. The culture was maintained at 37 °C until the D600 had reached 1.8–2.0, at which point cells were harvested by centrifugation (5000 g, 4 °C, 20 min). The cell pellet was resuspended in 15 ml of lysis buffer [300 mM NaCl, 20 mM Tris/HCl, pH 8.0, 1×EDTA-free Complete Protease Inhibitor (Roche)] for each litre of original culture. The resuspended pellet was frozen and stored at −70 °C. Pellets were thawed in a water bath (37 °C) before sonication to complete the cell lysis. The lysate was clarified by centrifugation (12000 g, 4 °C, 20 min), and then batch-bound to Ni2+–nitrilotriacetate resin (Qiagen) (pre-equilibrated in 300 mM NaCl, 20 mM Tris/HCl, pH 8.0) for 10 min at 4 °C. The resin was packed into a PD-10 column (Amersham Biosciences, Chalfont St. Giles, Bucks., U.K.), and then washed with solutions (300 mM NaCl, 20 mM Tris/HCl, pH 8.0) containing increasing concentrations of imidazole (pH 8.0) at 4 °C. The wash sequence was typically 10, 25, 125 and 250 mM imidazole (two 10 ml washes of each). Fractions containing pure PANAT were identified by SDS/PAGE [23], pooled and dialysed against 200 vol. of buffer (20 mM Tris/HCl, pH 8.0, 1 mM EDTA, 1 mM dithiothreitol) at 4 °C for 16 h.
Western blot analysis
Protein samples were separated by SDS/PAGE and transferred to a nitrocellulose membrane (Hybond-C; Roche) for Western blot analysis as described previously [8]. An antibody raised against STNAT [8] was used to detect both PANAT in the P. aeruginosa lysate (antibody dilution 1:10000) and recombinant purified PANAT (antibody dilution 1:50000).
Enzymic assays
For the assays described below, the concentrations of NAT enzyme samples were adjusted to ensure a linear initial rate of reaction. Kinetic analyses were performed by varying the substrate concentration, and kinetic constants were determined from Hanes plots [24].
Acetylation of arylamines
Detection of the acetylation of arylamines was performed as described previously [25]. Samples of enzyme (0.3–1.5 pmol) and arylamine (50 –200 μM) in assay buffer (20 mM Tris/HCl, 1 mM dithiothreitol, pH 8.0) were pre-incubated at 37 °C for 5 min. AcCoA (400 μM final concentration) was added and the reaction was allowed to proceed at 37 °C. The reaction (100 μl total volume) was quenched with 100 μl of aqueous trichloroacetic acid (20%, w/v) at 0 °C. 4-Dimethylaminobenzaldehyde [800 μl; 5% (w/v) in 9:1 (v/v) acetonitrile/water] was then added and the absorbance was measured in a 10 mm path length cuvette at λmax=450 nm (Hitachi U-2001 spectrophotometer). The amount of arylamine remaining was determined by reference to a standard curve.
Hydrolysis of AcCoA
The rate of hydrolysis of AcCoA by NAT in the presence of an arylamine or hydrazine substrate was determined as described previously [17]. PANAT was diluted in assay buffer (20 mM Tris/HCl, pH 8.0) immediately prior to use to ensure that the final concentration of dithiothreitol was less than 1 μM, which has been demonstrated previously not to interfere with the assay [17]. Samples of enzyme (0.3–1.5 pmol) and substrate (500 μM) in assay buffer were pre-incubated at 37 °C for 5 min in a 96-well plate. AcCoA (400 μM final concentration) was added to start the reaction and the plate was incubated at 37 °C. The reaction (100 μl total volume) was quenched with 25 μl of guanidine hydrochloride solution (6.4 M guanidine/HCl, 0.1 M Tris/HCl, pH 7.3) containing 5 mM 5,5′-dithiobis-(2-nitrobenzoic acid), and then the absorbance was measured at λmax=405 nm (Anthos 2020 plate reader). The amount of CoA produced was determined by reference to a standard curve.
X-ray crystallography and data collection
PANAT was concentrated to 7–14 mg/ml using Amicon ultracentrifugation concentrators (Millipore, Watford, Herts., U.K.). The crystals described were grown at 19 °C using the sitting-drop vapour diffusion technique by mixing equal volumes (1 μl) of the concentrated PANAT solution with mother liquor {Molecular Dimensions Screen II; condition number 28 [100 mM Hepes, pH 7.5, 20% poly(ethylene glycol) 10K]}. These crystals are grown reproducibly by both hanging- and sitting-drop vapour diffusion methods [26], and both from manual 24-well plates and using a protein crystallization robot (Tecan Genesis 150 robot; Laboratory of Molecular Biophysics, University of Oxford). Crystals of dimensions of 15 μm×15 μm×100 μm typically grew after 2–5 days.
Prior to data collection, crystals were transferred briefly to a cryo-protection solution of 6 M sodium formate, before flash freezing in a nitrogen cryo-stream at 100 K (Oxford Cryosystems). Data were collected on beamline ID29 at the European Synchrotron Radiation Facility (ESRF, Grenoble, France) with an ADSC Quantum-4 CCD detector. The space group was determined, and data were integrated, scaled and merged using the programs MOSFLM and SCALA [27].
RESULTS
In order to determine whether the panat gene, identified from the complete genome sequence of P. aeruginosa strain PA01 [28], is expressed endogenously, RNA was extracted from P. aeruginosa cultures and RT-PCR was performed with NAT-specific primers. A PCR product of the expected size (855 bp) was observed (Figure 1A), indicating that the panat gene is expressed endogenously by P. aeruginosa. A Western blot using a monospecific antiserum against STNAT [8] indicated the presence of a cross-reacting protein with an approximate molecular mass of 31 kDa in lysates of P. aeruginosa (Figure 1B). The antibody has subsequently been shown to recognize pure recombinant PANAT (approx. 33.5 kDa with a hexa-histidine tag; Figure 1B).
Figure 1. Endogenous expression of PANAT and purification of the recombinant enzyme.
(A) Ethidium bromide-stained 1.8% (w/v) agarose gel showing the 855 bp fragment obtained after RT-PCR using total RNA isolated from Pseudomonas aeruginosa PA01 (lane 2). The absence of genomic DNA contamination was confirmed using a negative control with no reverse transcriptase in the RT-PCR experiment (lane 1). (B) Western blot showing PANAT detected in P. aeruginosa lysate using an antibody raised against STNAT at 1:10000 dilution (lane 1). Recombinant pure PANAT with a hexa-histidine tag (1.0 μg) was detected using the anti-STNAT antibody at a 1:50000 dilution (lane 2). (C) Purification of PANAT. Coomassie Blue-stained SDS/12%-PAGE gel showing the single-step purification of PANAT as described in the Experimental section. Lanes: M, markers; 1, insoluble lysate; 2, soluble lysate; 3, unbound lysate; 4, 10 mM imidazole wash; 5, 25 mM imidazole wash; 6, 125 mM imidazole wash; 7, 250 mM imidazole wash; 8, PANAT after 2 months at 4 °C.
The panat gene was then amplified by PCR, producing the expected product of size 870 bp, and cloned into the pET28b(+) expression vector to facilitate the overexpression of PANAT with an N-terminal hexa-histidine tag. The protein was purified to apparent homogeneity using Ni2+-ion affinity chromatography (Figure 1C). Typically, 30 mg of PANAT was purified per litre of bacterial culture. The identity of PANAT was confirmed by tryptic digestion and subsequent analysis of the fragments by MALDI-TOF (matrix-assisted laser desorption ionization–time-of-flight) MS analysis (see Supplementary Table S1 at http://www.BiochemJ.org/bj/385/bj3850605add.htm).
The apparent Michaelis constant and maximum reaction velocity for AcCoA were determined by following the acetylation of p-anisidine [Km (app)=136 μM; Vmax=13280 nmol·min−1·mg PANAT−1]. Substrates that have been shown to be acetylated by MSNAT, STNAT and TBNAT, as well as by bacterial cell lysates [3], were screened for substrate activity with PANAT (Table 1). Where it has been possible to compare these specific activities with literature values for the pure recombinant enzymes STNAT and MSNAT [17], the relative specific activities are shown in Figure 2. The activity of PANAT for the arylamine substrates, including 2-aminofluorene, the 4-haloanilines (4-chloroaniline, 4-bromoaniline, 4-iodooaniline) and 5-AS, is typically an order of magnitude greater compared with the recombinant MSNAT and STNAT enzymes. This trend does not extend to the hydrazine substrates, which constitute the most rapidly acetylated structural class of substrates for both STNAT and MSNAT. Additionally, PANAT acetylates the folate precursor pABA (4-aminobenzoic acid) and the folate catabolite pABA-Glu (4-aminobenzoylglutamate), both of which are substrates for the human NAT1 enzyme [29,30]. pABA acetylation activity is rare in prokaryotes, having been identified only in P. aeruginosa PA01, Bacteroides sp. and S. typhimurium YG1024 [3]. Acetylation of pABA-Glu by prokaryotic NATs has only been reported for STNAT [31].
Table 1. Substrates of PANAT.
The rate of hydrolysis of AcCoA (nmol·min−1·mg of PANAT−1) was measured in the presence of PANAT, AcCoA (400 μM) and compound (500 μM) as described in the Experimental section. nd, no detectable activity.
| Class/compound | Short name | Rate (nmol·min−1·mg−1) |
|---|---|---|
| Arylamine drugs | ||
| Sulphacetamide | SAcA | nd |
| Sulphanilamide | SAnA | nd |
| Sulphadiazine | SDZ | nd |
| Sulphamethazine | SMZ | nd |
| Trimethoprim | TMP | nd |
| Sulphisoxazole | SIOZ | 1000±2 |
| Sulphamethoxazole | SMOZ | 1310±5 |
| 5-Aminosalicylate | 5-AS | 73300±3300 |
| Other arylamines | ||
| 4-Aminobenzoic acid L-glutamate | pABA-Glu | 532±60 |
| Aniline | AL | 629±40 |
| 4-Trifluoromethylaniline | TFMA | 2760±233 |
| 4-Chloroaniline | 4-CA | 4140±136 |
| 4-Bromoaniline | 4-BA | 5710±622 |
| 3,4-Dichloroaniline | DCA | 6760±583 |
| 4-Aminobenzoic acid | pABA | 8200±78 |
| 4-Iodoaniline | 4-IA | 13800±4860 |
| 4-Aminosalicylate | 4-AS | 18660±467 |
| 4-Phenoxyaniline | POA | 21970±972 |
| 2-Aminofluorene* | 2-AF | 44710±2720 |
| Alkoxyanilines | ||
| 4-Hexyloxyaniline* | HOA | 12050±1170 |
| 4-Anisidine | ANS | 13470±0 |
| 4-Ethoxyaniline | EOA | 19240±1750 |
| 4-Butoxyaniline* | BOA | 25660±1170 |
| 4-Aminoveratrole | AMV | 44510±3690 |
| Hydrazines | ||
| Phenylhydrazine | PHZ | nd |
| 4-Methoxyphenylhydrazine | MPZ | 641±603 |
| Isoniazid | INH | 2324±0 |
| 4-Chlorobenzoic hydrazide | CBZ | 14000±1940 |
| Hydralazine | HDZ | 29550±3110 |
* Assay performed in the presence of 5% (v/v) DMSO.
Figure 2. Comparison of the specific activities of PANAT with those of other bacterial NATs.
Shown is the rate of hydrolysis of AcCoA (nmol of CoA·min−1·mg of protein−1) in the presence of AcCoA (400 μM), substrate (500 μM) and NAT enzyme. PANAT from P. aeruginosa (black bars), MSNAT (grey bars) and STNAT (unshaded bars) were compared. MSNAT and STNAT data are literature values [17]. The same method was used for all three enzymes. Each bar represents the mean±S.D. from triplicate determinations. Abbreviations are as described in Table 1.
The 1.95 Å structure of PANAT was solved by molecular replacement using the program MOLREP [27]. The search model was created by modifying the high-resolution MSNAT structure (PDB identifier 1GX3), such that residues not conserved in PANAT were truncated to the Cβ atom. Using this model, molecular replacement identified a single copy of PANAT within the asymmetric unit, consistent with a Matthew's coefficient of 2.5 Å3·Da−1 and a solvent content of 49.7%. After manual rebuilding using program O [32], the PANAT structure was initially refined via a simulated-annealing step using the CNS suite of programs [33]. Subsequent refinement involved iterative manual rebuilding and maximum likelihood refinement using the program REFMAC5 [27]. A final refinement step was to solvate the protein, using the program ARP [27]. The stereochemical quality of the final model was verified using the program Procheck [34]. In the structure, 94.9% of residues were found in the most favoured regions, 4.7% were found in additionally allowed regions, and one residue (Asp225; 0.4%) was found in a generously allowed region. Clear electron density supports the conformation of Asp225. The final PANAT model has an Rfactor of 19.1% and an Rfree of 22.4%, consisting of a single molecule in the asymmetric unit, with an additional 133 water molecules. Electron density was attributable to residues 1–276, while the C-terminal three residues (Ile-Ser-Ala) could not be confidently located. Although the N-terminal hexa-histidine motif and linker residues (20 residues) could not be reliably traced, after 2 months at 4 °C there was no apparent degradation of the enzyme, as shown by SDS/PAGE (lane 8, Figure 1C) and no change in activity was observed (results not shown). This suggests that these residues are disordered and not degraded within the crystal. Co-ordinates have been submitted to the RSCB PDB under PDB identifier 1W4T (http://www.rcsb.org/pdb/). A summary of the final model statistics is presented in Supplementary Table S2 (http://www.BiochemJ.org/bj/385/bj3850605add.htm).
DISCUSSION
In this paper we have described the identification of the panat gene of Pseudomonas aeruginosa through its sequence identity with the NAT family of proteins. The panat open reading frame has been cloned and the gene has been expressed to generate sufficient levels of recombinant protein for both in vitro activity assays and X-ray crystallographic studies. Both our structural and biochemical data confirm the identification of the panat gene as encoding a NAT enzyme; PANAT is able to catalyse the in vitro acetylation of a broad range of arylamines and arylhydrazines (Table 1, Figure 2), and the X-ray structure of PANAT reveals a fold that is highly reminiscent of both MSNAT and STNAT structures (Figure 3). The anti-STNAT antibody cross-reacts with the PANAT recombinant protein (Figure 1B), as it does with MSNAT and, to a lesser extent, TBNAT [8].
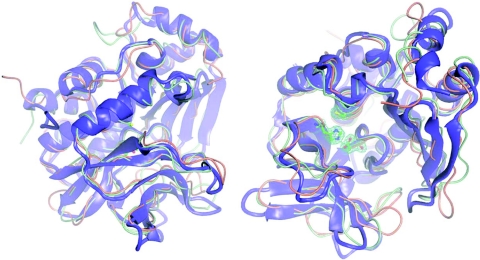
Figure 3. X-ray crystal structure of PANAT.
Orthogonal views of the X-ray crystal structures of PANAT (blue/purple), MSNAT (orange) and STNAT (green) superimposed using the program O [32]. All images were generated using Aesop [M. E. M. Noble, unpublished work; details available from M. E. M. N. on request (martin@biop.ox.ac.uk)]. Representative final 2Fobs−Fcal·αcalc electron density for the active-site triad of PANAT is contoured at 0.2e-Å-3 in green.
There are now three model NAT proteins that readily crystallize and for which X-ray crystallographic structures have been determined [14,15]. Crystals from two of these proteins routinely diffract to better than 2 Å, and so provide good model systems with which to investigate protein–ligand interactions. The aims are twofold: first the improvement and validation of our current TBNAT model [18], for which no structural data are currently available; and secondly to facilitate the rational structure-led ligand design of small-molecule inhibitors with potential therapeutic value. Following on from studies of other bacterial NATs [20], in the present paper we have compared both the enzymic rates of acetylation and the structural properties of PANAT with both those of MSNAT and STNAT.
The X-ray crystallographic structure reveals that, despite low sequence similarity (Figure 4), PANAT shares an almost identical fold with that of both STNAT and MSNAT (Figure 3) [13,14]. PANAT has an RMSD (root mean square deviation) of 1.5 Å with STNAT (over 248 equivalent residues) and of 1.3 Å with MSNAT (over 240 equivalent atoms). The structure may be divided into three domains of approximately equal size. The first two domains, a helical bundle and a β-barrel, are aligned in such a way that three residues (Cys73, His113 and Asp128; P. aeruginosa numbering) form a catalytic triad. The structural motif that these residues form is well documented in NATs and is identical with that of the cysteine protease superfamily [8,13,15,35–37]. The catalytic triad residues superpose with an RMSD of only 0.054 Å (Cα residues), while a more general comparison of active-site residues in the different NAT structures reveals a significantly higher level of sequence identity than suggested by the global similarity, and further reinforces the similarity between these proteins (Figure 3). The third domain is linked to the second via an interdomain helix (approx. residue 180). The interface formed between domains II and III and the lid region together form a substantial active-site cleft.
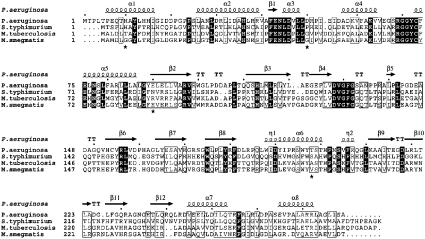
Figure 4. Multiple sequence alignment of selected bacterial NATs.
The sequence alignment was constructed using ClustalX [51] and ESPript [52]. The secondary structural elements were identified from the PANAT structure using ESPript. α-Helices, η-helices, β-sheets and strict β-turns are denoted α, η, β and TT respectively. Grey stars indicate side chains for which multiple conformations were modelled. SwissProt accession numbers: P. aeruginosa, Q9HUY3; M. smegmatis, O86309; S. typhimurium, Q00267; M. tuberculosis, P96848. Similar amino acids are highlighted in boxes, and completely conserved residues are indicated by white lettering on a dark background.
Minor differences between PANAT and other NAT structures may be categorized as either (a) single point insertions/deletions, which are accommodated within loop regions of the structure and result in little perturbation of the protein fold, and (b) mobile loop regions between more stable secondary structural elements. These are located between β7/β8, β9/β10, β12/β13 and β13/β14. Similar conformations for these regions are seen in a second PANAT crystal form (results not shown), suggesting that the differences are a property of PANAT, and not due to an artefact of packing within the crystal lattice. Such differences are minor, since PANAT clearly shares the NAT fold. The invariant phenylalanine contained within the highly conserved Pro-Phe-Glu-Asn-Leu motif (residues 41– 45; PANAT numbering) has been implicated in substrate binding [38], and so it is interesting to note that this sequence is Ala-Phe-Glu-Asn-Leu in PANAT. Surprisingly, the change from proline to alanine has no effect on the NAT fold, with both local secondary structural elements and individual side-chain conformations being identical in this region of MSNAT and PANAT. The change in residue leaves the main-chain nitrogen atom with an uncompensated hydrogen bond, which in the PANAT structure is compensated for by an additional water molecule forming hydrogen bonds with the carbonyl groups of both Arg38 and Phe256.
This is now the third example of a crystallographic structure of a bacterial NAT, and suggests that other bacterial NAT proteins are likely to share an identical fold. Whether this conservation extends to more phylogenetically distant species remains to be seen, but the high level of bacterial structural conservation serves to support the validity of our previously published model structure of TBNAT [18].
To investigate whether the structural similarity is extended to the enzymic properties of NAT proteins, we measured the acetylation of a broad range of substrates, including those for which N-acetylation activity has been reported previously for P. aeruginosa cultures [3,9]. These included 2-aminofluorene, 5-AS, 4-aminosalicylic acid and pABA. Although the specific activity profile of PANAT substrates is broadly similar to those of MSNAT and STNAT, we have identified some key differences. Firstly, PANAT is significantly more active against arylamine substrates than is MSNAT or STNAT (Figure 2). Secondly, the relationship between the rate of acetylation and hydrophobicity of alkoxyanilines by MSNAT {p-anisidine<4-aminoveratrole<4-ethoxyaniline<4-butoxyaniline<4-hexyloxyaniline (Figure 2) [17]} is not conserved for PANAT, for which the rate of acetylation is in the order 4-hexyloxyaniline<p-anisidine<4-ethoxyaniline<4-butoxyaniline<4-aminoveratrole (Figure 2). Lastly, the arylhydrazine substrate hydralazine, which for both MSNAT and STNAT is the most rapidly acetylated substrate tested, is acetylated more slowly when compared with other PANAT substrates.
We propose that differences in the distribution of surface hydrophobicity in the MSNAT and PANAT active sites are at least partially responsible for the differing specific activity profiles (Figures 2 and 5). The cores of both NAT active sites do not possess significant hydrophobic character, in keeping with the presence of several water molecules observed in this region of both crystal structures. However, regions of hydrophobicity are found on the edge of both active sites, albeit in differing locations. It is also interesting to note that, despite the overall fold conservation, subtle changes to side-chain conformations result in quite differing surface profiles. Co-crystallization experiments are currently under way in order to identify the binding site of these substrates, from which we hope to investigate these differences in more detail.
Figure 5. Molecular surface and active-site architecture of PANAT and MSNAT.
Molecular surfaces were calculated for both PANAT (A) and MSNAT (B) using the program Aesop [M. E. M. Noble, unpublished work; details available from M. E. M. N. on request (martin@biop.ox.ac.uk)]. Hydrophobicity was then calculated for each surface and underlying protein using the program GRID [50]. Surfaces are coloured on identical scales of increasing hydrophobicity from grey through green to yellow. Underlying secondary structural elements of PANAT (blue) and MSNAT (orange) and the active-site cysteine (ball and stick mode) are also shown.
Normal faecal microflora, among which P. aeruginosa is found, have been shown to contribute to the acetylation of 5-AS [22]. Of the substrates measured in the present study, 5-AS was the most rapidly acetylated, suggesting that PANAT may contribute to the in vivo acetylation, and hence therapeutic inactivation, of 5-AS [39]. Recent studies with human NAT1 and NAT2 [40] have shown that PANAT is an order of magnitude more active in the acetylation of 5-AS than the human NAT enzymes. Hence PANAT in gut flora is likely to be an important contributor to the therapeutic inactivation of 5-AS. Inhibition of the N-acetylation of 5-AS by intestinal microflora using an exogenous PANAT-specific ligand may therefore have combinatorial therapeutic value [41]. Similarly, inhibition of PANAT-mediated acetylation of the antihypertensive drug hydralazine may help to reduce the occurrence of systemic lupus erythematosus, which this drug is known to cause [42].
The growing problem of P. aeruginosa multidrug resistance has been accounted for largely by its plethora of regulatory genes and efflux pumps [43–48]. Previous studies have shown that the mexABoprM multidrug efflux system is responsible for resistance of P. aeruginosa to the antibacterial agents trimethoprim and sulphamethoxazole [45]. Sulphamethoxazole and sulphisoxazole were poor substrates for PANAT, and no acetylation of trimethoprim or any of the other sulphonamides tested was observed.
pABA is required for the synthesis of folic acid in P. aeruginosa, as shown by hypersensitivity to sulphonamides and trimethoprim when these agents are not removed by efflux, and so it was surprising to note that pABA is a substrate for PANAT. The literature apparent Km for acetylation of pABA in P. aeruginosa lysate (1200 μM) is considerably higher than the reported Km for the conversion of pABA into 7,8-dihydropteroate by dihydropteroate synthase from Streptococcus faecalis R (1.1 μM) [49]. Although the two enzymes are from different prokaryotes, we predict that the rate of removal of pABA by acetylation in vivo is insignificant compared with the rate of formation of 7,8-dihydropteroate in P. aeruginosa.
The data in the present paper present a picture of close structural similarity between bacterial NAT proteins. This is partially reflected in similar specific activity profiles, although we have identified a number of key differences that result from subtle structural differences. Current co-crystallization experiments are aimed at identifying the mode of substrate binding, from which we hope to understand further the nature of these differences. With its ease of preparation and crystallization, PANAT is ideal for use in high-throughput screening assays for inhibitors of bacterial NATs, to complement data already obtained for the MSNAT enzyme. The PANAT structure is being used within our laboratory in an iterative, rational ligand-design process to identify both TBNAT and broad-range bacterial NAT antagonists for use in a range of clinical applications.
We recommend that PANAT is designated NAT105*1, in accordance with current NAT nomenclature, as agreed by the International NAT Nomenclature Committee (http://www.louisville.edu/medschool/pharmacology/NAT.html).
Online data
Acknowledgments
We are grateful to the Wellcome Trust for continued financial support. I. M. W. is in receipt of an MRC studentship. We thank Professor Stephen G. Davies (Chemistry Research Laboratory, University of Oxford) for his continued supervision of I. M. W., and Andrew W. Mulvaney, Richard J. Vickers and Angela J. Russell (Chemistry Research Laboratory, University of Oxford) for advice and helpful discussion. We also thank Professor A. M. Chakrabarty (University of Illinois College of Medicine, Chicago, IL, U.S.A.) for his kind donation of Pseudomonas aeruginosa genomic DNA, and Andrew Spiers (Plant Sciences, University of Oxford) for helpful discussion. MALDI-TOF analysis was carried out by Tony Willis (Department of Biochemistry, University of Oxford).
References
- 1.Upton A., Johnson N., Sandy J., Sim E. Arylamine N-acetyltransferases – of mice, men and microorganisms. Trends Pharmacol. Sci. 2001;22:140–146. doi: 10.1016/s0165-6147(00)01639-4. [DOI] [PubMed] [Google Scholar]
- 2.Evans D. A. N-acetyltransferase. Pharmacol. Ther. 1989;42:157–234. doi: 10.1016/0163-7258(89)90036-3. [DOI] [PubMed] [Google Scholar]
- 3.Delomâenie C., Fouix S., Longuemaux S., Brahimi N., Bizet C., Picard B., Denamur E., Dupret J. M. Identification and functional characterization of arylamine N-acetyltransferases in eubacteria: evidence for highly selective acetylation of 5-aminosalicylic acid. J. Bacteriol. 2001;183:3417–3427. doi: 10.1128/JB.183.11.3417-3427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber W. W., Hein D. W. N-acetylation pharmacogenetics. Pharmacol. Rev. 1985;37:25–79. [PubMed] [Google Scholar]
- 5.Riddle B., Jencks W. P. Acetyl-coenzyme A:arylamine N-acetyltransferase. Role of the acetyl-enzyme intermediate and the effects of substituents on the rate. J. Biol. Chem. 1971;246:3250–3258. [PubMed] [Google Scholar]
- 6.Evans D. A. P., Manley K. A., McKuisick V. A. Genetic control of isoniazid acetylation in man. Br. Med. J. 1960;2:485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe M., Sofuni T., Nohmi T. Involvement of Cys69 residue in the catalytic mechanism of N-hydroxyarylamine O-acetyltransferase of Salmonella typhimurium. Sequence similarity at the amino acid level suggests a common catalytic mechanism of acetyltransferase for S. typhimurium and higher organisms. J. Biol. Chem. 1992;267:8429–8436. [PubMed] [Google Scholar]
- 8.Payton M., Auty R., Delgoda R., Everett M., Sim E. Cloning and characterization of arylamine N-acetyltransferase genes from Mycobacterium smegmatis and Mycobacterium tuberculosis: increased expression results in isoniazid resistance. J. Bacteriol. 1999;181:1343–1347. doi: 10.1128/jb.181.4.1343-1347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh S. E., Lo H. H., Chung J. G. The characteristics of arylamine N-acetyltransferase in Pseudomonas aeruginosa. Curr. Microbiol. 1998;36:353–360. doi: 10.1007/s002849900322. [DOI] [PubMed] [Google Scholar]
- 10.Bass J. B., Jr, Farer L. S., Hopewell P. C., O'Brien R., Jacobs R. F., Ruben F., Snider D. E., Jr, Thornton G. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am. J. Respir. Crit. Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 11.Moore M., Onorato I. M., McCray E., Castro K. G. Trends in drug-resistant tuberculosis in the United States, 1993–1996. JAMA, J. Am. Med. Assoc. 1997;278:833–837. [PubMed] [Google Scholar]
- 12.Sinclair J. C., Delgoda R., Noble M. E., Jarmin S., Goh N. K., Sim E. Purification, characterization, and crystallization of an N-hydroxyarylamine O-acetyltransferase from Salmonella typhimurium. Protein Expression Purif. 1998;12:371–380. doi: 10.1006/prep.1997.0856. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair J. C., Sandy J., Delgoda R., Sim E., Noble M. E. Structure of arylamine N-acetyltransferase reveals a catalytic triad. Nat. Struct. Biol. 2000;7:560–564. doi: 10.1038/76783. [DOI] [PubMed] [Google Scholar]
- 14.Sandy J., Mushtaq A., Kawamura A., Sinclair J., Sim E., Noble M. The structure of arylamine N-acetyltransferase from Mycobacterium smegmatis – an enzyme which inactivates the anti-tubercular drug, isoniazid. J. Mol. Biol. 2002;318:1071–1083. doi: 10.1016/S0022-2836(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 15.Mushtaq A., Payton M., Sim E. The COOH terminus of arylamine N-acetyltransferase from Salmonella typhimurium controls enzymic activity. J. Biol. Chem. 2002;277:12175–12181. doi: 10.1074/jbc.M104365200. [DOI] [PubMed] [Google Scholar]
- 16.Payton M., Gifford C., Schartau P., Hagemeier C., Mushtaq A., Lucas S., Pinter K., Sim E. Evidence towards the role of arylamine N-acetyltransferase in Mycobacterium smegmatis and development of a specific antiserum against the homologous enzyme of Mycobacterium tuberculosis. Microbiology. 2001;147:3295–3302. doi: 10.1099/00221287-147-12-3295. [DOI] [PubMed] [Google Scholar]
- 17.Brooke E. W., Davies S. G., Mulvaney A. W., Pompeo F., Sim E., Vickers R. J. An approach to identifying novel substrates of bacterial arylamine N-acetyltransferases. Bioorg. Med. Chem. 2003;11:1227–1234. doi: 10.1016/s0968-0896(02)00642-9. [DOI] [PubMed] [Google Scholar]
- 18.Upton A. M., Mushtaq A., Victor T. C., Sampson S. L., Sandy J., Smith D. M., van Helden P. V., Sim E. Arylamine N-acetyltransferase of Mycobacterium tuberculosis is a polymorphic enzyme and a site of isoniazid metabolism. Mol. Microbiol. 2001;42:309–317. doi: 10.1046/j.1365-2958.2001.02648.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhakta S., Besra G. S., Upton A. M., Parish T., Sholto-Douglas-Vernon C., Gibson K. J. C., Knutton S., Gordon S., daSilva R. P., Anderton M. C., Sim E. Arylamine N-acetyltransferase is required for synthesis of mycolic acids and complex lipids in Mycobacterium bovis BCG and represents a novel drug target. J. Exp. Med. 2004;199:1191–1199. doi: 10.1084/jem.20031956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payton M., Mushtaq A., Yu T. W., Wu L. J., Sinclair J., Sim E. Eubacterial arylamine N-acetyltransferases-identification and comparison of 18 members of the protein family with conserved active site cysteine, histidine and aspartate residues. Microbiology. 2001;147:1137–1147. doi: 10.1099/00221287-147-5-1137. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert J. P., Gomollon F., Mate J., Pajares J. M. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systematic review. Dig. Dis. Sci. 2002;47:471–488. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 22.van Hogezand R. A., Kennis H. M., van Schaik A., Koopman J. P., van Hees P. A., van Tongeren J. H. Bacterial acetylation of 5-aminosalicylic acid in faecal suspensions cultured under aerobic and anaerobic conditions. Eur. J. Clin. Pharmacol. 1992;43:189–192. doi: 10.1007/BF01740669. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning. A Laboratory Manual. [Google Scholar]
- 24.Cornish-Bowden A. London: Portland Press; 1995. Fundamentals of Enzyme Kinetics. [Google Scholar]
- 25.Coroneos E., Gordon J. W., Kelly S. L., Wang P. D., Sim E. Drug metabolizing N-acetyltransferase activity in human cell lines. Biochim. Biophys. Acta. 1991;1073:593–599. doi: 10.1016/0304-4165(91)90235-9. [DOI] [PubMed] [Google Scholar]
- 26.McPherson A. Crystallization of macromolecules: general principles. Methods Enzymol. 1985;114:112–120. doi: 10.1016/0076-6879(85)14007-3. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative Computational Project. The CCP4 Suite: programs for protein crystallography (1994) Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 28.Stover C. K., Pham X. Q., Erwin A. L., Mizoguchi S. D., Warrener P., Hickey M. J., Brinkman F. S., Hufnagle W. O., Kowalik D. J., Lagrou M., et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature (London) 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 29.Weber W. W., Vatsis K. P. Individual variability in p-aminobenzoic acid N-acetylation by human N-acetyltransferase (NAT1) of peripheral blood. Pharmacogenetics. 1993;3:209–212. doi: 10.1097/00008571-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Minchin R. F. Acetylation of p-aminobenzoylglutamate, a folic acid catabolite, by recombinant human arylamine N-acetyltransferase and U937 cells. Biochem. J. 1995;307:1–3. doi: 10.1042/bj3070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgoda R., Lian L. Y., Sandy J., Sim E. NMR investigation of the catalytic mechanism of arylamine N-acetyltransferase from Salmonella typhimurium. Biochim. Biophys. Acta. 2003;1620:8–14. doi: 10.1016/s0304-4165(02)00500-7. [DOI] [PubMed] [Google Scholar]
- 32.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 33.Brunger T., Adams P. D., Clore G. M., Delano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J.-S., Kuszewski J., Nilges M., Panu N. S., et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 34.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 35.Pompeo F., Mushtaq A., Sim E. Expression and purification of the rifamycin amide synthase, RifF, an enzyme homologous to the prokaryotic arylamine N-acetyltransferases. Protein Expression Purif. 2002;24:138–151. doi: 10.1006/prep.2001.1550. [DOI] [PubMed] [Google Scholar]
- 36.Kawamura A., Sandy J., Upton A., Noble M., Sim E. Structural investigation of mutant Mycobacterium smegmatis arylamine N-acetyltransferase: a model for a naturally occurring functional polymorphism in Mycobacterium tuberculosis arylamine N-acetyltransferase. Protein Expression Purif. 2003;27:75–84. doi: 10.1016/s1046-5928(02)00592-2. [DOI] [PubMed] [Google Scholar]
- 37.Butcher N. J., Boukouvala S., Sim E., Minchin R. F. Pharmacogenetics of the arylamine N-acetyltransferases. Pharmacogenomics J. 2002;2:30–42. doi: 10.1038/sj.tpj.6500053. [DOI] [PubMed] [Google Scholar]
- 38.Brooke E. W., Davies S. G., Mulvaney A. W., Okada M., Pompeo F., Sim E., Vickers R. J., Westwood I. M. Synthesis and in vitro evaluation of novel small molecule inhibitors of bacterial arylamine N-acetyltransferases (NATs) Bioorg. Med. Chem. Lett. 2003;13:2527–2530. doi: 10.1016/s0960-894x(03)00484-0. [DOI] [PubMed] [Google Scholar]
- 39.Ireland A., Priddle J., Jewell D. Comparison of 5-aminosalicylic acid and N-acetylaminosalicylic acid uptake by the isolated human colonic epithelial cell. Gut. 1992;33:1343–1347. doi: 10.1136/gut.33.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamura A., Sim E. Eukaryotic arylamine N-acetyltransferase: Investigation of substrate specificity by high-throughput screening. Biochem. Pharmacol. 2004 doi: 10.1016/j.bcp.2004.09.014. in the press. [DOI] [PubMed] [Google Scholar]
- 41.Buchman A. L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001;33:289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Sim E., Gill E. W., Sim R. B. Drugs that induce systemic lupus erythematosus inhibit complement component C4. Lancet. 1984;ii:422–424. doi: 10.1016/s0140-6736(84)92905-2. [DOI] [PubMed] [Google Scholar]
- 43.Poole K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 44.Pirnay J. P., De Vos D., Cochez C., Bilocq F., Pirson J., Struelens M., Duinslaeger L., Cornelis P., Zizi M., Vanderkelen A. Molecular epidemiology of Pseudomonas aeruginosa colonization in a burn unit: persistence of a multidrug-resistant clone and a silver sulfadiazine-resistant clone. J. Clin. Microbiol. 2003;41:1192–1202. doi: 10.1128/JCM.41.3.1192-1202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kèohler T., Kok M., Michea-Hamzehpour M., Plesiat P., Gotoh N., Nishino T., Curty L. K., Pechere J. C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1996;40:2288–2290. doi: 10.1128/aac.40.10.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carsenti-Etesse H., Cavallo J. D., Roger P. M., Ziha-Zarifi I., Plesiat P., Garrabe E., Dellamonica P. Effect of beta-lactam antibiotics on the in vitro development of resistance in Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2001;7:144–151. doi: 10.1046/j.1469-0691.2001.00225.x. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg E. P. Bacterial genomics. Pump up the versatility. Nature (London) 2000;406:947–948. doi: 10.1038/35023203. [DOI] [PubMed] [Google Scholar]
- 48.Whiteley M., Bangera M. G., Bumgarner R. E., Parsek M. R., Teitzel G. M., Lory S., Greenberg E. P. Gene expression in Pseudomonas aeruginosa biofilms. Nature (London) 2001;413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 49.Iwai K., Okinaka O. Radioassay for dihydropteroate-synthesizing enzyme activity. Methods Enzymol. 1980;66:560–564. doi: 10.1016/0076-6879(80)66507-0. [DOI] [PubMed] [Google Scholar]
- 50.Goodford P. J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 1985;28:849–857. doi: 10.1021/jm00145a002. [DOI] [PubMed] [Google Scholar]
- 51.Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gouet P., Courcelle E., Stuart D. I., Metoz F. ESPript: multiple sequence alignment in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.