Abstract
GPCRs (G-protein-coupled receptors) play an extremely important role in transducing extracellular signals across the cell membrane with high specificity and sensitivity. They are central to many of the body's endocrine and neurotransmitter pathways, and are consequently a major drug target. It is now clear that GPCRs interact with a range of proteins, including other GPCRs. Identifying and elucidating the function of such interactions will significantly enhance our understanding of cellular function, with the promise of new and improved pharmaceuticals. Biophysical techniques involving resonance energy transfer, namely FRET (fluorescence resonance energy transfer) and BRET (bioluminescence resonance energy transfer), now enable us to monitor the formation of dynamic GPCR–protein complexes in living cells, in real time. Their use has firmly established the concept of GPCR oligomerization, as well as demonstrating GPCR interactions with GPCR kinases, β-arrestins, adenylate cyclase and a subunit of an inwardly rectifying K+ channel. The present review examines recent technological advances and experimental applications of FRET and BRET, discussing particularly how they have been adapted to extract an ever-increasing amount of information about the nature, specificity, stoichiometry, kinetics and agonist-dependency of GPCR–protein interactions.
Keywords: arrestin, bioluminescence resonance energy transfer (BRET), fluorescence resonance energy transfer (FRET), G-protein-coupled receptor (GPCR), oligomerization
Abbreviations: AR, adrenergic receptor; BRET, bioluminescence resonance energy transfer; BRET1, original BRET methodology using coelenterazine h as the substrate for Renilla luciferase; BRET2, modified BRET methodology using DeepBlueC™ coelenterazine as the substrate for Renilla luciferase; CFP, cyan fluorescent protein; CHO, Chinese-hamster ovary; DAMGO, [D-Ala2-MePhe4-Gly(ol)5]enkephalin; D2R, dopamine D2 receptor; ER, endoplasmic reticulum; ET, endothelin; FRET, fluorescence resonance energy transfer; GABAB1R, γ-aminobutyric acid B1 receptor; GABAB2R, γ-aminobutyric acid B2 receptor; GFP, green fluorescent protein; GnRHR, gonadotropin-releasing hormone receptor; GPCR, G-protein-coupled receptor; GRK, GPCR kinase; HA, haemagglutinin; Kir channel, inwardly rectifying K+ channel; LH, luteinizing hormone; MTR, melatonin receptor; pbFRET, photobleaching FRET; PTHR, parathyroid hormone receptor; RET, resonance energy transfer; Rluc, Renilla luciferase; SSTR, somatostatin receptor; TM, transmembrane helix; TRHR, thyrotropin-releasing hormone receptor; YFP, yellow fluorescent protein
INTRODUCTION
A great deal of information is accumulating regarding the molecular properties, signalling pathways and functional regulation of GPCRs (G-protein-coupled receptors). These integral membrane proteins, characterized by a single polypeptide chain with seven TMs (transmembrane helices), represent the largest and most versatile of all the receptor families, with an essential role in the regulation of almost all physiological processes in both mammalian and non-mammalian species.
In addition to binding ligands and G-proteins, GPCRs interact with a diverse range of proteins that have potential roles in trafficking, signalling, desensitization, internalization and recycling. These include other GPCRs, GRKs (GPCR kinases), second-messenger-dependent kinases (such as protein kinases A and C), arrestins (particularly β-arrestins), a range of molecular chaperones that aid protein folding and transport to the plasma membrane, RAMPs (receptor-activity-modifying proteins) and PDZ-domain-containing proteins [1]. The potential importance of such protein interactions to GPCR function has only recently been realized, resulting in an explosion of interest in techniques for monitoring the formation of dynamic GPCR–protein complexes in living cells.
The present review focuses on the biophysical techniques of FRET (fluorescence resonance energy transfer) and BRET (bioluminescence resonance energy transfer). These techniques are highly sensitive and enable protein–protein interactions to be analysed in real time, in living cells [2]. Their use is currently revolutionizing academic GPCR research, and their potential for high-throughput pharmaceutical screening should not be underestimated [3–6].
RET (RESONANCE ENERGY TRANSFER)
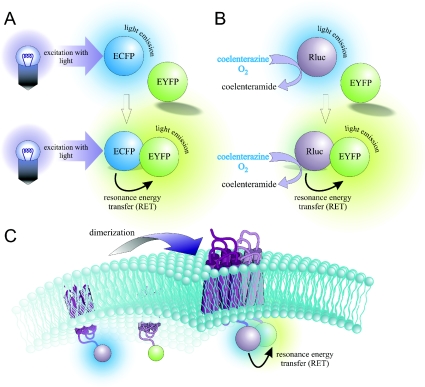
Energy transferred from a donor molecule to an acceptor molecule in a non-radiative manner as a result of dipole–dipole coupling is referred to as RET [7]. If the donor is a fluorescent molecule, exposure to light of a characteristic wavelength will result in excitation. Subsequent energy transfer to a fluorescent acceptor molecule is then referred to as FRET (Figure 1A). Alternatively, the donor molecule can be an enzyme, Rluc (Renilla luciferase), which causes energy to be released upon oxidization of a suitable substrate, namely coelenterazine. Resultant energy transfer to a fluorescent acceptor molecule is then referred to as BRET (Figure 1B). Both of these techniques are eminently suitable for investigating interactions involving GPCRs (Figure 1C).
Figure 1. Resonance energy transfer.
RET occurs between donor and acceptor molecules if they are in close proximity (less than 100 Å), resulting in energy emission from the acceptor at a characteristic wavelength. FRET involves excitation of a donor fluorophore with light (A), whereas BRET occurs when the donor Rluc catalyses the oxidation of coelenterazine to coelenteramide (B). Both of these techniques are particularly suitable for investigating GPCR–protein complexes in living cells, including oligomerization (C). Illustration created by Uli Schmidt (http://www.scigraphico.com.au/).
RET efficiency is dependent upon a number of factors, not least the spectral properties, relative distance and relative orientation of the donor and acceptor molecules that are involved [8–11]. The donor emission spectrum needs to overlap the acceptor excitation spectrum significantly. However, if the donor and acceptor emission spectra overlap significantly, then spectral resolution is lost, resulting in a low signal-to-noise ratio. RET efficiency is believed to be inversely proportional to the distance between donor and acceptor molecules by the sixth power [8]. This very high proximity-dependence means that, for interactions between proteins in living cells, significant energy transfer implies that donor and acceptor molecules are within 100 Å (1 Å=0.1 nm) of each other [9], a distance indicative of direct interaction. The relative orientation of donor and acceptor dipoles is critical. As a result, donor and acceptor molecules usually require significant freedom of movement, so that their relative orientation is favourable for at least part of the time. However, fusion with proteins, such as GPCRs, is likely to restrict this freedom, which may or may not be detrimental to RET efficiency [10]. This factor must be taken into account when interpreting results produced using both FRET and BRET techniques.
Both FRET and BRET can be detected by microscopy, scanning spectroscopy or a suitable plate reader that is capable of sequential or simultaneous detection of filtered light emitted within two distinct wavelength windows. Microscopy is often used for detecting FRET, being most suitable for studies involving photobleaching [7]. In contrast, microscopy is rarely used to detect BRET, with the majority of studies utilizing plate-reading instrumentation [11].
FRET
Fluorophore combinations
A number of variants of GFP (green fluorescent protein), fused to the proteins of interest, have now been used in FRET studies. The selection of a particular combination is dependent upon the compatibility of their excitation and emission spectra, as discussed above. Combinations used in the study of GPCR–protein complexes using FRET are shown in Table 1, and peak excitation and emission wavelengths of fluorophores can be obtained from the Zeiss Corporation website (http://www.zeiss.com). Usually the fusion constructs are co-expressed in the same cell; however, it is also possible to express them in separate cells that are subsequently fused [12].
Table 1. Combinations of fluorophores for studying GPCR–protein complexes using FRET.
Cy3, indocarbocyanine; RFP/DsRed, red fluorescent protein; TrITC, tetramethylrhodamine isothiocyanate.
| Donor | Acceptor | References | |
|---|---|---|---|
| Fusion proteins | CFP* | GFP* | [61] |
| CFP* | YFP* | [27,33,34,44,50,60,61,69,95] | |
| GFP* | RFP/DsRed | [26,69] | |
| GFP* | YFP* | [16,53,73,94] | |
| YFP* | RFP | [12] | |
| Conjugated to antibodies | Europium chelate | Allophycocyanin | [16,20–22] |
| Europium cryptate | Alexa Fluor 647 | [17,18] | |
| FITC/fluorescein | Rhodamine | [13–15,19] | |
| Fusion protein and conjugated to antibody | GFP* | Cy3 | [23] |
| Conjugated to ligands | FITC | Texas Red | [15] |
| FITC | TrITC | [24] |
* Note that GFP variants may or may not be ‘enhanced’ to improve expression, cellular solubility and/or stability, such as EGFP/GFP2, ECFP and EYFP. For simplicity, these proteins are referred to as GFP, CFP and YFP respectively, as commonly occurs in the FRET literature. Data on excitation and emission peaks can be obtained from the Zeiss Corporation website (http://www.zeiss.com).
An alternative to fusion proteins is the use of fluorophores conjugated to antibodies (Table 1). These are often against HA (haemagglutinin)-tagged proteins [13–19], although several studies have utilized anti-c-Myc and/or anti-FLAG™ antibodies [16–18,20–22]. Furthermore, receptor-specific polyclonal antibodies can be used in combination with fluorophore-conjugated secondary antibodies [14,15]. Indeed, this is a potential advantage of FRET over BRET, as, provided suitable antibodies are available, endogenously expressed protein can be studied [18], thereby avoiding the problems of exogenous expression. Fluorophores fused to proteins can be used in combination with fluorophore-conjugated antibodies against epitope-tagged receptors, an example being the use of a receptor–GFP fusion protein in combination with a Cy3 (indocarbocyanine)-conjugated anti-Myc antibody to Myc-tagged receptors [23].
Fluorophore-conjugated ligands
Although almost all FRET/BRET studies of GPCRs involve detection of tagged receptors, it is also possible to demonstrate oligomerization by using ligands conjugated to fluorophores [15,24] (Table 1). Obviously, this approach precludes the study of constitutive oligomerization, as ligand binding is a prerequisite of the detection process. However, it does have the potential for studying endogenous GPCR interactions without the need for receptor antibodies. Significantly higher FRET was observed between hCG (human chorionic gonadotropin)-occupied LH (luteinizing hormone) receptors than between LH-occupied LH receptors [24]. This may represent differences in the ability of particular agonists to induce oligomerization; however, it may also result from differences in the orientations of the fluorophores due to the differences in agonist structure. Significant FRET was observed between somatostatin molecules conjugated to FITC and Texas Red when added at low concentrations to cells containing SSTR (somatostatin receptor) 1, or both SSTR1 and SSTR5, indicating that these homo- and hetero-oligomers bind more than one ligand molecule [15].
pbFRET (photobleaching FRET)
FRET intensity can be measured directly; however, if standard fluorophores are used, substantial correction is required to overcome problems of direct acceptor excitation, contaminating donor fluorescence and varying levels of fluorophores in different samples [2,25]. Consequently, alternative methods are now commonly used, which use photobleaching to demonstrate FRET indirectly. Photobleaching of a fluorophore by prolonged exposure to excitation light results in its irreversible photochemical destruction at a rate comparable with FRET [7]. Donor photobleaching results in a decrease in fluorescence intensity, which is monitored in the presence and absence of an acceptor fluorophore. When an acceptor is in close enough proximity, FRET occurs and competes with the photobleaching process [13]. This competition is measured as an increase in the photobleaching time constant, which is particularly advantageous as it is independent of absolute signals [7].
An alternative to donor pbFRET is acceptor pbFRET. Donor and acceptor emissions are measured before and after acceptor photobleaching. An increase in donor fluorescence after acceptor destruction provides evidence that FRET was occurring between donor and acceptor molecules [26,27]. Examples of studies using both forms of pbFRET to study GPCR oligomerization are shown in Table 2.
Table 2. Studies of GPCR oligomerization using FRET.
ND, not determined.
| GPCRs | Constitutive signal? | Change in signal with agonist | Methods* | Reference |
|---|---|---|---|---|
| Homo-oligomerization | ||||
| Adenosine A2A receptor | Yes | ND | FRET, time-resolved FRET | [16] |
| α1a-AR | Yes | ND | FRET (scanning spectroscopy) | [61] |
| α1b-AR | Yes | No effect | FRET (scanning spectroscopy), competition, FRET between receptor fragments/chimaeras | [61] |
| Yes | ND | Time-resolved FRET | [21] | |
| Chemokine receptor CXCR4 | Yes | Increase | FRET microscopy, time-course, competition | [33] |
| Complement C5a receptor | Yes | No effect | FRET (scanning spectroscopy), time-course | [34] |
| D2R | Yes | Increase | FRET, dose–response, competition | [60] |
| α-Factor receptor | Yes | Increase | FRET (scanning spectroscopy), competition | [95] |
| Yes | ND | FRET (scanning spectroscopy), competition, FRET between receptor fragments | [50] | |
| GABAB1R | Yes | ND | Time-resolved FRET | [18] |
| GABAB2R | Yes | ND | Time-resolved FRET | [18] |
| GnRHR | No | Increase | Acceptor pbFRET microscopy, time-course | [26] |
| No | Increase | Donor pbFRET microscopy, dose–response | [73] | |
| ND | Increase | FRET microscopy, time-course | [94] | |
| H1 histamine receptor | Yes | ND | Time-resolved FRET | [21] |
| Yes | ND | Time-resolved FRET | [22] | |
| LH receptor | ND | Significant FRET observed | Donor pbFRET microscopy with labelled agonists | [24] |
| Neuropeptide Y1 receptor | Yes | No effect | FRET microscopy, FRET (scanning spectroscopy) | [69] |
| Neuropeptide Y2 receptor | Yes | No effect | FRET microscopy, FRET (scanning spectroscopy) | [69] |
| Neuropeptide Y5 receptor | Yes | No effect | FRET microscopy, acceptor pbFRET microscopy, FRET (scanning spectroscopy) | [69] |
| δ-Opioid receptor | Yes | No effect | Time-resolved FRET | [20] |
| SSTR2 | Yes | Decrease | Donor pbFRET microscopy, dose–response | [19] |
| SSTR5 | No | Increase | Donor pbFRET microscopy, dose–response | [13] |
| ND | Significant FRET observed | FRET microscopy with labelled agonists | [15] | |
| Thyrotropin (thyroid-stimulating hormone) receptor | Yes | ND | FRET microscopy | [12] |
| Yes | Decrease | FRET microscopy, acceptor pbFRET microscopy, dose–response | [23] | |
| Hetero-oligomerization | ||||
| Adenosine A2A receptor and D2R | Yes | ND | FRET, acceptor pbFRET microscopy | [53] |
| α1a and α1b-AR | Yes | ND | FRET (scanning spectroscopy) | [61] |
| α1b-AR and H1 histamine receptor | Yes | ND | Time-resolved FRET | [21] |
| D2R and SSTR5 | No | Increase | Donor pbFRET microscopy | [14] |
| Endothelin ETA and ETB receptors | Yes | No immediate effect, decrease after 30 min with ETB agonist | Acceptor pbFRET microscopy, competition, time-course | [27] |
| GABAB1R and GABAB2R | Yes | No effect (results not shown) | Time-resolved FRET, time-course | [18] |
| Yes | ND | Time-resolved FRET | [17] | |
| SSTR1 and SSTR5 | Small | Increase | Donor pbFRET microscopy, dose–response, FRET microscopy with labelled agonists | [15] |
* Unless stated otherwise, FRET is detected in a fluorimetric microplate reader by measuring filtered light emitted in two distinct wavelength windows.
Time-resolved FRET
Time-resolved FRET is yet another variation designed to overcome the problems of direct FRET measurement. Its success is due to the prolonged fluorescence characteristics of certain lanthanide compounds (such as europium compounds), which enable the processes of excitation and detection to be separated temporally [28]. Measurements are generally taken after a delay of 50 μs [16,20–22], by which time acceptor fluorescence should result from FRET, as opposed to direct excitation [28]. The time-resolved FRET methodology usually involves washing to remove free antibodies [16,20–22]; however, Maurel et al. [18] have demonstrated a homogeneous assay using europium-cryptate-labelled antibodies that dispenses with the need for a wash step. This makes the assay much more applicable to high-throughput screening. Again, examples of studies utilizing time-resolved FRET are shown in Table 2.
BRET
BRET involves the use of fusion proteins, with Rluc as the donor and a GFP variant as the acceptor [11] (BRET between firefly luciferase and DsRed has been demonstrated with purified proteins [29], but this combination has yet to be used to study GPCRs). The original BRET procedure, referred to in this review as BRET1, was pioneered by Xu et al. [30]. The Rluc substrate is coelenterazine h that, upon being oxidized to coelenteramide h, results in light emission with a peak wavelength of approx. 480 nm. Transfer of energy to YFP (yellow fluorescent protein) then results in energy emission peaking at approx. 530 nm. In contrast, BRET2 utilizes a modified form of coelenterazine called DeepBlueC™ (Packard Biosciences), oxidation of which results in energy emission peaking at approx. 400 nm. The acceptor for this method is usually GFP, emitting energy peaking at about 510 nm. The major advantage of BRET2 over BRET1 is that the increased separation of donor and acceptor emission spectra provides greater signal resolution [31]. However, possible disadvantages that may affect sensitivity are the reduced overlap of excitation spectra and the reduced quantum yield of DeepBlueC™ compared with coelenterazine h [32]. Examples of studies using both BRET1 and BRET2 to study GPCR oligomerization are shown in Table 3.
Table 3. Studies of GPCR oligomerization using BRET.
ND, not determined; NK1 receptor, neurokinin 1 (substance P) receptor.
| GPCRs | Constitutive signal? | Change in signal with agonist | Methods* | Reference |
|---|---|---|---|---|
| Homo-oligomerization | ||||
| Adenosine A1 receptor | Yes | No effect | BRET2 | [36] |
| Adenosine A2A receptor | Yes | No effect (results not shown) | BRET2 | [96] |
| Yes | No effect | BRET1, saturation curves | [16] | |
| β1-AR | Yes | No effect (results not shown) | BRET2 | [76] |
| Yes | No effect (results not shown) | BRET2, saturation curves | [32] | |
| β2-AR | Yes | Increase | BRET1 (scanning spectroscopy and plate reading), dose–response | [74] |
| Yes | ND | BRET1 (scanning spectroscopy) | [20] | |
| Yes | Increase | BRET1 (scanning spectroscopy) | [37] | |
| Yes | No effect (results not shown) | BRET2 | [76] | |
| Yes | No effect (results not shown) | BRET2, saturation curves | [32] | |
| Yes | ND | BRET1, single-cell BRET1 microscopy | [49] | |
| Yes | ND | BRET2 | [79] | |
| Yes | ND | BRET1 (scanning spectroscopy), competition | [64] | |
| Yes | ND | BRET2, saturation curves | [57] | |
| β3-AR | Yes | ND | BRET2 | [57] |
| Angiotensin AT1 receptor | Yes | No effect | BRET2, competition | [65] |
| Calcium-sensing receptor | Yes | No effect | BRET2 (scanning spectroscopy and plate reading), competition | [63] |
| Chemokine receptor CCR5 | Yes | No effect | BRET1, competition | [51] |
| Chemokine receptor CXCR4 | Yes | ND | BRET1 | [51] |
| Yes | Minimal increase | BRET2 | [92] | |
| Cholecystokinin CCKA receptor | Yes | Decrease | BRET1 (scanning spectroscopy), time-course, competition, dose–response | [37] |
| Yes | ND | BRET1 (scanning spectroscopy), competition | [64] | |
| Cholecystokinin CCKB receptor | Yes | No effect | BRET1 (scanning spectroscopy), competition | [64] |
| GnRHR | No (see text) | Increase | BRET1 | [35] |
| MTR1 | Yes | No effect | BRET1, competition curves, single-cell BRET1 microscopy | [49] |
| Yes | ND | BRET1, saturation curves | [56] | |
| MTR2 | Yes | Increase | BRET1, competition curves, single-cell BRET1 microscopy | [49] |
| Yes | Increase | BRET1 | [52] | |
| Yes | Generally increase | BRET1, saturation curves, dose–response | [56] | |
| Neuropeptide Y4 receptor | Yes | Decrease | BRET2, time-course, dose–response | [38] |
| δ-Opioid receptor | Yes | No effect | BRET1 (scanning spectroscopy) | [20] |
| Yes | ND | BRET1 (scanning spectroscopy) | [68] | |
| Yes | No effect | BRET2 (scanning spectroscopy) | [31] | |
| κ-Opioid receptor | Yes | No effect | BRET1 (scanning spectroscopy) | [31] |
| μ-Opioid receptor | Yes | ND | BRET1 (scanning spectroscopy) | [68] |
| Yes | ND | BRET2 | [97] | |
| Oxytocin receptor | Yes | No effect | BRET1, competition, saturation curves | [52] |
| Yes | No effect | BRET1 | [93] | |
| Yes | ND | BRET1, competition | [66] | |
| TRHR1 | Yes | Increase | BRET1, time-course, dose–response, competition | [35] |
| Yes | Increase | BRET1 | [62] | |
| TRHR2 | Yes | Increase | BRET1 | [62] |
| Vasopressin V1a receptor | Yes | No effect | BRET1, competition, saturation curves | [52] |
| Vasopressin V2 receptor | Yes | No effect | BRET1, competition, saturation curves | [52] |
| Hetero-oligomerization | ||||
| Adenosine A1 and purinergic P2Y1 receptors | Yes | Increase | BRET2, time-course | [36] |
| Adenosine A2A receptor and D2R | Yes | No effect (results not shown) | BRET2 | [96] |
| Yes | No effect | BRET1, competition with a chimaeric receptor, saturation curves | [53] | |
| Yes | ND | BRET1 | [16] | |
| β1-AR and β2-AR | Yes | No effect (results not shown) | BRET2 | [76] |
| Yes | No effect (results not shown) | BRET2, saturation curves | [32] | |
| β2-AR and β3-AR | Yes | ND | BRET2, saturation curves | [57] |
| β2-AR and δ-opioid receptor | Small | Increase | BRET1 (scanning spectroscopy) | [20] |
| Cholecystokinin CCKA and CCKB receptors | Yes | No effect | BRET1 (scanning spectroscopy), compitition | [64] |
| GABAB1R and GABAB2R | Yes | ND | BRET1, competition | [52] |
| MTR1 and MTR2 | Yes | Increase depends on tag configuration | BRET1 | [49] |
| Yes | Generally increase for MTR1–Rluc/MTR2–YFP | BRET1, saturation curves, dose–response | [56] | |
| δ- and κ-opioid receptors | Yes | ND | BRET1 (scanning spectroscopy) | [68] |
| Yes | ND | BRET1 (scanning spectroscopy) | [31] | |
| μ-Opioid and NK1 receptors | Yes | No effect (results not shown) | BRET2 | [97] |
| Oxytocin and vasopressin V1a receptors | Yes | No effect | BRET1, competition, saturation curves | [52] |
| Yes | ND | BRET1, competition | [66] | |
| Oxytocin and vasopressin V2 receptors | Yes | No effect | BRET1, competition, saturation curves | [52] |
| Yes | ND | BRET1, competition | [66] | |
| TRHR1 and TRHR2 | Yes | Increase | BRET1, competition | [62] |
| Vasopressin V1a and V2 receptors | Yes | No effect | BRET1, competition, saturation curves | [52] |
* BRET is generally detected in a microplate reader by measuring filtered light emitted in two distinct wavelength windows.
TEMPORAL AND SPATIAL INFORMATION OBTAINED USING RET
FRET and BRET kinetics
FRET has been evaluated over time using both microscopy [26,27,33] and scanning spectroscopy [34]. Such studies can reveal interesting information as to the stability of interactions. For example, no decrease in FRET between tagged endothelin ETA and ETB receptors was observed after 5 min of agonist treatment [27]. However, after 30 min with an ETB-receptor-specific agonist, there was a significant reduction in FRET that was dependent upon endocytosis (discussed below).
BRET time-courses have been produced by incubating with modulators for set periods of time before commencing the BRET assay. The agonist-induced increase in BRET between TRHRs (thyrotropin-releasing hormone receptors) was found to be time-dependent, reaching a maximum after approx. 20 min [35]. Likewise, agonist-induced BRET between adenosine A1 receptors and D2Rs (dopamine D2 receptors) was maximal after approx. 10 min [36]. In contrast, BRET between cholecystokinin CCKA receptors was found to decrease following agonist treatment, with a significant reduction after only 2 min [37]. Such studies can provide useful information; however, as readings are only taken at certain time points, crucial kinetic information may be missed. For example, the agonist-induced decrease in BRET between neuropeptide Y4 receptors was investigated for up to 180 min, but was already maximal at the first time point of 15 min [38].
BRET kinetic profiles have been produced for the interaction between neuropeptide Y receptors and the intracellular adaptor protein, β-arrestin 2 [39]. These profiles were again generated by pre-incubating with agonist. An alternative to pre-incubation is to take readings in real time. This has been done over short time periods to investigate agonist-induced interactions between the oxytocin receptor and both GRK2 and β-arrestin 2 (discussed below), using coelenterazine h and instrumentation capable of injecting agonist [40]. Coelenterazine h is not stable over time, therefore Hasbi et al. [40] plotted time points representing the means of ten or 40 consecutive measurements taken at 0.4–0.5 s intervals. The assay time span was also restricted to 10 min. Until recently, a stable Renilla luciferase substrate has not been available, preventing prolonged real-time kinetic profiles being produced using BRET. Following the introduction of EnduRen™ (Promega), combined with improved kinetics software for BRET instrumentation, such real-time profiles can now be produced over several hours. EnduRen™ is a protected form of coelenterazine h that is metabolized to the free substrate within the cell by endogenous esterases. Consequently, extracellular substrate degradation and autoluminescence is decreased substantially, resulting in stable luminescence over many hours. This is in stark contrast with unprotected coelenterazine h, which degrades rapidly [41]. Therefore the potential for investigating prolonged real-time kinetics of interactions involving GPCRs is enormous [42,43].
RET can be used to investigate the kinetics of conformational changes within GPCRs by incorporating donor and acceptor molecules into different regions of the same receptor molecule. FRET has been used to measure such changes upon agonist binding by incorporating CFP (cyan fluorescent protein) and YFP into the third intracellular loop and C-terminal tail respectively [44]. Agonist treatment resulted in a rapid decrease in FRET, whereas no change was observed with antagonist treatment. Comparison between the kinetics observed with the α2A-AR (adrenergic receptor) and the PTHR (parathyroid hormone receptor) suggested that the conformational change believed to be involved in receptor activation occurred 25-fold more rapidly for the former, a result in keeping with the physiological roles of these receptors [44].
Subcellular localization
The FRET technology can be used to identify the subcellular location of protein–protein interactions in living cells. For example, time-resolved FRET using N-terminal c-Myc- and FLAG™-tagged GPCRs, with membrane-impermeant Eu3+-labelled anti-c-Myc and allophycocyanin-labelled anti-FLAG™ antibodies, has shown oligomerization occurring at the cell surface [20–22]. Similar results were obtained using the combination of N-terminal FLAG™- and HA-tagged receptors [16]. By monitoring only receptors that are incorporated into the plasma membrane, this method also avoids the problem of detecting interactions between immature or incompletely processed receptors.
GABAB1R (γ-aminobutyric acid B1 receptor) binds ligands with considerably higher affinity than GABAB2R (γ-aminobutyric acid B2 receptor), but is not expressed on the cell surface, unless it forms a heterodimer with GABAB2R [45–47]. The interaction appears to mask an ER (endoplasmic reticulum) retention signal in the C-terminal tail, enabling the heterodimer to be translocated to the plasma membrane [48]. Using membrane-impermeant europium-cryptate-labelled anti-HA and Alexa-Fluor-647-labelled anti-Myc antibodies, Maurel et al. [18] showed that the time-resolved FRET signal between these receptor subtypes was directly proportional to the amount of GABAB1R at the cell surface, as measured by a radioligand binding assay [18]. Such a relationship validates this technique further for investigating cell-surface oligomerization. Furthermore, by using a mutant GABAB1R lacking the ER retention signal, these receptors were shown to homo-oligomerize on the cell surface [18]. Such mutant receptors are still not functional [48]; however, this does not appear to result from an inability to form homo-oligomers in the plasma membrane [18].
In contrast with FRET, BRET technology has so far not been successfully adapted for subcellular localization of protein–protein interactions in living cells; although, in studies where significant changes in BRET are observed following agonist treatment of GPCRs, it would appear that a significant proportion of BRET-tagged receptors are present at the plasma membrane in order to interact with the membrane-impermeant ligand. The use of single-cell BRET has been reported; however, the BRET signal was recorded from the entire cell, rather than from distinct subcellular locations [49].
FRET and BRET analysis has been carried out on cell fractions. Evidence for significant homo-oligomerization occurring in both plasma-membrane- and ER-enriched fractions has been provided for α-factor receptors [50] and complement C5a receptors [34] using the FRET technology. Furthermore, BRET has been detected in both fractions between tagged chemokine CCR5 receptors [51], and between each combination of tagged oxytocin, and vasopressin V1a and V2 receptors [52]. This provides evidence that both homo- and hetero-oligomerization can occur soon after synthesis in the ER. Therefore the phenomenon may have an important role in receptor trafficking, as has been demonstrated for GABAB1R/GABAB2R heterodimerization [45–48]. GPCR interactions in the ER resulting in a FRET/BRET signal support the theory that the majority of GPCR oligomers are constitutive, and that signal modulation caused by ligand binding is often likely to be due to changes in the relative distance and/or orientation of the FRET/BRET donor and acceptor molecules.
Estimation of distances
FRET can be used to estimate distance on a nanometre scale provided that other variables, such as relative donor/acceptor orientation, are taken into account. Such calculations also tend to assume that interactions are dimeric rather than oligomeric in nature [53]. Even so, useful information can be provided, for example, the smaller RET between two fluorescently labelled ligand molecules binding to the SSTR1/5 heterodimer, compared with the same two ligand molecules binding to the SSTR5 homodimer, implies that the homodimer is a more compact structure [15].
GPCR–GPCR INTERACTIONS
Within the field of GPCR research, the most extensive use of the biophysical techniques FRET and BRET has been in the study of GPCR–GPCR interactions (oligomerization), either between the same receptor subtype (homo-oligomerization) or between different receptor subtypes (hetero-oligomerization). Studies using biochemical techniques had previously provided evidence for such interactions [54]; however, the technical difficulties associated with such protocols, particularly that of artifactual aggregation, meant that this evidence was often dismissed [55]. The ability of RET techniques to analyse GPCR–GPCR interactions in live cells has finally enabled such criticisms to be answered, particularly as oligomerization is observed at physiologically relevant protein expression levels using FRET [13,14] and BRET [32,49,51,52,56,57].
The list of publications demonstrating GPCR oligomerization using RET techniques is now extensive and expanding rapidly. Studies demonstrating oligomerization using FRET and BRET are summarized in Tables 2 and 3 respectively. These illustrate the range of receptors that are now believed to homo/hetero-oligomerize and the popularity of these techniques to analyse such interactions. It should also be noted that, with some exceptions to be discussed later, the actual number of GPCRs interacting in a particular complex is unclear, and so oligomerization may actually be dimerization. For simplicity, we will use the term oligomerization in the present review, unless there is evidence to the contrary. Now that the concept of GPCR oligomerization is firmly established [58,59], researchers are employing increasingly innovative FRET and BRET methods to gain additional information about the nature and functional significance of these interactions.
FRET and BRET competition assays
RET competition assays have two forms, both of which demonstrate the specificity of the GPCR– GPCR interaction. The first involves the expression of donor- and acceptor-tagged receptors in the presence and absence of a single concentration of untagged receptor (usually excess). Co-expression of untagged receptor that interacts with one or both of the tagged receptors results in a reduction in RET. This is not the case in the presence of untagged receptors that do not interact specifically with either tagged receptor, as demonstrated using FRET [27,50,60,61] and BRET [35,37,52,62–65].
The second form involves expressing constant amounts of the donor- and acceptor-tagged receptors with increasing amounts of untagged receptor, which competes for interaction with the tagged receptors [49,66]. By using an adapted version of the dimer, trimer and tetramer models of energy transfer quenching proposed by Veatch and Stryer [67], and assuming oligomerization occurs randomly between tagged and untagged receptors, such data have provided information with regard to oligomerization states using BRET [49].
BRET saturation assays
BRET saturation assays involve expressing a constant amount of donor-tagged receptor with increasing amounts of acceptor-tagged receptor. Theoretically, the BRET signal should increase with increasing amounts of acceptor until all donor molecules are interacting with acceptor molecules. Therefore a saturation level is achieved, beyond which further increases in acceptor amount do not increase the BRET signal [32]. Indeed, in circumstances where acceptor-tagged receptors interact (homo-oligomerize), further increases will eventually result in a reduction in BRET signal, because acceptor–acceptor interactions will out-compete donor–acceptor interactions [68]. In contrast with the theoretical saturation curves resulting from specific receptor oligomerization, random collisions are predicted to result in a quasi-linear relationship between acceptor concentration and BRET level. Therefore the ability of experimental data to be fitted to theoretical saturation curves provides evidence for the specificity of GPCR–GPCR interactions. In a similar fashion to BRET competition assays, data derived from BRET saturation assays can be used to assess the oligomerization state of receptors [32,56,57], again using a modified form of the Veatch and Stryer model [67]. They can also be used to assess the relative affinity of receptors for other receptors, comparisons being expressed as BRET50 values (concentration of acceptor giving 50% of the maximal BRET level) [32,52,56,57].
Proportion and affinity of receptors existing as oligomers
Various RET studies have now provided evidence for the proportion of receptors in particular oligomerization states. Data from FRET studies imply that neuropeptide Y1 and Y5 receptor homo-oligomerization may be more likely to occur than neuropeptide Y2 receptor homo-oligomerization [69], and the formation of α1b-AR or H1 histamine receptor homo-oligomers may form more efficiently than hetero-oligomers of these receptors [21]. However, comparisons of absolute FRET/BRET signals in this way should be interpreted with caution, as RET signals are dependent upon a range of factors, not least relative protein expression levels and dipole orientation.
MTRs (melatonin receptors) appear to exist primarily as constitutive dimers as determined by BRET competition assays [49]. Data from BRET saturation assays imply that a similar situation occurs with β-ARs, an estimated 80% of which appear to exist in this state [32]. The affinity of β1- and β2-ARs for themselves and for each other appears to be similar, with BRET50 (the concentration of acceptor giving 50% of energy transfer) values for each combination being comparable [32]. β2-ARs also appear to have an affinity for β3-ARs similar to that for themselves [57]. Data from BRET saturation assays indicates that the various combinations of oxytocin, vasopressin V1a and V2 receptors occur with similar propensity [52]; however, data from BRET competition assays implies that oxytocin homo-oligomerization may be favoured over hetero-oligomerization with vasopressin V1a or V2 receptors [66]. The affinity of MTR1 for itself and MTR2 appears to be similar; however, the affinity of MTR2 for itself appears to be 3–4-fold lower [56]. This implies that, should MTR1 and MTR2 be expressed at similar levels in a physiological setting, heterodimerization would be favoured over MTR2 homodimerization.
Insights into the dimerization interface
An untagged chimaeric GnRHR (gonadotropin-releasing hormone receptor) with a TRHR C-terminal tail did not reduce the BRET signal between tagged TRHRs. As a reduction was observed following competition with untagged wild-type TRHR, the implication is that the C-terminal tail is not required for TRHR homo-oligomerization [35]. A similar conclusion was reached for α1b-AR homo-oligomerization, as a FRET signal was still observed between truncated receptor mutants [61]. That study also investigated the roles of both N-linked glycosylation and a putative GXXXG dimerization motif; however, neither of these appeared to influence α1b-AR homo-oligomerization [61].
The differences in FRET observed between various tagged fragments of the α-factor receptor provided evidence that, in this particular receptor, TM1 is required for oligomerization and the neighbouring N-terminal domain and TM2 may facilitate the interaction [50]. A similar investigation using α1b-AR fragments provided strong evidence for TMs 1 and/or 2 playing a significant role in homo-oligomerization [61]. Furthermore, the study of chimaeric receptors in which α1b-AR TMs were replaced with those from the β2-AR (which does not appear to interact significantly with the α1b-AR) indicated that TMs 1 and 7 are important [61]. Taken together, these findings agree with those derived from the α-factor receptor that suggest a major role for TM1, facilitated by neighbouring regions (TMs 1 and 7 are juxtapositioned in the plasma membrane).
A chimaeric D2R has been produced that has TM5, TM6, intracellular loop 3 and extracellular loop 3 replaced by the analogous regions of the D1R (dopamine D1 receptor) [70]. In a BRET competition assay, this untagged chimaeric receptor did not reduce the BRET signal between tagged adenosine A2A receptors and D2Rs, even though a significant reduction was observed by competition with untagged wild-type D2R [53]. This implies that sites required by the D2R for interaction with the adenosine A2A receptor are located within TM5, TM6, intracellular loop 3 and/or extracellular loop 3.
In the GABAB1R–GABAB2R heterodimer, it is apparent that the C-terminal tails interact via coiled-coil α-helices [71,72]. Using time-resolved FRET, evidence has recently been presented for an interaction between the extracellular domains of these receptors [17], implicating an additional dimerization interface. GABAB1R extracellular domains were also shown to homodimerize in the same study [17].
Effect of agonist on the FRET/BRET signal
The majority of GPCR oligomers appear to form constitutively; however, addition of agonist can result in an increase or decrease in FRET/BRET signal (Tables 2 and 3). This has been interpreted as an agonist-induced change in oligomerization state [19,27,36–38], a phenomenon which may well occur with some GPCRs. However, an alternative explanation in the majority of cases may be that alteration of receptor conformation causes the relative distance and/or orientation of the donor and acceptor molecules to change [49,51]. Treatment with agonist, antagonist or inverse agonist increased the BRET signal for the MTR2 homodimer, but did not alter the signal for the MTR1 homodimer [49]. This implies that, if oligomerization is regulated by ligand binding, it is independent of receptor activation. Ligands increased the signal resulting from the MTR1–Rluc–MTR2–YFP combination, but had no effect on the signal resulting from the MTR2–Rluc–MTR1–YFP combination. These data are consistent with changes in distance/orientation of the donor and acceptor molecules in constitutive oligomers.
Dose–response curves have been produced for different ligand types at different receptors using FRET [15,60,73] and BRET [35,37,38,56,74]. Comparison with ligand-binding affinities determined by classical radioligand-binding assays has led to some interesting conclusions for MTRs. The efficiency of a ligand to induce changes in the BRET signal within MTR2–MTR2 dimers appears to correlate with binding affinity; however, this is not the case with the MTR1–MTR2 heterodimer. Indeed, certain ligands seemed to show increased specificity for the heterodimer [56]. Evidence has now been presented for hetero-oligomerization contributing to clinical conditions [75]. Therefore the development of pharmaceuticals that specifically target hetero-oligomers has enormous potential benefits. The oligomerization state of MTRs, as determined by BRET competition assays, was not affected by ligand treatment [49].
A ligand-induced increase in BRET between the adenosine A1 and purine P2Y1 receptors was only observed following treatment with agonists of both receptors in combination, an effect blocked by P2Y1 antagonist [36]. It was suggested that this is consistent with the concept of agonists promoting the formation of hetero-oligomers [36]; however, it can also be explained by activation-induced conformational changes, which, in this case, may need to occur in both receptors to significantly alter the relative distance/orientation of the BRET tags. Intriguingly, agonist did not induce a change in the BRET signal for adenosine A1 receptor homo-oligomers [36]. An agonist-induced increase in BRET signal has been demonstrated for the β2-AR using BRET1 [37,74], but not BRET2 [32,76]. The reason for this is unclear; however, it has been suggested that BRET1 may be more sensitive than BRET2 for detecting small changes in relative distance between BRET tags [32].
The majority of studies investigating the effect of agonist on constitutive oligomerization using FRET/BRET have reported either an increase in signal or no effect (Tables 2 and 3). Exceptions include studies of cholecystokinin receptors [37], neuropeptide Y4 receptors [38] and SSTR2 [19], in which dose-dependent decreases were observed. The reduction in signal with the cholecystokinin receptors was observed for a variety of BRET tag combinations and positions. Therefore it was suggested that, in this case, an agonist-induced dissociation of oligomers is more likely than just a conformational change [37]. Similar conclusions by those investigating the neuropeptide Y4 receptors [38] and SSTR2 [19] were supported by Western blot analysis. However, a conformational change that influences the distance/orientation of BRET donor and acceptor molecules could possibly also influence accessibility to antibodies [58].
Investigation of endothelin ETA–ETB receptor hetero-oligomerization showed no effect of agonist treatment after 5 min, but a 50% decrease in FRET after 30 min with an ETB receptor-specific agonist [27]. This did not occur with ET1, which interacts with both receptors. Furthermore, this apparent dissociation of the hetero-oligomer was dependent upon endocytosis, as it did not occur when clathrin-mediated internalization was inhibited by sucrose treatment or co-expression with a dominant-negative dynamin mutant.
Some studies, particularly using FRET, have described RET between GPCRs that is agonist-dependent rather than constitutive (Tables 2 and 3). The significance of such observations is unclear, although they imply that activation of these particular receptors induces oligomerization [13,14,26,35,73]. However, it should be noted that the lack of a FRET/BRET signal does not necessarily mean that receptors do not interact. It is possible that the relative orientation and/or distance between the donor and acceptor molecules in the complex is unfavourable for RET. Furthermore, conformational changes following receptor activation may alter the positioning of these molecules such that FRET/BRET occurs. Such a situation may be observed with the mammalian type I GnRHR. This GPCR is unique in not possessing a C-terminal tail and so fusion with donor or acceptor molecules usually involves inclusion of a ‘C-terminal tail spacer’ to allow for freedom of movement [26,35]. We have observed a constitutive BRET signal with mammalian type I GnRHRs that possess a catfish GnRHR C-terminal tail as a ‘spacer’ (K. Kroeger, K. Pfleger, L. Miles and K. Eidne, unpublished work). This is in contrast with a spacer of just ten amino acids, whereupon an agonist-induced rather than constitutive signal is observed [35].
A further interesting observation, the caveat above notwithstanding, is that no FRET signal was observed between SSTR1s regardless of agonist treatment [15]. This is particularly intriguing as, in the same study, a FRET signal was observed between SSTR1 and SSTR5. The implication is that SSTR1 functions as a monomer in the absence of other receptor subtypes. Therefore, despite increasing evidence for oligomerization playing a crucial role in GPCR function in general, the phenomenon is not necessarily universal. Indeed, SSTRs illustrate the diversity of GPCRs with regard to oligomerization, even between receptor subtypes. In contrast with the lack of FRET signal observed between SSTR1s, constitutive FRET is observed between SSTR2s that decreases with agonist [19], whereas FRET between SSTR5s is only seen in the presence of agonist [13]. Such differences, in addition to the variety of functions being attributed to such interactions [58], demonstrates the need to investigate GPCR oligomerization on a case-by-case basis. At this stage, any generalizations pertaining to GPCR–GPCR interactions should be made with extreme caution.
Validating the physiological/pathophysiological relevance of oligomerization
As more and more GPCRs are shown to oligomerize, it is clearly important to establish the physiological/pathophysiological relevance of these interactions. FRET and BRET are excellent tools for identifying oligomerization, but their use should be complemented by other assays to establish the functional roles of particular interactions. FRET and BRET tend to use exogenous protein expression systems; however, as discussed previously, FRET can potentially be used to monitor endogenously expressed GPCRs using fluorophore-conjugated ligands or receptor-specific antibodies. Further improvements in experimental design, detection instrumentation and reagents should result in such studies becoming more common in the future.
An important first step in establishing physiological/pathophysiological relevance of hetero-oligomerization is to demonstrate in vivo co-expression of the different GPCRs in the same tissue, and, ideally, in the same cell. Secondly, it is important to show functional cross-talk between the receptor signalling systems, thereby providing an explanation for a particular interaction in vivo. Thirdly, demonstration of novel pharmacological and/or functional properties resulting from hetero-oligomerization provides evidence for the mechanism by which the GPCR–GPCR interaction modulates cellular activity. This has recently been reviewed extensively elsewhere [58].
A number of studies provide good examples of the relevance of GPCR hetero-oligomerization. The best example of a physiological role for this phenomenon is probably the interaction between GABAB1R and GABAB2R, which appears to be critical for trafficking functional receptors to the plasma membrane [45–48]. With respect to a pathophysiological situation, strong in vivo evidence has been provided for increased hetero-oligomerization between angiotensin AT1 receptors and bradykinin B2 receptors being at least partly responsible for the increased response to angiotensin II observed in pre-eclampsia, a major complication of pregnancy [75]. These hetero-oligomers seem to be resistant to inactivation by oxidative stress, resulting in maintained angiotensin II signalling in pre-eclamptic women, in contrast with normotensive women.
Establishing the physiological/pathophysiological relevance of homo-oligomerization is much more difficult, although a number of suggestions have been made as to the possible role of such interactions. The importance of hetero-oligomerization to GABAB1R–GABAB2R function indicates that GPCR–GPCR interactions can be critical for trafficking to the plasma membrane. It therefore seems likely that homo-oligomerization can play a similar role, particularly as most of these interactions appear to be initiated at or soon after the ER as discussed above. A second concept that is currently gaining momentum is that of multiprotein signalling complexes, of which GPCR oligomers are a part [77]. Such complexes have the potential to increase the speed and efficiency of signal transduction as a result of co-operativity [58]. An example of homo-oligomerization potentially being exploited in the clinic involves co-treatment of patients with morphine and DAMGO {[D-Ala2-MePhe4-Gly(ol)5]enkephalin} [78]. Morphine is able to activate the μ-opioid receptor without promoting desensitization and endocytosis. This has severe consequences, as chronic use results in tolerance. The μ-opioid receptor agonist DAMGO, unlike morphine, is able to desensitize the receptor and can do so at sub-analgesic doses. Significant internalization of morphine-bound receptor is observed following co-treatment with morphine and sub-analgesic DAMGO, implying that the DAMGO-bound receptors ‘drag’ the morphine-bound receptors into the cell as a result of homo-oligomeric interactions [78]. This phenomenon could well be utilized in the future to reduce the problem of tolerance in chronic morphine treatment.
INTERACTIONS BETWEEN GPCRs AND OTHER MEMBRANE PROTEINS
BRET has been shown to occur between the β2-AR and a subunit of a Kir channel (inwardly rectifying K+ channel), namely the Kir3.1 subunit [79]. A significant BRET signal was only obtained with co-expression of either a Kir3.2c or Kir3.4 subunit. As such co-expression is required for functional ion channel production, this implies that interaction with the GPCR is improved or stabilized by the presence of a functional channel [79]. In the same study, a BRET signal was also observed between the β2-AR and adenylate cyclase [79]. There was no change in the BRET signal with agonist treatment in either case, implying that these interactions are not dependent upon receptor activation. Consequently, these observations provide evidence for the emerging concept of signalling complex pre-assembly [80].
INTERACTIONS OF GPCRs WITH GRKs AND β-ARRESTINS
Following agonist binding, GPCRs are generally phosphorylated by GRKs. This enables adapter proteins, known as β-arrestins, to bind to the receptor, blocking the interaction with G-protein and targeting the GPCR to clathrin-coated pits for endocytosis [81]. Therefore β-arrestins have a critical role in the sequestration and down-regulation of most GPCRs. The ubiquitously expressed β-arrestins have two forms, β-arrestin 1 [82] and β-arrestin 2 [83]. Oakley et al. [84] proposed a receptor classification scheme based on β-arrestin usage, designating receptors that interact preferentially with β-arrestin 2 as ‘Class A’ and receptors that interact with both β-arrestins with similar affinity as ‘Class B’. Class A receptors appear to rapidly dissociate from β-arrestin upon internalization. Trafficking to an acidified endosomal vesicle is followed by ligand dissociation, receptor dephosphorylation and recycling to the plasma membrane. In contrast, Class B receptors appear to form stable complexes with β-arrestins, accumulating in endocytic vesicles, whereupon they are targeted for degradation or slowly recycled to the plasma membrane [81].
FRET has been used to show the kinetics of interaction between PTHR and β-arrestin 2 [44]. Following agonist treatment, an increase in FRET was observed with a t1/2 (half-life) of 150 s. Furthermore, varying expression of β-arrestin or GRKs was found to modulate this rate of association [44].
The β2-AR, unlike the β3-AR [57], interacted with β-arrestin 2 in an agonist-dependent manner that was shown to be dose-dependent using BRET [74]. Co-expression of untagged β3-AR reduced the BRET signal between tagged β2-AR and β-arrestin 2, implying that the ability of the β2-AR–β3-AR hetero-oligomer to interact with β-arrestin 2 was lower than that of the β2-AR homo-oligomer [57]. This conclusion was supported by dose–response curves that were biphasic when wild-type β3-AR was co-expressed, probably due to a component of high-affinity interaction between the β2-AR homo-oligomer and β-arrestin 2, and a component of low-affinity interaction between the β2-AR–β3-AR hetero-oligomer and β-arrestin 2.
Using BRET, TRHR has been shown to interact with both β-arrestin 1 [35,62] and β-arrestin 2 [62] in an agonist-dependent manner, thus designating it a Class B GPCR according to the classification scheme described above [84]. In contrast, TRHR2 appears to interact with β-arrestin 2 much more strongly than with β-arrestin 1 [62], thereby designating it a Class A GPCR according to the scheme [84]. No BRET signal was observed between either β-arrestin and a truncated form of TRHR [62] that had been shown previously not to recruit β-arrestins or undergo agonist-stimulated internalization [85–87]. This supports the conclusion that BRET between these GPCRs and β-arrestins results from specific interactions.
BRET has been used to demonstrate that hetero-oligomerization can result in altered β-arrestin interactions. A small BRET signal was observed between tagged TRHR2 and β-arrestin 1; however, upon co-expression with untagged TRHR, this signal increased significantly (3-fold) [62]. The untagged truncated TRHR had a similar effect despite being unable to interact directly with β-arrestins. This demonstrates that the increase in BRET signal results from an increased interaction between TRHR2 and β-arrestin 1 as a result of hetero-oligomerization, and not because an interaction between TRHR and β-arrestin 1 brings the donor molecule fused to TRHR2 within close proximity of the acceptor molecule fused to β-arrestin 1 [62].
No BRET signal was observed between the GnRHR and β-arrestin 1 [35], a result that correlates with previous studies, indicating that this receptor internalizes via a β-arrestin-independent mechanism [88,89].
More recently, the oxytocin, and vasopressin V1a and V2 receptors [52], the neuropeptide Y1, Y2, Y4 and Y5 receptors [39], and the angiotensin AT1 receptor [65] were all shown to interact with β-arrestin 2 in an agonist-dependent manner using BRET. The BRET kinetic profiles for the neuropeptide Y receptors correlated with their known internalization profiles, as the rapidly internalizing Y1 receptor associated with β-arrestin 2 most rapidly, and the slowly internalizing Y2 receptor associated with β-arrestin 2 least rapidly [39]. BRET dose–response curves were also produced for neuropeptide Y receptors interacting with β-arrestin 2, comparing agonist potency as well as observing the rightward shift of curves with increasing concentrations of antagonists. Comparison with ligand-binding data enabled conclusions to be drawn as to the relative affinity of these receptors for β-arrestin 2 [39].
The agonist-induced interaction of the oxytocin receptor with both GRK2 and β-arrestin 2 has been analysed over time using BRET [40]. The BRET signal between tagged receptor and GRK2 was inhibited by competition with untagged GRK2, demonstrating the specificity of the interaction. The BRET signal observed between tagged receptor and β-arrestin was reduced significantly by co-expression of a GRK2 dominant-negative mutant, indicating the importance of GRK2-mediated phosphorylation for subsequent interaction with β-arrestin 2 [40]. A comparison of time-courses supports the concept of GRK2-mediated phosphorylation preceding β-arrestin association, with the GRK2 interaction occurring within 4 s, and the β-arrestin interaction occurring after a 10 s lag [40].
Monitoring of the GPCR–β-arrestin interaction by BRET is likely to have significant utility as a platform for drug screening [4], particularly as most GPCRs interact strongly with β-arrestin 2 in an agonist-dependent manner [84]. The almost universal nature of the interaction is more convenient for functional screening than successively assaying coupling to the various different G-proteins [4].
INTERACTIONS BETWEEN G-PROTEIN SUBUNITS
Although biophysical studies measuring GPCR interactions with G-proteins directly have not yet been published, GPCR-mediated dissociation of the α and β G-protein subunits has been investigated using FRET [90]. Gα2 and Gβ of Dictyostelium discoideum were tagged with CFP and YFP respectively. The loss of RET resulting from subunit dissociation was maximal within 10 s of GPCR stimulation with the agonist, cAMP. The FRET signal was restored within 2 min as the activating cAMP was removed by endogenous phosphodiesterases. Furthermore, the reduction in FRET signal occurred in a dose-dependent manner [90].
Again using the combination of CFP and YFP fluorophores, Bünemann et al. [91] also investigated G-protein subunit interactions with FRET. However, in contrast with the generally accepted dogma, they concluded that Gi activation involves rearrangement rather than dissociation [91]. The FRET signal between Gαi1–YFP and N-terminally tagged Gβ1γ2–CFP or Gγ2–CFP was expected to decrease upon receptor activation as a result of subunit dissociation. However, the signal increased, probably reflecting a decrease in distance between fluorophores following subunit rearrangement. To test this theory further, the combination of Gαi1–YFP and C-terminally tagged Gγ2–CFP was assessed. The distance between fluorophores in this configuration would be expected to increase following the predicted rearrangement, and the observed decrease in FRET signal was in keeping with this hypothesis. The authors suggest that the contradictory results observed with Gα2 and Gβ of D. discoideum (described above) may be due to the different G-protein investigated [91]. The G-protein activation mediated via the α2A-AR was found to be complete within 1–2 s, at least five times slower than activation of the actual GPCR [91].
ADDRESSING CRITICISMS OF FRET/BRET
Receptor overexpression
A major criticism of FRET/BRET studies is that protein overexpression can result in RET attributed to a high incidence of random collisions, rather than direct protein–protein interactions. For example, investigation of SSTR5 homo-oligomerization using pbFRET showed significant basal energy transfer in CHO-K1 (Chinese-hamster ovary) cells expressing high levels of receptor [13]. However, in the same study, a second CHO-K1 cell line expressing a 5-fold lower receptor concentration showed insignificant basal energy transfer between receptors, which increased with agonist in a dose-dependent manner. Therefore it is critical for studies using these techniques to include adequate controls to demonstrate the specificity of interactions and establish levels of RET considered to be background in any given experiment.
Using approximately equimolar concentrations of β2-AR–Rluc and β2-AR–GFP, BRET data were produced for a range of protein expression levels (total β2-AR protein concentration of 1.4 to 87.2 pmol/mg of protein). It was found that, for total concentrations of between 1.4 and 26.3 pmol/mg of protein, the BRET signal did not increase. However, concentrations of 47.3 pmol/mg of protein and higher did exhibit greater BRET [32]. This demonstrates that there is a substantial range for which non-specific interactions do not contribute significantly to the BRET signal; however, at high protein expression levels, artifactual interactions do occur. Therefore BRET assays should be carried out with low expression levels, and/or suitable negative controls should be included to correct for the signal component resulting from random collisions. The BRET signal was stable for receptor expression levels ranging from 40 to 100 fmol of receptor/mg of membrane proteins for CCR5 receptors [51], from 200 to 1000 fmol/mg of protein for opioid receptors [68], and from 580 to 6570 fmol/mg of protein for β2-AR–β3-AR hetero-oligomers [57]. BRET between CXCR4 receptors did not change over a 30-fold range in total amount of DNA transfected (1–30 μg of DNA) [92]. Therefore, as long as expression levels are kept relatively low, the BRET signal does appear to be independent of receptor density.
Transient transfection of cells with BRET-tagged receptors results in a mixed population of cells with a spectrum of protein expression levels [52]. Therefore, if BRET can occur as a result of receptor overexpression, perhaps the signal results from this subpopulation of highly expressing cells [49,52]. This problem highlights the need for good negative controls, such as the parallel analysis of similar proteins that do not produce a FRET/BRET signal under the same conditions, despite similar expression profiles. Studies of GPCR oligomerization, for example, often include an unrelated GPCR as such a control [16,34,35,37,38,49,51–53,56,57,61,62,74,92,93]. Evidence has been presented supporting the use of transient transfection in BRET assays. Fractionation of the cell population according to YFP expression using flow cytofluorimetry enabled subpopulations of cells to be evaluated. BRET signals from these fractions were not found to differ significantly from that resulting from the total population, demonstrating independence from receptor density [52]. Data from single-cell BRET assays evaluated microscopically have also been shown to correlate with those derived from transiently transfected cell populations analysed using a plate reader [49]. Of course, the use of stably transfected clonal cell lines circumvents the issue, as there is a homogeneous population of cells expressing receptor at the same level. An alternative is to use the baculovirus expression system in insect cells. This enables protein expression levels to be controlled more closely than with transient transfection, as protein expression can be titrated by adjusting the multiplicity of viral infection [94].
Membrane microdomains
It has been suggested that RET between GPCRs could be an artifact of clustering in membrane microdomains, such as clathrincoated pits [35] or membrane rafts [16,53]. However, a dominant-negative dynamin mutant that inhibits clathrin-mediated endocytosis did not affect the agonist-induced BRET between TRHRs [35]. Furthermore, disruption of membrane rafts by cyclodextrin treatment did not alter the BRET signal between adenosine A2A receptors [16], or between A2A receptors and D2Rs [53]. Furthermore, these BRET signals were not affected by repletion with cholesterol after cyclodextrin treatment. Such artifactual FRET/BRET signals would also be expected to occur between unrelated receptors; however, as discussed above, the lack of signal with unrelated receptors is often used as a negative control for oligomerization studies.
CONCLUDING REMARKS
FRET and BRET techniques and technologies are constantly being improved with regard to experimental design, instrumentation and reagents. The result is a transformation in the field of GPCRs that promises to answer many of the questions previously beyond our reach. The demonstration of GPCR oligomerization exemplifies the power of these methods, and, as studies expand to investigate interactions with other proteins, further exciting revelations will undoubtedly follow. Furthermore, as our understanding of cellular complexity improves, the need for drug-discovery programmes to incorporate such methods is becoming increasingly clear.
Acknowledgments
We are grateful to Uli Schmidt for contributing the artwork, Lisa Coleman for help with manuscript preparation and Matthew Dalrymple for proofreading before submission. This work was supported by a grant from the National Health and Medical Research Council (NHMRC) of Australia (project grant #212065). K.D.G.P. and K.A.E. are supported by a WAIMR Postdoctoral Fellowship and an NHMRC Principle Research Fellowship respectively. We also acknowledge Berthold Australia, Berthold Technologies and Promega.
References
- 1.Brady A. E., Limbird L. E. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signalling. 2002;14:297–309. doi: 10.1016/s0898-6568(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 2.Eidne K. A., Kroeger K. M., Hanyaloglu A. C. Applications of novel resonance energy transfer techniques to study dynamic hormone receptor interactions in living cells. Trends Endocrinol. Metab. 2002;13:415–421. doi: 10.1016/s1043-2760(02)00669-0. [DOI] [PubMed] [Google Scholar]
- 3.Boute N., Jockers R., Issad T. The use of resonance energy transfer in high-throughput screening: BRET versus FRET. Trends Pharmacol. Sci. 2002;23:351–354. doi: 10.1016/s0165-6147(02)02062-x. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand L., Parent S., Caron M., Legault M., Joly E., Angers S., Bouvier M., Brown M., Houle B., Menard L. The BRET2/arrestin assay in stable recombinant cells: a platform to screen for compounds that interact with G protein-coupled receptors (GPCRS) J. Recept. Signal Transduct. Res. 2002;22:533–541. doi: 10.1081/rrs-120014619. [DOI] [PubMed] [Google Scholar]
- 5.Roda A., Guardigli M., Pasini P., Mirasoli M. Bioluminescence and chemiluminescence in drug screening. Anal. Bioanal. Chem. 2003;377:826–833. doi: 10.1007/s00216-003-2096-6. [DOI] [PubMed] [Google Scholar]
- 6.Milligan G. Applications of bioluminescence and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur. J. Pharm. Sci. 2004;21:397–405. doi: 10.1016/j.ejps.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Patel R. C., Lange D. C., Patel Y. C. Photobleaching fluorescence resonance energy transfer reveals ligand-induced oligomer formation of human somatostatin receptor subtypes. Methods. 2002;27:340–348. doi: 10.1016/s1046-2023(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 8.Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Annu. Phys. 1948;2:54–75. [Google Scholar]
- 9.Wu P., Brand L. Resonance energy transfer: methods and applications. Anal. Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 10.Overton M. C., Blumer K. J. Use of fluorescence resonance energy transfer to analyze oligomerization of G-protein-coupled receptors expressed in yeast. Methods. 2002;27:324–332. doi: 10.1016/s1046-2023(02)00090-7. [DOI] [PubMed] [Google Scholar]
- 11.Pfleger K. D., Eidne K. A. New technologies: bioluminescence resonance energy transfer (BRET) for the detection of real time interactions involving G-protein coupled receptors. Pituitary. 2003;6:141–151. doi: 10.1023/b:pitu.0000011175.41760.5d. [DOI] [PubMed] [Google Scholar]
- 12.Latif R., Graves P., Davies T. F. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 2001;276:45217–45224. doi: 10.1074/jbc.M103727200. [DOI] [PubMed] [Google Scholar]
- 13.Rocheville M., Lange D. C., Kumar U., Sasi R., Patel R. C., Patel Y. C. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 14.Rocheville M., Lange D. C., Kumar U., Patel S. C., Patel R. C., Patel Y. C. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 15.Patel R. C., Kumar U., Lamb D. C., Eid J. S., Rocheville M., Grant M., Rani A., Hazlett T., Patel S. C., Gratton E., Patel Y. C. Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3294–3299. doi: 10.1073/pnas.042705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canals M., Burgueno J., Marcellino D., Cabello N., Canela E. I., Mallol J., Agnati L., Ferré S., Bouvier M., Fuxe K., et al. Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Neurochem. 2004;88:726–734. doi: 10.1046/j.1471-4159.2003.02200.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Maurel D., Etzol S., Brabet I., Ansanay H., Pin J. P., Rondard P. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J. Biol. Chem. 2004;279:15824–15830. doi: 10.1074/jbc.M313639200. [DOI] [PubMed] [Google Scholar]
- 18.Maurel D., Kniazeff J., Mathis G., Trinquet E., Pin J. P., Ansanay H. Cell surface detection of membrane protein interaction with homogeneous time-resolved fluorescence resonance energy transfer technology. Anal. Biochem. 2004;329:253–262. doi: 10.1016/j.ab.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Grant M., Collier B., Kumar U. Agonist-dependent dissociation of human somatostatin receptor 2 dimers: a role in receptor trafficking. J. Biol. Chem. 2004;279:36179–36183. doi: 10.1074/jbc.M407310200. [DOI] [PubMed] [Google Scholar]
- 20.McVey M., Ramsay D., Kellett E., Rees S., Wilson S., Pope A. J., Milligan G. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer: the human δ-opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J. Biol. Chem. 2001;276:14092–14099. doi: 10.1074/jbc.M008902200. [DOI] [PubMed] [Google Scholar]
- 21.Carrillo J. J., Pediani J., Milligan G. Dimers of class A G protein-coupled receptors function via agonist-mediated trans-activation of associated G proteins. J. Biol. Chem. 2003;278:42578–42587. doi: 10.1074/jbc.M306165200. [DOI] [PubMed] [Google Scholar]
- 22.Bakker R. A., Dees G., Carrillo J. J., Booth R. G., López-Gimenez J. F., Milligan G., Strange P. G., Leurs R. Domain swapping in the human histamine H1 receptor. J. Pharmacol. Exp. Ther. 2004;311:131–138. doi: 10.1124/jpet.104.067041. [DOI] [PubMed] [Google Scholar]
- 23.Latif R., Graves P., Davies T. F. Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J. Biol. Chem. 2002;277:45059–45067. doi: 10.1074/jbc.M206693200. [DOI] [PubMed] [Google Scholar]
- 24.Roess D. A., Horvat R. D., Munnelly H., Barisas B. G. Luteinizing hormone receptors are self-associated in the plasma membrane. Endocrinology. 2000;141:4518–4523. doi: 10.1210/endo.141.12.7802. [DOI] [PubMed] [Google Scholar]
- 25.van Roessel P., Brand A. H. Imaging into the future: visualizing gene expression and protein interactions with fluorescent proteins. Nat. Cell Biol. 2002;4:E15–E20. doi: 10.1038/ncb0102-e15. [DOI] [PubMed] [Google Scholar]
- 26.Cornea A., Janovick J. A., Maya-Nunez G., Conn P. M. Gonadotropin-releasing hormone receptor microaggregation: rate monitored by fluorescence resonance energy transfer. J. Biol. Chem. 2001;276:2153–2158. doi: 10.1074/jbc.M007850200. [DOI] [PubMed] [Google Scholar]
- 27.Gregan B., Jürgensen J., Papsdorf G., Furkert J., Schaefer M., Beyermann M., Rosenthal W., Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J. Biol. Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 28.Selvin P. R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- 29.Arai R., Nakagawa H., Kitayama A., Ueda H., Nagamune T. Detection of protein–protein interaction by bioluminescence resonance energy transfer from firefly luciferase to red fluorescent protein. J. Biosci. Bioeng. 2002;94:362–364. doi: 10.1263/jbb.94.362. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y., Piston D. W., Johnson C. H. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc. Natl. Acad. Sci. U.S.A. 1999;96:151–156. doi: 10.1073/pnas.96.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay D., Kellett E., McVey M., Rees S., Milligan G. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem. J. 2002;365:429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercier J. F., Salahpour A., Angers S., Breit A., Bouvier M. Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 33.Toth P. T., Ren D., Miller R. J. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1α and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J. Pharmacol. Exp. Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 34.Floyd D. H., Geva A., Bruinsma S. P., Overton M. C., Blumer K. J., Baranski T. J. C5a receptor oligomerization. II. Fluorescence resonance energy transfer studies of a human G protein-coupled receptor expressed in yeast. J. Biol. Chem. 2003;278:35354–35361. doi: 10.1074/jbc.M305607200. [DOI] [PubMed] [Google Scholar]
- 35.Kroeger K. M., Hanyaloglu A. C., Seeber R. M., Miles L. E., Eidne K. A. Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor: detection in living cells using bioluminescence resonance energy transfer. J. Biol. Chem. 2001;276:12736–12743. doi: 10.1074/jbc.M011311200. [DOI] [PubMed] [Google Scholar]
- 36.Yoshioka K., Saitoh O., Nakata H. Agonist-promoted heteromeric oligomerization between adenosine A1 and P2Y1 receptors in living cells. FEBS Lett. 2002;523:147–151. doi: 10.1016/s0014-5793(02)02965-4. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z. J., Miller L. J. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J. Biol. Chem. 2001;276:48040–48047. doi: 10.1074/jbc.M105668200. [DOI] [PubMed] [Google Scholar]
- 38.Berglund M. M., Schober D. A., Esterman M. A., Gehlert D. R. Neuropeptide Y Y4 receptor homodimers dissociate upon agonist stimulation. J. Pharmacol. Exp. Ther. 2003;307:1120–1126. doi: 10.1124/jpet.103.055673. [DOI] [PubMed] [Google Scholar]
- 39.Berglund M. M., Schober D. A., Statnick M. A., McDonald P. H., Gehlert D. R. The use of bioluminescence resonance energy transfer 2 to study neuropeptide Y receptor agonist-induced β-arrestin 2 interaction. J. Pharmacol. Exp. Ther. 2003;306:147–156. doi: 10.1124/jpet.103.051227. [DOI] [PubMed] [Google Scholar]
- 40.Hasbi A., Devost D., Laporte S. A., Zingg H. H. Real-time detection of interactions between the human oxytocin receptor and G protein-coupled receptor kinase-2. Mol. Endocrinol. 2004;18:1277–1286. doi: 10.1210/me.2003-0440. [DOI] [PubMed] [Google Scholar]
- 41.Pfleger K. D., Lim E., Dalrymple M. B., Schmidt U., Szefczyk S. M., Eidne K. A. A comparative study of Renilla luciferase substrates and their application to real-time bioluminescence resonance energy transfer (BRET); 2004. 47th Annual Meeting of the Endocrine Society of Australia, Sydney, Australia. Abstract #140. [Google Scholar]
- 42.Pfleger K. D., Lim E., Miles L. E., Seeber R. M., Eidne K. A. Prolonged real-time kinetics of protein–protein interactions involving G-protein coupled receptors determined using bioluminescence resonance energy transfer (BRET); 2004. 12th International Congress of Endocrinology, Lisbon, Portugal. Abstract P1134. [Google Scholar]
- 43.Eidne K. A., Dalrymple M. B., Schmidt U., Kroeger K. M., Pfleger K. D. GPCR oligomerization – monitoring the formation of dynamic protein complexes in living cells; 2004. 12th International Congress of Endocrinology – 12th ICE, pp. 113–118, Medimond International Proceedings, Bologna. [Google Scholar]
- 44.Vilardaga J. P., Bünemann M., Krasel C., Castro M., Lohse M. J. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 2003;21:807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- 45.Jones K. A., Borowsky B., Tamm J. A., Craig D. A., Durkin M. M., Dai M., Yao W. J., Johnson M., Gunwaldsen C., Huang L. Y., et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 46.White J. H., Wise A., Main M. J., Green A., Fraser N. J., Disney G. H., Barnes A. A., Emson P., Foord S. M., Marshall F. H. Heterodimerization is required for the formation of a functional GABAB receptor. Nature (London) 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 47.Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., Mosbacher J., Bischoff S., Kulik A., Shigemoto R., et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature (London) 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 48.Margeta-Mitrovic M., Jan Y. N., Jan L. Y. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 49.Ayoub M. A., Couturier C., Lucas-Meunier E., Angers S., Fossier P., Bouvier M., Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 50.Overton M. C., Blumer K. J. The extracellular N-terminal domain and transmembrane domains 1 and 2 mediate oligomerization of a yeast G protein-coupled receptor. J. Biol. Chem. 2002;277:41463–41472. doi: 10.1074/jbc.M205368200. [DOI] [PubMed] [Google Scholar]
- 51.Issafras H., Angers S., Bulenger S., Blanpain C., Parmentier M., Labbe-Jullie C., Bouvier M., Marullo S. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J. Biol. Chem. 2002;277:34666–34673. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 52.Terrillon S., Durroux T., Mouillac B., Breit A., Ayoub M. A., Taulan M., Jockers R., Barberis C., Bouvier M. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol. Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 53.Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S. R., Neve K., Fuxe K., Agnati L. F., Woods A. S., et al. Adenosine A2A–dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 54.Kroeger K. M., Pfleger K. D. G., Eidne K. A. Biophysical and biochemical methods to study GPCR oligomerization. In: Devi L. A., editor. G-Protein Coupled Receptors: Signaling, Dimerization and Neuropharmacology. Totowa: Humana Press; 2004. in the press. [Google Scholar]
- 55.Angers S., Salahpour A., Bouvier M. Biochemical and biophysical demonstration of GPCR oligomerization in mammalian cells. Life Sci. 2001;68:2243–2250. doi: 10.1016/s0024-3205(01)01012-8. [DOI] [PubMed] [Google Scholar]
- 56.Ayoub M. A., Levoye A., Delagrange P., Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- 57.Breit A., Lagace M., Bouvier M. Hetero-oligomerization between β2- and β3-adrenergic receptors generates a β-adrenergic signaling unit with distinct functional properties. J. Biol. Chem. 2004;279:28756–28765. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- 58.Kroeger K. M., Pfleger K. D., Eidne K. A. G-protein coupled receptor oligomerization in neuroendocrine pathways. Front. Neuroendocrinol. 2003;24:254–278. doi: 10.1016/j.yfrne.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol. Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 60.Wurch T., Matsumoto A., Pauwels P. J. Agonist-independent and -dependent oligomerization of dopamine D2 receptors by fusion to fluorescent proteins. FEBS Lett. 2001;507:109–113. doi: 10.1016/s0014-5793(01)02969-6. [DOI] [PubMed] [Google Scholar]
- 61.Stanasila L., Perez J. B., Vogel H., Cotecchia S. Oligomerization of the α1a- and α1b-adrenergic receptor subtypes: potential implications in receptor internalization. J. Biol. Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- 62.Hanyaloglu A. C., Seeber R. M., Kohout T. A., Lefkowitz R. J., Eidne K. A. Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes: differential regulation of β-arrestins 1 and 2. J. Biol. Chem. 2002;277:50422–50430. doi: 10.1074/jbc.M209340200. [DOI] [PubMed] [Google Scholar]
- 63.Jensen A. A., Hansen J. L., Sheikh S. P., Brauner-Osborne H. Probing intermolecular protein-protein interactions in the calcium-sensing receptor homodimer using bioluminescence resonance energy transfer (BRET) Eur. J. Biochem. 2002;269:5076–5087. doi: 10.1046/j.1432-1033.2002.03218.x. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Z. J., Harikumar K. G., Holicky E. L., Miller L. J. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J. Biol. Chem. 2003;278:52972–52979. doi: 10.1074/jbc.M310090200. [DOI] [PubMed] [Google Scholar]
- 65.Hansen J. L., Theilade J., Haunso S., Sheikh S. P. Oligomerization of wild type and nonfunctional mutant angiotensin II type I receptors inhibits Gαq protein signaling but not ERK activation. J. Biol. Chem. 2004;279:24108–24115. doi: 10.1074/jbc.M400092200. [DOI] [PubMed] [Google Scholar]
- 66.Devost D., Zingg H. H. Homo- and hetero-dimeric complex formations of the human oxytocin receptor. J. Neuroendocrinol. 2004;16:372–377. doi: 10.1111/j.0953-8194.2004.01188.x. [DOI] [PubMed] [Google Scholar]
- 67.Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J. Mol. Biol. 1977;113:89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- 68.Gomes I., Filipovska J., Jordan B. A., Devi L. A. Oligomerization of opioid receptors. Methods. 2002;27:358–365. doi: 10.1016/s1046-2023(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 69.Dinger M. C., Bader J. E., Kobor A. D., Kretzschmar A. K., Beck-Sickinger A. G. Homodimerization of neuropeptide Y receptors investigated by fluorescence resonance energy transfer in living cells. J. Biol. Chem. 2003;278:10562–10571. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- 70.Kozell L. B., Neve K. A. Constitutive activity of a chimeric D2/D1 dopamine receptor. Mol. Pharmacol. 1997;52:1137–1149. doi: 10.1124/mol.52.6.1137. [DOI] [PubMed] [Google Scholar]
- 71.Kammerer R. A., Frank S., Schulthess T., Landwehr R., Lustig A., Engel J. Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil α-helices. Biochemistry. 1999;38:13263–13269. doi: 10.1021/bi991018t. [DOI] [PubMed] [Google Scholar]
- 72.Kuner R., Kohr G., Grünewald S., Eisenhardt G., Bach A., Kornau H. C. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 73.Horvat R. D., Roess D. A., Nelson S. E., Barisas B. G., Clay C. M. Binding of agonist but not antagonist leads to fluorescence resonance energy transfer between intrinsically fluorescent gonadotropin-releasing hormone receptors. Mol. Endocrinol. 2001;15:695–703. doi: 10.1210/mend.15.5.0633. [DOI] [PubMed] [Google Scholar]
- 74.Angers S., Salahpour A., Joly E., Hilairet S., Chelsky D., Dennis M., Bouvier M. Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc. Natl. Acad. Sci. U.S.A. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.AbdAlla S., Lother H., el Massiery A., Quitterer U. Increased AT1 receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 76.Lavoie C., Mercier J. F., Salahpour A., Umapathy D., Breit A., Villeneuve L. R., Zhu W. Z., Xiao R. P., Lakatta E. G., Bouvier M., Hebert T. E. β1/β2-Adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J. Biol. Chem. 2002;277:35402–35410. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 77.Edwards S. W., Tan C. M., Limbird L. E. Localization of G-protein-coupled receptors in health and disease. Trends Pharmacol. Sci. 2000;21:304–308. doi: 10.1016/s0165-6147(00)01513-3. [DOI] [PubMed] [Google Scholar]
- 78.He L., Fong J., von Zastrow M., Whistler J. L. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 79.Lavine N., Ethier N., Oak J. N., Pei L., Liu F., Trieu P., Rebois R. V., Bouvier M., Hebert T. E., Van Tol H. H. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J. Biol. Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 80.Bray D. Signaling complexes: biophysical constraints on intracellular communication. Annu. Rev. Biophys. Biomol. Struct. 1998;27:59–75. doi: 10.1146/annurev.biophys.27.1.59. [DOI] [PubMed] [Google Scholar]
- 81.Luttrell L. M., Lefkowitz R. J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 82.Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. β-Arrestin: a novel protein that regulates β-adrenergic-receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 83.Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. β-Arrestin2, a novel member of the arrestin/β-arrestin gene family. J. Biol. Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 84.Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. Differential affinities of visual arrestin, βarrestin1, and βarrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 85.Nussenzveig D. R., Heinflink M., Gershengorn M. C. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J. Biol. Chem. 1993;268:2389–2392. [PubMed] [Google Scholar]
- 86.Yu R., Hinkle P. M. Signal transduction and hormone-dependent internalization of the thyrotropin-releasing hormone receptor in cells lacking Gq and G11. J. Biol. Chem. 1999;274:15745–15750. doi: 10.1074/jbc.274.22.15745. [DOI] [PubMed] [Google Scholar]
- 87.Drmota T., Milligan G. Kinetic analysis of the internalization and recycling of [3H]TRH and C-terminal truncations of the long isoform of the rat thyrotropin-releasing hormone receptor-1. Biochem. J. 2000;346:711–718. [PMC free article] [PubMed] [Google Scholar]
- 88.Vrecl M., Anderson L., Hanyaloglu A., McGregor A. M., Groarke A. D., Milligan G., Taylor P. L., Eidne K. A. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: effect of β-arrestin on internalization kinetics. Mol. Endocrinol. 1998;12:1818–1829. doi: 10.1210/mend.12.12.0207. [DOI] [PubMed] [Google Scholar]
- 89.Heding A., Vrecl M., Hanyaloglu A. C., Sellar R., Taylor P. L., Eidne K. A. The rat gonadotropin-releasing hormone receptor internalizes via a β-arrestin-independent, but dynamin-dependent, pathway: addition of a carboxyl-terminal tail confers β-arrestin dependency. Endocrinology. 2000;141:299–306. doi: 10.1210/endo.141.1.7269. [DOI] [PubMed] [Google Scholar]
- 90.Janetopoulos C., Jin T., Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 91.Bünemann M., Frank M., Lohse M. J. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Babcock G. J., Farzan M., Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J. Biol. Chem. 2003;278:3378–3385. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 93.Devost D., Zingg H. H. Identification of dimeric and oligomeric complexes of the human oxytocin receptor by co-immunoprecipitation and bioluminescence resonance energy transfer. J. Mol. Endocrinol. 2003;31:461–471. doi: 10.1677/jme.0.0310461. [DOI] [PubMed] [Google Scholar]
- 94.Cheung T. C., Hearn J. P. Development of a baculovirus-based fluorescence resonance energy transfer assay for measuring protein–protein interaction. Eur. J. Biochem. 2003;270:4973–4981. doi: 10.1046/j.1432-1033.2003.03899.x. [DOI] [PubMed] [Google Scholar]
- 95.Overton M. C., Blumer K. J. G-protein-coupled receptors function as oligomers in vivo. Curr. Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 96.Kamiya T., Saitoh O., Yoshioka K., Nakata H. Oligomerization of adenosine A2A and dopamine D2 receptors in living cells. Biochem. Biophys. Res. Commun. 2003;306:544–549. doi: 10.1016/s0006-291x(03)00991-4. [DOI] [PubMed] [Google Scholar]
- 97.Pfeiffer M., Kirscht S., Stumm R., Koch T., Wu D., Laugsch M., Schroder H., Hollt V., Schulz S. Heterodimerization of substance P and μ-opioid receptors regulates receptor trafficking and resensitization. J. Biol. Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]