Abstract
Background
Preliminary data suggests that obesity might hasten the decline in mRNA vaccine-induced immunity against SARS-CoV-2. However, whether this renders individuals with obesity more susceptible to long COVID symptoms post-vaccination remains uncertain. Given sleep’s critical role in immunity, exploring the associations between obesity, probable long COVID symptoms, and sleep disturbances is essential.
Methods
We analyzed data from a survey of 5919 adults aged 18 to 89, all of whom received two SARS-CoV-2 mRNA vaccinations. Participants were categorized into normal weight, overweight, and obesity groups based on ethnicity-specific BMI cutoffs. The probability of long COVID was evaluated using the Post-Acute Sequelae of SARS-CoV-2 (PASC) score, as our survey did not permit confirmation of acute SARS-CoV-2 infection through methods such as antibody testing. Additionally, sleep patterns were assessed through questionnaires.
Results
Participants with obesity exhibited a significantly higher adjusted odds ratio (OR) of having a PASC score of 12 or higher, indicative of probable long COVID in our study, compared to those with normal weight (OR: 1.55, 95% CI: 1.05, 2.28). No significant difference was observed for overweight individuals (OR: 0.92 [95% CI: 0.63, 1.33]). Both obesity and probable long COVID were associated with increased odds of experiencing a heightened sleep burden, such as the presence of obstructive sleep apnea or insomnia (P < 0.001). However, no significant interaction between BMI and probable long COVID status was found.
Conclusions
Even post-vaccination, individuals with obesity may encounter a heightened risk of experiencing prolonged COVID-19 symptoms. However, confirming our observations necessitates comprehensive studies incorporating rigorous COVID infection testing, such as antibody assays - unavailable in our anonymous survey. Additionally, it is noteworthy that the correlation between probable long COVID and sleep disturbances appears to be independent of BMI.
Subject terms: Epidemiology, Immunological disorders
Introduction
According to the World Health Organization, since December 2019, over 760 million humans have contracted the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in Coronavirus Disease 2019 (COVID-19), with a recorded global death toll of 6.9 million attributed to the infection [1]. A meta-analysis indicates that approximately 43% of individuals diagnosed with COVID-19 have reported experiencing lingering symptoms or health issues following the acute phase of infection, also named long COVID [2]. Numerous medications have been introduced to mitigate the severe and long-lasting health effects associated with COVID-19, including antiviral agents such as remdesivir [3]. However, vaccinations against SARS-CoV-2, particularly utilizing the recently developed mRNA vaccines, have emerged as the most potent measure in combating the virus [4]. For instance, mRNA vaccines have been associated with a reduced likelihood of contracting SARS-CoV-2, being hospitalized, or dying from COVID-19 [5]. Furthermore, they may lower the risk of experiencing long COVID symptoms, such as chest pain, breathing difficulties, ageusia, or anosmia [6].
People with obesity face an increased risk of developing a more severe course of COVID-19. For instance, in a study of COVID-19 cases among patients aged 18 years and younger, individuals with obesity had a 3.1 times higher risk of hospitalization and a 1.4 times higher risk of severe illness when hospitalized, which includes admission to the intensive care unit, need for invasive mechanical ventilation, or death [7]. Moreover, obesity has been identified as a risk factor for persistent symptoms following SARS-CoV-2 infection [8–11]. Given that mRNA vaccination against SARS-CoV-2 reduces both mortality and morbidity of COVID-19 [4, 5], including long COVID [6], it may be particularly relevant for people with obesity. However, a recent study suggests that two doses of SARS-CoV-2 mRNA vaccine may provide less protection in people with obesity. In this study, it was observed that breakthrough infections occurred more frequently and developed more rapidly in individuals with obesity who had received two doses of mRNA vaccine, compared to those with normal weight [12]. However, it remains unclear whether this weight-related effect also influences the risk of experiencing long COVID symptoms in individuals who have received two doses of the SARS-CoV-2 mRNA vaccine.
In our present multinational anonymous survey study, we included 5919 adults who had completed a two-dose regimen of SARS-CoV-2 mRNA vaccination. Our primary objective was to investigate whether individuals with obesity have a higher odds ratio (OR) of experiencing symptoms resembling long COVID, thus rejecting the null hypothesis that BMI status does not affect the likelihood of experiencing such symptoms. Given that only a small proportion of respondents reported a positive COVID test result, the assessment of long COVID likelihood relied on the Post-Acute Sequelae of SARS-CoV-2 (PASC) score [13]. This scoring system incorporates various typical symptoms associated with long COVID to ascertain its presence. Additionally, we explored the potential impact of gender on the relationship between BMI status and long COVID-like symptoms, considering gender-based differences in long COVID risk [14].
Notably, research indicates a link between obesity and sleep, encompassing issues such as insomnia [15], obstructive sleep apnea (OSA; [16]), and presence of short sleep duration [17]. Given sleep’s pivotal role in managing viral infections [18, 19], including SARS-CoV-2 [20–23], it is reasonable to hypothesize that individuals with obesity may be more prone to experiencing symptoms akin to long COVID compared to those with normal weight, owing to heightened sleep burden. Consequently, we investigated whether the occurrence of insomnia, OSA, and both short and long sleep durations is more prevalent among participants with probable long COVID and obesity.
Study design and statistical methods
Study population
In this study, self-reported data were collected from 5919 participants aged 18 to 89, with 63% of participants being women. The data were obtained through the second wave of the International COVID Sleep Study survey (ICOSS-2), as described in detail in reference [24]. The survey was conducted anonymously between May and December 2021 and was accessible through various online platforms, including RedCap and Qualtrics. To reach a diverse audience, the survey was advertised on university web pages, newspapers, television, Facebook, and Twitter (since 2023 named X). Additionally, it was made available in multiple languages, including German, Portuguese, Brazilian Portuguese, English, French, Bulgarian, Croatian, Chinese, Finnish, Hebrew, Italian, Japanese, Norwegian, and Swedish. After applying the exclusion criteria outlined in Supplemental Table S1, 5919 participants who reported receiving two doses of mRNA SARS-CoV-2 vaccine (Moderna and BioNTech/Pfizer) had complete data for analysis. During the survey period in 2021, a third dose of SARS-CoV-2 mRNA vaccine was not yet standard. Therefore, we only inquired about participants receiving up to two doses of SARS-CoV-2 mRNA vaccine.
This study adhered to the principles outlined in the Helsinki Declaration and obtained either ethical approval or waivers in all participating countries, in accordance with their respective national research governance and regulations. Notably, ethical approval was not mandated by national law in Austria, Brazil, Finland, France, Norway, and Sweden due to the anonymous nature of the survey collection. The anonymity of the data was preserved throughout the study. Prior to accessing the questionnaire, participants were required to provide their consent to participate, with a minimum age requirement of 18 years. No monetary compensation was provided to participants. Further details regarding ethical approval in each country can be found in Supplemental Table S2.
Definition of BMI cut-off points
The BMI ranges used to define normal weight, overweight, and obesity vary for Asians compared to other ethnicities [25]. Hence, we employed the following BMI thresholds for non-Asians: normal weight (reference group) <25 kg/m2, overweight 25-29.9 kg/m2, and obesity ≥30 kg/m2. Meanwhile, for participants of Asian ethnicity, their BMI status was determined as follows: normal weight <23 kg/m2, overweight ≥23 to <27.5 kg/m2, and obesity ≥27.5 kg/m2.
Definition of composite risk score for long COVID
A recent study [13] identified 12 symptoms that persist for at least six months post-infection with SARS-CoV-2 that can be used to assess the likelihood of experiencing long COVID, using a PASC score. Specifically, each symptom was assigned a score based on its predictive ability for long COVID: loss of or change in smell or taste (8 points), post-exertional malaise (7 points), chronic cough (4 points), brain fog (3 points), thirst (3 points), palpitations (2 points), chest pain (2 points), fatigue (1 point), dizziness (1 point), gastrointestinal tract symptoms (1 point), changes in sexual desire or capacity (1 point), and abnormal movements (1 point).
We included eight symptoms that lasted for at least six months at the time of the survey to calculate the PASC score for each participant. These symptoms were: loss of or change in smell or taste, post-exertional malaise, brain fog, palpitations, chest pain, fatigue, dizziness, and gastrointestinal tract symptoms. Based on a previous study’s proposal [13], participants with a PASC score of 12 or higher were classified as having a high likelihood of experiencing long COVID, regardless of whether they reported a previous SARS-CoV-2 infection.
To address the potential bias of misclassifying participants as unlikely to suffer from probable long COVID due to the absence of survey questions about chronic cough, thirst, changes in sexual desire or capacity, and abnormal movements, a sensitivity analysis was performed (see statistical section for more details).
Assessment of sleep
Based on participants’ sleep duration reports, we evaluated if participants usually slept less than six or more than nine hours per night (in the following, referred to as short or long sleep duration, respectively). The literature often uses these thresholds to discriminate short and long sleep duration from normal sleep duration [26, 27]. Participants’ insomnia risk was determined through the Insomnia Severity Index (ISI) [28], a validated questionnaire of seven items assessing the severity of insomnia symptoms and their impact on daily functioning. A score greater than 14 is indicative of moderate-to-severe insomnia. To assess the presence of OSA, we used the STOP scale [29]. Specifcally, participants were asked to respond on a 5-point Likert scale to the following four questions: (a) Do you snore loudly, surpassing the volume of talking or being audible through closed doors? Response options ranged from “Not at all” to “Every night/almost every night.” (b) Do you frequently experience daytime tiredness, fatigue, or excessive sleepiness? Response options spanned from “Not at all” to “Every day/almost every day.” (c) Has anyone ever witnessed you ceasing to breathe or choking during your sleep? Response options varied from “No, never” to “Every day/almost every day.” Participants’ answers were dichotomized into two categories for the first three questions: 0 = Less than three nights per week and 1 = Three nights per week or more. (d) We also surveyed whether participants currently had or received treatment for high blood pressure. A “Yes”-response was counted as one score. The risk of OSA was considered high if participants scored two or greater on the STOP scale.
Statistical analysis
Data are presented as mean ± SD unless otherwise specified. Group characteristics were compared using the Chi-Square test for categorical variables and generalized linear models for continuous variables. Logistic regression analyses were conducted to examine the associations between BMI group as a predictor and probable long COVID status as a dependent variable using SPSS 28.0 (IBM Corp., Armonk, NY, USA).
In addition to conducting an unadjusted logistic regression analysis, we employed one additional regression model to examine the robustness of the association between BMI status and probable long COVID. The adjusted analysis incorporated self-reported positive SARS-CoV-2 test, age, sex, race/ethnicity, smoking status, the time elapsed since the first vaccination ( ≤ six vs. > six months), urbanicity, and weekly physical activity level score (ranging from 0 to 7; higher score indicating higher physical activity; for more details, see [22]). We additionally considered a medical history encompassing hypertension, type 2 diabetes, depression, and attention deficit hyperactivity disorder. These were defined as instances where individuals had been diagnosed with or received treatment for these conditions either prior to or at the time of the survey. This inclusion was motivated by the recognition that each of these conditions commonly co-occurs with obesity [30–33].
To ensure the robustness of the hypothesized association between BMI status and probable long COVID, we conducted several sensitivity analyses:
1. We examined individuals who reported testing positive for SARS-CoV-2 before the survey (n = 515).
2. We separately analyzed data for men and women because previous findings suggest that the risk of long COVID is higher among women than men [14]. In this context, we assessed multiplicative interactions between BMI status and sex.
3. Participants from the USA were excluded from the analysis as they were significantly younger than participants from other countries (Supplemental Table S3).
4. We excluded individuals whose PASC score fell between 3 and 11, as our study did not survey four of the twelve symptoms used to calculate the PASC score in a previous study [13]. As mentioned earlier, if experienced for at least six months, the total sum of these symptoms corresponds to 9 points.
5. We excluded participants (n = 412) who were underweight from the normal weight reference group. Underweight was defined as having a BMI < 18.5 kg/m2 across all ethnicities.
To assess the potential variability in the risk of inadequate sleep associated with BMI and probable long COVID status, we conducted logistic regression analyses, both unadjusted and adjusted. Our binary outcome variables included moderate-to-severe insomnia, a high risk of OSA, short nighttime sleep duration (less than 6 hours), and long nighttime sleep duration (more than 9 hours). In the adjusted regression model, both BMI group and probable long COVID status were entered together to account for mutual adjustment. Additionally, for the sleep outcomes, we assessed multiplicative interactions between BMI group and probable long COVID status. Overall, a P value less than 0.05 was considered significant.
Results
Cohort characteristics
A comprehensive overview of characteristics, categorized by BMI status, is provided in Table 1. Compared to participants with normal weight, those with obesity exhibited several distinctive features. Specifically, they were more likely to be White/Caucasian and reside in urban areas. Additionally, they were more likely to report a history of type 2 diabetes, hypertension, depression, and attention deficit hyperactivity disorder. Additionally, they more frequently met the criteria for OSA and moderate-to-severe insomnia and reported shorter sleep durations. Finally, participants in the obesity group had significantly higher PASC scores, indicating a greater likelihood of experiencing persistent symptoms commonly associated with a prior COVID-19 infection. The participant’s country of origin is specified in Supplemental Table S3.
Table 1.
Characteristics of ICOSS-2 participants categorized by body mass index status (N = 5919).
| Variable | Normal weight | Overweight | Obesity | P value |
|---|---|---|---|---|
| Participants, total number | 3406 | 1710 | 803 | -- |
| PASC score, mean (SD) | 1.0 (3.0) | 1.2 (3.6) | 2.9 (5.2) | <0.001 |
| PASC score ≥12 | 93 (2.7) | 58 (3.4) | 74 (9.2) | <0.001 |
| Age (years), mean (SD) | 43.2 (17.7) | 48.4 (16.4) | 47.5 (15.8) | <0.001 |
| Sex | <0.001 | |||
| Male | 1097 (32.2) | 828 (48.4) | 294 (36.6) | |
| Female | 2309 (67.8) | 882 (51.6) | 509 (63.4) | |
| Race/ethnicity | <0.001 | |||
| White/Caucasian | 1580 (46.4) | 837 (48.9) | 535 (66.6) | |
| Asian | 1557 (45.7) | 757 (44.3) | 201 (25.0) | |
| Other | 269 (7.9) | 116 (6.8) | 67 (8.3) | |
| Smoking status | 0.19 | |||
| Never or less than once per month | 2736 (80.3) | 1351 (79.0) | 633 (78.8) | |
| Less than once per week | 88 (2.6) | 52 (3.0) | 15 (1.9) | |
| 1–5 days per week | 96 (2.8) | 38 (2.2) | 19 (2.4) | |
| Every day or almost daily | 486 (14.3) | 269 (15.7) | 136 (16.9) | |
| Weekly physical activity score, mean (SD) | 1.9 (2.4) | 1.9 (2.3) | 1.5 (2.1) | <0.001 |
| Urbanicity | 1871 (54.9) | 976 (57.1) | 525 (65.4) | <0.001 |
| ≤6 months have elapsed since the first mRNA vaccination | 2818 (82.7) | 1336 (78.1) | 648 (80.7) | <0.001 |
| Body mass index (kg/m2), mean (SD) | 21.0 (2.1) | 26.0 (1.7) | 33.4 (3.7) | <0.001 |
| Reported a positive SARS-CoV-2 test result | 270 (7.9) | 145 (8.5) | 100 (12.5) | <0.001 |
| Regularly sleeping less than 6 hours per night | 474 (13.9) | 314 (18.4) | 180 (22.4) | <0.001 |
| Regularly sleeping more than 9 hours per night | 97 (2.8) | 69 (4.0) | 25 (3.1) | 0.08 |
| Insomnia severity index score, mean (SD) | 7.5 (5.6) | 8.3 (5.9) | 10.1 (6.8) | <0.001 |
| High risk for moderate-to-severe insomnia | 420 (12.3) | 279 (16.3) | 230 (28.6) | <0.001 |
| High risk for obstructive sleep apnea | 119 (3.5) | 159 (9.3) | 166 (20.7) | <0.001 |
| History of type 2 diabetes | 75 (2.2) | 72 (4.2) | 64 (8.0) | <0.001 |
| History of hypertension | 299 (8.8) | 357 (20.9) | 272 (33.9) | <0.001 |
| History of attention deficit hyperactivity disorder | 271 (8.0) | 152 (8.9) | 118 (14.7) | <0.001 |
| History of depression | 341 (10.0) | 235 (13.7) | 217 (27.0) | <0.001 |
Data are presented as numbers (percentages) unless otherwise stated. Group comparisons were conducted using Chi-Square tests for categorical variables and generalized linear models for continuous variables (where P values refer to the main effect of BMI status). A significance level of P < .05 was used to determine statistical significance. PASC post-acute sequelae of SARS-CoV-2.
Association between obesity and long COVID status
The frequency of PASC symptoms among double-vaccinated ICOSS-2 participants, categorized by BMI group alone, as well as by combined BMI group and probable long COVID status, is presented in Supplemental Table S4 and Supplemental Table S5, respectively. As depicted in Fig. 1, participants with obesity had 1.55 times higher OR of having a PASC score ≥12 [95% CI: 1.05, 2.28], compared to those with normal weight (adjusted P = 0.028). However, there were no significant differences in OR of having a PASC score ≥12 between participants with overweight and those with normal weight, both in the unadjusted (P = 0.188) and the adjusted analyses (P = 0.650; Fig. 1).
Fig. 1. Association of body mass index with probable long COVID status.
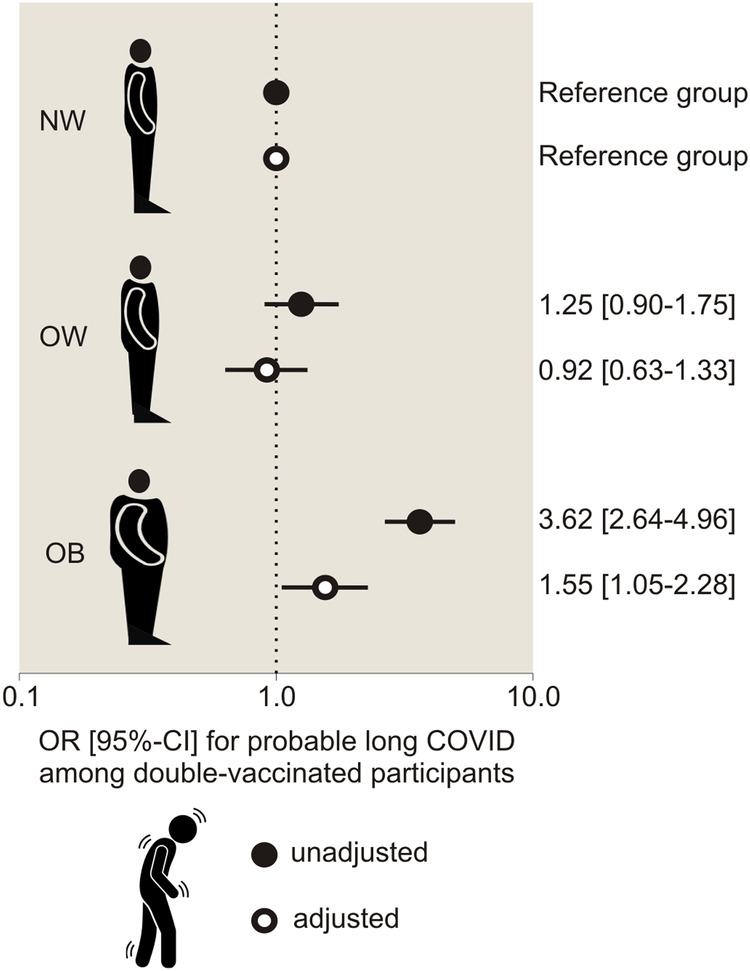
OR [95% CI] indicates odds ratio [95% Confidence Interval]. NW participants with normal weight, OW participants with overweight; and OB, participants with obesity.
In the subgroup analysis of ICOSS-2 participants who indicated that they had tested positive for SARS-CoV-2 before the survey, the unadjusted OR of having a PASC score ≥12 was 2.61 times higher in participants with obesity (n = 100) compared to the normal weight reference group (n = 270; [95% CI: 1.49, 4.58], P < 0.001). However, the unadjusted OR of having a PASC score ≥12 between participants with overweight (n = 145) and those with normal weight did not significantly differ (OR: 1.01 [95% CI: 0.56, 1.84], P = 0.968). After adjusting for potential confounders, the OR of having a PASC score ≥12 did not significantly differ among the BMI groups (P = 0.808 for obesity vs. normal weight and P = 0.381 for overweight vs. normal weight, respectively).
As summarized in Supplemental Table S6, the association between obesity and a having a PASC score ≥12 was present in both men and women in the unadjusted analysis (P < 0.001). Following adjustment, OR of having a PASC score ≥12 were only significantly higher among women (adjusted P = 0.032). However, no significant multiplicative interaction between participants’ sex and BMI status was found (adjusted P = 0.518).
Ultimately, the correlation between obesity and an increased OR of having a PASC score ≥12 remained significant even after excluding participants from the USA (Supplemental Table S7), those from the group without probable long COVID whose PASC score ranged from 3 to 11 (Supplemental Table S8), and individuals who were underweight (Supplemental Table S9).
Association between obesity, probable long COVID, and inadequate sleep
As presented in Table 2, a significant correlation between overweight and obesity and elevated adjusted ORs ranging from 1.33 to 5.12 [95% CI range: 1.11, 6.70] for experiencing moderate-to-severe insomnia, OSA, and short sleep duration was found (adjusted P ≤ 0.002). Furthermore, participants with obesity had lower OR of sleeping more than 9 h (OR: 0.60 [95% CI: 0.37, 0.98], P = 0.042). Additionally, irrespective of their BMI status, participants with probable long COVID exhibited a heightened likelihood of experiencing moderate-to-severe insomnia, OSA, and prolonged sleep duration (adjusted ORs ranging from 1.98 to 2.88 [95% CI range: 1.32, 4.78]; adjusted P ≤ 0.008).
Table 2.
Association between probable long COVID, body mass index, and sleep in ICOSS-2.
| Group status | OR [95% CI] for being at high risk for moderate-to-severe insomnia | OR [95% CI] for having a high risk for obstructive sleep apnea | OR [95% CI] for sleeping regularly less than 6 hours per night | OR [95% CI] for sleeping regularly more than 9 hours per night | ||||
|---|---|---|---|---|---|---|---|---|
| unadjusted | adjusteda | unadjusted | adjusteda,b | unadjusted | adjusteda | unadjusted | adjusteda | |
|
Normal weight (N = 3406) |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
Overweight (N = 1710) |
1.39 [1.18–1.63] | 1.33 [1.11–1.59] | 2.83 [2.22–3.62] | 2.26 [1.75–2.91] | 1.39 [1.19–1.63] | 1.35 [1.15–1.59] | 1.43 [1.05–1.96] | 1.33 [0.96–1.86] |
|
Obesity (N = 803) |
2.85 [2.37–3.43] | 1.83 [1.48–2.26] | 7.20 [5.61–9.25] | 5.12 [3.91–6.70] | 1.79 [1.48–2.17] | 1.63 [1.32–2.00] | 1.10 [0.70–1.71] | 0.60 [0.37–0.98] |
|
PASC score <12 (N = 5964) |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
PASC score ≥12 (N = 225) |
6.15 [4.69–8.07] | 2.26 [1.64–3.10] | 4.18 [3.02–5.78] | 1.98 [1.32–2.96] | 1.65 [1.21–2.26] | 1.06 [0.75–1.51] | 4.82 [3.15–7.38] | 2.88 [1.73–4.78] |
PASC post-acute sequelae SARS-CoV-2.
aIndependent variables included in the adjusted logistic regression model: body mass index status, probable long COVID status, age, sex, ethnicity, SARS-CoV-2 test positivity report, the time elapsed since first vaccination, smoking status, living area, weekly physical activity level status score, presence of hypertension, presence of type 2 diabetes, presence of attention deficit hyperactivity disorder, and presence of depression.
bThe adjusted logistic regression model did not contain hypertension as an independent variable, as it is a component of the STOP score used to assess the risk for obstructive sleep apnea [29].
A separate logistic regression analysis, incorporating the multiplicative interaction term between BMI and probable long COVID status, indicated that the association between participants’ probable long COVID status and sleep outcomes remained consistent across different BMI statuses (adjusted P ≥ 0.141 for the multiplicative interaction between BMI and probable long COVID status).
Discussion
In our international survey study, encompassing 5919 participants aged 18 to 89 years who had received two doses of SARS-CoV-2 mRNA vaccine, we found that participants with obesity exhibited a higher likelihood of experiencing multiple probable long COVID symptoms, as determined by a composite risk score for long COVID, compared to those with normal weight. This finding challenges the null hypothesis, indicating that BMI status indeed influences the probability of experiencing long COVID symptoms.
While our survey study did not directly investigate the mechanisms underlying the association between obesity and experiencing probable long COVID, despite being double-vaccinated with SARS-CoV-2 mRNA vaccine, several potential explanations exist. For instance, a previous study [12] revealed that vaccinated participants with severe obesity had a 76% higher likelihood of experiencing hospitalization or death from COVID-19. This finding may be attributed to the fact that 55% of participants with severe obesity had undetectable levels of neutralizing antibodies against SARS-CoV-2, compared to only 12% of those with normal BMI six months after their second vaccine dose. Additionally, the neutralizing capacity of SARS-CoV-2-specific antibodies was lower in participants with obesity compared to those with a BMI in the normal weight range.
In our study, participants with probable long COVID exhibited elevated odds of experiencing sleep disorders, including moderate-to-severe insomnia and a high risk for OSA, as well as prolonged sleep durations, compared to participants with a low likelihood of long COVID. It is noteworthy that participants with obesity presented with a heightened sleep burden, manifesting as a higher likelihood of suffering from insomnia, OSA, and short sleep duration. However, associations between sleep outcomes and probable long COVID did not vary by BMI status. This suggests that although improving sleep in patients with persistent COVID symptoms may hold promise for reducing the frequency, duration, and severity of such symptoms - given that sleep enhances innate and adaptive immunity [18, 19, 34] - the heightened sleep burden due to probable long COVID may occur regardless of a participant’s BMI status.
Limitations
When interpreting our results, it is important to consider several factors. Firstly, we relied on self-reported data in this study, which could have introduced recall bias. Secondly, to mitigate the risk of misclassifying participants experiencing potential long COVID symptoms, our survey study utilized the PASC score, incorporating a cluster of post-COVID infection symptoms documented in the literature [13]. However, concerns remain regarding participants with a PASC score of 12 or higher, especially if previous COVID infection has not been confirmed, as this could stem from conditions beyond those considered in our analysis. Thus, comprehensive studies, which include rigorous testing for COVID infections such as antibody assays and polymerase chain reaction testing, are necessary to confirm our findings. Another limitation is that various factors such as limited access to SARS-CoV-2 tests, timing issues related to test sensitivity, and reluctance among respondents to undergo testing may contribute to the fact that only 9% of participants reported a positive SARS-CoV-2 result. Additionally, previous research has highlighted that a significant number of SARS-CoV-2 cases go undetected [35], with some individuals potentially unaware of their infection. It is also worth mentioning that our analysis focused exclusively on SARS-CoV-2 mRNA vaccine, as the majority of respondents received this type of vaccine. Finally, future studies could enhance their comprehensiveness by incorporating additional long COVID symptoms, as demonstrated in previous research [36]. This approach would contribute to a deeper understanding of how BMI status may influence both the risk and severity of long COVID symptoms despite vaccination against SARS-CoV-2.
Conclusions
Participants with obesity may face an increased likelihood of experiencing multiple symptoms attributed to long COVID, even after receiving two doses of the SARS-CoV-2 mRNA vaccine, compared to individuals with normal weight. This finding from our study aligns well with previous results suggesting that participants with obesity exhibit a faster decline in immunity against SARS-CoV-2 following vaccination compared to those with normal weight [12]. The association between probable long COVID and disrupted sleep, irrespective of BMI, may be significant from a therapeutic standpoint, as sleep can enhance immunity [18, 19, 34, 37] and thus aid in the recovery from long COVID. However, given the limitations of our study, such as reliance on self-reported data and potential confounding factors, our findings should be viewed as hypothesis-generating rather than definitive conclusions.
Supplementary information
Supplementary Information: Associations Between Obesity, a Composite Risk Score for Probable Long COVID, and Sleep Problems in SARS-CoV-2 Vaccinated Individuals
Acknowledgements
We express our sincere gratitude to the anonymous participants who generously donated their data and dedicated their time to contribute to the success of this study. Without their valuable participation, this research would not have been possible. Figure 1 was created with BioRender.com.
Author contributions
YD, BB, BH, CMM, CAE, FC, IM, LDeG, MP, TP, and YKW contributed to the conceptualization and design of the survey. All authors were involved in data collection. IM and MP compiled the master file containing all raw data from the participating countries. PX and CB formulated the study hypothesis, conducted the analyses, and drafted the manuscript. All co-authors provided guidance on data interpretation and made critical revisions to the text. Lastly, all authors reviewed and approved the final manuscript.
Funding
CB receives financial support from the Novo Nordisk Foundation (NNF23OC0081873) and the Swedish Brain Research Foundation (FO2023-0292). P.X. research is financially supported by the Åke Wiberg Foundation (M23-0040) and Tore Nilsson Foundation (2023-079). IM and MP’s research is financially supported by the Signe and Arne Gyllenberg Foundation (IM: #5815 and MP: #5961). The funding sources had no involvement in the design, implementation, data analysis, interpretation, or manuscript preparation of this study. Open access funding provided by Uppsala University.
Data availability
Raw data from the ICOSS surveys are accessible to researchers affiliated with universities upon approval from the ICOSS core group. For inquiries, please contact Bjørn Bjorvatn at Bjorn.Bjorvatn@uib.no.
Competing interests
FC reports grants from the Ontario Ministry of Health Innovation Grant, ResMed Foundation, and University Health Network Foundation, as well as consultation fees from Takeda Pharma outside the submitted work. CAE reports grants from the Wellcome Trust and NIHR (HTA) and is a co-founder and shareholder in Big Health outside the submitted work. YI reports personal fees and others from Astellas Pharma, personal fees from Eisai, others from Idorsia Pharmaceuticals Japan, grants from Koike Medical, personal fees from Otsuka Pharmaceutical, and grants from Philips Japan, outside the submitted work. AML reports personal fees from Takeda, Jazz Pharmaceuticals, and UCB, as well as a research grant from Aoporphan drugs. DL reports grants from Philips (Netherlands), grants from Vanda (USA), grants from Sanofi, grants from VitalAire International, grants from Merck, and grants from Janssen, Jazz, and TYTHM outside the submitted work. KM reports personal fees outside the submitted work from Eisai, Meiji Seika Pharma, Mochida, MSD, Otsuka Pharmaceutical, and Yoshitomi Pharmaceutical. CMM reports research grants from the Canadian Institutes for Health Research, Eisai, Idorsia, and Lallemand Health, consulting fees from Eisai, Idorsia, Pear Therapeutics, and Sunovion, as well as royalties from Mapi Research Trust, all outside the submitted work. MP reports personal fees and others from Bioprojet, others from Jazz Pharmaceuticals, personal fees from UCB Pharma, personal fees from GSK, personal fees from Takeda, personal fees and others from MSD, personal fees from Orion, and personal fees and others from Umecrine, outside the submitted work. TP reports personal fees from Jazz Pharmaceuticals, personal fees from Bayer Healthcare, personal fees from Neuwirth, and personal fees from Löwenstein Medical, outside the submitted work, and is a shareholder of The Siestagroup GmbH, Advanced Sleep Research GmbH, and Nukute. GP reports personal fees from UCB Pharma, personal fees from Jazz Pharmaceuticals, personal fees from Bioprojet, personal fees from Idorsia, and personal fees from Takeda, outside the submitted work. YKW reports grants from Research Grant Council General Research Fund, grants from Health and Medical Research Fund, personal fees from Eisai Co., Ltd., and others from Lundbeck HK Limited, outside the submitted work. LDeG reports personal fees from Idorsia outside the submitted work. BH reports minor funding from the City of Vienna, Department for Cultural Affairs, Science and Research. YD is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Avadel, Harmony Biosciences, Idorsia, Orexia, Takeda, Paladin, and Bioprojet. All other authors have nothing to disclose.
Ethics
This study was carried out in accordance with the Helsinki Declaration and was either ethically approved or waived in all countries, according to the national research governance and regulations. According to national research governance and regulations, the anonymous nature of survey collection did not require ethical evaluation or approval in Austria, Brazil, Finland, France, Norway, and Sweden. The data remained anonymous from the outset. Informed consent was obtained from the participants prior to their involvement in the study. Detailed information on ethical approval in each country is shown in Supplemental Table S2.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pei Xue, Email: pei.xue@uu.se.
Christian Benedict, Email: christian.benedict@uu.se.
Supplementary information
The online version contains supplementary material available at 10.1038/s41366-024-01556-w.
References
- 1.Coronavirus disease (COVID-19). https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19); date of retrieval: 24/05/24
- 2.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-Coronavirus Disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593–607. 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–15. 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–61. 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18:2027160. 10.1080/21645515.2022.2027160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. 10.1136/bmj-2021-069676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw Open. 2021;4:e2111182. 10.1001/jamanetworkopen.2021.11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, Zhang J, Tang HT, Wong HK, Lyu A, Cheung CH, et al. Prevalence and risk factors of long COVID 6-12 months after infection with the Omicron variant among nonhospitalized patients in Hong Kong. J Med Virol. 2023;95:e28862. 10.1002/jmv.28862 [DOI] [PubMed] [Google Scholar]
- 9.Loosen SH, Jensen BO, Tanislav C, Luedde T, Roderburg C, Kostev K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: a cross-sectional study from 50,402 COVID-19 patients. Infection. 2022;50:1165–70. 10.1007/s15010-022-01784-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heubner L, Petrick PL, Güldner A, Bartels L, Ragaller M, Mirus M, et al. Extreme obesity is a strong predictor for in-hospital mortality and the prevalence of long-COVID in severe COVID-19 patients with acute respiratory distress syndrome. Sci Rep. 2022;12:18418. 10.1038/s41598-022-22107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vimercati L, De Maria L, Quarato M, Caputi A, Gesualdo L, Migliore G, et al. Association between long COVID and overweight/obesity. J Clin Med. 2021;10:4143. 10.3390/jcm10184143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Klaauw AA, Horner EC, Pereyra-Gerber P, Agrawal U, Foster WS, Spencer S, et al. Accelerated waning of the humoral response to COVID-19 vaccines in obesity. Nat Med. 2023;29:1146–54. 10.1038/s41591-023-02343-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 Infectio. JAMA. 2023;329:1934–1946. 10.1001/jama.2023.8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166:1775–82. 10.1001/archinte.166.16.1775 [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869–94. 10.1016/S0889-8529(03)00069-0 [DOI] [PubMed] [Google Scholar]
- 17.van den Berg JF, Knvistingh Neven A, Tulen JH, Hofman A, Witteman JC, Miedema HM, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond). 2008;32:1083–90. 10.1038/ijo.2008.57 [DOI] [PubMed] [Google Scholar]
- 18.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. 10.1007/s00424-011-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel K, Rey AE, Cheylus A, Ayling K, Benedict C, Lange T, et al. A meta-analysis of the associations between insufficient sleep duration and antibody response to vaccination. Curr Biol. 2023;33:998–1005.e2. 10.1016/j.cub.2023.02.017 [DOI] [PubMed] [Google Scholar]
- 20.Izuhara M, Matsui K, Yoshiike T, Kawamura A, Utsumi T, Nagao K, et al. Association between sleep duration and antibody acquisition after mRNA vaccination against SARS-CoV-2. Front Immunol. 2023;14:1242302. 10.3389/fimmu.2023.1242302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin YN, Zhou LN, Liu ZR, Wang Y, Li SQ, Lu FY, et al. Short sleep duration is associated with prolonged virus shedding in SARS-CoV-2 omicron-infected patients. Nat Sci Sleep. 2023;15:547–54. 10.2147/NSS.S411677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue P, Merikanto I, Chung F, Morin CM, Espie C, Bjorvatn B, et al. Persistent short nighttime sleep duration is associated with a greater post-COVID risk in fully mRNA-vaccinated individuals. Transl Psychiatry. 2023;13:32. 10.1038/s41398-023-02334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athanasiou N, Baou K, Papandreou E, Varsou G, Amfilochiou A, Kontou E, et al. Association of sleep duration and quality with immunological response after vaccination against severe acute respiratory syndrome coronavirus-2 infection. J Sleep Res. 2023;32:e13656. 10.1111/jsr.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merikanto I, Dauvilliers Y, Chung F, Wing YK, De Gennaro L, Holzinger B, et al. Sleep symptoms are essential features of long-COVID - Comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II). J Sleep Res. 2023;32:e13754. 10.1111/jsr.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Carbonell L, Mignot E, Leschziner G, Dauvilliers Y. Understanding and approaching excessive daytime sleepiness. Lancet. 2022;400:1033–46. 10.1016/S0140-6736(22)01018-2 [DOI] [PubMed] [Google Scholar]
- 27.Ohayon MM, Reynolds CF 3rd, Dauvilliers Y. Excessive sleep duration and quality of life. Ann Neurol. 2013;73:785–94. 10.1002/ana.23818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 29.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 30.El Meouchy P, Wahoud M, Allam S, Chedid R, Karam W, Karam S. Hypertension related to obesity: pathogenesis, characteristics and factors for control. Int J Mol Sci. 2022;23:12305. 10.3390/ijms232012305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 32.Lanoye A, Adams E, Fuemmeler BF. Obesity and attention-deficit hyperactivity disorder. Curr Top Behav Neurosci. 2022;57:221–41. 10.1007/7854_2022_337 [DOI] [PubMed] [Google Scholar]
- 33.Jokela M, Laakasuo M. Obesity as a causal risk factor for depression: systematic review and meta-analysis of Mendelian Randomization studies and implications for population mental health. J Psychiatr Res. 2023;163:86–92. 10.1016/j.jpsychires.2023.05.034 [DOI] [PubMed] [Google Scholar]
- 34.Christoffersson G, Vågesjö E, Pettersson US, Massena S, Nilsson EK, Broman JE, et al. Acute sleep deprivation in healthy young men: impact on population diversity and function of circulating neutrophils. Brain Behav Immun. 2014;41:162–72. 10.1016/j.bbi.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Lau H, Khosrawipour T, Kocbach P, Ichii H, Bania J, Khosrawipour V. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology. 2021;27:110–5. 10.1016/j.pulmoe.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer A, Badier N, Zhang L, Elbéji A, Wilmes P, Oustric P, et al. Long COVID classification: findings from a clustering analysis in the predi-COVID cohort study. Int J Environ Res Public Health. 2022;19:16018. 10.3390/ijerph192316018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–180. 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information: Associations Between Obesity, a Composite Risk Score for Probable Long COVID, and Sleep Problems in SARS-CoV-2 Vaccinated Individuals
Data Availability Statement
Raw data from the ICOSS surveys are accessible to researchers affiliated with universities upon approval from the ICOSS core group. For inquiries, please contact Bjørn Bjorvatn at Bjorn.Bjorvatn@uib.no.


