Abstract
COVID-19 has been associated with high mortality in patients treated with Chimeric Antigen Receptor (CAR) T-cell therapy for hematologic malignancies. Here, we investigated whether the outcome has improved over time with the primary objective of assessing COVID-19-attributable mortality in the Omicron period of 2022 compared to previous years. Data for this multicenter study were collected using the MED-A and COVID-19 report forms developed by the EBMT. One-hundred-eighty patients were included in the analysis, 39 diagnosed in 2020, 35 in 2021 and 106 in 2022. The median age was 58.9 years (min-max: 5.2–78.4). There was a successive decrease in COVID-19-related mortality over time (2020: 43.6%, 2021: 22.9%, 2022: 7.5%) and in multivariate analysis year of infection was the strongest predictor of survival (p = 0.0001). Comparing 2022 with 2020–2021, significantly fewer patients had lower respiratory symptoms (21.7% vs 37.8%, p = 0.01), needed oxygen support (25.5% vs 43.2%, p = 0.01), or were admitted to ICU (5.7% vs 33.8%, p = 0.0001). Although COVID-19-related mortality has decreased over time, CAR T-cell recipients remain at higher risk for complications than the general population. Consequently, vigilant monitoring for COVID-19 in patients undergoing B-cell-targeting CAR T-cell treatment is continuously recommended ensuring optimal prevention of infection and advanced state-of-the art treatment when needed.
Subject terms: Immunotherapy, Haematological cancer
Introduction
It was previously reported that patients with hematologic malignancies treated with B-cell-directed Chimeric Antigen Receptor (CAR) T-cell therapy are facing a substantially increased morbidity and mortality following an infection with severe adult respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (COVID-19) when compared to the general population [1, 2]. Factors considered to be associated with a higher mortality risk were ongoing B-cell aplasia, T-cell depletion, hypogammaglobulinemia, and cytopenias; all leading to a severely immunocompromised state. During the pre-Omicron period, when only a minority of CAR T-cell recipients were vaccinated, we, as the European Society for Blood and Marrow Transplantation Infectious Disease Working Party (EBMT IDWP) and European Hematology Association (EHA) Lymphoma Group reported a COVID-19-attributable mortality rate of 41% [1]. Since then, much has changed as patients have been offered multiple vaccine doses and new therapies have become available. In addition, many patients probably have had prior SARS-CoV-2 infections and as has been shown in several studies, hybrid immunity most likely confers a lower risk for severe COVID-19 [3, 4]. Furthermore, SARS-CoV-2 variants less prone to cause severe lower respiratory tract disease have emerged [5, 6]. In an analysis from the EBMT registry of allogeneic hematopoietic stem cell transplantation recipients (allo-HCT), a major improvement in outcome was shown. The mortality during the early Omicron period in 2022 was only 4.5% compared to almost 25% during the first phase of the pandemic. Moreover, the mortality in vaccinated allo-HCT patients was only 1% [7]. On the other hand CAR T-cell recipients have shown substantially impaired humoral responses to vaccination whilst the exact role of cellular mediated immunity remains uncertain [8–11]. Given this decreased vaccine immunogenicity, additional therapeutic interventions are strongly recommended [12, 13]. The aim of this study was to analyze whether the outcome of SARS-CoV-2 infection in CAR T-cell recipients has improved over time, particularly between March 2020 and December 2022, information very much needed for optimized clinical decision making based on appropriate risk stratification.
Subjects and methods
The COVID-19 report form developed by the EBMT was updated and used for this multicenter survey study (www.ebmt.org). All patients gave informed consent to have their data reported to the EBMT registry. The Swedish central Ethical Board (EPM 2020-01731, 2021-04692) approved the study and other approvals, if required, were obtained according to national regulations.
CAR T-cell recipients treated for hematological B-cell malignancies with either a positive SARS-CoV-2 PCR or antigen-test diagnosed before January 2023 and at least six weeks of follow-up after initial SARS-CoV-2 diagnosis (unless the patient had died) were included. Data on baseline clinical characteristics, COVID-19 infection, management, and outcome were collected. Patients were split in three calendar years (2020, 2021, 2022). The period since the beginning of the pandemic until June 2021 includes patients previously reported [1]. Patients were considered fully vaccinated after receiving at least 3 doses of any COVID-19 vaccine. Virologic resolution of SARS-CoV-2 infection was defined as the time from the first positive PCR or antigen test until the first negative PCR test. Clinical resolution was defined as the time from the first positive PCR or antigen test until the first day when no clinical COVID-19-related signs were present. Metabolic comorbidity was defined as the presence of one or more of the following conditions: obesity, hypertension, diabetes, hypercholesterolemia, active smoking, and cardiovascular disease. The primary objective of this study was to assess the COVID-19-attributable mortality in the Omicron period of 2022 and compare it to previous years. Secondary objectives were to evaluate the frequencies of lower respiratory tract symptoms, oxygen support, hospital- and ICU admission and to explore factors associated with mortality.
Statistics
Descriptive statistics were used for clinical characteristics. For continuous variables the median, minimum and maximum values were used and for categorical variables the absolute and percentage frequencies. The continuous variables between groups were compared using the Kruskal-Wallis or one-way-ANOVA test and the categorical variables between groups were compared using the Chi-Square or Fisher-exact test (as appropriate). The overall survival (OS) was estimated using the Kaplan-Meier method, considering death due to any cause as an event and time from SARS-CoV-2 infection to the last date of follow-up as survival time. Risk factors were evaluated using a Cox proportional hazard model. We applied multiple significance testing in several 3-factor multivariable models due to the limited number of events. A p-value < 0.05 was considered statistically significant. All p-values are two-sided. The statistical software SAS v. 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform the main analyses.
Results
Patients
One hundred eighty patients from 12 different countries were included. Thirty-nine patients were reported in 2020, 35 in 2021 and 106 in 2022. Table 1 displays demographic and clinical characteristics. The median age was 58.9 years (min-max: 5.2–78.4), including 178 adults and 2 children (<18-years old). Patients in 2022 were older (median 61.3, min-max: 20.2–78.4) than in 2020–2021 (median 56.2, min-max: 5.2–72.8). Sixty-eight patients were female (37.8%). The majority of patients received CAR T-cell therapy for B-cell-non-Hodgkin lymphoma (82.2%). Most patients were in complete remission (CR) at time of SARS-CoV-2 infection (72.2%) and this proportion did not significantly change between 2020–2021 and 2022. Additional characteristics (e.g. type of CAR T-cell therapy, prior allo-HCT, IVIG substitution for hypogammaglobulinemia) can be found in Supplementary Table 1.
Table 1.
Baseline demographics and clinical characteristics of patients infected by SARS-CoV-2 per year of inclusion and differences over the years.
| Characteristics | Year | Total | P-value | ||
|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | Δ 2020–2021 versus 2022# | ||
| N = 39 | N = 35 | N = 106 | N = 180 | ||
| Age, median age in years (min-max) at COVID-19 | 58.5 (5.2–72.8) | 55.2 (17.6–72.6) | 61.3 (20.2–78.4) | 58.9 (5.2–78.4) | |
| 56.2 (5.2–72.8) | 61.3 (20.2–78.4) | 0.01** | |||
| Female sex, N (%) | 17 (43.6) | 17 (48.6) | 34 (32.1) | 68 (37.8) | 0.06 |
| CAR T-cell therapy indication, N (%) | 0.7 | ||||
| Acute leukemia | 5 (12.8) | 5 (14.3) | 10 (9.4) | 20 (11.1) | |
| B-NHL | 32 (82.1) | 27 (77.1) | 89 (84.0) | 148 (82.2) | |
| Multiple myeloma | 2 (5.1) | 3 (8.6) | 7 (6.6) | 12 (6.7) | |
| Disease status at time of COVID-19 diagnosis, N (%) | 0.09 | ||||
| Relapse/Progression | 8 (20.5) | 7 (20) | 13 (12.3) | 28 (15.5) | |
| Partial response | 4 (10.3) | 5 (14.3) | 9 (8.5) | 18 (10) | |
| Complete response | 27 (69.2) | 22 (62.9) | 81 (76.4) | 130 (72.2) | |
| Unknown | 0 (0.0) | 1 (2.9) | 3 (2.8) | 4 (2.2) | |
| Metabolic comorbidity*, N (%) | 11 (28.2) | 10 (28.6) | 36 (34.0) | 57 (31.7) | 0.3 |
| Unknown | 0 (0.0) | 5 (14.3) | 10 (9.4) | 15 (8.3) | |
| Lansky/Karnofsky-score < 70 at COVID-19 diagnosis, N (%) | 9 (23.1) | 3 (8.6) | 8 (7.5) | 20 (11.1) | 0.1 |
| Unknown | 0 (0.0) | 1 (2.9) | 15 (14.2) | 16 (8.9) | |
| Median time from CAR-T to COVID-19 diagnosis, months (min days-max months) | 7.7 (1–17.5) | 7.4 (6–37.1) | 6.4 (5–42.5) | 7.2 (1–42.5) | 0.6 |
| Symptoms at time of COVID-19 diagnosis, N (%) | |||||
| Asymptomatic | 5 (12.8) | 3 (9.1) | 14 (13.5) | 22 (12.2) | 0.6 |
| Fever | 26 (66.7) | 19 (54.3) | 46 (43.4) | 91 (50.6) | |
| 45 (60.8) | 46 (43.4) | 0.02** | |||
| Upper respiratory symptoms | 17 (43.6) | 13 (37.1) | 64 (60.4) | 94 (52.2) | |
| 30 (40.5) | 64 (60.4) | 0.02** | |||
| Lower Respiratory symptoms | 15 (38.5) | 13 (37.1) | 23 (21.7) | 51 (28.3) | |
| 28 (37.8) | 23 (21.7) | 0.01** | |||
| Oxygen support, N (%) | 18 (46.2) | 14 (40.0) | 27 (25.5) | 59 (32.8) | |
| 32 (43.2) | 27 (25.5) | 0.01** | |||
| Missing | 0 (0.0) | 3 (8.6) | 3 (2.8) | 6 (3.3) | |
| Invasive mechanical ventilation | 8/18 (44.4) | 7/14 (50.0) | 3/27 (11.1) | 18/59 (30.5) | |
| Hospital admission, N (%) | 32 (82.1) | 23 (65.7) | 46 (43.4) | 101 (56.1) | |
| 55 (74.3) | 46 (43.4) | <0.0001** | |||
| Missing | 0 (0.0) | 2 (5.7) | 0 (0.0) | 2 (1.1) | |
| Median hospital admission duration, days (min-max) | 23.5 (1–93) | 18 (2–82) | 11 (1–280) | 17.5 (1–280) | |
| 21(1–93) | 11 (1–280) | ||||
| ICU admission, N (%) | 15 (38.5) | 10 (28.6) | 6 (5.7) | 31 (17.2) | |
| 25 (33.8) | 6 (5.7) | <0.0001** | |||
| Missing | 1 (2.6) | 1 (2.9) | 0 (0.0) | 2 (1.1) | |
| Median ICU admission duration, days (min-max) | 20.0 (2–68) | 17.5 (7–38) | 7.5 (3–57) | 13.5 (2–68) | |
| 19 (2–68) | 7.5 (3–57) | ||||
B-NHL B-cell Non-Hodgkin Lymphoma.
*Metabolic comorbidities include obesity, hypertension, diabetes, hypercholesterolemia, smoking and cardiovascular disease ** significant. # The P values given in this table are for the comparison of the years 2020 and 2021 combined with the year 2022 ** significant P value.
Previous vaccination
Seventy-four of 180 patients were reported to have received at least one vaccine dose prior to the SARS-CoV-2 diagnosis (41.1%). Forty-seven of these patients (26.1%) received at least 3 vaccine doses and were considered fully vaccinated. Twenty of the 74 vaccinated patients had received at least 2 vaccine doses before their CAR T-cell infusion. The percentage of fully vaccinated patients significantly increased in 2022 compared to 2021 (no vaccine available in 2020; Table 2).
Table 2.
COVID-19 vaccination status prior to SARS-CoV-2 diagnosis.
| 2020 (N = 39) | 2021 (N = 35) | 2022 (N = 106) | Total (N = 180) | P value Δ 2021 versus 2022 | |
|---|---|---|---|---|---|
| COVID-19 vaccination status | |||||
| No vaccination | 39 (100.0) | 21 (60.0) | 15 (14.2) | 75 (41.7) | |
| 1–2 vaccinations | 0 (0.0) | 7 (20.0) | 20 (18.9) | 27 (15.0) | |
| ≥3 vaccinations | 0 (0.0) | 1 (2.9) | 46 (43.4) | 47 (26.1) | <0.0001 |
| Unknown | 0 (0.0) | 6 (17.1) | 25 (23.6) | 31 (17.2) | |
SARS-CoV-2 infection
The median time from CAR-T-cell infusion to SARS-CoV-2 infection was 7.2 months (min-max: 1 day - 42.5 months) and did not significantly differ between 2020–2021 and 2022. 12.2% of the patients were asymptomatic and this proportion of patients did not significantly differ between 2020–2021 and 2022. At the time of diagnosis, fewer patients had lower respiratory tract symptoms in 2022 compared to 2020 and 2021 (2020–2021: 37.8%, 2022: 21.7%, p = 0.01) and fever (2020–2021: 60.8%, 2022: 43.4%, p = 0.02). Conversely, more patients had upper respiratory symptoms in 2022 compared to 2020 and 2021 (2020–2021: 40.5%, 2022; 60.4%, p = 0.02). The proportion of patients hospitalized during a COVID-19 episode decreased over time (2020–2021: 74.3%, 2022: 43.4%, p < 0.0001) as did the proportion of patients needing admission to the ICU (2020–2021: 33.8%, 2022: 5.7%, p < 0.0001). In 2022, the duration of hospital admission was shorter (11 days versus 21 days) as was the time in an ICU (19 days versus 7.5 days). Furthermore, fewer patients needed oxygen support (2020–2021: 43.2%, 2022: 25.5%, p = 0.01). Available laboratory values at time of SARS-CoV-2 diagnosis can be found in Supplementary Table 2.
COVID-19 treatment
The proportion of patients treated with monoclonal antibodies (Moabs) increased over time (0% in 2020, 8.6% in 2021 and 14.2% in 2022) and 3.8% of patients had received pre-exposure tixagevimab/cilgavimab in 2022. The proportion of patients receiving antiviral drugs remained stable over time (28.2% in 2020, 31.4% in 2021 and 34.6% in 2022). However, the drugs given changed over time (11.1% of the patients received nirmatrelvir/ritonavir in 2022 compared with none during 2020 and 2021; Table 3 and Supplementary Table 3).
Table 3.
COVID-19 drugs used for treatment.
| 2020 (N = 39) | 2021 (N = 35) | 2022 (N = 106) | Total (N = 180) | |
|---|---|---|---|---|
| Drug | ||||
| Corticosteroids | 8 (20.5) | 7 (20) | 20 (18.9) | 35 (19.4) |
| Anti-inflammatory drugs* | 6 (15.4) | 6 (17.1) | 11 (10.4) | 23 (12.8) |
| Antiviral therapy | ||||
| Remdesivir | 11 (28.2) | 11 (31.4) | 24 (22.6) | 46 (25.6) |
| Molnupiravir | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (0.6) |
| Nirmatrelvir/Ritonavir | 0 (0.0) | 0 (0.0) | 20 (11.1) | 20 (11.1) |
| Monoclonal antibodies | 0 (0.0) | 3 (8.6) | 15 (14.2) | 18 (10) |
| Convalescent plasma | 9 (23.1) | 8 (22.9) | 12 (11.3) | 29 (16.1) |
| Pre-exposure monoclonal antibodies | 0 (0.0) | 0 (0.0) | 4 (3.8) | 4 (2.2) |
*The given anti-inflammatory drugs were Tocilizumab, Siltuximab, Anakinra, Baricitinib and Eculizumab and no use of Ruxolitinib, Sarilumab or Colchicine was reported.
Mortality
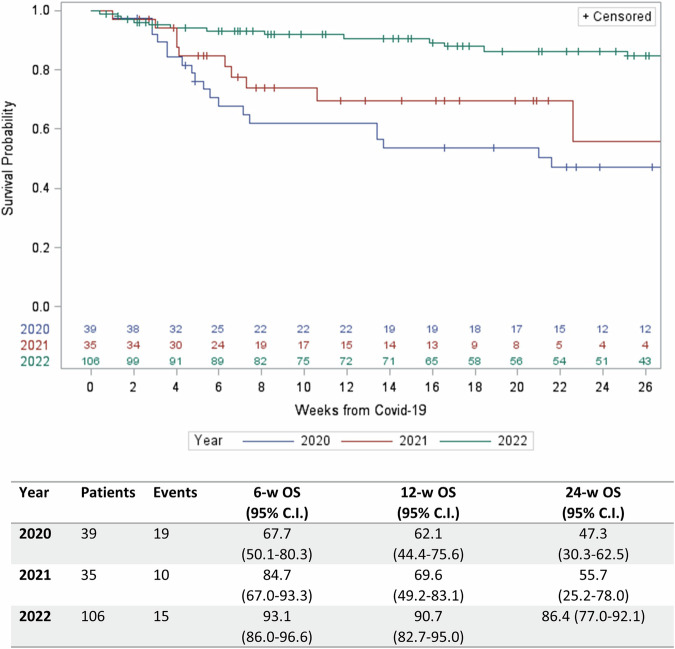
At the time of analysis 44 of 180 patients had died (24.4%) of whom 33 (75%) died from COVID-19. The COVID-19-attributable mortality decreased in 2022 to 7.5% (2020: 43.6% and 2021: 22.9%; Table 4). OS per year is shown in Fig. 1. Three of 47 fully vaccinated patients, died due to COVID-19 (6.4%). Risk factors associated with mortality in univariate analysis were older age (10 year effect, HR 1.46, 95% CI 1.14–1.86, p = 0.003), having a metabolic comorbidity (HR 2.53, 95% CI 1.39–4.62, p = 0.003), shorter time between CAR T-cell infusion and SARS-CoV-2 infection (≤3 months, HR 2.41, 95% CI 1.29–4.51, p = 0.006), remission status (no CR, HR 4.99, 95% CI 2.74–9.09, p < 0.0001), platelet count (>75 × 109/L, HR 0.32, 95% CI 0.17–0.61, p < 0.001), performance status, (10 points effect, HR 0.68, 95% CI 0.60-0.77, p < 0.0001), not being fully vaccinated before SARS-CoV-2 infection (HR 5.64, 95% CI 1.72-18.47, p = 0.004) and diagnosis of SARS-CoV-2 infection in the years 2020–2021 (year 2020, HR 3.92, 95% CI 1.99-7.74, year 2021, HR 2.65, 95% CI 1.18-5.93, year 2020–2021, HR 3.37, 95% CI 1.80–6.31, p = 0.0001; Supplementary Table 4). For the period with the highest mortality, the first 3 months after CAR T-cell infusion, mortality decreased in 2021 and 2022, (2020): 86% (6/7); 2021: 20% (1/5); 2022: 31% (8/26). Several 3-factor multivariate models were constructed testing for age, performance status, remission status, year of SARS-CoV-2 infection and time between CAR T-cell infusion and SARS-CoV-2 infection. In these models, all factors remained significant (Supplementary Table 5).
Table 4.
COVID-19-attributable mortality and resolution of COVID-19.
| 2020 (N = 39) | 2021 (N = 35) | 2022 (N = 106) | Total (N = 180) | |
|---|---|---|---|---|
| Non-COVID-19 related mortality, N (%) | 2 (5.1) | 2 (5.7) | 7 (6.6) | 11 (6.1) |
| COVID-19 related mortality, N (%) | 17 (43.6) | 8 (22.9) | 8 (7.5) | 33 (18.3) |
| Median time between COVID-19 until death, days (min-max) | 37 (7–151) | 37 (7–158) | 57 (3–283) | 39 (3–283) |
| Median follow-up time from SARS-CoV-2 diagnosis, days (min-max) | 96 (7–425) | 60 (7–332) | 158 (3–343) | 119 (3–425) |
| Virologic (PCR) resolution of SARS-CoV-2 in surviving patients, N (%) | 14 (70.0) | 15 (60.0) | 47 (51.6) | 76 (55.9) |
| Median duration virologic SARS-CoV-2 infection, days (min-max) | 24 (6–94) | 54 (13–332) | 51 (5–221) | 50 (5–332) |
| Clinical resolution COVID-19 in surviving patients, N (%) | 6 (30.0) | 9 (36.0) | 38 (41.8) | 53 (39.0) |
| Median duration clinical COVID-19, days (min-max) | 31 (7–157) | 13 (4-82) | 22 (5–218) | 21 (4–218) |
| Missing data COVID-19 resolution, N (%) | 0 (0.0) | 1 (2.9) | 6 (5.7) | 7 (3.9) |
Fig. 1. Overall survival of patients after COVID-19 diagnosis per year.
Overall survival was estimated using the Kaplan-Meier method, considering death due to any cause as an event and time from COVID-19 infection to the last date of follow-up as survival time.
Resolution
For patients surviving SARS-CoV-2 infection, the median time to clinical resolution was 21 days (min–max 4-218) and the median time to virologic (PCR) resolution 50 days (min–max 5-332; Table 4).
Discussion
The emergence of the COVID-19 pandemic had a tremendous impact on CAR T-cell therapy both in clinical trials and in standard of care. Previous reports, describing the pre-Omicron and the pre-vaccination period, showed clearly that these patients were one of the most vulnerable groups in the population with high risks for severe and prolonged disease and death in up to 50% of patients [1, 2]. Since these early reports, there have been several important developments. These include the introduction of population-based vaccination, introduction of new COVID-19 therapies, and the emergence of new SARS-CoV-2 variants, possibly all contributing to the lower mortality observed in allo-HCT- and lymphoid malignancy patients (4.5–7%) [7, 8, 14]. A cohort study of 64 CAR T-cell recipients that looked at the impact of COVID-19 vaccination and Moab use, reported a COVID-19-attributable mortality of 13% in 2022 [15]. In a retrospective analysis of 75 children and young adults receiving CAR T-cell therapy the mortality rate was 4.3%. The admission rate for SARS-CoV-2 infections was nearly 10 times higher in the pre-Omicron period (40.4%) compared to the Omicron period (4.3%), with 95.7% of patients having asymptomatic or mild SARS-CoV-2 infection after the emergence of the Omicron variant [16].
In this study, describing the largest cohort of CAR T-cell recipients with SARS-CoV-2 infection to date, we report that the COVID-19-related mortality was significantly reduced over time, with a COVID-19-attributable mortality rate of 43.6% at the beginning of the pandemic that had decreased to 7.5% in 2022 (the Omicron period). Furthermore, significantly fewer patients had lower respiratory symptoms, needed oxygen support, or had to be admitted to the hospital or ICU, reflecting a much lower severity of COVID-19. Increasing age has been one of the most important factors associated with worse outcome of COVID-19 in the general population [17]. Restricted by a limited number of events, we explored whether the year of SARS-CoV-2 infection and time from CAR T-cell therapy were associated with OS in different 3-factor multivariate models together with previously identified factors such as age, performance status and tumor remission status [1]. All these factors had significant impact on OS. The impact of SARS-COV-2 infection occurring early after CAR T cell therapy is similar to the finding in allo-HCT recipients [7]. As the Omicron period coincided with more patients being (fully) vaccinated and the availability of new therapeutic modalities, it is difficult to disentangle the relative contribution of the Omicron variant, previous SARS-CoV-2 infections, vaccinations, and new COVID-19 therapies. In the general population, newer SARS-CoV-2 variants have been associated with progressively less lung involvement and decreasing mortality, even in unvaccinated patients [18]. This has been particularly shown comparing the Omicron with the Delta variants [19]. Although vaccination is significantly associated with better survival after SARS-CoV-2 infection, vaccine effectiveness can’t be assessed in this study since we only assessed breakthrough infections.
Patients with ongoing B-cell aplasia such as patients treated with CAR T-cell therapy still have a diminished humoral response to COVID-19 vaccination with multiple dose regimens [10, 20]. These findings are in line with patients with B-cell aplasia due to primary immunodeficiency syndromes, such as XLP. However, from a large study in immunocompromised patients including patients with inborn B-cell defects it became evident that these patients have the principle capacity to mount significant T-cell responses against COVID-19 [21]. Robust cellular responses can also be detected in the majority of CAR T-cell recipients and proportions of spike-specific CD8 + T-cells even seem to be significantly higher in the absence of a humoral response [22, 23]. Although, CD8 + T-cells are associated with improved survival in B-cell depleted patients with hematologic malignancies and COVID-19, the true protective value of T-cell responses remains uncertain [24]. The finding that patients have an increased risk of dying during the first 3 months after CAR T-cell therapy can possibly be explained by the fact that patients in this period do not only have B-cell aplasia, but are also severely T-cell depleted due to the lymphodepleting chemotherapy preceding CAR T-cell infusion. The recommendations regarding timing of vaccination vary. Some groups recommend starting as early as 3 months after CAR T-cell infusion, while others recommend individual consideration based on the immune status of the patient [12, 25]. Furthermore, revaccination is advised given the apprehension about potential immunity loss after CAR T-cell infusion. However, it is important to note that the optimal revaccination regimen remains to be determined in studies [9, 26]. The potentially very long time to virologic COVID-19 resolution clearly shows that prolonged viral shedding remains a problem in CAR T-cell recipients. B-cell aplasia with diminished neutralizing antibodies seems to be the main risk factor [27]. The optimal duration and isolation measures for infection prevention are still undetermined due to the absence of standardized tests capable of differentiating between infectious virus and non-viable RNA [28]. The combination of convalescent plasma with remdesivir, as well as antiviral combinations, have shown possible effects on SARS-CoV-2 clearance in immunocompromised patients [29–31].
Limitations of this study is its retrospective nature, difficulties to ensure that all patients especially those with mild infections were reported, the lack of information about the specific SARS-CoV-2 variants infecting the patients, and missing data regarding B- and T-cell recovery after CAR T-cell therapy at time of SARS-CoV-2 diagnosis.
We conclude that COVID-19 related morbidity and mortality has been significantly reduced over time. Nonetheless CAR T-cell recipients remain at much higher risk than the general population. Therefore, the vulnerability of patients after B-cell directed CAR T-cell therapy warrants further careful monitoring together with access to and the development of preventive measures and COVID-19 treatments, including vaccinations, antivirals and monoclonal antibodies. In addition, the potential effects of COVID-19 infections on off-target side-effects associated with CAR T-cell therapy such as cytokine-release-syndrome (CRS), neurotoxicity (ICANS), hematological toxicity (ICAHT) and in particular macrophage-activation syndrome (MAS) are poorly understood and need to be studied much more extensively.
Supplementary information
Acknowledgements
We are grateful to all physicians, nurses, and other staff treating these patients under very challenging circumstances and still being able to help with providing data for this manuscript.
Author contributions
PL, RdeC, AS, MJK and SM designed the study, analyzed the data and formed the writing committee. GT is the study statistician. NSK managed the registry data. All others provided data and critically reviewed and approved first a preliminary and then the final version of the manuscript.
Funding
In October 2023 this work was presented in part at the IDWeek in Boston, USA (Ljungman P, Spanjaart AM, Tridello G, Knelange N, de la Camara R, Kersten MJ, Mielke S. 485. Improved Outcome of COVID-19 in CAR T Cell Treated Patients; results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Open Forum Infect Dis. 2023 Nov 27;10 (Suppl 2):ofad500.555.). Open access funding provided by Karolinska Institute.
Competing interests
There is no financial support for this work that could have influenced the outcomes described in the manuscript. However, several authors report a potential conflict of interest, which is described below. PL has received speaker fees for Kite/Gilead and Pfizer, PB received advisory board and consultancy fees from Allogene, Amgen, BMS/Celgene, Kite/Gilead, Incyte, Miltenyi Biomedicine, Novartis, Nektar, Pfizer and Pierre Fabre, JM has received research support from Pfizer and Gilead and has served as a consultant or speaker for Gilead, Pfizer, MSD, Cidara, Mundipharma, F2G, Amplyx, Basilea, Shionoghi, Synexis and Takeda, AB has received fees for conference attendance and advisory boards from Kite/Gilead and consultancy fees from NovartisSLP participated in advisory boards for Novartis, Kite-Gilead and Janssen, MJK reports honoraria from BMS/Celgene, Kite/Gilead, Novartis, and Roche; consulting or advisory roles for BMS/Celgene, Kite Gilead, Miltenyi Biotec, Novartis, Takeda Pharmaceuticals, and Adicet Bio; and research funding from Kite Gilead, all to her institution. RC has received speaker fees for MSD, GSK, Novartis, Pfizer, Gilead and received advisory board and consultancy fees from MSD, Astellas, Roche; SM has received speaker’s fees via his institution from Celgene/BMS, Novartis, Janssen and Pfizer, received travel support and fees via his institution for participation in an expert panel from Kite/Gilead, served on DSMB for Miltenyi and Mendes (via his institution), is the founder of SWECARNET (via his institution) and his spouse is the founder of ScientifyResearch.
Ethics approval and consent to participate statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Swedish central Ethical Board (EPM 2020-01731, 2021-04692). All patients gave informed consent to have their data reported to the EBMT registry.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anne Mea Spanjaart, Per Ljungman, Marie José Kersten, Stephan Mielke.
Deceased: Riccardo Saccardi.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-024-02336-1.
References
- 1.Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. 10.1038/s41375-021-01466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busca A, Salmanton-García J, Corradini P, Marchesi F, Cabirta A, Di Blasi R, et al. COVID-19 and CAR T cells: a report on current challenges and future directions from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv. 2022;6:2427–33. 10.1182/bloodadvances.2021005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl J Med. 2022;386:2201–12. 10.1056/NEJMoa2118946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911. 10.1016/j.envres.2022.112911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Mølbak K, Møller CH, et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13:5760. 10.1038/s41467-022-33498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;17:386.e14 [DOI] [PubMed] [Google Scholar]
- 7.Ljungman P, Tridello G, Piñana JL, Ciceri F, Sengeloev H, Kulagin A, et al. Improved outcomes over time and higher mortality in CMV seropositive allogeneic stem cell transplantation patients with COVID-19; An infectious disease working party study from the European Society for Blood and Marrow Transplantation registry. Front Immunol. 2023;14:1125824. 10.3389/fimmu.2023.1125824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P, Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, et al. Real-world assessment of immunogenicity in immunocompromised individuals following SARS-CoV-2 mRNA vaccination: a one-year follow-up of the prospective clinical trial COVAXID. EBioMedicine. 2023;94:104700. 10.1016/j.ebiom.2023.104700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggenburg S, Hofsink Q, Rutten CE, Nijhof IS, Hazenberg MD, Goorhuis A. SARS-CoV-2 vaccine-induced humoral and cellular immunity in patients with hematologic malignancies. Semin Hematol. 2022;59:192–7. 10.1053/j.seminhematol.2022.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haggenburg S, Hofsink Q, Lissenberg-Witte BI, Broers AEC, van Doesum JA, van Binnendijk RS, et al. COBRA KAI Study Team. Antibody Response in Immunocompromised Patients With Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19. JAMA Oncol. 2022;8:1477–83. 10.1001/jamaoncol.2022.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138:1278–81. 10.1182/blood.2021012769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesaro S, Mikulska M, Hirsch HH, Styczynski J, Meylan S, Cordonnier C, et al. Update of recommendations for the management of COVID-19 in patients with haematological malignancies, haematopoietic cell transplantation and CAR T therapy, from the 2022 European Conference on Infections in Leukaemia (ECIL 9). Leukemia. 2023;37:1933–8. 10.1038/s41375-023-01938-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33:259–75. 10.1016/j.annonc.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Pagano L, Salmanton-García J, Marchesi F, Blennow O, Gomes da Silva M, Glenthøj A, et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: results from the EPICOVIDEHA survey. Blood. 2022;140:2773–87. 10.1182/blood.2022017257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doesum JA, Salmanton-García J, Marchesi F, Di Blasi R, Falces-Romero I, Cabirta A, et al. Impact of SARS-CoV-2 vaccination and monoclonal antibodies on outcome post-CD19-directed CAR T-cell therapy: an EPICOVIDEHA survey. Blood Adv. 2023;7:2645–55. 10.1182/bloodadvances.2022009578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNerney KO, Richards RM, Aguayo-Hiraldo P, Calkoen FG, Talano JA, Moskop A, et al. SARS-CoV-2 infections in pediatric and young adult recipients of chimeric antigen receptor T-cell therapy: an international registry report. J Immunother Cancer. 2023;11:e005957. 10.1136/jitc-2022-005957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, China: A retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TD, Subramanian A, Chakraborty R, Cotton SA, Herrera M, Huang Y, et al. The effect of SARS-CoV-2 variant on respiratory features and mortality. Sci Rep. 2023;13:4503. 10.1038/s41598-023-31761-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–12. 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofsink Q, Haggenburg S, Lissenberg-Witte BI, Broers AEC, van Doesum JA, van Binnendijk RS, et al. Fourth mRNA COVID-19 vaccination in immunocompromised patients with haematological malignancies (COBRA KAI): a cohort study. EClinicalMedicine. 2023;61:102040. 10.1016/j.eclinm.2023.102040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller TR, Sekine T, Trubach D, Niessl J, Chen P, Bergman P, et al. Additive effects of booster mRNA vaccination and SARS-CoV-2 Omicron infection on T cell immunity across immunocompromised states. Sci Transl Med. 2023;15:eadg9452. 10.1126/scitranslmed.adg9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez MA, Bhatti AM, Fitzpatrick K, Boonyaratanakornkit J, Huang ML, Campbell VL, et al. Humoral and cellular responses to SARS-CoV-2 vaccines before and after chimeric antigen receptor-modified T-cell therapy. Blood Adv. 2023;7:1849–53. 10.1182/bloodadvances.2022008338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atanackovic D, Luetkens T, Omili D, Iraguha T, Lutfi F, Hardy NM, et al. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell-depleted lymphoma after CART therapy. Blood. 2022;140:152–6. 10.1182/blood.2022016175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–9. 10.1038/s41591-021-01386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khawaja F, Papanicolaou G, Dadwal S, Pergam SA, Wingard JR, Boghdadly ZE, et al. Frequently Asked Questions on Coronavirus Disease 2019 Vaccination for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T-Cell Recipients From the American Society for Transplantation and Cellular Therapy and the American Society of Hematology. Transpl Cell Ther. 2023;29:10–18. 10.1016/j.jtct.2022.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kampouri E, Hill JA, Dioverti V. COVID-19 after hematopoietic cell transplantation and chimeric antigen receptor (CAR)-T-cell therapy. Transpl Infect Dis. 2023;25:e14144. 10.1111/tid.14144 [DOI] [PubMed] [Google Scholar]
- 27.Dispinseri S, Secchi M, Pirillo MF, Tolazzi M, Borghi M, Brigatti C, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12:2670. 10.1038/s41467-021-22958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killingley B, Mann AJ, Kalinova M, Boyers A, Goonawardane N, Zhou J, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28:1031–41. 10.1038/s41591-022-01780-9 [DOI] [PubMed] [Google Scholar]
- 29.Mikulska M, Sepulcri C, Dentone C, Magne F, Balletto E, Baldi F, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients. Clin Infect Dis. 2023;77:280–6. 10.1093/cid/ciad181 [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Simmons W, Karmarkar EN, Yoke LH, Braimah AB, Orozco JJ, et al. Successful treatment of prolonged, severe coronavirus disease 2019 lower respiratory tract disease in a B cell acute lymphoblastic leukemia patient with an extended course of Remdesivir and Nirmatrelvir/Ritonavir. Clin Infect Dis. 2023;76:926–9. 10.1093/cid/ciac868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown LK, Moran E, Goodman A, Baxendale H, Bermingham W, Buckland M, et al. Treatment of chronic or relapsing COVID-19 in immunodeficiency. J Allergy Clin Immunol. 2022;149:557–61. 10.1016/j.jaci.2021.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.