Abstract
We have reported previously that gp91phox, expressed in CHO (Chinese hamster ovary) cells, functions as a voltage-dependent proton channel. However, others have reported that COS-7 cells expressing gp91phox failed to exhibit outward proton currents, and concluded that gp91phox does not function as a proton channel. To investigate this clear difference in findings, we have examined the expression and cellular localization of the fusion protein EGFP-C–91, in which gp91phox is fused to the C-terminus of enhanced green fluorescent protein. EGFP-C–91 was observed in the plasma membrane and intracellular membranes of 30% of the transfected COS-7 cells. In the remaining COS-7 cells, EGFP-C–91 was detected in the intracellular membranes only. In CHO cells EGFP-C–91 was present in both the plasma membrane and the intracellular membranes of all transfected cells. Under the whole-cell configuration, outward currents were recorded from COS-7 cells expressing gp91phox. These increased in magnitude and lost their ‘droop’ over time as the pipette solution equilibrated with the cell cytoplasm (50 min). The threshold activation voltage for the currents was shifted by ∼60 mV for a 1 unit difference in bath pH. Zn2+ inhibited the outward currents observed in COS-7 cells expressing gp91phox. The tail current reversal potential was −64 mV at a pHo (external pH) of 8.0, −40 mV at pHo 7.4 and −8 mV at pHo 7.0, indicating that the current arises from the movement of protons. Outward currents were exhibited by 37.5% of the COS-7 cells expressing gp91phox. Proton currents were recorded following the excision of inside-out patches from cells transfected with gp91phox. The presence of outward proton currents in COS-7 cells expressing gp91phox provides further support for our proposed role for gp91phox as the NADPH oxidase-associated proton channel.
Keywords: confocal microscopy, COS-7 cell, green fluorescent protein, NADPH oxidase, Nox2, proton current
Abbreviations: CGD, chronic granulomatous disease; CHO, Chinese hamster ovary; CHO-91 cells, cells expressing Nox2/gp91phox; DMEM, Dulbecco's modified Eagle's medium; EGFP, enhanced green fluorescent protein; EGFP-C–91, fusion protein in which gp91phox is fused to the C-terminus of EGFP; pHi, internal pH; pHo, external pH
INTRODUCTION
NADPH oxidase, an enzyme present in myeloid cells, generates superoxide (O2•−) in an electrogenic manner [1]. The efflux of electrons from NADPH, through NADPH oxidase, to oxygen generates an electric current of 15–20 pF [2], which results in a rapid depolarization of the membrane potential immediately following the activation of the enzyme [1,3]. It has been proposed that the electrogenic activity of NADPH oxidase is required to drive K+ ions across the phagosome membrane to generate a high intraphagosome potassium concentration. The hypertonic intraphagosome environment is suggested to be required for the release of the granule proteins cathepsin G and elastase, which participate in the killing of the engulfed bacterium [4]. The activity of this Ca2+-activated K+ channel is reported to provide charge compensation for ∼5% of the superoxide generated [4,5]. An efflux of protons provides the major charge compensation for the movement of the electron through the NADPH oxidase [6].
NADPH oxidase is a multi-protein enzyme complex. Mutations in any one of four subunits, i.e. gp91phox (Nox2), p47phox, p67phox or p22phox, results in the absence of a functional NADPH oxidase in myeloid cells, as observed in patients with CGD (chronic granulomatous disease) [7]. A role for a fifth subunit, p40phox, has been proposed, although mutations in this protein have yet to be detected in a patient with CGD. Genes encoding proteins with sequence similarity to gp91phox have been identified in the genomes of a wide range of organisms [8]. The products of these genes in the human genome have been termed Nox proteins (for NADPH oxidase; Nox1–Nox5). Gp91phox, the originally identified protein of this family, has been renamed Nox2. In addition, genes encoding proteins with similarity in their domain structure to p47phox and p67phox, i.e. Nox organizer (NOXO1/p41) and Nox activator (NOXA1/p51) respectively, have been reported to be expressed in stomach, small intestine and uterus [9–11].
We have previously reported the presence of proton currents in CHO (Chinese hamster ovary) cells transfected with and expressing Nox2/gp91phox, but not in non-transfected CHO cells [12–14]. Mutation of histidine residues within the third transmembrane domain resulted in the loss of proton conduction through gp91phox [15,16]. Therefore we concluded that Nox2/gp91phox functions as a voltage-dependent proton channel. Expression of Nox2 [17], Nox1S (a shortened transmembrane domain region of Nox1) [17,18] or Nox5 [19] in HEK-293 cells have all been reported to be associated with the expression of proton currents.
An alternative hypothesis for the role of Nox2 in proton channels has been proposed by DeCoursey et al. [20]. They suggest that Nox2 is not itself the proton channel, but may function as a modulator of an as yet unidentified protein, which is the proton channel [20,21]. They reported a failure to detect proton currents in COS-7 cells transfected with Nox2 [21], and therefore suggested that the voltage-dependent currents reported following expression of Nox2 in either CHO or HEK cells [13,14,17,18] resulted from enhancement of the activity of an existing proton channel by the overexpression of Nox2/gp91phox [20,21]. They therefore concluded that Nox2/gp91phox itself is not a proton channel [21].
Nox2 is a protein consisting of 570 amino acids, and contains at least three Asn residues that are utilized for N-linked glycosylation [22]. The expression of Nox2 in COS-7 cells reported previously [23] resulted in a protein product that appeared to lack extensive glycosylation, as it was detected as a discreet band at 55 kDa on a Western blot, rather than the broad smear (average molecular mass ∼91 kDa) that is observed in blots of myeloid cells. The absence of glycosylation may prevent the expressed protein leaving the endoplasmic reticulum, hence altering its cellular localization. A failure of the expressed protein to localize to the plasma membrane may account for the reported absence of proton currents in COS-7 cells expressing Nox2 [21].
The recording of outward currents in CHO-91 cells (cells expressing Nox2/gp91phox) was reported previously not to be observed immediately upon establishment of the whole-cell configuration, but only after allowing time for perfusion of the cell with the pipette solution [12]. The rate of perfusion is inversely related to the size of the cell. As COS-7 cells have a larger diameter than either CHO or myeloid cells, then insufficient perfusion of the cell cytosol by the pipette solution, and hence buffering of pHi (internal pH), might also have accounted for the absence of proton currents in COS-7 cells expressing Nox2 [21].
To determine whether Nox2/gp91phox functions as a proton channel when expressed in COS-7 cells, we have determined the cellular localization of Nox2, introduced into COS-7 cells through the expression of EGFP (enhanced green fluorescent protein)-tagged Nox2, in both COS-7 and CHO cells. We report here that, unlike in CHO cells (in which it showed a predominantly plasma membrane localization), EGFP-tagged Nox2/gp91phox expressed in COS-7 cells was present either in both the exterior and internal membranes (in some cells) or exclusively in the internal membranes (in other cells). We recorded outward currents in some of the COS-7 cells expressing Nox2, and found that the threshold of activation and the reversal potential of the tail currents were dependent upon the pH gradient, and therefore must be carried by protons. We thus conclude that the expression of Nox2 in cell lines, including COS-7 cells, is associated with the presence of outward proton currents.
MATERIALS AND METHODS
Maintenance of COS-7 cells
COS-7 cells were maintained in DMEM (Dulbecco's modified Eagle's medium; Sigma) supplemented with 10% (v/v) fetal calf serum, 50 units·ml−1 penicillin and 50 μg·ml−1 streptomycin. The cells were split (one flask into four flasks) and harvested every 3–4 days, as described previously for CHO cells [13]. CHO cells were maintained in F-12/Ham's nutrient medium containing 10% (v/v) fetal calf serum, 50 units·ml−1 penicillin and 50 μg·ml−1 streptomycin, as described previously [13].
Construction of the COS-7 EGFP-C–91 cell line
The pEGFP-C1 vector (Clontech) encodes a red-shifted variant of GFP. The full-length cDNA for human gp91phox (Nox2) was inserted into the pEGFP-C1 vector as a HindIII/BamHI fragment, placing gp91phox after the C-terminus of EGFP. The COS-7 cells were harvested and resuspended in 0.6 ml of serum-free DMEM. They were transformed with 10 μg of pEGFP-C1-gp91phox DNA by electroporation (220 V, 960 μF) in a Bio-Rad Gene Pulser. The cells were diluted with 6 ml of DMEM/10% (v/v) fetal calf serum and plated out into six-well plates.
The CMV (cytomegalovirus) promoter, in the pEGFP-C1 vector, drives the constitutive expression of the EGFP fusion protein. The expression of the EGFP-C–91 fusion protein (in which gp91phox is fused to the C-terminus of EGFP) was examined from 24 h to 72 h after transformation, in non-fixed, non-permeabilized living cells, using an Inverted Leica TCS-NT confocal laser scanning microscope equipped with krypton/argon laser, with excitation lines at 488, 568 and 647 nm.
Construction of the COS-7/pMEP4-gp91phox cell line
Full-length gp91phox had previously been inserted behind the metallothionein-inducible promoter of the plasmid pMEP4 to generate pMEP4-91 [13]. COS-7 cells (107) were resuspended in 0.8 ml of serum-free DMEM with 10 μg of pMEP4-91 DNA contained in a 0.4 cm Gene Pulser cuvette. The cells were transformed by electroporation (230 V, 960 μF) and allowed to stand at room temperature for 20 min before being diluted with 20 ml of DMEM/10% (v/v) fetal calf serum and placed in a T75 tissue culture flask. The non-adherent/dead cells were removed after 24 h and the tissue culture medium replaced. Hygromycin B (150 μg·ml−1) was added 48 h after electroporation to select for transformants. The cells were split three times in the presence of 150 μg·ml−1 hygromycin B prior to their initial use.
Whole-cell recordings
Non-transformed and transformed COS-7 cells were grown in 2.5 cm-diameter Corning Petri dishes in DMEM supplemented with 10% (v/v) fetal calf serum, 50 units·ml−1 penicillin and 50 μg·ml−1 streptomycin. The whole-cell currents were recorded 24–48 h after seeding the Petri dish with the cells. In cells transfected with pMEP4–91, the expression of gp91phox was induced by a 16 h (overnight) incubation in the presence of 10 μM Cd2+. Control, non-transfected cells were treated similarly. Cells not incubated in the presence of Cd2+ (both transfected and non-transfected) were also examined. Whole-cell recording was performed as described previously for CHO and CHO-91 cells [12], with a pipette pH of 6.5 and a bath solution pH of 8.0, 7.4 or 7.0. The pipette solution contained 119 mM tetramethylammonium hydroxide, 3.7 mM EGTA, 0.74 mM CaCl2 and 120 mM Mes buffer, pH 6.5. The bath solutions contained 110 mM tetramethylammonium methane sulphonate, 2 mM Ca(OH)2, 2 mM Mg(OH)2, 5 mM glucose and 100 mM buffer (Epps for pH 8.0 and 7.4, and Hepes for pH 7.0). The bath temperature was maintained at 22 °C throughout the recordings using a PETC80-15npi temperature regulator. Perfusion of the pipette solutions into the cell was followed optically, by the inclusion of Trypan Blue (0.2%, w/v) in the pipette solution. No recordings were made from cells that failed to fill with the blue dye.
Excised-patch recording
CHO cells expressing EGFP-C–91 were utilized in preference to COS-7 cells, as CHO cells express EGFP-C–91 at their external cell membrane, compared with the few COS-7 cells within the population that do so. The cells were allowed to attach and grow for 24–48 h prior to use. The pipette [pHo (external pH) 8.0] and bath (pHi 7.0) solutions contained 100 mM Epps (in the pipette) or Hepes (bath solution), 80 mM tetramethylammonium methane sulphonate, 1 mM EGTA and 2 mM MgCl2. Following obtainment of a GΩ seal, the patch was excised by rapid upward deflection of the pipette holder, and currents were recorded immediately in the patches where a stable seal was maintained. The bath temperature was kept at 22 °C throughout.
RESULTS
We have reported previously the presence of voltage-dependent outward proton currents in CHO cells associated with the expression of human gp91phox [12], and therefore concluded that gp91phox, the product of the X-linked CGD gene, functions as a voltage-dependent proton channel. COSphox cells are a cell line generated through the transfection of COS-7 cells with four of the subunits of the NADPH oxidase, i.e. gp91phox, p67phox, p47phox and p22phox [24]. In contrast with our hypothesis, Morgan et al. [21] failed to record outward proton currents from COSphox cells. To investigate the reported difference in results between gp91phox expressed in different cell types (CHO and COS-7), we have generated COS-7 cells that express gp91phox and have utilized these cells to determine the cellular localization of the protein and to record voltage-dependent whole-cell currents.
Expression and cellular localization of human gp91phox in COS-7 cells
In the previous study reporting expression of gp91phox in COS-7 cells, the protein appeared to lack extensive N-linked glycosylation [23]. The absence of appropriate glycosylation may prevent the expressed protein leaving the endoplasmic reticulum and/or alter its cellular localization. A failure of gp91phox to localize to the plasma membrane could account for the absence of proton currents in COS-7phox cells.
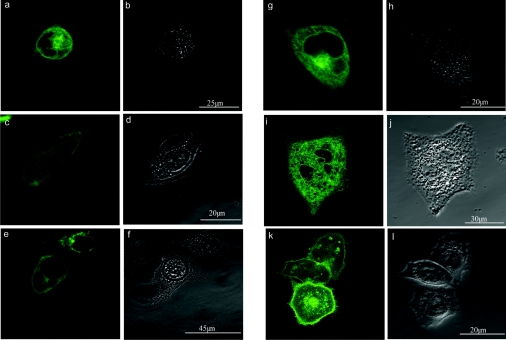
To determine the cellular localization of expressed gp91phox, we transformed COS-7 and CHO cells with EGFP-C–91 and determined the localization of the fluorescent fusion protein using confocal microscopy. At 24 h following transformation, EGFP-C–91 was observed to be located in both intracellular membranes and the plasma membrane of the cells (Figures 1a, 1c and 1e). Of the transformed COS-7 cells expressing EGFP-C–91, the protein could be visualized in both the plasma membrane and intracellular membranes in only 30% of them. In the remaining 70%, the protein was located in intracellular membranes only (Figure 1g), being absent from the plasma membrane. At 72 h post-transformation, EGFP-C–91 was still only observed within the intracellular membranes (Figure 1i) in the majority of COS-7 cells, suggesting that the failure to observe the protein in the plasma membrane at 24 h was not due to insufficient time being allowed for the synthesis, processing and trafficking of the protein through the cell to the plasma membrane. Cells transformed with the pEGFP-C1 vector alone showed EGFP fluorescence throughout the cytoplasm (results not shown). Therefore insertion of EGFP-C–91 into the membrane is driven by membrane insertion signals within the gp91phox protein sequence. In contrast with COS-7 cells, transformation of CHO cells with pEGFP-C–91 resulted in the expressed protein being detected in both the plasma membrane and intracellular membranes in 100% of the cells. The expressed protein was located predominantly in the plasma membrane of the cell (Figure 1k) at 24–72 h post-transformation. The apparent difference in the localization of EGFP-C–91 between CHO and COS-7 cells suggests that retention of EGFP-C–91 in the intracellular membranes in COS-7 cells is not due to misdirection of the protein within the cell resulting from the attachment of EGFP to gp91phox, but rather is specific to this cell type. EGFP-C–91 also shows a predominant localization to the external membrane in HeLa cells (results not shown), further supporting the observation that the localization of gp91phox in COS-7 cells is a cell-line-specific feature. COS-7 cells transfected with p22phox, the second transmembrane component of NADPH oxidase, showed cellular localization of the expressed protein (results not shown) that was comparable with that reported for gp91phox (Figure 1). In additiion, p22phox did not enhance the exterior expression of gp91phox, in agreement with the results of others [23].
Figure 1. Localization of EGFP-C–91 in COS-7 and CHO cells.
The expression and cellular localization of the EGFP-C–91 fusion protein in COS-7 (a, c, e, g, i) and CHO (k) cells was monitored using a Lecia inverted confocal laser scanning microscope. The images were collected either 24 h (a, c, e, g, k) or 72 h (i) after transformation. Panels (b), (d), (f), (h), (j) and (l) are the corresponding transmitted light images for panels (a), (c), (e), (g), (i) and (k) respectively, and include scale bars that are applicable to both the fluorescence and light images.
The localization of gp91phox in COS-7 cells was shown previously to have an annular pattern, with no apparent intracellular protein [23]. However, the expression of gp91phox was determined by Yu et al. [23] in non-permeabilized cells, using the monoclonal antibody 7D5, which recognizes an external epitope [25]. The lack of access of the antibody to intracellular antigens would result in any gp91phox located in intracellular membranes going undetected. Similarly, the antibody would not bind to COS-7 cells not expressing gp91phox on the exterior of the cell. Morgan et al. [21] used Fscan analysis and the monoclonal antibody 7D5 to detect gp91phox expression in COS-7 cells. They reported that 93% of the COS-7 cells expressed gp91phox. We also observed that a high percentage of COS-7 cells expressed gp91phox, even with transient transfection. However, we have used confocal microscopy to establish the cellular localization of the expressed protein. Therefore we can conclude that gp91phox can be expressed in COS-7 cells; however, in only a fraction of the cells (∼30%) is a proportion of the expressed protein located in the plasma membrane. To record a current under the whole-cell configuration, the ion channel protein should be located in the plasma membrane. Therefore, if gp91phox is the proton channel, it would be predicted that in only ∼30% of the COS-7 cells that express the protein would an outward current be recorded.
Whole-cell currents in COS-7 cells
The consequence of a low cytoplasmic concentration of protons and the high buffering power of the cell cytosol is that, unlike other ion currents, sustained proton currents are not recorded in a cell immediately following the establishment of the whole-cell configuration; rather, they occur after perfusion of the pH buffer from the pipette into the cell to regulate/clamp pHi and to act as a source of protons. Even in the presence of a high concentration of buffer (120 mM) in the pipette solution, the pHi of the cell does not necessarily fall to the pH of the pipette solution. We have shown previously that after complete perfusion of CHO cells with pipette solution containing 120 mM Mes at pH 6.5, or even pH 5.5, the pHi of the CHO cell was 6.8 after 30–40 min [12].
The perfusion of fluorescent dyes from a pipette into a CHO cell has been reported to occur over 10–15 min following establishment of the whole-cell configuration [12]. The recording of an outward current from CHO-91 cells has been reported previously to reach a maximum 10–25 min after establishment of the whole-cell conformation. As COS-7 cells are larger (diam. 25–70 μm) than CHO cells (diam. 20–25 μm) (Figure 1), it would therefore be predicted that perfusion of the cell cytosol would require a longer time for COS-7 than for CHO cells [12]. It is therefore possible that Morgan et al. [21] failed to observe outward proton currents as a result of there being insufficient time for perfusion and regulation of the pHi arising from the large cellular volume of COS-7 cells compared with CHO and myeloid (diam. ∼10 μm) cells in particular [26].
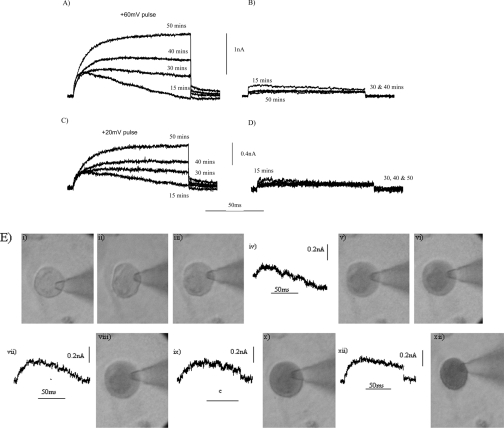
To determine whether the expression of gp91phox in COS-7 cells is associated with the presence of proton currents, we recorded whole-cell currents elicited by depolarizing voltage pulses to +20 mV and +60 mV over time following establishment of the whole-cell configuration. Recordings were made infrequently to avoid draining the pH buffering of the cell. Outward currents were observed from 15 min after obtaining the whole-cell configuration in COS-7 cells expressing gp91phox in a bath solution of pH 8.0 (Figures 2A and 2C). The current recorded at 15 min was not sustained, and ‘drooped’ during both depolarizing voltage pulses. The droop suggests that there was an insufficient concentration of protons to sustain the current, and thus a failure, at this time point, of the pipette solution pH buffer to fully clamp the cytosolic pHi of the cell. The droop in the current was less pronounced at later time points (30–50 min). In addition, the size of the current recorded at both +20 mV and +60 mV increased with time (Figures 2A and 2C).
Figure 2. Time course for the development of an outward current in COS-7 cells expressing gp91phox and in non-transfected COS-7 cells.
The currents elicited by a depolarizing voltage pulse to +60 mV (A, B) or +20 mV (C, D) were recorded in COS-7 cells expressing gp91phox (A, C) and in non-transfected COS-7 cells (B, D) at the time points shown after establishment of the whole-cell conformation. Between pulses, the cells were maintained at −60 mV. The time course for the equilibration of the pipette solution with the cell cytoplasm was monitored through the inclusion of 0.2% (w/v) Trypan Blue in the pipette solution (E). The development of the current was recorded during the time course (E). Still photographs of the progress of equilibration were taken at 0 min (i), 5 min (ii), 12 min (iii), 25 min (v), 32 min (vi), 42 min (viii), 52 min (x) and 65 min (xii) following the establishment of the whole-cell configuration. Recordings of the outward current elicited by a depolarizing pulse to +20 mV were obtained at 15 min (iv), 35 min (vii), 45 min (ix) and 55 min (xi) following establishment of the whole-cell configuration. The bath solution was at pH 8.0, and the bath temperature was maintained at 22 °C throughout.
To demonstrate that the observed time course for development of the currents paralleled that for equilibration of the pipette solution with the cell cytoplasm, the progression of Trypan Blue into the cell was monitored along with the development of the currents (Figure 2E). For the cell shown, complete equilibration with the cytoplasm took over 50 min (Figure 2E). The development of the current occurred with the same time course as that required for filling of the cell with the pipette solution (Figure 2E). Therefore outward currents developed with time as the pipette solution pH buffer perfused the cell, as would be predicted for proton currents. The larger the cell, the longer the time required to achieve full equilibration. Small outward currents were recorded from non-transfected COS-7 cells in response to the same depolarizing voltage pulses and over the same time course (Figures 2B and 2D).
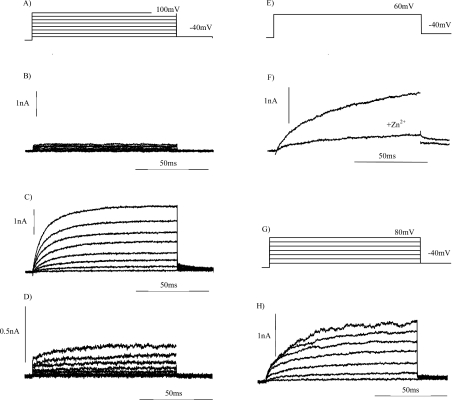
Figure 3 shows the outward currents elicited by a series of depolarizing voltage pulses from a holding potential of −60 mV to −40 mV through to +100 mV, in 20 mV steps (Figure 3A) for both a non-transformed COS-7 cell (Figure 3B) and a COS-7 cell expressing Nox2 (Figures 3C and 3D). These currents were recorded after allowing adequate time for perfusion of the pipette solution. In keeping with that reported previously for CHO-91 cells at pHo 8.0, outward currents were first observed at depolarizing voltage pulses to −20 mV (Figure 3C). Retaining the same pipette solution but placing the cells in a bath solution at pHo 7.0 decreased the transmembrane pH gradient by 1 pH unit. Under these conditions, currents were not observed until depolarization to +40 mV and greater (Figure 3D). At +80 mV the amplitude of the current elicited by the depolarizing pulse was ∼0.02 nA for non-transformed COS-7 cells, 2 nA for COS-7 cells expressing Nox2 in a bath solution at pH 8.0, and 0.2 nA when the bath solution pH was 7.0. Of all the COS-7 cells expressing gp91phox for which whole-cell configuration and dialysis of the cytosol with pipette solution were obtained, 37.5% exhibited outward currents. If we include in the total those COS-7 cells in which complete filling with Trypan Blue was not obtained, then outward currents (as shown in Figure 3) were recorded in only 21% of cells.
Figure 3. Whole-cell currents recorded from non-transformed COS-7 cells, COS-7 cells expressing gp91phox and CHO cells expressing EGFP-C–91.
Whole-cell currents were elicited by the depolarizing stepped-voltage protocol for COS-7 (A, E) and CHO (G) cells. The outward currents were recorded from non-transformed COS-7 cells (B), COS-7 cells expressing gp91phox (C, D, F) and COS-7 cells expressing EGFP-C–91 (H), as described in the Materials and methods section. The bath solution was at pH 8.0 (B, C, F) or pH 7.0 (D). Outward currents in the presence and absence of 200 μM Zn2+ are shown in (F). The holding potential was −60 mV and the bath temperature was maintained at 22 °C.
The threshold voltage for proton current activation has been reported previously to be dependent upon the pH gradient established across the plasma membrane. The larger the pH gradient, the lower the threshold voltage necessary for activation of the current and/or establishment of the proton motive force that is necessary for the efflux of protons currents [12,27]. The shift in the threshold voltage of 60 mV per pH unit reported here (Figure 3) is slightly larger than that of ∼40 mV/pH unit reported by Cherny et al. [27]. This shift in activation voltage in response to a decrease in the transmembrane concentration gradient for protons strongly suggests that protons are the charge carrier of the outward currents. The inhibition of proton currents by Zn2+ or Cd2+ has been reported previously [12]. The observation that the amplitude of the outward current elicited from COS-7 cells expressing gp91phox was markedly decreased by the presence of 200 μM Zn2+ (Figure 3F) provides further evidence that the charge-carrying species for these currents is the proton.
EGFP-C–91 expressed in COS-7 and CHO cells may not retain the three-dimensional folding and hence function(s) described previously for non-tagged gp91phox. The incorrect folding of a protein could result in its mis-targeting within the cell. To determine whether EGFP-C–91 retained the proton channel function described for gp91phox, we recorded outward currents elicited from cells expressing the EGFP-tagged protein. In a bath solution at pH 8.0, outward currents were elicited by the depolarizing voltage step protocol (Figure 3E) from COS-7 cells expressing EGFP-C–91 (Figure 3F). Therefore addition of the tag did not alter the ability of gp91phox to function as a proton conduction pathway, suggesting that incorrect folding of the protein had not arisen following the addition of the GFP tag.
The average whole-cell capacitance was 35.96 pF for COS-7 cells expressing Nox2, and 39 pF for non-transformed cells. This is higher than that for CHO cells (24–28 pF) or PLB-985 cells (8–14 pF), a myeloid cell line. Outward currents are observed at ∼5 min from PLB-985 cells [26]. The time lag before outward proton currents were first observed in COS-7 cells expressing gp91phox was longer than that reported previously for either CHO-91 or PLB-985 cells [12,26], as would be predicted for the perfusion of a large cell (Figure 1).
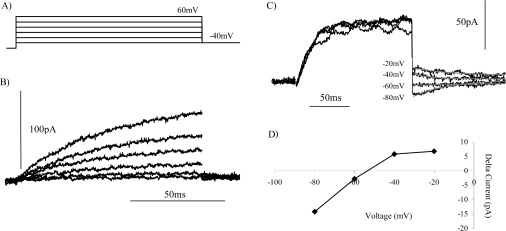
pH-dependence of the reversal potential of tail currents
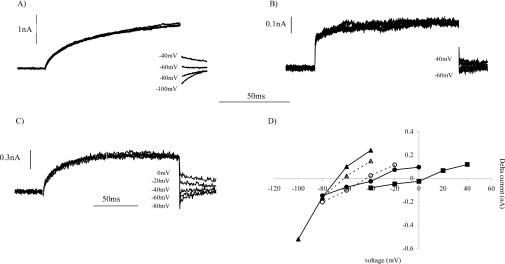
The bath and pipette solutions were designed to minimize the contribution of ions other than protons to the outward current. With a bath solution of pHo 8.0 and a pHi of 6.8, the tail currents would be predicted to reverse at −70 mV (1.2×58 mV) if the charge carriers are protons, and to show a 58 mV positive shift per pH unit decrease in the membrane pH gradient. In COS-7 cells, as in CHO cells [12], outward currents were activated at potentials more positive than −40 mV (Figure 3C); therefore, after the end of the activating depolarizing pulse, the direction of the tail currents could only be determined at voltage steps ≤−40 mV (Figure 4A). Figure 4(B) shows the tail currents observed following a depolarizing voltage pulse to +20 mV to activate the outward current. For COS-7 cells expressing Nox2/gp91phox at pHo 8.0, the reversal potential of the tail currents was −68 mV (Figure 4D). This is slightly more positive than the value of −70 mV predicted from the Nernst potential for protons. Under similar conditions, the reversal potential of the tail currents in CHO-91 cells has been reported to be −55±6 mV [12]. In a bath solution at pHo 7.0, the currents activated, and therefore the tail currents, were much smaller than those recorded at pHo 8.0 (Figure 4B). In a bath solution at pH 7.0 the tail current reversal potential shifted in a positive direction to −8 mV (Figure 4D), a change of 56 mV per pH unit. In addition, at pHo 7.4 (Figure 4C), the tail reversal potential was −40 mV (Figure 4D), comparable with that reported previously for CHO-91 cells [12]. We have previously reported a shift of 56 mV per pH unit in the tail current reversal potential for CHO-91 cells [12]. The reversal potentials of the tail currents and the current–voltage profile for the outward currents, and the observed shift in the threshold voltage for activation per pH unit change, for COS-7 cells expressing Nox2/gp91phox are comparable with the characteristics of proton currents described previously in CHO-91 cells [12]. Therefore the outward currents recorded in a subpopulation of COS-7 cells expressing gp91phox were proton currents. We thus conclude that COS-7 cells expressing gp91phox exhibit time- and voltage-dependent outward proton currents, as reported previously for CHO cells expressing gp91phox.
Figure 4. Dependence of the reversal potential of tail currents upon the pH of the bath solution.
To determine the pH dependence of the reversal potential, the repolarizing tail currents were recorded from COS-7 cells expressing gp91phox in bath solutions at pH 8.0 (A), pH 7.0 (B) and pH 7.4 (C). Outward currents were activated by depolarizing pulse to +20 mV (A), +100 mV (B) or +60 mV (C). The time-dependent tail currents were recorded upon repolarization of the membrane to (A) −40 mV through to −100 mV, (B) +40 mV through to −60 mV or (C) 0 mV through to −80 mV, in incremental 20 mV steps. (D) The difference in the tail current amplitude between the end and start of the repolarization pulse step was plotted against the repolarization command voltage for pHo 8.0 (▲), pHo 7.4 (●) and pHo 7.0 (■). The reversal potential of the tail currents obtained for CHO cells expressing EGFP-C–91 at either pHo 8.0 (▲) or pHo 7.4 (○) is also shown (broken lines). To allow plotting of the data for all three external bath solutions, the values for pHo 7.0 have been scaled up by a factor of 5.
CHO cells expressing EGFP-C–91 exhibited outward currents (Figure 3F) similar to those described with the non-tagged protein. We also determined the tail reversal potentials for CHO cells expressing EGFP-tagged gp91phox in bath solutions at pH 8.0 and pH 7.4 (open symbols in Figure 4D). The reversal potential at pHo 8.0 of −64 mV for the EGFP-tagged protein is comparable with that obtained with the non-tagged protein expressed in COS-7 and CHO cells (Figures 4A and 4D), while a reversal potential of −44 mV at pHo 7.4 is more negative than that reported for the non-tagged protein. Therefore a 20 mV shift in the reversal potential of the tail currents for a 0.5 pH unit shift is smaller than the 28 mV shift observed for gp91phox in COS-7 cells.
Inside-out patches
Excision of a patch of membrane from a cell results in the exposure of the inside, cytosolic face of the plasma membrane to the pH buffer of the bath solution. Currents should therefore be recordable immediately following excision, without the time lag required for a pipette solution to equilibrate with the cell cytoplasm. Inside-out patches were excised from CHO cells expressing EGFP-C–91 (Figure 5), CHO-91 cells (results not shown) and COS-7 cells expressing gp91phox (results not shown). Outward currents (Figure 5B) were elicited in excised patches by a stepped voltage protocol (Figure 5A). With a 1 pH unit difference across the membrane (pipette pH 8.0), outward currents were recorded at 0 mV and higher depolarizing voltage pulses, and in a few patches at −20 mV. These currents were recorded immediately following excision. The reversal potential of a set of tail currents was −54 mV (Figures 5C and 5D), which is comparable with the value of −58 mV predicted for a 1 pH unit difference. The tail current reversal potential for proton currents in excised patches from rat alveolar epithelial cells was reported to be −34 mV for 1 pH unit difference and −87 mV for a 2 pH unit difference [28], both of which are more positive than the predicted values of −58 and −116 mV respectively. Considering all patches in which the seal was stable and maintained, currents were recorded in 14% of the patches excised. Byerly and Suen [29] have reported previously that proton currents were detected in five out of 38 (13%) inside-out patches from neurons of the snail Lymnaea stagnalis, whereas 85% of the patches demonstrated K+ currents. Their suggested non-uniform distribution of proton currents in the membrane is in keeping with our observed low percentage of patches demonstrating currents.
Figure 5. Outward current in excised patches.
The bath (pH 7.0) and pipette (pHo 8.0) solutions were as described in the Materials and methods section. Excised patches were detached from CHO cells expressing EGFP-C–91 following formation of a GΩ seal. The outward currents (B) were recorded immediately after excision, in response to the voltage step protocol (A). The reversal potential of the outward current in a second excised patch was determined following an activation pulse to +100 mV (C). The first-order exponential fit of the tail currents is overlaid in grey (C). The amplitude of the tail currents (−20 mV to −80 mV) was plotted against the voltage of the repolarization step (D).
DISCUSSION
We have generated COS-7 cell lines that express gp91phox. Using GFP-tagged gp91phox (EGFP-C–91), we demonstrate that a minority of the COS-7 cells (∼30%) expressed the protein at the plasma membrane. In the remaining cells the expressed protein was present in intracellular membranes only, and not at the exterior of the cell. Time- and voltage-dependent proton currents were observed in 37.5% of COS-7 cells expressing gp91phox. These currents were absent or small in non-transformed COS-7 cells. The reversal potential of the tail currents at pHo 8.0 was −64 mV for COS-7 cells expressing gp91phox, which showed a 56 mV shift for a 1 pH unit fall in bath solution pH. These are compared with the values reported previously for CHO-91 cells [12].
Our detection of outward currents from COS-7 cells expressing gp91phox is in contrast with the absence of such currents reported by Morgan et al. [21]. We suggest that the failure of these authors to observe proton currents in COS-7phox cells may be explained by our observation that in only a minority of the cells is the protein in the plasma membrane, and therefore capable of exhibiting a proton channel. In Morgan et al. [21], the authors reported currents recorded over only 24 min after establishment of the whole-cell configuration. In addition, a failure to observe voltage-dependent outward currents may arise from there being insufficient time for perfusion of the pipette solution, and therefore failure to control pHi of the cells. For a number of cells we observed large inward current at early time points, which then disappeared with time, in agreement with Morgan et al. [21], although we did not determine the charge-carrying species. COS-7 cells are larger than both CHO (Figure 1) and myeloid cells. We suggest that the large cellular volume of COS-7 cells makes them an unsuitable cell line for the recording of proton currents.
Control of pHi in mollusc neurons has been reported previously to require a pipette with diameter of at least one-third of the cell diameter, even with pH buffer concentrations of 120 mM [30]. The tip diameter of the pipette used in our studies [12] and those of others was 1–2 μm, while cell diameters are ∼10 μm for myeloid cells, 20–25 μm for CHO cells and 30–50 μm for COS-7 cells. Therefore optimal conditions for rapid perfusion and control of pHi are not achieved.
For the inside-out patches that exhibited proton currents, the currents were recorded immediately after excision of the patch from the gp91phox-expressing cell, and not after a lag. The absence of a time delay demonstrates that exposure of at least the cytosolic face of the membrane to a strongly buffered pH solution is required to measure proton currents. This is in agreement with our observation that proton currents under the whole-cell configuration can be recorded only following filling of the cell with the pipette solution. As we have demonstrated, filling is time dependent, i.e. the bigger the cell the longer required.
Proton currents have been reported previously to be absent from non-transformed COS-7 cells [21]. However, we observed small outward currents at 30–50 min (Figure 1b), which require further investigation to determine the ion(s) involved. In recent years the expression of mRNAs for members of the Nox family has been reported for a wide range of cells and species [8,31]. The COS-7 cell line was derived from African Green Monkey kidney cells. The expression of Nox4 in kidney cells, including HEK-293 cells, has been described previously [31–33]. Therefore absence of the expression of the monkey equivalent of Nox protein should be determined to establish if COS-7 cells do lack proton channels.
The detection of outward currents in COS-7 cells transformed with gp91phox has been reported previously [17]. However, questions as to the identity of the charge carrier of the currents has been raised, based on the direction of the tail currents. The length of time that the cell had been in the whole-cell conformation prior to recording of the currents was not reported [17]. In light of the data presented here, the identity of the charge carrier of these currents cannot be established, as the properties, and hence the reversal potential, of the tail currents is determined by the pHi of the cell. The efficiency and extent of dialysis of the cell, and therefore the pHi at the time of recording of the currents, was not reported [17].
It has been suggested that gp91phox is not itself the proton channel, but is a modulator of a pre-existing proton channel [20], i.e. the proton channel is another, as yet unidentified, protein that is constitutively expressed in the cells. This hypothesis appeared to be supported by the failure of these authors to record proton currents in COS-7 cells expressing gp91phox. However, our recording of outward currents in COS-7 cells transformed with gp91phox is compatible with our proposed role for gp91phox as a proton channel. The observation that only ≤30% of our COS-7 cells expressed the protein at the plasma membrane, and the time course of the detection of the currents, provide explanation(s) for the reported failure of Morgan et al. [21] to observe currents. The possibility that gp91phox acts as modulator subunit for an unidentified proton channel is of course not excluded by the recording of current in COS-7 cells expressing gp91phox.
In conclusion, we report the recording of outward proton currents from COS-7 cells expressing gp91phox that are absent from non-transformed COS-7 cells. The currents were not apparent immediately after establishment of the whole-cell configuration, but developed over time. The re-examination of the cellular localization of Nox2 expressed in COS-7 cells and the detection of outward voltage-dependent proton currents provides support for, rather than conflicting with, our previously suggested role for gp91phox as a voltage-dependent proton channel.
Acknowledgments
The work on the EGFP-C–91 was funded by a grant from the BBSRC. The equipment utilized to record the whole-cell outward currents was purchased with a grant from the Arthritis Research Campaign. We thank Dr Bob Meech (Department of Physiology, University of Bristol) for useful discussions and for his continuing interest in this project.
References
- 1.Henderson L. M., Chappell J. B., Jones O. T. G. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrenzel J., Serrander L., Banfi B., Nusse O., Fouyouzi R., Lew D. P., Demaurex N., Krause K. H. Electron currents generated by the human phagocyte NADPH oxidase. Nature (London) 1998;392:734–737. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 3.Jankowski A., Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. quantification of the NADPH oxidase induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 4.Reeves E. P., Lu H., Jacobs H. L., Messina C. G., Bolsover S., Gabella G., Potma E. O., Warley A., Roes J., Segal A. W. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature (London) 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 5.Ahluwalia J., Tinker A., Clapp L. H., Duchen M. R., Abramov A. Y., Pope S., Nobles M., Segal A. W. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature (London) 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Henderson L. M., Chappell J. B., Jones O. T. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 1988;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- 7.Roos D., De Boer M., Kuribayashi F., Meischl C., Weening R. S., Segal A. W., Ahlin A., Nemet K., Hossle J. P., Bernatowska-Matuszkiewicz E., Middleton-Price H. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 8.Lambeth J. D., Cheng G., Arnold R. S., Edens W. A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000;10:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 9.Banfi B., Clark R. A., Steger K., Krause K. H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 10.Geiszt M., Lekstrom K., Witta J., Leto T. L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 11.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidase. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 12.Henderson L. M., Meech R. W. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 1999;114:771–786. doi: 10.1085/jgp.114.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson L. M., Banting G., Chappell J. B. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91phox functions as an essential part of the channel. J. Biol. Chem. 1995;270:5909–5916. [PubMed] [Google Scholar]
- 14.Henderson L. M., Thomas S., Banting G., Chappell J. B. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem. J. 1997;325:701–705. doi: 10.1042/bj3250701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson L. M. Role of histidines identified by mutagenesis in the NADPH oxidase-associated H+ channel. J. Biol. Chem. 1998;273:33216–33223. doi: 10.1074/jbc.273.50.33216. [DOI] [PubMed] [Google Scholar]
- 16.Mankelow T. J., Henderson L. M. Proton conduction through full-length gp91phox requires histidine 115. Protoplasma. 2003;221:101–108. doi: 10.1007/s00709-002-0056-1. [DOI] [PubMed] [Google Scholar]
- 17.Maturana A., Arnaudeau S., Ryser S., Banfi B., Hossle J. P., Schlegel W., Krause K. H., Demaurex N. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 2001;276:30277–30284. doi: 10.1074/jbc.M010438200. [DOI] [PubMed] [Google Scholar]
- 18.Banfi B., Maturana A., Jaconi S., Arnaudeau S., Laforge T., Sinha B., Ligeti E., Demaurex N., Krause K. H. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 19.Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K. H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 20.DeCoursey T. E., Cherny V. V., Morgan D., Katz B. Z., Dinauer M. C. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 2001;276:36063–36066. doi: 10.1074/jbc.C100352200. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D., Cherny V. V., Price M. O., Dinauer M. C., DeCoursey T. E. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 2002;119:571–580. doi: 10.1085/jgp.20018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallach T. M., Segal A. W. Analysis of glycosylation sites on gp91phox the flavocytochrome of the NADPH oxidase, by site directed mutagenesis and translation in vitro. Biochem. J. 1997;321:583–585. doi: 10.1042/bj3210583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L. X., Quinn M. T., Cross A. R., Dinauer M. C. Gp91phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price M. O., McPhail L. C., Lambeth J. D., Han C. H., Knaus U. G., Dinauer M. C. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99:2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]
- 25.Burritt J. B., DeLeo F. R., McDonald C. L., Prigge J. R., Dinauer M. C., Nakamura M., Nauseef W. M., Jesaitis A. J. Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J. Biol. Chem. 2001;276:2053–2061. doi: 10.1074/jbc.M006236200. [DOI] [PubMed] [Google Scholar]
- 26.Henderson L. M., Meech R. W. Proton conduction through gp91phox. J. Gen. Physiol. 2002;120:1–8. doi: 10.1085/jgp.20028708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherny V. V., Markin V. S., DeCoursey T. E. The voltage activated hydrogen ion conductances in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCoursey T. E., Cherny V. V. Voltage-activated proton currents in membrane patches of rat alveolar epithelial cells. J. Physiol. (Cambridge, U.K.) 1995;489:299–307. doi: 10.1113/jphysiol.1995.sp021051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byerly L., Suen Y. Characterization of proton currents in neurones of the snail, Lymnaea stagnalis. J Physiol. (Cambridge, U.K.) 1989;413:75–89. doi: 10.1113/jphysiol.1989.sp017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byerly L., Moody W. J. Membrane currents of internally perfused neurons of the snail, Lymnaea stagnalis, at low intracellular pH. J. Physiol. (Cambridge, U.K.) 1986;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng G., Cao Z., Xu X., Meir E. G., Lambeth J. D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 32.Geiszt M., Kopp J. B., Varnai P., Leto T. L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]