Abstract
In C9 cells, LPA (lysophosphatidic acid) induced inositol phosphate production, increased intracellular calcium concentration and inhibited adenylate cyclase activity. These responses were abolished in cells challenged with active phorbol esters. Action of phorbol esters was blocked by inhibitors of PKC (protein kinase C) and by its down-regulation. LPA1 receptor phosphorylation was observed in response to phorbol esters. The effect was rapid (t1/2∼1 min), intense (2-fold) and sustained (at least 60 min). PKC inhibitors markedly decreased the LPA1 receptor phosphorylation induced by phorbol esters. LPA1 receptor tagged with the green fluorescent protein internalized in response to PKC activation. In addition, LPA and angiotensin II were also capable of inducing LPA1 receptor phosphorylation, showing that LPA1 receptor can be subjected to homologous and heterologous desensitization.
Keywords: LPA1 receptor, lysophosphatidic acid (LPA), protein kinase C, receptor desensitization, receptor phosphorylation
Abbreviations: [Ca2+]i, intracellular calcium concentration; EGFP, enhanced green fluorescent protein; GPCR, G-protein-coupled receptor; GRK, G-protein coupled receptor kinase; GTP[S], guanosine 5′-[γ-thio]triphosphate; LPA, lysophosphatidic acid; PKC, protein kinase C; RT, reverse transcriptase
INTRODUCTION
LPA (lysophosphatidic acid), in addition to being a key intermediate in de novo lipid synthesis, is an important intercellular messenger. Originally reported to be the major phospholipid growth factor in mammalian serum [1,2], it is now a known mediator of diverse cellular processes, such as migration [3–6], proliferation and cell survival [7,8], aggregation of platelets [9,10], smooth-muscle contraction [11,12], cytoskeletal reorganization [13–15], myelination [16,17], neurogenesis [18,19] and neurotransmitter release [20,21]. It has also been proposed that LPA is involved in the pathogenesis of some clinical disorders, including atherosclerosis and myocardial injury [22,23], cancer [2,24,25] and neurodegenerative and psychiatric diseases [26,27].
It is generally accepted that the diverse effects of LPA are mediated by three distinct receptors [28,29]: LPA1 [18,30], LPA2 [31] and LPA3 [28,32]. A fourth LPA receptor was recently identified, which seems to be distant from the Edg family [33]. Nevertheless, all these receptors share the common GPCR (G-protein-coupled receptor) structure: an extracellular N-terminal domain, a C-terminal intracellular tail and seven transmembrane helices connected by three extracellular and three intracellular loops [34,35].
Stimulation of GPCRs triggers the exchange of GDP for GTP on the Gα-subunits of G-proteins and, consequently, the dissociation of α-subunits from the βγ-dimers. Such subunits can modulate the activity of downstream effectors such as adenylate cyclase, phospholipases, phosphodiesterases, ionic channels or protein kinases. The activity of these effector enzymes and ion channels regulates the intracellular concentration of second-messenger molecules or ions, which elicit cellular responses [34,36,37].
Rapid modulation of the function of many of these receptors takes place through phosphorylation/dephosphorylation cycles. Receptor phosphorylation occurs mainly on serine and threonine residues located in the third cytoplasmic loop or C-terminal tail of the receptors [38,39]. Receptor phosphorylation increases the affinity of the receptor for a family of cytoplasmic inhibitory proteins known as arrestins [40]. Phosphorylation of the receptors and recruitment of arrestins attenuates signalling by blocking G-proteins from further interaction with the receptors [41]. In addition, arrestins act as adapters to facilitate the endocytosis of GPCR mediated by clathrin-coated pits [41–45]. Internalized receptors are ultimately either dephosphorylated by a membrane-associated phosphatase [46,47] and recycled back to the plasma membrane or are ubiquitin-targeted for degradation (down-regulation) or both. Several lines of evidence support the hypothesis that receptor internalization is required for the re-sensitization of many GPCRs [42,43,45,48].
The cellular response to a given agonist may be desensitized by cellular exposure to that agonist itself, in a process known as homologous desensitization. Desensitization of the response can also be generated by cellular exposure to agonists for unrelated receptors, in a process termed as heterologous desensitization [49].
Current ideas indicate that homologous desensitization is mediated through receptor phosphorylation by GRKs (G protein coupled receptor kinases) and subsequent binding of β-arrestin. In contrast, heterologous desensitization involves the phosphorylation of GPCRs by second-messenger-dependent kinases, such as cAMP-dependent kinase and PKC (protein kinase C).
For the LPA receptors, relatively little is known concerning their regulation. The diverse actions of LPA receptors, ubiquitous expression and evolutionary conservation suggest that they play a critical role in a number of fundamental processes. The existence of several receptor subtypes suggests distinct receptor functions in vivo and raises questions about possible differences in their regulation.
We tested the effect of direct activation of PKC by PMA (also known as TPA) on the phosphorylation and function of LPA1 receptor expressed in the rat hepatic epithelial cell line C9. The results indicate that PKC induces LPA1 receptor phosphorylation at the same time as it inhibits LPA cell response. Our results also indicate that LPA1 receptor is rapidly internalized into cells in response to PMA in a process that occurs independent of agonist occupation.
EXPERIMENTAL
L-α-LPA (oleoyl-sn-glycero-3-phosphate), angiotensin II, PMA, bradykinin, staurosporine, bisindolylmaleimide I and protease inhibitors were purchased from Sigma–Aldrich. Ham's F12 medium (Kaighn's modification, F12K), fetal bovine serum, trypsin, antibiotics and other reagents used for cell culture were obtained from Gibco BRL (Gaithersburg, MD, U.S.A.). Fura 2/AM (fura 2 acetoxymethyl ester) was from Molecular Probes. Myo-[2-3H]inositol (22.9 Ci/mmol) and [32P]Pi (8500–9120 Ci/mmol) were from PerkinElmer Life Sciences (Boston, MA, U.S.A.). Protein A–Sepharose beads were from Upstate Biotechnology (Lake Placid, NY, U.S.A.). Pertussis toxin was purified from vaccine concentrates [50,51].
Cell culture
The rat hepatic epithelial cell line C9 was purchased from the A.T.C.C. (Manassas, VA, U.S.A.). C9 cells were cultured in F12K medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, 100 units/ml penicillin and 0.25 μg/ml amphotericin B at 37 °C under a 95% air and 5% CO2 atmosphere. The growth medium was removed and replaced with F12K, 1% fetal bovine serum, 12–16 h before the experiment. Where indicated, cells were incubated with pertussis toxin (100 ng/ml) for 12–16 h.
[Ca2+]i (intracellular calcium concentration) determination
Cells were loaded with 2.5 μM of the fluorescent Ca2+ indicator fura 2/AM in Krebs–Ringer–Hepes containing 0.05% BSA (pH 7.4) for 1 h at 37 °C. Cells were washed three times to eliminate the unincorporated indicator. Fluorescence measurements were carried out at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm, chopper interval set at 0.5 s using an AMINCO-Bowman Series 2 luminescence spectrometer. [Ca2+]i was calculated by the method of Grynkiewicz et al. [52].
Inositol phosphate determination
Cells approaching confluence growing in 35-mm plates were labelled with [3H]myo-inositol (6 μCi/ml) for 12–16 h. The cells were washed twice with Krebs–Ringer–Hepes buffer containing 1.3 mM CaCl2 and incubated for 20 min in 2 ml of the same buffer containing 10 mM LiCl at 37 °C in a 5% CO2 atmosphere. Agonist stimulation was stopped by the addition of chloroform/methanol (1:2, v/v). Total inositol phosphates were separated by Dowex AG1-X8 chromatography [53].
Molecular receptor characterization by RT (reverse transcriptase)–PCR
Total RNA was isolated using TRIzol® reagent (Qiagen) according to the manufacturer's instructions. LPA receptor cDNAs were generated using the Access RT–PCR System (Promega). For RT–PCRs, a 50 μl reaction mixture consisting of 0.1 unit/μl RT AMV (avian myeloblastosis virus), 0.1 unit/μl Tf1 DNA polymerase, reaction buffer AMV/TF1, 1 mM MgSO4, 0.2 mM of each dNTP, 1 μM of each primer and 0.1 μg of total RNA were incubated at 48 °C for 45 min, followed by 2 min at 95 °C and 35 cycles of 30 s at 95 °C, 30 s at 56 °C and 2 min at 72 °C.
We used the primers reported by Möller et al. [54] to amplify LPA receptor genes. The primers are as follows: to amplify LPA1, 5′-TCTTCTGGGCCATTTTCAAC-3′ and 5′-TGCCTRAAGGTGGCGCTCAT-3′ (349 bp); to amplify LPA2, 5′-CCTACCTCTTCCTCATGTTC-3′ and 5′-TAAAGGGTGGAGTCCATCAG-3′ (798 bp); to amplify LPA3, 5′-GGAATTGCCTCTGCAACATCT-3′ and 5′-GAGTAGATGATGGGGTTCA-3′ (382 bp). All primers were purchased from Sigma.
Receptor constructs and stable expression in C9 cells
The full-length cDNA encoding the mouse LPA1 (generously provided by Dr K. Lynch, Department of Pharmacology, University of Virginia, Charlottesville, VA, U.S.A.) was amplified and its stop codon removed by PCR before insertion into pEGFP-N1 vector (Clontech BD Biosciences) using KpnI–BamHI to generate a receptor tagged at the C-terminus with the EGFP (enhanced green fluorescent protein) fusion protein. The presence of the correct nucleotide sequences was verified by double-stranded DNA sequencing.
C9 cells were stably transfected with these constructs using the LIPOFECTAMINE™ 2000 (Invitrogen) following the manufacturer's instructions. Clones expressing high levels of EGFP-tagged LPA receptors were selected by resistance to Geneticin (G418) as well as by FACS using an FACSCalibur (BD Biosciences) and Cell Quest software (BD Biosciences). As a negative control, C9 cells were also stably transfected with the pEGFP-N1 vector.
Immunoprecipitation and metabolic labelling of receptors
Cells grown in 35-mm plates and approaching confluence were incubated in phosphate-free Dulbecco's modified Eagle's medium for 1 h and labelled with [32P]Pi (75 μCi/ml) in the same culture medium for 4 h at 37 °C. Labelled cells were stimulated with agents as indicated and then washed twice with ice-cold PBS and solubilized with 0.5 ml of ice-cold lysis buffer containing 100 mM NaCl, 20 mM NaF, 10 mM sodium pyrophosphate, 50 mM Tris (pH 8), 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 10 mg/ml deoxycholic acid (sodium salt), 100 nM okadaic acid and protease inhibitors (20 μg/ml leupeptin, 20 μg/ml aprotinin, 100 μg/ml PMSF, 500 μg/ml bacitracin, 20 μg/ml benzamidin, 20 μg/ml pepstatin and 50 μg/ml trypsin inhibitor).
The plates were maintained on ice for 1 h. Insoluble material was pelleted by centrifugation at 12000 g for 10 min at 4 °C. The supernatants were precleaned with Protein A–agarose, followed by incubation overnight with constant agitation at 4 °C with 1:250 anti-EGFP antiserum (Clontech BD Biosciences) and Protein A–agarose. On the next day, samples were centrifuged and the pellets were washed three times with buffer WB1 [50 mM Tris/HCl, 150 mM NaCl, 1% Triton X-100 and 12 mM deoxycholic acid (sodium salt), pH 7.5], twice with buffer WB2 [50 mM Tris/HCl, 500 mM NaCl, 0.1% Triton X-100 and 1.2 mM deoxycholic acid (sodium salt), pH 7.5] and once with buffer WB3 [50 mM Tris/HCl, 0.1% Triton X-100 and 1.2 mM deoxycholic acid (sodium salt), pH 7.5]. Finally, the immunocomplexes were denatured by boiling on loading buffer [120 mM Tris (pH 6.8), 4% SDS, 0.2% glycerol, 5% 2-mercaptoethanol, 7 M urea, 1 mM dithiothreitol and 10 mg/ml Bromophenol Blue] and subjected to SDS/PAGE. The gels were dried and the level of receptor phosphorylation was assessed using a Molecular Dynamics PhosphorImager and the Imagequant software.
Confocal microscopy
Confocal images were obtained using an MCR 1024 Bio-Rad confocal system (Bio-Rad, Hercules, CA, U.S.A.) attached/interfaced to a TMD 300 Nikon Diaphot inverted light microscope with a 100×/1.3 NA glycerol-immersion objective. EGFP was excited using the 488 nm line of a krypton/argon laser and the emitted fluorescence detected with a 515–540 nm band pass filter. All images were obtained using a numerical aperture of 1.3 and the same laser percentage, iris aperture and gain. Operating the laser at a low power setting (97–99% attenuation) substantially reduced photobleaching and photodamage. Confocal images were viewed, processed and converted into TIFF format using Todd Clark's program Confocal Assistant 4.2. Further analysis was performed using Scion Image (Scion, Frederick, MD, U.S.A.). Assays were performed with cells growing on a coverglass-based chamber. When agonists were added, the solution was pipetted directly into the chamber while pictures were taken simultaneously.
All data were analysed and plotted by using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, U.S.A.). Statistical analysis between comparable groups was performed using ANOVA with Bonferroni's analysis and was effected with software included in the GraphPad Prism program.
RESULTS
Signalling and desensitization of the LPA response in C9 cells
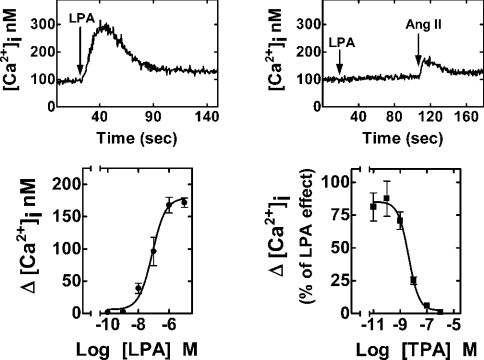
Our main goal was to study LPA effects and receptor regulation in a mammalian cell model that naturally expresses LPA receptors; hence, we used the rat hepatic epithelial cell line C9, which has been shown to mobilize calcium in response to LPA [55]. Since LPA is present in serum, we maintained the cells in the medium with minimum amount of serum, which allowed us to keep the cells in good condition. We first characterized the calcium response induced by LPA in this cell line. LPA induced an almost immediate increase in [Ca2+]i (Figure 1, upper left panel), and this effect was completely abolished in cells previously challenged with PMA (1 μM), a direct activator of PKC (Figure 1, upper right panel). The effect of LPA was concentration-dependent with an EC50 of 78±3 nM (means±S.E.M., n=12; Figure 1, lower left panel). The phorbol ester PMA (TPA) blocked the action of LPA in a concentration-dependent manner (IC50=5.0±0.3 nM; means±S.E.M., n=6; Figure 1, lower right panel). The effect of PMA was not due to a general deterioration of the calcium response since the effect of a different agent, angiotensin II, was clearly observed (Figure 1, upper right panel). These results suggest that depletion of calcium stores is not taking place and the action of PMA on LPA-mediated increase in [Ca2+]i might be explained on the basis of receptor desensitization.
Figure 1. Effects of LPA and PMA on [Ca2+]i.
C9 cells endogenously expressing LPA receptors were loaded with fura 2. Representative [Ca2+]i traces are presented in cells challenged with 1 μM LPA (upper left panel) or treated with 1 μM PMA for 5 min and then challenged with 1 μM LPA and subsequently with 100 nM angiotensin II (upper right panel). Lower left panel: effect of different concentrations of LPA on [Ca2+]i. Lower right panel: effect of different concentrations of PMA (TPA) on the increase in [Ca2+]i induced by 1 μM LPA. In the lower panels, mean values are plotted and vertical lines represent the S.E.M. for 6–12 experiments using different cell preparations. Where no vertical lines are shown, they are within the symbols.
Functional coupling with G-proteins
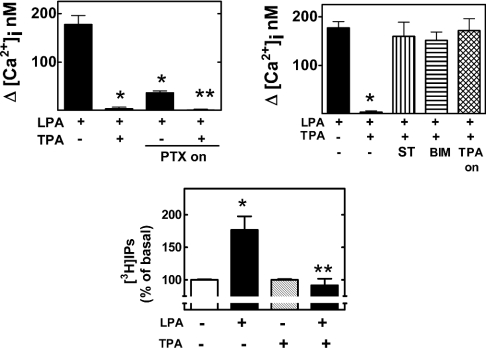
LPA receptors are known to couple with heterotrimeric G-proteins mainly of the Gq and Gi subfamilies. We then asked whether LPA-induced calcium response was mediated through Gq and/or Gi. C9 cells were incubated overnight with pertussis toxin (100 ng/ml); such a treatment inactivated pertussis toxin-sensitive G-proteins [55]. As is shown in Figure 2 (upper left panel), pertussis toxin markedly decreased but did not abolish the LPA-mediated calcium response, suggesting that both pertussis-toxin-sensitive and -insensitive G-proteins participate in the general effect, albeit the role of Gi was bigger than that of Gq. Interestingly, both were completely abolished by the activation of PKC with PMA (Figure 2, upper left panel). The coupling of LPA receptors with Gi protein in C9 cells was further confirmed by the observation that LPA markedly inhibited the cAMP accumulation induced by 1 μM forskolin and that such an effect of LPA was completely blocked by PTX treatment (results not shown). LPA (1 μM) markedly increased the production of [3H]inositol phosphates in 3H-inositol-labelled cells. PMA (1 μM) did not alter basal production of inositol phosphates but completely blocked the effect of LPA (Figure 2, lower panel).
Figure 2. Effects of PKC and the roles of pertussis-toxin-sensitive G-proteins in the actions of LPA.
Cells were incubated with the agents indicated as described in the Materials and methods section. LPA, 1 μM LPA; TPA, 1 μM PMA; TPA-on, PMA (1 μM, incubated overnight); PTX-on, pertussis toxin 100 ng/ml overnight; ST, preincubation with 300 nM staurosporine before the addition of other agents; BIM, preincubation with 1 μM bisindolylmaleimide I before the addition of other agents. Mean values are plotted and vertical lines represent the S.E.M. for 6–12 experiments using different cell preparations. Upper left panel: *P<0.001 versus LPA alone, **P<0.001 versus LPA alone and P<0.05 versus LPA treated with pertussis toxin. Upper right panel: *P<0.001 versus all the other treatments. Lower panel: *P<0.01 versus basal (no treatment); **P<0.01 versus LPA without PMA.
Use of PKC inhibitors to block LPA desensitization
To confirm the role of PKC in LPA desensitization induced by PMA, we used two different approaches to reverse PKC actions. One of them was the use of two PKC inhibitors, bisindolylmaleimide I and staurosporine; the other approach was to induce the down-regulation of classic (α, β and γ) and novel (δ, ε, θ, ν, η and μ) PKC isoforms [56,57] by overnight incubation with 1 μM PMA. Staurosporine and bisindolylmaleimide I did not alter the ability of LPA to increase [Ca2+]i (results not shown), but completely abolished the inhibitory action of PMA (Figure 2, upper right panel). Similarly, overnight incubation of the cells with PMA did not alter the effect of LPA on [Ca2+]i (results not shown); however, under these conditions, acute administration of PMA was without effect on the action of LPA (Figure 2, upper right panel).
To document further the action of PMA on the coupling of LPA receptors with GTP-binding proteins, we determined the ability of LPA to stimulate the binding of [35S]GTP[S] (where GTP[S] stands for guanosine 5′-[γ-thio]triphosphate) to untreated cell membranes (control) and PMA-treated cells membranes. In control membranes, LPA clearly increased [35S]GTP[S] binding in vitro (∼40%), whereas LPA induced a small increase in radiolabelled nucleotide binding (∼15–20%) when membranes from PMA-treated C9 cells were used (results not shown).
LPA receptor gene expression in a C9 cell line
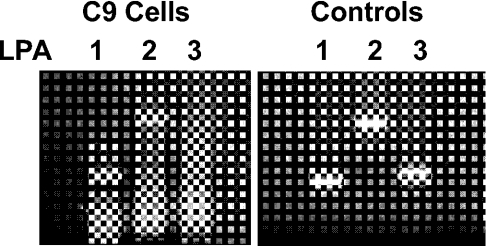
To identify which LPA receptor subtypes were mediating LPA responses in C9 cells, we characterized LPA receptor subtype gene expression by RT–PCR using specific primers for each one [54]. Robust cDNA amplification was obtained for LPA1 and LPA2 receptor subtypes, whereas no LPA3 cDNA amplification was observed in C9 cells (Figure 3). To confirm the correct size of the expected products for each gene, we used the plasmid containing LPA receptors (Figure 3). The identity of the RT–PCR product for LPA1 receptor was confirmed by sequencing. Expression of LPA4 receptor was not explored since this receptor subtype is coupled with adenylate cyclase in a stimulatory manner [33].
Figure 3. Expression of mRNA of LPA receptors in C9 cells.
RT–PCR products LPA1 (349 pb), LPA2 (798 pb) and LPA3 (382 pb) using total RNA from C9 cells as template (left panel) or plasmid cDNA of mLPA1, hLPA2 and hLPA3 (right panel).
Immunoprecipitation of phosphorylated LPA1 receptor
It was clear that PKC activation by PMA induced LPA signal desensitization, but it remained to be determined whether LPA receptors were phosphorylated. Given the rapid nature of LPA desensitization, receptor phosphorylation was examined as a probable mechanism for signal silencing. We attempted the immunoprecipitation of endogenous LPA receptors without success using commercial antibodies and others generated in our laboratory using different receptor peptides as antigens. Two main reasons could explain the failure: a low receptor density and/or that the antibodies were not suitable for immunoprecipitation. To circumvent these problems, we generated a fusion protein of LPA1 receptor targeted with the EGFP, concentrating our efforts on this receptor at the present time. The addition of EGFP protein to the C-terminus of the LPA1 receptor provides the opportunity to overexpress the receptor and demonstrate unequivocal LPA1 receptor phosphorylation by immunoprecipitation using a commercial high-affinity polyclonal anti-EGFP antibody. The LPA1–EGFP receptor was transfected into C9 cells to maintain the ‘cellular context’.
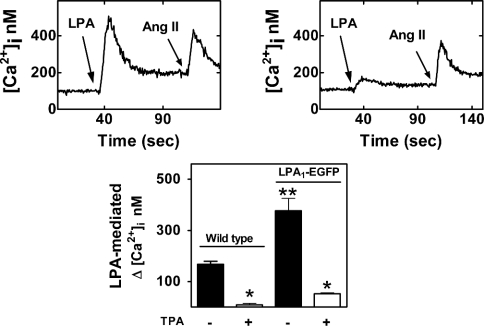
Stably transfected cells expressing LPA1–EGFP were sorted by flow cytometry and grown under selection by G418. When these cells were challenged with LPA, the increase in [Ca2+]i was consistently greater than that observed in the parental cells and the effect was also markedly attenuated by PMA (Figure 4).
Figure 4. Effects of LPA and PMA on [Ca2+]i in cells stably expressing LPA1–EGFP.
C9 cells expressing LPA1–EGFP receptors were loaded with fura 2. Representative [Ca2+]i traces are presented in cells challenged with 1 μM LPA and subsequently with 100 nM angiotensin II (upper left panel) or treated with 1 μM PMA for 5 min and then challenged with 1 μM LPA and subsequently with 100 nM angiotensin II (upper right panel). Lower panel, comparison of the effects of 1 μM LPA in wild-type cells or cells expressing LPA1–EGFP receptors, and pretreated without or with 1 μM PMA for 5 min. Mean values are plotted and vertical lines represent the S.E.M. for six experiments using different cell preparations. *P<0.001 versus absence of PMA; **P<0.001 versus wild-type.
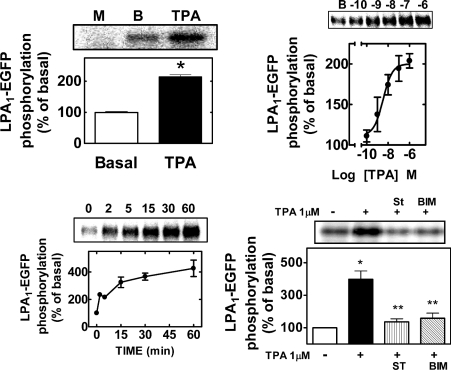
We use polyclonal rabbit antibodies directed against the EGFP protein to immunoprecipitate LPA1–EGFP receptor. It was observed that this antibody immunoprecipitated a single band with a molecular mass of approx. 90 kDa from LPA1–EGFP C9 cells, metabolically labelled with [32P]Pi (Figure 5, upper left panel). A commercial antibody against the LPA1 receptor (Santa Cruz Biotechnology) confirmed the identity of this band in Western-blot assays (results not shown). As expected, no immunoprecipitation was detected in non-transfected cells (Figure 5, upper left panel, M) and no phosphorylation was observed when cells were transfected with EGFP (results not shown). The band corresponding to the LPA1–EGFP was labelled in the basal state, suggesting that the receptor is a phosphoprotein. Such basal receptor phosphorylation was markedly increased (∼2-fold) in cells treated for 5 min with 1 μM PMA (Figure 5). The effect of PMA was concentration-dependent with an apparent EC50 of 11±2 nM (mean±S.E.M., n=4; Figure 5, upper right panel). LPA receptor phosphorylation was relatively rapid (t1/2∼1 min) and was sustained up to 60 min (Figure 5, lower left panel). LPA1 phosphorylation induced by PMA was completely inhibited by staurosporine and bisindolylmaleimide I (Figure 5, lower left panel). No effect of the inhibitors on LPA1 receptor basal phosphorylation was detected (results now shown). These results demonstrate that PKC induces LPA desensitization associated with LPA receptor phosphorylation.
Figure 5. LPA1–EGF receptor phosphorylation.
C9 cells stably expressing LPA1–EGFP receptor were metabolically labelled with [32P]Pi and the LPA1–EGFP receptor was immunoprecipitated using anti-EGFP polyclonal rabbit antibodies. Upper left panel: LPA1–EGFP basal phosphorylation (BASAL) or that induced by 1 μM PMA during a 5 min incubation (TPA), *P<0.001 versus basal. A representative autoradiograph of samples from cells expressing the EGFP alone (M) or from cells expressing LPA1–EGFP receptor treated with vehicle (B) or 1 μM PMA (TPA). Upper right panel: concentration–response curve for the effect of PMA on LPA1–EGFP receptor phosphorylation; a representative autoradiograph is shown (B, basal). Lower left panel, time course of the effect of 1 μM PMA on LPA1–EGFP phosphorylation. Results are expressed as the percentage of LPA1–EGFP receptor basal phosphorylation; mean values are plotted and vertical lines represent the S.E.M. for 4–5 determinations using different cell preparations. Lower right panel: effect of 300 nM staurosporine (ST) or 1 μM bisindolylmaleimide I (BIM) on PMA-induced LPA1–EGFP receptor phosphorylation; *P<0.001 versus basal, **P<0.001 versus PMA alone; a representative autoradiograph is shown.
Effect of activation of angiotensin II and LPA receptors on LPA1 receptor phosphorylation
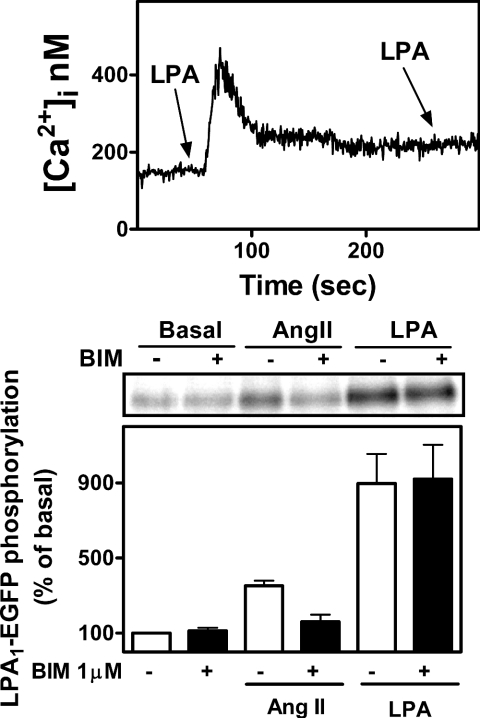
It has already been reported that the LPA response is subjected to agonist-induced regulation involving GRK2 and β-arrestin-1 [58] and that the LPA1 receptor is endocytosed via a dynamin2- and Rab5-dependent pathway [59]; however, it has not been reported that LPA receptor phosphorylation is a step in these processes. To test the possibility that the natural agonist might in fact promote LPA1–EGFP receptor phosphorylation, we challenged LPA1–EGFP C9 cells for 5 min with 1 μM LPA. LPA1 receptor phosphorylation was very robust (5–8-fold) and stronger than that observed with PMA (Figure 6). As shown in Figure 6 (upper panel), LPA induced functional desensitization with respect to calcium release. Bradykinin (results not shown) and angiotensin II also induced LPA1–EGFP receptor phosphorylation but to a smaller extent (Figure 6). Interestingly, the effect of angiotensin II was markedly decreased by bisindolylmaleimide I, but that of LPA was not (Figure 6).
Figure 6. Homologous desensitization and effects of LPA and angiotensin II on LPA1–EGFP receptor phosphorylation.
Upper panel: representative [Ca2+]i trace of cells challenged with 1 μM LPA followed by a second 1 μM LPA stimulation. Lower panel: cells were preincubated in the absence or presence of 1 μM bisindolylmaleimide for 30 min and then challenged with 100 nM angiotensin II (Ang II) or 1 μM LPA. Results are expressed as the percentage of LPA1–EGFP receptor basal phosphorylation. Mean values are plotted and vertical lines represent the S.E.M. for three determinations using different cell preparations. A representative autoradiograph is presented.
LPA1–EGFP receptor internalization induced by PMA
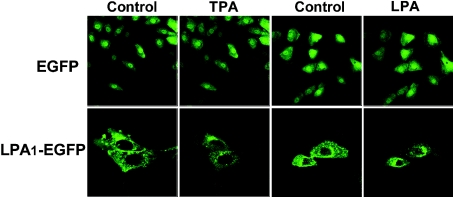
Finally, we asked whether PKC activation by PMA could affect the subcellular localization of LPA1–EGFP receptors. Fluorescence confocal microscope images showed that LPA1–EGFP is localized at the plasma membrane and intracellularly (Figure 7); the intracellular signal may be generated by receptors in the process of being inserted into the plasma membrane, being internalized or recycled. In contrast, EGFP alone showed a homogeneous cytosolic distribution (Figure 7).
Figure 7. Fluorescence confocal microscopy images of cells stably expressing the EGFP or the LPA1–EGFP.
Cells were incubated for 5 min in the absence of any agent and then further incubated for another 5 min in the presence of 1 μM PMA or LPA.
Treatment of cells with PMA (1 μM) induced LPA1–EGFP internalization as evidenced by a marked reduction of fluorescence at the level of the plasma membrane and accumulation of vesicles around the nucleus (Figure 7). The process was visualized in real time, showing that the internalization process occurs very fast; similarly, internalization of LPA1–EGFP receptors was induced by the agonist LPA (Figure 7). Altogether, these results demonstrate a temporal correlation between PMA-induced phosphorylation, desensitization and internalization of LPA1 receptors and strongly support the participation of PKC in all these processes. The role of PKC in the phosphorylation of these receptors induced by LPA is not clear; probably, GRKs may play a predominant role.
DISCUSSION
In C9 cells, LPA inhibits adenylate cyclase, induces inositol phosphate production and increases [Ca2+]i signalling through both pertussis toxin-sensitive and -insensitive G-proteins, albeit pertussis toxin-sensitive (probably Gi) proteins are mainly responsible for LPA receptor-mediated effect in these cells. It is well established that Gβγ subunits of Gi proteins activate phospholipase C β [60]. The ability of LPA to activate Gi proteins is consistent with previous reports in many other cells [1,61–64].
LPA-induced calcium response was completely abolished in C9 cells previously challenged with PMA. Such an action is selective for LPA response and cannot be attributed to calcium depletion or cell deterioration since the subsequent stimulation by a different agent induces calcium mobilization. The action of PMA was blocked by pretreatment with PKC inhibitors (bisindolylmaleimide I or staurosporine), as well as by PKC down-regulation. Our functional data indicate that LPA signalling is regulated by PKC activation in these cells. Moreover, the [35S]GTP[S] binding data suggest that PKC activation induced LPA receptor–G-protein uncoupling.
Pharmacological quantification and identification of LPA receptor subtypes is extremely difficult due to high non-specific radioligand binding and that selective agonists and antagonist for these receptors are just being developed [6,65,66]. Using RT–PCR, we documented that LPA1 and LPA2 receptors are expressed in C9 cells. In the present study, we did not explore the presence of LPA4 receptors, a new isoform recently identified and structurally distant from the Edg family [33].
The C-terminal EGFP-tagged LPA1 receptor allowed us direct detection of LPA1 receptor phosphorylation. LPA receptor phosphorylation correlates with the desensitization in these cells. Use of a PKC inhibitor markedly decreased LPA1–EGFP receptor phosphorylation induced by PMA. Altogether, these results indicate that PKC activation induced a marked desensitization of the LPA receptors, associated with a very fast receptor phosphorylation. Previous reports have shown the ability of PMA to block LPA signalling [67,68], but to the best of our knowledge this is the first report showing phosphorylation of LPA receptors and its association with desensitization. Analysis of the third intracellular loop and the C-terminus of LPA1 receptors showed several PKC putative phosphorylation sites [69] such as Thr-236, Ser-312, Thr-321 and Ser-341. Whether these residues are the actual sites of phosphorylation and their functional significance remain to be established.
There is a large amount of evidence associating phosphorylation of GPCRs with desensitization [49,55,70], and the role of PKC in the modulation of GPCRs' function has also been extensively documented [71–73]. Our confocal microscopy studies also gave interesting data. In C9 cells, PMA induced a relatively rapid and strong decrease in surface receptors, suggesting that PKC-mediated phosphorylation might play a role in LPA receptor internalization. This is consistent with the results obtained with other GPCRs [74].
Although the effects of PMA are clear when compared with LPA1–EGFP basal phosphorylation, the signal intensity is less than the LPA1–EGFP phosphorylation induced by LPA. Our result indicates that agonist-occupied LPA1 receptors are phosphorylated and desensitized. This process could involve some isoform(s) of GRKs, as observed for many other receptors, but this remains to be defined experimentally.
We showed in the present study that activation of GPCRs endogenously expressed in C9 cells, such as those for angiotensin II, can also induce LPA1–EGFP receptor phosphorylation. Several reports have documented the importance of cross-regulation between receptors coupled with the same or different signal-transduction pathways [70,75,76]. This cross-regulation seems to be important when considered in a physiological context, where cells are constantly exposed to mixtures of messengers present in the extracellular media. There are variations in the signalling pathways participating in such cross-talks [72,73,77].
In summary, our results indicate that (i) activation of PKC by PMA induced the phosphorylation of LPA1 receptor; (ii) this effect was associated with receptor desensitization and internalization and, finally, (iii) LPA1 receptor could be phosphorylated by agonist stimulation and through the activation of unrelated receptors.
Multiple LPA receptors could provide cells with overlapping properties for essential functions and/or could have different specialized functions. The existence of several LPA receptor subtypes suggests different properties in their action and/or regulation. In addition, it is possible that receptor combinatorial functions or synergisms may exist in cells expressing more than one LPA receptor subtype. The phosphorylation and desensitization of LPA receptors may have physiological importance, given that persistent LPA receptor activity could participate in the genesis of pathological conditions. Information concerning the tissue expression, their specific functions and their regulation is only just emerging.
Acknowledgments
This research was partially supported by grants from Dirección General de Asuntos del Personal Académico (IN206302 and IX247004) and Consejo Nacional de Ciencia y Tecnología (36230-N). We thank Dr K. Lynch for making available to us plasmids expressing the LPA receptors, Dr F. Recillas, C. Aranda and V. Mendoza (Instituto de Fisiología Celular, UNAM) for their help and advice with molecular biology techniques and Dr L. F. Oropeza and Dr L. Vaca (Instituto de Fisiología Celular, UNAM) for help with confocal microscopy. J.A.G.-S. dedicates this paper to his former advisor Dr J. N. Fain on his 70th birthday.
References
- 1.van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell (Cambridge, Mass.) 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 2.Moolenaar W. H. Mitogenic action of lysophosphatidic acid. Adv. Cancer Res. 1991;57:87–102. doi: 10.1016/s0065-230x(08)60996-3. [DOI] [PubMed] [Google Scholar]
- 3.Gerrard J. M., Clawson C. C., White J. G. Lysophosphatidic acids: III. Enhancement of neutrophil chemotaxis. Am. J. Pathol. 1980;100:609–618. [PMC free article] [PubMed] [Google Scholar]
- 4.Lummen G., Virchow S., Rumenapp U., Schmidt M., Wieland T., Otto T., Rubben H., Jakobs K. H. Identification of G protein-coupled receptors potently stimulating migration of human transitional-cell carcinoma cells. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:769–776. doi: 10.1007/pl00005117. [DOI] [PubMed] [Google Scholar]
- 5.Lynch K. R., Im D. S. Life on the edg. Trends Pharmacol. Sci. 1999;20:473–475. doi: 10.1016/s0165-6147(99)01401-7. [DOI] [PubMed] [Google Scholar]
- 6.Durieux M. E., Lynch K. R. Signalling properties of lysophosphatidic acid. Trends Pharmacol. Sci. 1993;14:249–254. doi: 10.1016/0165-6147(93)90021-b. [DOI] [PubMed] [Google Scholar]
- 7.Swarthout J. T., Walling H. W. Lysophosphatidic acid: receptors, signaling and survival. Cell. Mol. Life Sci. 2000;57:1978–1985. doi: 10.1007/PL00000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye X., Ishii I., Kingsbury M. A., Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- 9.Gerrard J. M., Kindom S. E., Peterson D. A., Peller J., Krantz K. E., White J. G. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am. J. Pathol. 1979;96:423–438. [PMC free article] [PubMed] [Google Scholar]
- 10.Sandmann G., Siess W., Essler M. Lysophosphatidic acid is the unique platelet-activating substance in human malignant ascites. Eur. J. Med. Res. 2003;8:397–404. [PubMed] [Google Scholar]
- 11.Toews M. L., Ustinova E. E., Schultz H. D. Lysophosphatidic acid enhances contractility of isolated airway smooth muscle. J. Appl. Physiol. 1997;83:1216–1222. doi: 10.1152/jappl.1997.83.4.1216. [DOI] [PubMed] [Google Scholar]
- 12.Mori M., Tsushima H. Activation of Rho signaling contributes to lysophosphatidic acid-induced contraction of intact ileal smooth muscle of guinea-pig. Can. J. Physiol. Pharmacol. 2000;78:729–736. [PubMed] [Google Scholar]
- 13.Jalink K., Eichholtz T., Postma F. R., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 14.Tigyi G., Fischer D. J., Sebok A., Yang C., Dyer D. L., Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 15.Manning T. J., Jr, Rosenfeld S. S., Sontheimer H. Lysophosphatidic acid stimulates actomyosin contraction in astrocytes. J. Neurosci. Res. 1998;53:343–352. doi: 10.1002/(SICI)1097-4547(19980801)53:3<343::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Weiner J. A., Hecht J. H., Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- 17.Li Y., González M. I., Meinkoth J. L., Field J., Kazanietz M. G., Tennekoon G. I. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J. Biol. Chem. 2003;278:9585–9591. doi: 10.1074/jbc.M213244200. [DOI] [PubMed] [Google Scholar]
- 18.Hecht J. H., Weiner J. A., Post S. R., Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida A., Ueda H. Neurobiology of the Edg2 lysophosphatidic acid receptor. Jpn. J. Pharmacol. 2001;87:104–109. doi: 10.1254/jjp.87.104. [DOI] [PubMed] [Google Scholar]
- 20.Shiono S., Kawamoto K., Yoshida N., Kondo T., Inagami T. Neurotransmitter release from lysophosphatidic acid stimulated PC12 cells: involvement of lysophosphatidic acid receptors. Biochem. Biophys. Res. Commun. 1993;193:667–673. doi: 10.1006/bbrc.1993.1676. [DOI] [PubMed] [Google Scholar]
- 21.Renback K., Inoue M., Ueda H. Lysophosphatidic acid-induced, pertussis toxin-sensitive nociception through a substance P release from peripheral nerve endings in mice. Neurosci. Lett. 1999;270:59–61. doi: 10.1016/s0304-3940(99)00464-4. [DOI] [PubMed] [Google Scholar]
- 22.Goetzl E. J., Graeler M., Huang M. C., Shankar G. Lysophospholipid growth factors and their g protein-coupled receptors in immunity, coronary artery disease, and cancer. Sci. World J. 2002;2:324–338. doi: 10.1100/tsw.2002.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta. 2002;1582:204–215. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., Fang X. J., Casey G., Mills G. B. Lysophospholipids activate ovarian and breast cancer cells. Biochem. J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills G. B., Moolenaar W. H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 26.Ye X., Fukushima N., Kingsbury M. A., Chun J. Lysophosphatidic acid in neural signaling. Neuroreport. 2002;13:2169–2175. doi: 10.1097/00001756-200212030-00002. [DOI] [PubMed] [Google Scholar]
- 27.Harrison S. M., Reavill C., Brown G., Brown J. T., Cluderay J. E., Crook B., Davies C. H., Dawson L. A., Grau E., Heidbreder C., et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell. Neurosci. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Chun J., Goetzl E. J., Hla T., Igarashi Y., Lynch K. R., Moolenaar W., Pyne S., Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 29.Lynch K. R. Lysophospholipid receptor nomenclature. Biochim. Biophys. Acta. 2002;1582:70–71. doi: 10.1016/s1388-1981(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 30.An S., Dickens M. A., Bleu T., Hallmark O. G., Goetzl E. J. Molecular cloning of the human Edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochem. Biophys. Res. Commun. 1997;231:619–622. doi: 10.1006/bbrc.1997.6150. [DOI] [PubMed] [Google Scholar]
- 31.An S., Bleu T., Hallmark O. G., Goetzl E. J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 32.Bandoh K., Aoki J., Hosono H., Kobayashi S., Kobayashi T., Murakami-Murofushi K., Tsujimoto M., Arai H., Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi K., Ishii S., Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 34.Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987;26:2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin J. M., Schertler G. F., Unger V. M. An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J. Mol. Biol. 1997;272:144–164. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]
- 36.Gutkind J. S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J. Biol. Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 37.Karnik S. S., Gogonea C., Patil S., Saad Y., Takezako T. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol. Metab. 2003;14:431–437. doi: 10.1016/j.tem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Bouvier M., Hausdorff W. P., De Blasi A., O'Dowd B. F., Kobilka B. K., Caron M. G., Lefkowitz R. J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature (London) 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- 39.Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J. Biol. Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 40.Lohse M. J., Andexinger S., Pitcher J., Trukawinski S., Codina J., Faure J. P., Caron M. G., Lefkowitz R. J. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J. Biol. Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 41.Luttrell L. M., Lefkowitz R. J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 43.Lefkowitz R. J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 44.Pitcher J. A., Freedman N. J., Lefkowitz R. J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 45.Koenig J. A., Edwardson J. M. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol. Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- 46.Pitcher J. A., Payne E. S., Csortos C., DePaoli-Roach A. A., Lefkowitz R. J. The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alcantara-Hernandez R., Vázquez-Prado J., García-Sáinz J. A. Protein phosphatase-protein kinase interplay modulates alpha 1b-adrenoceptor phosphorylation: effects of okadaic acid. Br. J. Pharmacol. 2000;129:724–730. doi: 10.1038/sj.bjp.0703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sibley D. R., Strasser R. H., Benovic J. L., Daniel K., Lefkowitz R. J. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lefkowitz R. J. G protein-coupled receptor kinases. Cell (Cambridge, Mass.) 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 50.Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J. Biol. Chem. 1983;258:14647–14651. [PubMed] [Google Scholar]
- 51.García-Sáinz J. A., Romero-Ávila M. T., Ruiz-Arriaga A., Ruiz-Puente J., Agundis C., Ortiz V., Isibasi A. Characterization and detoxification of an easily prepared acellular pertussis vaccine. Antigenic role of the A protomer of pertussis toxin. Vaccine. 1992;10:341–344. doi: 10.1016/0264-410x(92)90375-t. [DOI] [PubMed] [Google Scholar]
- 52.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 53.Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem. J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moller T., Contos J. J., Musante D. B., Chun J., Ransom B. R. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J. Biol. Chem. 2001;276:25946–25952. doi: 10.1074/jbc.M102691200. [DOI] [PubMed] [Google Scholar]
- 55.García-Caballero A., Olivares-Reyes J. A., Catt K. J., García-Sáinz J. A. Angiotensin AT(1) receptor phosphorylation and desensitization in a hepatic cell line. Roles of protein kinase C and phosphoinositide 3-kinase. Mol. Pharmacol. 2001;59:576–585. doi: 10.1124/mol.59.3.576. [DOI] [PubMed] [Google Scholar]
- 56.Standaert M. L., Cooper D. R., Hernandez H., Arnold T. P., Farese R. V. Differential down-regulation of insulin-sensitive protein kinase-C isoforms by 12-O-tetradecanoylphorbol-13-acetate in rat adipocytes and BC3H-1 myocytes. Endocrinology. 1993;132:689–692. doi: 10.1210/endo.132.2.8425487. [DOI] [PubMed] [Google Scholar]
- 57.Newton A. C. Regulation of protein kinase C. Curr. Opin. Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 58.Iacovelli L., Capobianco L., D'Ancona G. M., Picascia A., De Blasi A. Regulation of lysophosphatidic acid receptor-stimulated response by G-protein-coupled receptor kinase-2 and beta-arrestin1 in FRTL-5 rat thyroid cells. J. Endocrinol. 2002;174:103–110. doi: 10.1677/joe.0.1740103. [DOI] [PubMed] [Google Scholar]
- 59.Murph M. M., Scaccia L. A., Volpicelli L. A., Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J. Cell Sci. 2003;116:1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 60.Clapham D. E. Calcium signaling. Cell (Cambridge, Mass.) 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 61.van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toman R. E., Spiegel S. Lysophospholipid receptors in the nervous system. Neurochem. Res. 2002;27:619–627. doi: 10.1023/a:1020219915922. [DOI] [PubMed] [Google Scholar]
- 63.Fukushima N., Chun J. The LPA receptors. Prostaglandins. 2001;64:21–32. doi: 10.1016/s0090-6980(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 64.Contos J. J., Ishii I., Chun J. Lysophosphatidic acid receptors. Mol. Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 65.Hasegawa Y., Erickson J. R., Goddard G. J., Yu S., Liu S., Cheng K. W., Eder A., Bandoh K., Aoki J., Jarosz R., et al. Identification of a phosphothionate analogue of lysophosphatidic acid (LPA) as a selective agonist of the LPA3 receptor. J. Biol. Chem. 2003;278:11962–11969. doi: 10.1074/jbc.M209168200. [DOI] [PubMed] [Google Scholar]
- 66.Lynch K. R., Macdonald T. L. Structure-activity relationships of lysophosphatidic acid analogs. Biochim. Biophys. Acta. 2002;1582:289–294. doi: 10.1016/s1388-1981(02)00183-x. [DOI] [PubMed] [Google Scholar]
- 67.Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2+-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J. Biol. Chem. 1990;265:12232–12239. [PubMed] [Google Scholar]
- 68.Plevin R., MacNulty E. E., Palmer S., Wakelam M. J. Differences in the regulation of endothelin-1- and lysophosphatidic-acid-stimulated Ins(1,4,5)P3 formation in rat-1 fibroblasts. Biochem. J. 1991;280:609–615. doi: 10.1042/bj2800609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blom N., Gammeltoft S., Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 70.Vázquez-Prado J., Medina L. C., García-Sáinz J. A. Activation of endothelin ETA receptors induces phosphorylation of alpha1b-adrenoreceptors in Rat-1 fibroblasts. J. Biol. Chem. 1997;272:27330–27337. doi: 10.1074/jbc.272.43.27330. [DOI] [PubMed] [Google Scholar]
- 71.Diviani D., Lattion A. L., Cotecchia S. Characterization of the phosphorylation sites involved in G protein-coupled receptor kinase- and protein kinase C-mediated desensitization of the alpha1B-adrenergic receptor. J. Biol. Chem. 1997;272:28712–28719. doi: 10.1074/jbc.272.45.28712. [DOI] [PubMed] [Google Scholar]
- 72.García-Sáinz J. A., Vázquez-Prado J., Medina L. C. Alpha 1-adrenoceptors: function and phosphorylation. Eur. J. Pharmacol. 2000;389:1–12. doi: 10.1016/s0014-2999(99)00896-1. [DOI] [PubMed] [Google Scholar]
- 73.Vázquez-Prado J., Casas-González P., García-Sáinz J. A. G protein-coupled receptor cross-talk: pivotal roles of protein phosphorylation and protein-protein interactions. Cell. Signal. 2003;15:549–557. doi: 10.1016/s0898-6568(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 74.Rapacciuolo A., Suvarna S., Barki-Harrington L., Luttrell L. M., Cong M., Lefkowitz R. J., Rockman H. A. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 75.Hadcock J. R., Port J. D., Gelman M. S., Malbon C. C. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of beta 2-adrenergic receptors in response to insulin. J. Biol. Chem. 1992;267:26017–26022. [PubMed] [Google Scholar]
- 76.Romero-Ávila M. T., Flores-Jasso C. F., García-Sáinz J. A. alpha1B-Adrenergic receptor phosphorylation and desensitization induced by transforming growth factor-beta. Biochem. J. 2002;368:581–587. doi: 10.1042/BJ20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casas-González P., Ruiz-Martínez A., García-Sáinz J. A. Lysophosphatidic acid induces alpha1B-adrenergic receptor phosphorylation through G beta gamma, phosphoinositide 3-kinase, protein kinase C and epidermal growth factor receptor transactivation. Biochim. Biophys. Acta. 2003;1633:75–83. [PubMed] [Google Scholar]