Abstract
Mediatorless, electrochemically driven, redox transformations of T1 (type 1) and T2 copper sites in Trametes hirsuta laccase were studied by cyclic voltammetry and spectroelectrochemical redox titrations using bare gold electrode. DET (direct electron transfer) between the electrode and the enzyme was observed under anaerobic conditions. From analysis of experimental data it is concluded that the T2 copper site is in DET contact with gold. It was found that electron transfer between the gold surface and the T1 copper site progresses through the T2 copper site. From EPR measurements and electrochemical data it is proposed that the redox potential of the T2 site for high-potential ‘blue’ laccase is equal to about 400 mV versus NHE (normal hydrogen electrode) at pH 6.5. The hypothesis that the redox potentials of the T2 copper sites in low- and high-potential laccases/oxidases from totally different sources might be very similar, i.e. approx. 400 mV, is discussed.
Keywords: gold electrode, laccase, redox potential, Trametes hirsuta, T1 site, T2 site
Abbreviations: DET, direct electron transfer; ET, electron transfer; NHE, normal hydrogen electrode; T1, T2 etc, type1, type 2
INTRODUCTION
Studies of DET (direct electron transfer) between proteins and electrodes yield important information on the thermodynamics and kinetics of biological redox processes. The knowledge of the heterogeneous reactions facilitates application of biomolecules in biosensors, biofuel cells, bio-electro-organic synthesis etc. DET at electrodes has been discovered for many proteins (for a recent review, see [1]) including large enzymes, for example, laccases from different origins [2,3]. As we will discuss later the mechanism of DET between electrodes and laccase enzymes is not yet understood.
Laccase (p-diphenol:oxygen oxidoreductase, EC 1.10.3.2) is a multi-copper oxidase, which catalyses the oxidation of various aromatic and some inorganic compounds with the concomitant reduction of dioxygen to water [4]. Due to a great variety of reactions catalysed by laccases, this enzyme has gained considerable interest in many fields of research, for example, development of oxygen cathodes in biofuel cells [5], green biodegradation of xenobiotics, including pulp bleaching [6], bioremediation [7], biosensors [8], organic synthesis [9] and labelling in immunoassays [3].
It has been shown that one fungal species can produce several laccase isoenzymes with different enzymic and physico-chemical properties [10], as exemplified by laccase isoenzymes from the white rot basidiomycete Trametes hirsuta (Coriolus hirsutus) [11–14]. T. hirsuta laccase isoenzymes have molecular masses ranging from 55 to 82 kDa, a pI between 3.5 and 7.4, pH optima between 2.5 and 4.5, and a carbohydrate composition from 11 to 15%. The catalytic site of the laccase consists of four copper ions per enzyme molecule, which can be classified into three types: one type 1 (T1), one type 2 (T2) and two type 3 (T3) copper ions. Laccase has strong absorbances at 610 nm and 330 nm due to the T1 and T3 coppers respectively. The T1 and T2 coppers have also been characterized by the following EPR parameters g⊥=2.046 mT, g‖=2.200, A‖=81×10−4 cm−1 for T1, and g‖=2.222, A‖=198×10−4 cm−1 for T2 [13,14]. The T3 copper shows fluorescence emission near 418 nm and an excitation maximum at 332 nm [13]. The T2 and T3 copper form a trinuclear cluster site, which is responsible for oxygen binding and its reduction to water. The function of the T1 site in this type of enzyme involves electron abstraction from reducing substrates (electron donors) with a subsequent ET (electron transfer) to the T2–T3 copper cluster. Some of the catalytic characteristics of T. hirsuta laccase towards organic and inorganic compounds have been summarized elsewhere [11–15].
The redox potential of the T1 site has been determined for many laccases [10,16–22] using different mediators. The redox potential [versus NHE (normal hydrogen electrode)] varies from 430 mV for tree laccase from Rhus vernicifera [19,20] up to 780 mV for fungal laccase from Polyporus versicolor [19]. It was found previously that the catalytic efficiency (kcat/KM) of laccases for some reducing substrates depends linearly on the redox potential of the T1 copper in the sense that the higher the potential of the T1 site the higher the catalytic efficiency [17,18]. That is why laccases with a high redox potential of the T1 site are of special interest in biotechnology, for example, for efficient bleaching and bioremediation processes. The potential of the T3 site has been determined for R. vernicifera and P. versicolor laccases by Reinhammar and Vänngård [19,20] to be equal to 480 and 780 mV respectively. The redox potential of the T2 site was, however, estimated only approximately for the low-potential laccase from R. vernicifera and was found to be about 390 mV [19,20].
The first publication reporting on DET between laccase and electrodes appeared in 1978 [2]. Measurement of the current originating from bioelectrocatalytic reduction of oxygen to water at carbon electrode modified with adsorbed laccase served as undisputed evidence of the heterogeneous ET process. In 1998 DET for two different laccases (fungal laccase from P. versicolor [23] and tree laccase from R. vernicifera [24]) was demonstrated under anaerobic conditions. In a study by Thuesen et al. [23] cyclic voltammetric peaks with a midpoint potential 790 mV versus NHE were assigned to DET between pyrolytic graphite and the T1 copper site of high-potential P. versicolor laccase. In the case of low-potential tree laccase at gold electrodes [24–26] cyclic voltammetry revealed a midpoint potential for DET being equal close to 400 mV. The observed heterogeneous ET process has also been assigned to mediatorless electrochemistry of the T1 site. Despite these data, the molecular mechanism of DET between laccases and electrodes under anaerobic and aerobic conditions remains largely unknown. Specifically, in a number of cases heterogeneous ET processes recorded by cyclic voltammetry do not correlate with the formal potential values of the T1 copper site in high-potential fungal laccases [27]. This issue is more extensively addressed in the Results and Discussion sections.
The objective of the present work was to determine some biochemical properties of T. hirsuta laccase, including the redox potentials of the T1 and T2 sites. The main efforts were concentrated on investigating the mechanism of DET between gold and laccase under anaerobic conditions.
EXPERIMENTAL
Chemicals
Sulphuric acid, 2,2′-biquinoline, EDTA, Na2HPO4, K3Fe(CN)6, K4Fe(CN)6, KH2PO4, NaOH, KCl and NaF were obtained from Merck (Darmstadt, Germany). Ampholine was from Pharmacia LKB Biotechnology (Bromma, Sweden). Coomassie G-250 and R-250 and markers for electrophoresis and chromatography were from Serva (Heidelberg, Germany). 2-Mercaptoethanol, Tris and glycine were from ICN Biomedical (Aurora, OH, U.S.A.). Acrylamide, N,N-methylenebisacrylamide, ammonium persulphate were from Reanal (Budapest, Hungary). Catechol was from Sigma (St. Louis, MO, U.S.A.). Glycerol, acetic acid, methanol, ethanol, MoO2, HCl, H3PO4, Na2CO3, (NH4)2SO4 and H3PO4 were of the highest purity available from Russian sources. All chemicals were of analytical grade. Buffers were prepared using water (18 MΩ) purified with a Milli-Q system (Millipore, Milford, CT, U.S.A.). The mediators {K4[Mo(CN)8] and K3[Mo(CN)8]} were synthesized and purified as described previously [28].
Enzyme
The basidiomycete T. hirsuta (Wulf. ex Fr.) Quél. [C. (Polyporus) hirsutus], strain T. hirsuta 56 producing extracellular laccase, was obtained from the laboratory collection of the Federal State Unitary Enterprise ‘GosNIISintezBelok’ (Moscow, Russia). Laccase was isolated from a culture medium of the basidiomycete T. hirsuta. The enzyme was purified using the following steps: precipitation of the enzyme from the cultural medium with saturated ammonium sulphate, FPLC ion-exchange chromatography on Servacel DEAE 52 (Reanal), then on DEAE-Toyopearl 650M (Toyo Soda, Tokyo, Japan) performed on standard equipment purchased from LKB Biotechnology, and HPLC size-exclusion chromatography on Bio Sep-SEC-S 2000 (Phenomenex, Torrance, CA, U.S.A.), using a Stayer-system (Acvilon, Moscow, Russia). The final purification was performed by means of HPLC on a TSK DEAE-2SW column (LKB Biotechnology). Homogeneous preparations of laccase (approx. 14 mg/ml) were stored frozen in 0.1 M phosphate buffer, pH 6.5, at −18 °C. The enzyme preparations were homogeneous as verified by SDS/PAGE. The laccase concentrations were measured spectrophotometrically at two wavelengths (228.5 nm and 234.5 nm) using BSA as standard for the calibration curve [29].
The molecular mass of laccase was determined by gel-filtration on a TSK G2000 SWXL (Tosohaas, Japan) using a Stayer-HPLC system (Acvilon), calibrated with a standard set of markers from Serva and by SDS/PAGE. The determination of carbohydrate content involved the derivatization of the monosaccharides, released after acid hydrolysis, by N-(4-methylcoumarin-7-yl)-glycamines, and their subsequent analysis by reverse-phase HPLC with fluorimetric quantification using a spectrofluorimeter MPF-4 (Hitachi, Tokyo, Japan).
The enzyme activity was measured spectrophotometrically as described previously [17]. The absorbance spectra were recorded with a Hitachi-557 spectrophotometer. Laccase-bound copper assay was performed with 2,2′-biquinoline [30]. The apoenzyme and partially T2 depleted T. hirsuta laccase were prepared as described previously [30–32]. The T2 copper content was measured by EPR spectroscopy. EPR spectra were registered with an EPR spectrometer ESC-106 (Bruker Ettlingen, Germany) in the X-band (microwave power, 20 mW; modulation amplitude, 0.5 mT; 4 accumulations; temperature, 77 K).
Electrochemical measurements
For determination of the redox potential of the T1 site of laccase two different methods of redox titration were used. For the T1 copper site titration a common redox titration method was used exploiting reduced and oxidized mediator, i.e. K4[Mo(CN)8] and K3[Mo(CN)8] respectively [17]. For simultaneous determination of the potential of the T1 and T2 sites of laccase a redox titration method with simultaneous measurement of the solution potential was used exploiting two couples of mediators, i.e. K4[Mo(CN)8]/K3[Mo(CN)8] and K4[Fe(CN)6]/K3[Fe(CN)6]. The difference between the two procedures is that in the first case the redox potential of the solution after equilibrium is calculated according to the Nernst equation using data from the spectrophotometric measurements only [17]. In the second case, besides calculation, direct measurement of the potential using a platinum electrode was carried out [19,22,33]. For the electrochemical measurement in this case, a two-electrode voltmeter (Ionomer I-130.1, Russia) was used. The reference electrode was an EVL-1MZ Ag|AgCl|KClsat electrode (201±2 mV versus NHE). Platinum wires were used as the counter and working electrodes. In all these experiments a 3 ml spectroelectrochemical cell was used. The platinum electrodes were rinsed with sulphuric acid, followed by electrochemical cycling in 0.2 M sulphuric acid, with a scan rate of 50 mV·s−1 between 0 and 1000 mV versus Ag|AgCl|KClsat (5 scans), and finally rinsed with Millipore water. The titration procedure was started with addition of 1 ml of laccase solution (14 mg/ml) into the cell (under anaerobic conditions) containing 1 ml of the mediator (0.02 M) and 1 ml of 0.3 M phosphate buffer at pH 6.5. The redox potential was registered on the platinum electrode after equilibrium. The cell was kept under anaerobic conditions before and during the redox titration experiments. For this argon was flushed through the cell. Traces of oxygen were removed by passing the gas through a solution containing VCl2. The design of the cell and the technique of titration have been described previously [34].
For redox titration of the T2 site EPR measurements were exploited. After the redox equilibrium in the cell containing laccase and mediators was observed by measuring a stable spectrum (spectrophotometrical measurement of the redox state of the T1 site between 450 and 800 nm) a portion of the solution was anaerobically removed from the cell, immediately frozen using liquid nitrogen, and the EPR-spectra were recorded at 77 K.
Electrochemical mediatorless potentiometric titrations were carried out using a spectroelectrochemical cell comprising a gold capillary (with a volume of 0.7 μl). The design of the cell has been described elsewhere [35]. The potential of the gold capillary of the cell was controlled by a three-electrode potentiostat (BAS LC-3E; Bioanalytical Systems, West Lafayette, IN, U.S.A.). Cyclic voltammograms of laccase in the gold capillary electrode were recorded using a three-electrode potentiostat (BAS CV-100W Electrochemical Analyser with BAS CV-100W software version 2.1.). In these measurements an Ag|AgCl|0.1 M KCl reference electrode (281 mV versus NHE) and a platinum counter electrode were used. The absorbance spectra were monitored with PC2000-UV-VIS, a miniature fibre optic spectrometer from Ocean Optics (Dunedin, FL, U.S.A.) with an effective range between 200 and 1100 nm. The pre-treatment of the gold capillary working electrode of the spectroelectrochemical cell was carried out as described previously [35], but without thiol-modification. Briefly, it comprised washing the cell capillary with a peroxide/sulphuric acid mixture followed by rinsing with Millipore water.
Cyclic voltammetry of laccase solution was recorded also with planar ‘BAS’ gold electrode in a 1-ml volume electrochemical cell. In these measurements an Ag|AgCl|3 M NaCl reference electrode (BAS) and a platinum counter electrode were used. The electrode surface of the working gold electrode was polished on DP Suspension and on alumina FF slurry (0.25 μm and 0.1 μm; Stuers, Copenhagen, Denmark), rinsed with Millipore water, and sonicated between and after polishing for 10 min. Then the electrode was kept in concentrated H2SO4 with 10% H2O2 for 1 h, subjected to 30 cycles in 0.5 M H2SO4 and rinsed with Millipore water.
RESULTS AND DISCUSSION
Main biochemical properties of T. hirsuta laccase
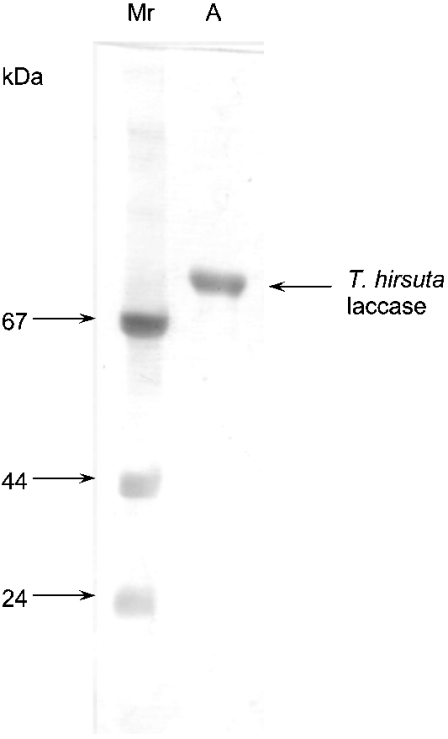
A homogeneous preparation of laccase from T. hirsuta has been obtained and characterized. The key biochemical properties such as molecular mass, pH optimum, thermal stability, copper content, pI and specific activity were determined (Table 1). It was found that the preparation of the enzyme contained one form of the monomeric protein with a molecular mass of 69±3 kDa determined by SDS/PAGE (Figure 1) and HPLC. The laccase contains 4 copper ions per molecule, and has a pH optimum of 3.5 for oxidation of ferrocyanide. The laccase has not been sequenced yet. However, two different amino acid sequences of T. hirsuta laccase isoforms have been published (GenBank accession numbers Q02497 [36] and AAL89554). The biochemical properties of the T. hirsuta laccase studied in this work are closer to those previously reported in [36] than to properties of the AAL89554 isoform [14,15]. Thus the sequence published in [36] (GenBank accession number Q02497) is the most likely to be that of the enzyme studied here (Table 2). Like all known laccases, the laccase from T. hirsuta is a glycoprotein and contains 12% carbohydrate per molecule of protein. It was found that the carbohydrate moieties of T. hirsuta laccase comprised mannose and N-acetylglucosamine. The T. hirsuta laccase is a highly stable enzyme retaining about 71% of its activity after incubation at 40 °C for 48 h.
Table 1. Some physico-chemical characteristics of the multiforms of the laccase from the basidiomycete T. hirsuta.
ND, not determined.
Figure 1. SDS/PAGE of the preparation of T. hirsuta laccase.
Lane A, the laccase preparation; lane Mr, molecular mass markers (shown in kDa on the left).
Table 2. Comparison of the redox potentials, E0′, and the amino acid sequence alignment of the T1 site for oxidases from different sources.
Underlined, ligand to T1 copper; bold and italic, amino acids which can or cannot form hydrogen bonds between each other (in [41]) respectively.
| Origin of oxidase | GenBank accession number | Sequence alignment | E0′, T1 (mV) | Reference | |
|---|---|---|---|---|---|
| T. hirsuta laccase | Q02497 | 111GHSFL115… | 473HCHIDFHLEAGF484 | 780 | Present study |
| T. versicolor laccase | A35883 | 111GHSFL115… | 473HCHIDFHLEAGF484 | 785 | [19] |
| T. villosa laccase | JC5356 | 112GNSFL116… | 473HCHIDFHLEAGF484 | 775 | [17] |
| C. cinereus laccase | AAD30964 | 108GHAFL112… | 473HCHIEFHLMNGL484 | 550 | [16] |
| Melanocarpus albomyces laccase | 1GW0A | 502HCHIAWHVSGGL513 | 460 | [17] | |
| R. vernicifera laccase | BAB63411 | 492HCHFERHTTEGM503 | 430 | [19] | |
| Zucchini ascorbate oxidase | A51027 | 506HCHIEPHLHMGM517 | 340 | [21] |
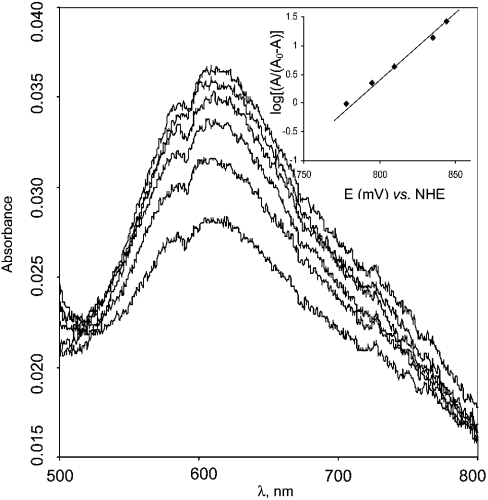
The potential of the T1 copper was measured by redox titration using mediators. The reduction of the T1 copper was followed by the disappearance of the blue absorbance band at 610 nm (Figure 2). From the titration curve (Figure 2, inset), it can be concluded that the redox potential of the T1 copper site is about +780 mV versus NHE. T1 titration is a one-electron process, since E versus log[A610/(A0610−A610)] has a slope of 49 mV, being close to the expected 59 mV.
Figure 2. Redox titration of the T1 copper site in laccase from T. hirsuta using the redox mediator couple K4[Mo(CN)8]/K3[Mo(CN)8].
The experiments were performed in 0.1 M phosphate buffer pH 6.0. Inset, potentiometric titration curve.
From an electrochemical point of view, as well as from analysis of the primary structures of the enzymes, all laccases can be divided into three groups in view of the potential of the T1 site: high-, middle- and low-potential laccases. The low-potential group includes enzymes from trees, for example, R. vernicifera laccase with a potential of the T1 site of about 430 mV versus NHE [19,20]. The middle group includes laccases from basidiomycetes, such as Myceliophthora thermophila [17], basidiomycete C30 [10], Rhizoctonia solany [17] and Coprinus cinereus [16]. These enzymes have a potential of the T1 site ranging from 470 to 710 mV versus NHE. The high-potential laccases, for example those from T. hirsuta, T. versicolor and T. villosa, all have a potential of the T1 site of about 780 mV versus NHE [17,19,38]. It was previously suggested that the value of the redox potential of the T1 site for the copper-containing oxidases depends on the ligands of the T1 copper and the amino acids, which form the T1 pocket [36,39]. Evidently, as can be seen from Table 2, all high-potential laccases have the same amino acid sequences ligating the T1 copper and forming the T1 pocket. Unexpectedly, studies of laccases obtained through site-directed mutagenesis studies have shown only little correlation between T1 copper-ligating residues and E01 of the T1 site [21,39,40]. On the basis of the structural data of the wild-type high-potential laccase (T. versicolor), Piontek et al. [41] suggested a new mechanism by which laccase and possibly other redox metallo-enzymes, can increase their redox potential. Such a mechanism assumes a modulation of the electron-density contribution at the metal and the ligating amino acid by their proximity. In high-potential laccase the distance between the T1 Cu and one of the histidines is longer if compared with the distance for laccases of the middle-potential group, for example, C. cinereus laccase [41,42]. The hydrogen bond between His-479 and Ser-113 (114 in case of T. villosa laccase) seems to be responsible for this (Table 2). Currently, however, the differences between redox potentials of the T1 site for laccases are not fully understood, therefore calling for further investigations. The two different isoforms of the T. hirsuta laccase, with known amino acid subsequences possess a good alignment (GenBank accession numbers Q02497 and AAL89554) at the T1 pocket, except for one amino acid in position number 482 (glutamic acid, Q02497) versus 477 (glutamine, AAL89554). Thus from this comparison and from the titration data (the determined redox potential of the T1 is about 780 mV versus NHE) it is reasonable to describe the T1 site of as having the structure of a high-potential laccase (Table 2).
The other properties such as molecular mass, activity and carbohydrate composition of this laccase differ to a greater or lower extent from previously reported laccases originating from the same fungus (see Table 1 and also [13,14,36,38]). Previously, it was already noticed that two laccases from T. hirsuta with different biochemical properties have very similar primary structures ([36] and GenBank accession number AAL89554), as well as that the isoenzymes of laccase from T. hirsuta can have different primary structures [13]. At the moment it is not clear whether T. hirsuta has several genes encoding laccases or the difference is due to posttranslational modification or proteolysis during cultivation and purification. Comparing the properties of several laccases from different sources, including multiple forms of laccase from basidiomycete T. hirsuta (see Table 1 and [4,11–14,17,22,36,38]), it can be concluded that T. hirsuta laccase has some superior characteristics, such as high stability, high activity and low content of carbohydrates, which make this laccase an attractive object for biochemical and electrochemical investigations.
Heterogeneous ET of laccase at gold electrodes
The possibility of electrochemical control of the redox reactions of different enzymes at electrodes is very important both for fundamental research and applications in biotechnology. In this respect gold as a noble metal is often used as electrode material. At present, a few reports exist concerning electrochemical studies of laccase at gold electrodes [2,24–27]. However, to our best knowledge, only two papers describe electrochemical studies of fungal high-potential laccases [2,27]. It was shown that the presence of the enzyme shifted the steady-state potential of bare gold in oxygenated buffer to a value of about 100 mV more positive than that without laccase [2]. After that, only in 1999 [27] were cyclic voltammograms of recombinant T. versicolor laccase (but not of wild-type laccase from the fungus) on 4,4′-pyridine thiolate modified gold characterized as having a formal potential of around 410 mV versus NHE. The authors [27] concluded that the T1 site is in DET with gold, despite a considerable difference between the formal potential measured by cyclic voltammetry (410 mV) and the redox potential of the T1 site (780 mV) determined by redox titration. Such a considerable shift of the redox potential could be possible only if the enzyme acquires a dramatically changed (denatured) conformation at the gold surface or if proper folding does not occur during its production by recombinant technology. In any case the data on the ET process between laccases and gold are very limited. Whatever the electrode material is, there is an enormous interest in understanding and controlling the heterogeneous ET of laccase at electrodes, as can be exemplified by the recent developments and interest in biofuel cells (for example, see [43]).
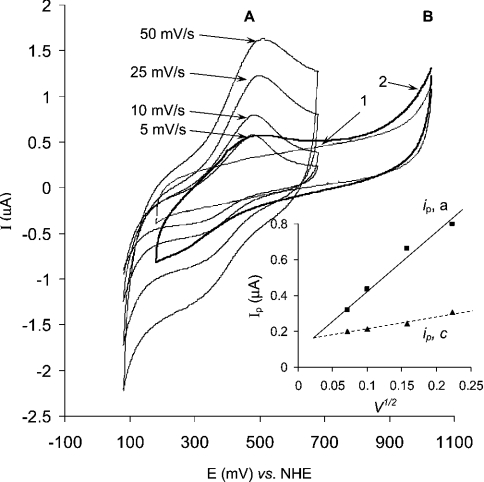
In the present work, for the first time we show evidence of DET between native high-potential laccase and gold electrode as recorded by cyclic voltammetry (Figure 3). The Figure shows cyclic voltammograms of the high redox potential laccase registered using a bare gold electrode. The cyclic voltammograms (Figure 3) show a quasi-reversible ET process with a midpoint potential of 405 mV versus NHE. However, the absence of any current peaks (Figure 3, curve 2) at a potential close to 780 mV (redox potential of the T1 site) indicates that the observed processes cannot be undisputedly assigned to a heterogeneous ET process of the T1 site (see below). The ratio of the anodic to cathodic peak current was approx. 1.5 to 1, and the difference between the anodic and cathodic peak potentials (ΔEp) was equal to about 170 mV. Both facts point to a quasireversible character of the observed ET process between laccase and gold. The scan rate dependence of the peak current in the cyclic voltammograms was found to be linear (Figure 3, inset). Such a dependency is due to the electrolysis of soluble (not immobilized) laccase molecules in the gold capillary.
Figure 3. Cyclic voltammograms of laccase in the capillary gold electrode.
The experiments were performed in 0.1 M phosphate buffer, pH 6.5, and in (A) and (B) the solution contained 10 mg/ml of T. hirsuta laccase. (A) Cyclic voltammogramms recorded at different scan rates; start potential, 80 mV versus NHE. Inset, the dependence of anodic and cathodic peak current on the square root of the potential scan rate (V/s). (B) Cyclic voltammograms recorded at 10 mV/s in a broader potential window; start potential, 1030 mV versus NHE. Curve 1, control measurement in the absence of the laccase; curve 2, with laccase.
The voltammograms (Figure 3) have definite similarity to voltammograms previously recorded for recombinant high-potential T. versicolor laccase [27]. To quantitatively compare the ET process recorded with wild-type T. hirsuta laccase (Figure 3) against that previously described for recombinant T. versicolor [27], a mathematical treatment of the cyclic voltammetry data (Figure 3) was performed, similarly to the case of T. versicolor [27]. Specifically, the diffusion coefficient, the rate of the heterogeneous ET process and the number of electrons characterizing the heterogeneous ET have been calculated and compared. In order to extract the mentioned information from cyclic voltammograms, mathematical formulae derived for the case of linear diffusion were used [27,44]. This might be possible in the case of our gold capillary if the electrochemical processes proceed only in the vicinity of the surface of the capillary wall. To estimate if the radial geometry of the capillary does not dramatically disturb the shape of the cyclic voltammograms the diffusion coefficient for laccase in the capillary and the thickness of the diffusion layer (i.e. the extent of penetration of the oxidation–reduction reaction from the gold surface to bulk of the solution) have been calculated. The diffusion coefficient was calculated from the linear peak current (ip,c) dependence on v1/2 (scan rate 1/2) (Figure 3, inset) by using the Randles–Sevcik equation [44]:
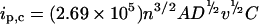 |
(1) |
where ip,c is the value for the cathodic peak current at a given scan rate, v is the scan rate, A is the area of the electrode (in our case equal to 0.19 cm2). The concentration, C, of the laccase in the capillary was equal to 1.36×10−7 mol/cm3. Using the mentioned values the diffusion coefficient (D) of laccase was found to be equal to 3.6(±3.3)×10−7 cm2·s−1. The value is similar to the diffusion coefficients determined for T. versicolor laccase, cytochrome c and BSA, which are 1.5×10−6, 1×10−6 and 6×10−7 cm2·s−1 respectively [27,45]. The similarity of the calculated diffusion coefficient with the previously reported values indicates that the geometry of the diffusion layer at the gold capillary walls can still be considered as linear. Using the value of the diffusion coefficient, we also estimated that for sweep rates of 5–50 mV/s the diffusion layer {δ=(2Dt)1/2 [44]} could penetrate into the bulk of the capillary from its wall to a maximum distance of 25–90 μm (25 μm at 50 mV/s) if the heterogeneous redox process were diffusion limited, i.e. electrochemically characterized as reversible. Since the process is quasireversible (determined from that ΔEp=170 mV≫59 mV), the electrochemically driven redox process penetrates into the solution to a much lower extent than our estimate based on a diffusion limited electrochemical process. These considerations justify the treatment of the cyclic voltammograms by using formulae derived for ordinary planar electrodes, as well as the comparison of our results with previously reported heterogeneous characteristics of recombinant T. versicolor laccase on gold [27].
The value of the rate constant for heterogeneous ET, khet, between the electrode and laccase was calculated using the method of Nicholson (eqn 2) [27,44]:
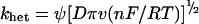 |
(2) |
where Ψ is a function dependent on ΔEp. The values of Ψ can be found from tables in [44] corresponding to ΔEp determined from the cyclic voltammograms (Figure 3). At the potential scan rates used, ΔEp was equal to about 0.17 V. Using this value khet was determined to be equal to 0.7(±0.5)×10−4 cm·s−1. The value is slightly lower than the khet previously found for truncated T. versicolor laccase (1.3×10−4 [27]). The absence of glycosylation on the recombinant laccase could be responsible for the higher value of khet. Such influence of glycosylation on khet was previously shown for horseradish peroxidase [46]. The difference in the ET rates for native and recombinant laccase is, however, not significant and in general the heterogeneous ET rates for native laccase from T. hirsuta and recombinant T. versicolor could be considered similar.
The number of electrons transferred in the electrochemically driven redox process was determined by analysis of the shape of the peaks of the cyclic voltammograms presented in Figure 3. For a quasireversible ET process the following equation is valid [27,44]:
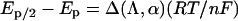 |
(3) |
where Ep is the potential at which the current is at a maximum in the cyclic voltammogram, Ep/2 is the potential at which the current is at half maximum in the cyclic voltammogram, Δ(Λ,α) is a function [44] depending on khet and D, and n is the number of electrons involved in the electrochemical reaction. Assuming α=0.5 and using khet=0.7×10−4 cm·s−1 and D=3.6×10−7 cm2·s−1 as calculated above, Δ(Λ,α) can be found from plots in [44]. Calculation leads to a result indicating that for a one-electron redox process the difference Ep/2−Ep should be in the range between 65 and 94 mV. From cyclic voltammograms (Figure 3) the mean value for this difference is equal to 82 mV and 87 mV for the anodic and the cathodic process respectively. The values fall into the estimated Ep/2−Ep range meaning that the electrochemically observed redox process of the laccase involves one electron. Such a calculation has given about 0.5 electron for truncated laccase from T. versicolor [27], probably pointing to a more complicated mechanism of heterogeneous ET of the recombinant laccase in comparison with the electrochemistry of the wild-type laccase from T. hirsuta.
In conclusion, the results from cyclic voltammetry in the gold capillary electrode can be summarized in that we have observed a heterogeneous one-ET process of wild-type high-potential laccase from T. hirsuta at the surface of a gold electrode. The process appeared to be centred at 405 mV versus NHE. Obviously, this value coincides well with the potential of the ET process previously found for recombinant high-potential T. versicolor laccase (410 mV) [27], as well as for low-potential R. vernicifera laccase (330 or 410 mV) [24–26]. However, this potential deviates by about 380 mV from the redox potential of the T1 site (780 mV). This discrepancy motivated us to exploit spectroelectrochemistry for a deeper study of the heterogeneous ET process. The first question is, of course, whether the ET process is a heterogeneous anaerobic oxidation–reduction of the blue copper sites in T. hirsuta laccase.
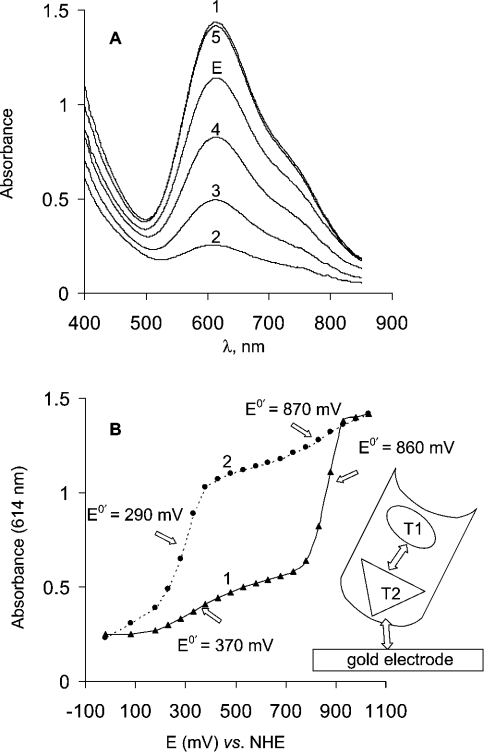
ET between a bare gold electrode and the T1 copper site for laccase in solution was undisputedly confirmed by our spectroelectrochemical measurements. From the spectroelectrochemical recordings (see Figure 4A) of the laccase solution in the gold capillary spectroelectrochemical cell it is easy to conclude that the blue colour vanishes when the applied potential is switched from +1030 mV to −20 mV versus NHE (absorbance spectra 1 and 2, respectively in Figure 4A). The process can be explained by reduction of the T1 site of T. hirsuta laccase in the gold capillary electrode under anaerobic conditions. The redox reaction is reversible and the enzyme can be reoxidized by applying an oxidizing potential (Figure 4A, curves 3, 4, and 5 correspond to applied potentials of 530, 830 and 1030 mV respectively) or by oxygen (results not shown). It should be mentioned that more than 80% of the initial enzyme activity is retained after being exposed to a long (up to 48 h) electrochemical titration procedure. The laccase activity was measured before and after redox titration using two enzyme substrates, namely ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)] and catechol, by a commonly used spectrophotometrical procedure.
Figure 4. Mediatorless spectroelectrochemical redox titration of T. hirsuta laccase by using a gold capillary cell.
(A) Absorbance spectra recorded during redox titration of T. hirsuta laccase in the spectroelectrochemical gold capillary cell. The experiments were performed in 0.1 M phosphate buffer, pH 6.5. Curves E and 1–5 are absorbance spectra of initially injected enzyme and at applied potentials −20, 530, 830 and 1030 mV respectively. (B) Spectroelectrochemical titration curves reflecting the dependence of absorbance of the laccase solution at 614 nm versus the applied potential. Titration curves recorded by changing the applied potential (curve 1) from −20 to 1030 mV and (curve 2) from 1030 to −20 mV.
The spectroelectrochemical data undoubtedly provide evidence of direct, unmediated heterogeneous ET between the laccase and gold. However, the mechanism of this process seems more complicated than a simple direct heterogeneous oxidation–reduction of the T1 site. The complexity of the mechanism is reflected by the spectroelectrochemical titration curves (Figure 4B). Two features in the titration curves are obvious. Firstly, hysteresis of the spectroelectrochemical titration curves is observed when changing the direction of the titration, i.e. the titration curves definitely do not coincide when the titration is made by changing the applied potential from −20 to +1030 mV (Figure 4B, curve 1) and from +1030 to −20 mV (Figure 4B, curve 2). Secondly, this complexity of the heterogeneous ET is, first of all, reflected by two noticeable ET processes. Two steps in the titration curves (Figure 4B) where a relatively strong dependence of the absorbance on the applied potential is seen, account for this conclusion. From Figure 4(B) it follows that the redox potentials of these two redox processes are in the range 290–370 and 860–870 mV. A few explanations can be considered to account for the observed hysteresis and the two-step process of DET of the laccase at the gold surface. The most probable is, however, that we observe ET to/from the T1 site (process at 860 mV versus NHE), which is mediated by some other redox site in the laccase. Hysteresis of the spectroelectrochemical titration indicates that ET to T1 goes through the redox site with a formal potential close to 290–370 mV versus NHE.
From the spectroelectrochemical and cyclic voltammetry data it is not possible to determine the structure of this site. It is important to note that the observed peaks in the cyclic voltammograms (Figure 3) are very close to this low-potential (290–370 mV versus NHE) redox process observed by spectroelectrochemical titration (Figure 4B). Moreover, it should be emphasized that cyclic voltammetry measurements done with other laccases from different sources (including low- and high-potential laccases) using gold electrodes have shown so far the heterogeneous ET process in the potential region between 330 and 410 mV versus NHE [24–27]. Such a coincidence of our and previously published cyclic voltammetry data suggests that low and high-potential laccases might have a common redox site with a redox potential value of about 330–410 mV. This definitely cannot be assigned to T1 or T3 in high-potential laccases, however, such a possibility might exist for the T2 copper.
As early as 1971–1972 Reinhammar and Vänngård concluded in two publications that the redox potential of the T2 site of low-potential laccase from R. vernicifera is approximately equal to 390 mV [19,20]. To the best of our knowledge, these two papers are the first and the last publications about potentiometric redox titrations of the T2 site of laccases in which the redox potential of the site was measured. The value of the redox potential of the T2 site of high-potential laccases has so far not been determined [19,20,47,48]. The absence of data on the redox potential of the T2 copper in high-potential laccases (from T. hirsuta in our case) encouraged us to examine the possibility of the T2 redox centre establishing an electronic contact with gold (observed by cyclic voltammetry, Figure 3) and mediating the ET to the T1 site (recorded by mediatorless spectroelectrochemical titration, Figure 4B). In this context we carefully examined additional experimental evidence pointing to a possibility for a redox potential of the T2 site being close to 400 mV versus NHE for high-potential laccases. Firstly, a simultaneous redox titration of the T1 and the T2 sites were carried out by spectroelectrochemical and EPR methods, and, secondly, electrochemistry of the T2 depleted enzyme and native laccases in the presence of F− ions was studied and compared.
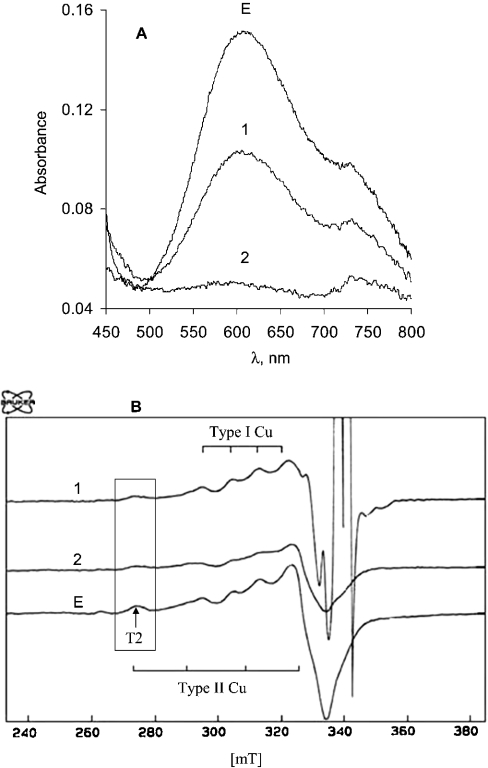
Spectroelectrochemical and EPR registration of the redox titration of the T1 and T2 copper centres in laccase from T. hirsuta was first attempted by using K4[Mo(CN)8]/K3[Mo(CN)8] with an E0′ of 778 mV. With a high concentration (4 mM, or ratio of mediator/laccase, 60:1) of reduced Mo-cyanide complex {K4[Mo(CN)8]} it was possible to partly reduce the T1 copper (Figure 5A, curve 1). At the same time, the T2 copper signal in the EPR spectra decreased by 34% from its initial value if the mediator/laccase mixture was kept for 10 min before freezing to −70 °C for the following EPR measurements (Figure 5B, curve 1). However, if the laccase/mediator mixture was kept for 30 min before freezing and EPR measurements, it was found that the T2 signal in the EPR spectra decreased by 65% (while the spectra from the T1 site was stable). Similar experiments have been repeated with the K4[Fe(CN)6]/K3[Fe(CN)6] redox couple characterized by an E0′ of 430 mV. From spectrophotometric measurements it was found that the T1 site was reduced immediately (Figure 5A, curve 2). However, the disappearance of the T2 component from the EPR spectra was dependent on time allowed for the laccase/mediator mixture to stay at room temperature before freezing and EPR measurements. The T2 component in EPR spectra showed that 55% of the laccase molecules in solution still had oxidized T2 if the mediator/laccase mixture was kept for 10 min before freezing to −70 °C (Figure 5B, curve 2). Keeping the laccase/K4[Fe(CN)6] mixture at room temperature for 20 and 30 min resulted in an EPR spectrum that indicated the presence of oxidized T2 copper in about 40% and 18% of the laccase molecules respectively. These results give rise to questions that need to be answered. Firstly, how can such a tremendous difference in the reduction rate of T1 (almost immediate) and T2 (at least 0.5 h) copper by the K4[Fe(CN)6]/K3[Fe(CN)6] redox couple be explained? Secondly, can the disappearance of the T2 component from the EPR spectra be solely ascribed to T2 copper reduction process?
Figure 5. Absorption (A) and EPR (B) spectra of T. hirsuta laccase during the redox titration by different mediators.
The experiments were performed in 0.1 M phosphate buffer, pH 6.5. Spectrum E characterizes the solution of laccase before addition of mediators. Spectrum 1 was recorded after mixing the enzyme with K3Mo(CN)8 and K4Mo(CN)8 (the redox potential after equilibrium, 770 mV versus NHE). Spectrum 2 was recorded after mixing the enzyme with K3Fe(CN)6 and K4Fe(CN)6 (the redox potential after equilibrium, 450 mV versus NHE).
The answer to the second question is unfortunately negative, i.e. our experiments indicated that the T2 copper changed co-ordination by being, probably, complexed by the cyanide ions always present in solutions of metal–cyanide complexes {K4[Fe(CN)6] in our case}. The evidence for this came from experiments designed to recover the T2 (as well as the T1) copper spectra by oxygenation of the laccase/K4[Fe(CN)6]/K3[Fe(CN)6] mixture after complete disappearance of the T2 component from the EPR spectra. The EPR spectral characteristics of the T2 copper were not recovered. This indicates that at least the T2 copper has irreversibly changed its co-ordination after the prolonged contact with the reducing metal–cyanide complex. The copper site was no longer reactive towards oxygen. It might be that the T2 copper was even depleted. Strong binding of CN−, F− and azide to the T2 site of the enzyme has been registered previously by EPR, as well as in the crystal structures of laccases [4,47–49]. Additionally, the reduction of the T2 copper and its complexation with cyanide is a well-known method for preparing T2-depleted laccase without enzymic activity or even the apoenzyme [30–32]. All this leads to the conclusion that redox titration of the copper centres in laccase by using metal–cyanide complex based redox compounds invokes at least two processes, i.e. redox transformation and changes of the T2 copper co-ordination.
Although the disappearance of the T2 component from the EPR spectra cannot be assigned solely to the T2 copper reduction process, a few following considerations allow the proposal that the driving force between the T2 copper site and the K4[Fe(CN)6]/K3[Fe(CN)6] couple is small under anaerobic conditions. To support this conclusion a number of facts should be taken into account. Firstly, our experiments of mixing laccase with the K4[Fe(CN)6]/K3[Fe(CN)6] redox couple show almost immediate reduction of all T1 copper (as observed by spectrophotometry) and only about 45% of T2 copper (observed by EPR within 10 min). Second, there are a few very early publications, which also support our conclusion regarding the redox potential of the T2 copper. Redox titration of high-potential enzyme from T. versicolor with K4Fe(CN)6, K4W(CN)8 (E0′≈490 mV) and quinol (E0′≈360 mV) reduced only approx. 60–70% of the T2 copper [48]. In the same study it was found that only ascorbate (E0′≈150 mV versus NHE) could totally reduce all the copper sites in this laccase [19,47,48]. Thus our and previously published data indicate that the formal potential of the T2 copper might be considerably more negative than the redox potential of the T1 site in high-potential laccases.
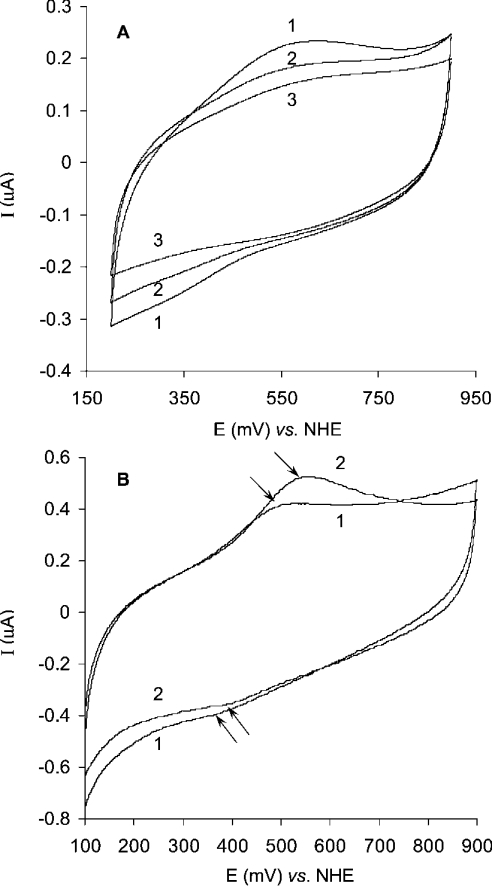
Additional evidence that the heterogeneous ET process at around 400 mV versus NHE is related to the T2 copper in T. hirsuta laccase was obtained by cyclic voltammetry comparing partly T2 copper-depleted with native laccase, as well as laccase incubated with F− ions. As can be seen from Figure 6(A) the peak current in cyclic voltammograms of different T. hirsuta laccase preparations is proportional to the T2 copper content. Specifically, approx. 50% T2-depleted preparation of the enzyme gives about 50% of the peak current (Figure 6A, curve 2) if compared with the current (Figure 6A, curve 1) for native laccase preparation. No current peaks are observed for copper depleted apoenzyme (Figure 6A, curve 3). This experimental evidence finalizes the conclusion that the current peaks in the cyclic voltammograms (Figure 3 and Figure 6) of the enzyme in the capillary or on planar gold electrodes correspond to the copper ion(s) of the laccase. In the presence of fluoride ions, which bind to the T2 site of the enzyme, the potentials of the cathodic and especially the anodic peaks of the voltammogram increased (Figure 6B). As the result of fluoride interaction, the midpoint potential of fungal laccase increased by approx. 35 mV (Figure 6B). Such behaviour is in agreement with previously published data concerning the change in the redox potential of the T2 site in the presence of F− ions for low-potential laccase [19]. It was shown that in the presence of F− the redox potential of the T2 site of Rhus vernicifera laccase insignificantly increased (by about 25 mV), but the redox potential of the T3 site significantly decreased (by 210 mV), whereas the redox potential of the T1 site was independent of the fluoride–enzyme interaction [19].
Figure 6. Cyclic voltammograms of laccase on the planar gold electrode.
The experiments were performed in 0.1 M phosphate buffer, pH 6.5. (A) Curve 1, native enzyme; curve 2, partly T2-depleted laccase; curve 3, apoenzyme. Laccase concentration, 4 mg/ml; scan rate, 10 mV/s; start potential, 900 mV versus NHE. (B) Curve 1, native enzyme; curve 2, after interaction with F− ions (10 mM) for 10 min. Laccase concentration, 7 mg/ml; scan rate, 50 mV/s; start potential, 100 mV versus NHE.
All these experimental results indicate that the redox potential of the T2 copper site at pH 6.5 is close to 400 mV versus NHE in high-potential T. hirsuta laccase. Taking into account all the previously published results and our experimental data we conclude that the redox process observed with cyclic voltammetry on gold (Figure 3) close to 400 mV is an electrochemically driven redox transformation of the T2 copper in the laccase from T. hirsuta. Our spectroelectrochemical measurements (Figure 4B) additionally indicate that this process at 400 mV is definitely not a transformation of the T1 copper. We state that the T2 copper (E0′≈400 mV versus NHE at pH 6.5) in the laccase from T. hirsuta mediates heterogeneous ET between gold and the T1 site. The proposed hypothesis of the ET between laccase and gold is schematically illustrated in the inset of Figure 4(B); however, the molecular mechanism of such a bio-electrochemical redox process must be much more complex, since it should involve the entire trinuclear T2–T3 copper centre.
Additionally, our results, in combination with previously published data [19,47,48], suggest that the T2 copper centre in many multicopper oxidases might have a formal potential value close to 400 mV versus NHE. Such a conclusion is supported by the fact that the formal redox potential of the T2 site under anaerobic conditions for laccases from totally different sources are very close to each other, for example, for the low-potential laccase from R. vernicifera it is known that E0′≈390 mV versus NHE [19] and from cyclic voltammetry data we found E0′≈405 mV versus NHE for the high-potential laccase from T. hirsuta. The conservation of the redox potential of the T2 site to ≈400 mV versus NHE would require that the structure of the T2 redox centres in laccases is conserved in terms of ligands and copper–ligand distances. From crystallographic data [41,49,50] for a few laccases and other copper oxidases, it can be found that such conservation is really retained (Table 3). All oxidases listed in Table 3 have no significant difference in their structure of the T2 site (e.g., ligand environment and distances between ligands and copper ions or copper–copper distances in T2–T3 cluster between the T2 copper and the T3 coppers), whereas the structures (ligands) and the redox potentials of the T1 sites for these enzymes are very different (Table 2).
Table 3. Copper–copper and copper–ligand distance (Å) for the T2 site of T. versicolor laccase (TvL), Melanocarpus albomyces laccase (MaL), and Zucchini ascorbate oxidase (AO).
Cu2, Cu3 and Cu4 correspond to two copper ions of the T3 and one copper ion of the T2 sites respectively.
In summary, we conclude that the redox potential of the T2 copper centre in the laccase from T. hirsuta is equal to about 400 mV versus NHE at pH 6.5. We present experimental evidence that the heterogeneous DET between the T2 copper and the surface of the gold electrode can be recorded by cyclic voltammetry. Spectroelectrochemically, we confirm that the heterogeneous ET between the surface of a bare gold electrode and the T1 copper proceeds through the T2 centre. These observations lead to the additional conclusion that the laccase molecules are oriented at the interface (gold electrode–solution) with the T2 centre being closer to the electrode surface than the T1 centre. Further studies of the electrochemical characteristics and mediatorless titration of the redox potentials of the T1, T2 and T3 copper sites in laccases from different sources are in the scope of further investigations by our laboratories.
Acknowledgments
We thank Dr Elena Gorshina for the cultivation of basidiomycete T. hirsuta. This work was supported by INCO-Copernicus grant (ICA2-CT-2000-10050), the Russian Foundation for Basic Research (02-04-48885 and 03-04-48937), the Russian programme ‘Biocatalytic Technologies’ (43.073.1.1.2505) and the Swedish Research Council.
References
- 1.Gorton L., Lindgren A., Larsson T., Munteanu F. D., Ruzgas T., Gazaryan I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta. 1999;400:91–108. [Google Scholar]
- 2.Berezin I. V., Bogdanovskaya V. A., Varfolomeev S. D., Tarasevich M. R., Yaropolov A. I. Bioelectrocatalysis. Equilibrium oxygen potential in the presence of laccase. Dokl. Akad. Nauk SSSR. 1978;240:615–618. [Google Scholar]
- 3.Kuznetsov B. A., Shumakovich G. P., Koroleva O. V., Yaropolov A. I. On applicability of laccase as label in the mediated and mediatorless electroimmunoassay: effect of distance on the direct electron transfer between laccase and electrode. Biosens. Bioelectron. 2001;16:73–84. doi: 10.1016/s0956-5663(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 4.Solomon E. I., Sundaram U. M., Machonkin T. E. Multicopper oxidases and oxygenases. Chem. Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 5.Barton S. C., Kim H.-H., Binyamin G., Zhang Y., Heller A. The ‘wired’ laccase cathode: high current density electroreduction of O2 to water at +0.7 V (NHE) at pH 5. J. Am. Chem. Soc. 2001;123:5802–5803. doi: 10.1021/ja010408b. [DOI] [PubMed] [Google Scholar]
- 6.Call H. P., Mucke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems. J. Biotechnol. 1997;53:163–202. [Google Scholar]
- 7.Mayer A. M., Staples R. C. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- 8.Haghighi B., Gorton L., Ruzgas T., Jönsson L. J. Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta. 2003;487:3–14. [Google Scholar]
- 9.Karamyshev A. V., Shleev S. V., Koroleva O. V., Yaropolov A. I., Sakharov I. Y. Laccase-catalyzed synthesis of conducting polyaniline. Enzyme Microb. Technol. 2003;33:556–564. [Google Scholar]
- 10.Klonowska A., Gaudin C., Fournel A., Asso M., Le Petit J., Giorgi M., Tron T. Characterization of a low redox potential laccase from the basidiomycete C30. Eur. J. Biochem. 2002;269:6119–6125. doi: 10.1046/j.1432-1033.2002.03324.x. [DOI] [PubMed] [Google Scholar]
- 11.Gindilis A. L., Zhazhina E. O., Baranov Y. A., Karyakin A. A., Gavrilova V. P., Yaropolov A. I. Isolation and properties of laccase from the basidial fungus Coriolus hirsutus (Fr.) Quel. Biochemistry (Moscow) 1988;53:735–739. [Google Scholar]
- 12.Shin K.-S., Kim C.-J. Properties of laccase purified from nitrogen limited culture of white-rot fungus Coriolus hirsutus. Biotechnol. Technol. 1998;12:101–104. [Google Scholar]
- 13.Shin K.-S., Lee Y.-J. Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch. Biochem. Biophys. 2000;384:109–115. doi: 10.1006/abbi.2000.2083. [DOI] [PubMed] [Google Scholar]
- 14.Koroljova-Skorobogat'ko O. V., Stepanova E. V., Gavrilova V. P., Morozova O. V., Lubimova N. V., Dzchafarova A. N., Jaropolov A. I., Makower A. Purification and characterization of the constitutive form of laccase from the basidiomycete Coriolus hirsutus and effect of inducers on laccase synthesis. Biotechnol. Appl. Biochem. 1998;28:47–54. [PubMed] [Google Scholar]
- 15.Smirnov S. A., Koroleva O. V., Gavrilova V. P., Belova A. B., Klyachko N. L. Laccases from basidiomycetes: physicochemical characteristics and substrate specificity towards methoxyphenolic compounds. Biochemistry (Moscow) 2001;66:774–779. doi: 10.1023/a:1010216829856. [DOI] [PubMed] [Google Scholar]
- 16.Schneider P., Caspersen M. B., Mondorf K., Halkier T., Skov L. K., Ostergaard P. R., Brown K. M., Brown S. H., Xu F. Characterization of a Coprinus cinereus laccase. Enzyme Microb. Technol. 1999;25:502–508. [Google Scholar]
- 17.Xu F., Shin W., Brown S. H., Wahleithner J. A., Sundaram U. M., Solomon E. I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 18.Xu F., Kulys J. J., Duke K., Li K., Krikstopaitis K., Deussen H. J., Abbate E., Galinyte V., Schneider P. Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds. Appl. Environ. Microbiol. 2000;66:2052–2056. doi: 10.1128/aem.66.5.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhammar B. R. M. Oxidation–reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim. Biophys. Acta. 1972;275:245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- 20.Reinhammar B., Vänngård T. I. Electron-accepting sites in Rhus vernicifera laccase as studied by anaerobic oxidation–reduction titrations. Eur. J. Biochem. 1971;18:463–468. doi: 10.1111/j.1432-1033.1971.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu F., Palmer A. E., Yaver D. S., Berka R. M., Gambetta G. A., Brown S. H., Solomon E. I. Targeted mutations in a Trametes villosa laccase. Axial perturbations of the T1 copper. J. Biol. Chem. 1999;274:12372–12375. doi: 10.1074/jbc.274.18.12372. [DOI] [PubMed] [Google Scholar]
- 22.Koroleva O. V., Yavmetdinov I. S., Shleev S. V., Stepanova E. V., Gavrilova V. P. Isolation and study of some properties of laccase from the basidiomycetes Cerrena maxima. Biochemistry (Moscow) 2001;66:618–622. doi: 10.1023/a:1010299012591. [DOI] [PubMed] [Google Scholar]
- 23.Thuesen M. H., Farver O., Reinhammar B., Ulstrup J. Cyclic voltammetry and electrocatalysis of the blue copper oxidase Polyporus versicolor laccase. Acta Chem. Scand. 1998;52:555–562. [Google Scholar]
- 24.Santucci R., Ferri T., Morpurgo L., Savini I., Avigliano L. Unmediated heterogeneous electron transfer reaction of ascorbate oxidase and laccase at a gold electrode. Biochem. J. 1998;332:611–615. doi: 10.1042/bj3320611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyung K. H., Jun K. Y., Hong H.-G., Kim H. S., Shin W. Immobilization of laccase onto the gold electrode using β-mercaptopropionate. Bull. Korean Chem. Soc. 1997;18:564–566. [Google Scholar]
- 26.Johnson D. L., Thompson J. L., Brinkmann S. M., Schuller K. A., Martin L. L. Electrochemical Characterization of purified Rhus vernicifera laccase: voltammetric evidence for a sequential four-electron transfer. Biochemistry. 2003;42:10229–10237. doi: 10.1021/bi034268p. [DOI] [PubMed] [Google Scholar]
- 27.Gelo-Pujic M., Kim H. H., Butlin N. G., Palmore G. T. Electrochemical studies of a truncated laccase produced in Pichia pastoris. Appl. Environ. Microbiol. 1999;65:5515–5521. doi: 10.1128/aem.65.12.5515-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gercog C., Gustav K., Shtrele I. Moscow: Mir; 1985. Manual on Inorganic Synthesis (in Russian) [Google Scholar]
- 29.Ehresmann B., Imbault P., Weil J. H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA and rRNA. Anal. Biochem. 1973;54:454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- 30.Malkin R., Malmström B. G., Vänngård T. Reversible removal of one specific copper(II) from fungal laccase. Eur. J. Biochem. 1969;7:253–259. doi: 10.1111/j.1432-1033.1969.tb19600.x. [DOI] [PubMed] [Google Scholar]
- 31.Ando K. Preparations and properties of apo- and reconstructed Rhus-laccases. J. Biochem. (Tokyo) 1970;68:501–508. doi: 10.1093/oxfordjournals.jbchem.a129380. [DOI] [PubMed] [Google Scholar]
- 32.Graziani M. T., Morpurgo L., Rotilio G., Mondovi B. Selective removal of Type 2 copper from Rhus vernicifera laccase. FEBS Lett. 1976;70:87–90. doi: 10.1016/0014-5793(76)80732-6. [DOI] [PubMed] [Google Scholar]
- 33.Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation–reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- 34.Shleev S. V., Kuznetsov S. V., Topunov A. F. A cell for bioelectrochemical studies with parallel spectrophotometric monitoring of the studied substance. Appl. Biochem. Microbiol. (Moscow) 2000;36:354–358. [PubMed] [Google Scholar]
- 35.Larsson T., Lindgren A., Ruzgas T. Spectroelectrochemical study of cellobiose dehydrogenase and diaphorase in a thiol-modified gold capillary in the absence of mediators. Bioelectrochemistry. 2001;53:243–249. doi: 10.1016/s0302-4598(01)00099-x. [DOI] [PubMed] [Google Scholar]
- 36.Kojima Y., Tsukuda Y., Kawai Y., Tsukamoto A., Sugiura J., Sakaino M., Kita Y. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J. Biol. Chem. 1990;265:15224–15230. [PubMed] [Google Scholar]
- 37. Reference deleted.
- 38.Shlev S. V., Zaitseva E. A., Gorshina E. S., Morozova O. V., Serezhenkov V. A., Burbaev D. S., Kuznetsov B. A., Yaropolov A. I. Spectral and electrochemical study of laccases from basidiomycetes. Moscow University Chem. Bull. 2003;44:35–39. [Google Scholar]
- 39.Xu F., Berka R. M., Wahleithner J. A., Nelson B. A., Shuster J. R., Brown S. H., Palmer A. E., Solomon E. I. Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem. J. 1998;334:63–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer A. E., Szilagyi R. K., Cherry J. R., Jones A., Xu F., Solomon E. I. Spectroscopic characterization of the Leu513His variant of fungal laccase: effect of increased axial ligand interaction on the geometric and electronic structure of the type 1 Cu site. Inorg. Chem. 2003;42:4006–4017. doi: 10.1021/ic026099n. [DOI] [PubMed] [Google Scholar]
- 41.Piontek K., Antorini M., Choinowski T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90 Å resolution containing a full complement of coppers. J. Biol. Chem. 2002;277:37663–37669. doi: 10.1074/jbc.M204571200. [DOI] [PubMed] [Google Scholar]
- 42.Ducros V., Brzozowski A. M., Wilson K. S., Brown S. H., Ostergaard P., Schneider P., Yaver D. S., Pedersen A. H., Davies G. J. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat. Struct. Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. [DOI] [PubMed] [Google Scholar]
- 43.Mano N., Heller A. A miniature membraneless biofuel cell operating at 0.36 V under physiological conditions. J. Electrochem. Soc. 2003;150:A1136–A1138. [Google Scholar]
- 44.Bard A. J., Faulkner L. R. New York: Wiley; 1980. Electrochemical Methods: Fundamentals and Applications. [Google Scholar]
- 45.Sanderson D. G., Anderson L. B., Gross E. L. Determination of the redox potential and diffusion coefficient of the protein plastocyanin using optically transparent filar electrodes. Biochim. Biophys. Acta. 1986;852:269–278. doi: 10.1016/0005-2728(86)90232-x. [DOI] [PubMed] [Google Scholar]
- 46.Lindgren A., Tanaka M., Ruzgas T., Gorton L., Gazaryan I., Ishimori K., Morishima I. Direct electron transfer catalysed by recombinant forms of horseradish peroxidase: insight into the mechanism. Electrochem. Commun. 1999;1:171–175. [Google Scholar]
- 47.Malkin R., Malmström B. G., Vänngård T. Spectroscopic differentiation of the electron-accepting sites in fungal laccase. Association of a near ultraviolet band with a two electron-accepting unit. Eur. J. Biochem. 1969;10:324–329. doi: 10.1111/j.1432-1033.1969.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 48.Fee J. A., Malkin R., Malmström B. G., Vänngård T. Anaerobic oxidation–reduction titrations of fungal laccase. Evidence for several high potential electron-accepting sites. J. Biol. Chem. 1969;244:4200–4207. [PubMed] [Google Scholar]
- 49.Hakulinen N., Kiiskinen L.-L., Kruus K., Saloheimo M., Paananen A., Koivula A., Rouvinen J. Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat. Struct. Biol. 2002;9:601–605. doi: 10.1038/nsb823. [DOI] [PubMed] [Google Scholar]
- 50.Messerschmidt A., Ladenstein R., Huber R., Bolognesi M., Avigliano L., Petruzzelli R., Rossi A., Finazzi-Agro A. Refined crystal structure of ascorbate oxidase at 1.9 Å resolution. J. Mol. Biol. 1992;224:179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]