Abstract
The addition of metastasis-directed radiotherapy (MDRT) to immunotherapy in patients with advanced urothelial carcinoma (aUC) has shown promising results. We report the real-world data from the ARON-2 study (NCT05290038) on the impact of conventional (CRT) or stereotactic body radiotherapy (SBRT) on the outcome of aUC patients receiving pembrolizumab after platinum-based-chemotherapy. Medical records of 837 patients were reviewed from 60 institutions in 20 countries. Two hundred and sixty-two patients (31%) received radiotherapy (cohort A), of whom 193 (23%) received CRT and 69 (8%) received SBRT. Patients were assessed for overall survival (OS), progression-free survival (PFS), and overall response rate (ORR). Univariate and multivariate analyses were used to explore the association of variables of interest with OS and PFS. With a median follow-up of 22.7 months, the median OS was 10.2 months, 6.8 months and 16.0 months in no RT, CRT and SBRT subgroups (p = 0.005), with an 1y-OS rates of 47%, 34% and 61%, respectively (p < 0.001). The 1y-OS rate in the SBRT subgroup were significantly higher for both lower (63%) and upper tract UC (68%), for pure urothelial histology (63%) and variant histologies (58%), and for patients with bone (40%) and lymph-node metastases (61%). Median PFS was 4.8 months, 9.6 months and 5.8 months in the CRT, SBRT and no RT subgroups, respectively (p = 0.060). The 1y-PFS rate was significantly higher (48%) in the SBRT population and was confirmed in all patient subsets. The difference in terms of ORR was in favour of SBRT. Our real-world analysis showed that the use of SBRT/pembrolizumab combination may play a role in a subset of aUC patients to increase disease control and possibly overall survival.
Keywords: ARON-2 study, Pembrolizumab, Radiation therapy, Stereotactic radiation therapy, Urothelial carcinoma, Real-world data
Subject terms: Medical research, Oncology
Introduction
In recent years, the therapeutic landscape of metastatic urothelial carcinoma (mUC) has been revolutionised by the advent of immune checkpoint inhibitors (ICIs), tyrosine kinase inhibitors (TKIs) and antibody–drug conjugates (ADCs)1,2.
For patients with mUC progressing after platinum-based chemotherapy, the PD-1 inhibitor pembrolizumab represents the standard of care, based on the results of the phase III KEYNOTE 045 study3. In this study, median OS was 3 months longer in the pembrolizumab group than in the chemotherapy group (10.3 vs. 7.4 months)3. However, at a median follow-up of more than two years, the KEYNOTE-045 study demonstrated only a modest benefit in progression-free survival (2.1 months, 95% CI 2.0–2.2 months), ORR (21.1%, 95% CI 16.4–26.5%) and disease control rate (DCR) (38.5%, 95% CI 32.7–44.6%)4. This reinforces the hypothesis that immunotherapy is not effective in stimulating a systemic anti-tumor immune response in all treated patients. A recent exploratory analysis of both the aforementioned KEYNOTE-045 trial3 and the phase II KEYNOTE-052 trial (evaluating pembrolizumab monotherapy in patients without previous exposure to platinum-based chemotherapy)5, suggested that multiple biomarkers characterizing the tumor microenvironment (TME) may play a role in response/resistance to anti-PD-1/PD-L1 monotherapy6.
Indeed, a proportion of patients require additional immune-stimulating therapies. Early clinical and preclinical evidence suggests that radiotherapy could synergize with immunotherapy and act as a tolerance switch. This action occurs by disseminating tumor-specific antigens and stimulating an inflammatory microenvironment7,8.
Tumor regression in non-irradiated fields, known as the abscopal effect, achieved with radiotherapy and concomitant ICI, has been hypoyhesised and reported in several real-world experiences7–9.
Traditionally, metastasis-directed radiotherapy (MDRT), especially to the bone and the brain, has been limited to disease palliation in mUC. Over recent years, MDRT with ablative intent has emerged as a valid strategy in mUC patients10.
A retrospective cohort reported by Fukushima et al.11 described the outcomes of pembrolizumab monotherapy combined with radiotherapy on the primary tumor in 14 pre-treated mUC patients. Compared to patients who did not receive irradiation, the radiotherapy group showed a significantly higher objective response rate (65% vs 19%; p < 0.001) and a higher 1y-PFS rate (52% vs 28%; p = 0.078). One retrospective analysis from the National Cancer Database12 included 4,459 patients and compared the outcomes of patients being treated with chemotherapy alone or with chemotherapy and radiation to the primary site. The median OS was 13.8 months (95% confidence interval [CI] 12.1–15.5) for the chemoradiation group versus 8.4 months (95% CI 7.5–9.4) for the chemotherapy group (p < 0.0001). In a multivariable analysis, chemo-radiation was found to be independently associated with better overall survival (hazard ratio 0.70, 95% CI 0.62–0.79; p < 0.0001) after adjusting for all clinico-demographic variables. This data indicates that more effective control of the primary tumor may lead to improved outcomes in the metastatic setting.
Sano et al. retrospectively evaluated the role of concurrent radiotherapy (palliative or ablative) in 27 patients with mUC who received pembrolizumab as a second-line treatment13. When compared to patients receiving pembrolizumab alone, these patients showed longer OS and PFS, although the data were not statistically significant. Nakamori and colleagues14 retrospectively analyzed a multi-institutional dataset of 235 patients with platinum-refractory mUC treated with pembrolizumab as second-line treatment. A significantly longer OS was observed in patients who received concomitant palliative radiation (39 patients, median OS: 21 months) compared to both patients who received palliative radiotherapy prior to ICI (32 patients, median OS: 9 months) (p = 0.001) and those not receiving palliative radiation (164 patients: median OS: 13 months) (p = 0.019)14.
In a randomized phase I trial, 18 pretreated mUC patients received concurrent (9 patients) or sequential (9 patients) pembrolizumab plus stereotactic body radiotherapy (SBRT)15. All objective responses were observed in the concomitant ICI/SBRT arm (ORR was 0% and 44% in non-irradiated metastatic lesions in the sequential and concomitant SBRT groups). The median OS was 4.5 months for the sequential SBRT group and 12.0 months for the concomitant SBRT group15.
The randomised trials SABR-COMET16 and ORIOLE17 demonstrated in patients with various metastatic tumors, including mUC, improved oncological outcomes. The randomized trials SABR-COMET16 and ORIOLE17 demonstrated improved oncological outcomes with MDRT in patients with various metastatic tumors, including mUC.
Based on the above results, a synergy between pembrolizumab and radiotherapy is extremely promising and concomitant administration should be preferred.
Indeed, there are currently 25 ongoing clinical trials investigating the efficacy of immunotherapy and radiotherapy combination in mUC18.
The ARON-2 study (NCT05290038)19–23 was designed to globally share real-world data on the efficacy of pembrolizumab in patients who had recurred or progressed after platinum-based chemotherapy. In this sub-analysis, we evaluated the impact of palliative or stereotactic radiation therapy in this population.
Patients and methods
Study population
This retrospective study included patients aged ≥ 18 years with a cytological and/or histological confirmed diagnosis of advanced UC progressing or recurring after platinum-based therapy and treated with pembrolizumab between January 1st 2016 to April 1st 2023. The study was conducted using a multicenter cohort composed of 60 Institutions from 20 Countries.
Cohort A included pembrolizumab-treated patients who received radiation therapy, while cohort B patients did not.
The following variables were assessed in the present study: age, gender, Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), tumor histology, type and time of surgery, time and setting of prior chemotherapy, site of metastases, and response to immunotherapy. Patients with missing data on tumor response to therapy were excluded from this analysis.
The follow-up consisted of periodic physical examinations, laboratory analyses, and computed tomography (CT) of the chest and the abdomen or magnetic resonance imaging (MRI) scans of the abdomen at baseline and every 2–4 months thereafter, according to the physicians’ practice, or when progressive disease was clinically suspected. Radiologists at each institution evaluated response to pembrolizumab based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.124.
CRT and SBRT regimens were selected and administered according to the local guidelines of each institution and based on the evidence available in the literature25–28. CRT regimens consisted of the administration of 1.8–2 Gy per fraction in a variable number of fractions depending on the organ irradiated and the intent of radiotherapy. SBRT was administered with a minimum daily dose of 5–6 Gy, which could be increased depending on the size/location of the irradiated lesion(s), and in a number of fractions ranging from 1 to 10.
The study protocol was approved on February 17, 2022 by the Ethical Committee of the coordinating center (Marche Region – Italy—No. 2022 39, Study Protocol “ARON 2 Project”) and by the Institutional Review Boards of participating centers. The study was conducted according to Good Clinical Practice (GCP) and International Ethical Guidelines for Biomedical Research, and the protocol has been designed with the ethical principles laid down in the Declaration of Helsinki on human experimentation. Informed consent was obtained from all participants.
Study endpoints
According to the RECIST 1.1 criteria, disease response to treatment was defined as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Overall Response Rate (ORR) was calculated by the sum of CR and PR.
OS was calculated from the time of the first pembrolizumab administration until death. PFS was calculated as the time from the first pembrolizumab administration to documented disease progression or death from any cause, whichever occurred first. Duration of response (DoR) was defined as the time from the start of pembrolizumab therapy to disease progression or death in patients who achieved CR or PR. Patients without disease progression or death or lost at follow-up at the time of the analysis were censored at the last follow-up visit. The following subgroups were planned for analysis: tumors tract (lower or upper), histology (pure UC or variants), and site of metastases (lymph node or bone metastases).
Statistical analysis
The Kaplan–Meier method with Rothman’s 95% confidence intervals (CI) was used to estimate survival curves for OS and PFS. Comparisons between survival curves were performed by using the log-rank test. Cox proportional hazards models were adopted to compare the multivariable effects on patients’ survival and to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). For the time point events, the 1-y OS rate and 1-y PFS rate, were defined as the percentage of patients alive or not progressed after one year from RT. Chi2 method has been used to compare the data. The level of significance was set at 0.05, and all p values were two-sided.
MedCalc version 19.6.4 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium) was used for the statistical analyses.
Results
Baseline characteristics
Eight hundred and thirty-seven patients were included in our analysis. The median follow-up period was 22.7 months (95% CI 14.1–59.5). 618 patients (74%) were male, and 219 (26%) were female. Median age was 71 years (range 26–95). The ECOG-PS was ≥ 2 in 165 patients (20%). Upper urinary tract carcinomas accounted for the 26% of all cases. Tumor histology was pure UC in 679 patients (81%), while variant histologies included: squamous in 83 (10%), poorly differentiated in 20 (2%), plasmacytoid in 12 (1%), neuroendocrine in 7 (1%), sarcomatoid in 7 (1%), clear cell in 6 (1%), glandular in 6 (1%), micropapillary in 6 (1%), nested in 5 (1%), microcystic in 2 (< 1%), lymphoepithelioma-like in 2 (< 1%), and giant cell in 2 (< 1%).
Two hundred and sixty-two patients (31%) received radiation therapy (cohort A); 193 patients (23%) received conventional radiotherapy (CRT) while 69 patients (8%) received stereotactic body radiotherapy (SBRT).
A total of 533 patients (64%) received pembrolizumab following progression to first-line platinum-based chemotherapy, while 304 patients (36%) received pembrolizumab after recurrence within 12 months since the completion of adjuvant or neoadjuvant chemotherapy. At the time of the analysis, 476 patients (57%) had died, while 301 patients (36%) were undergoing treatment with pembrolizumab. Of the 536 patients who had progressed to pembrolizumab, 156 patients had received further therapies. The baseline characteristics of the patients are presented in Table 1.
Table 1.
Patients’ characteristics.
| Patients | Overall | no RT | CRT | SBRT | p |
|---|---|---|---|---|---|
| 837 (%) | 575 (%) | 193 (%) | 69 (%) | ||
| Gender | 0.611 | ||||
| Male | 618 (74) | 426 (74) | 138 (72) | 54 (78) | |
| Female | 219 (26) | 149 (26) | 55 (28) | 15 (22) | |
| Median age, years (y) | 71 | 71 | 70 | 71 | – |
| Range | 26–95 | 26–95 | 29–88 | 29–88 | |
| ECOG Performance Status | 0.223 | ||||
| 0–1 | 672 (80) | 473 (82) | 142 (74) | 57 (83) | |
| ≥ 2 | 165 (20) | 102 (18) | 51 (26) | 12 (17) | |
| Primary tumor location | 0.518 | ||||
| Upper urinary tract | 214 (26) | 150 (26) | 50 (26) | 14 (20) | |
| Lower urinary tract | 623 (74) | 425 (74) | 143 (74) | 55 (80) | |
| Tumor histology | 0.063 | ||||
| Pure urothelial carcinoma | 679 (81) | 467 (81) | 163 (84) | 49 (71) | |
| Variants | 158 (19) | 108 (19) | 30 (16) | 20 (29) | |
| Metastatic disease | 0.03 | ||||
| Synchronous | 264 (32) | 191 (33) | 61 (32) | 12 (18) | |
| Metachronous | 573 (68) | 384 (67) | 132 (68) | 57 (82) | |
| Common sites of metastasis | |||||
| Lymph nodes | 594 (71) | 418 (73) | 127 (66) | 49 (71) | 0.539 |
| Lung | 285 (34) | 202 (35) | 61 (32) | 22 (21) | 0.073 |
| Bone | 254 (30) | 134 (23) | 89 (46) | 31 (45) | < 0.001 |
| Liver | 157 (19) | 157 (27) | 0 (0) | 0 (0) | < 0.001 |
| Brain | 18 (2) | 9 (2) | 5 (3) | 4 (6) | 0.293 |
| Patients progressed during first-line platinum-based therapy | 533 (64) | 341 (59) | 142 (74) | 50 (72) | 0.047 |
| Patients relapsed within < 1y since neoadjuvant/adjuvant therapy | 304 (36) | 234 (41) | 51 (26) | 19 (18) | |
| Radiation to: | |||||
| Lymph nodes | 83 (10) | – | 57 (30) | 26 (38) | - |
| Bone | 102 (12) | – | 74 (38) | 28 (41) | - |
| Lung | 7 (1) | – | 2 (1) | 5 (7) | - |
| Bladder | 25 (3) | – | 24 (12) | 1 (1) | - |
| Local recurrence | 23 (3) | – | 18 (9) | 5 (7) | - |
| Soft tissues | 12 (1) | – | 10 (5) | 2 (3) | - |
| Brain | 13 (2) | – | 7 (4) | 6 (9) | - |
| Other | 10 (1) | – | 9 (5) | 1 (2) | - |
| Therapies after pembrolizumab | |||||
| Paclitaxel | 47 (6) | 24 (4) | 19 (10) | 6 (9) | 0.232 |
| Vinflunine | 44 (5) | 30 (5) | 9 (5) | 5 (7) | 0.779 |
| Carboplatin and gemcitabine | 14 (2) | 8 (1) | 4 (2) | 2 (3) | 0.6 |
| Clinical trials | 9 (1) | 4 (1) | 3 (2) | 2 (3) | 0.6 |
| Docetaxel | 12 (1) | 7 (1) | 3 (2) | 2 (3) | 0.6 |
| Enfortumab vedotin | 10 (1) | 5 (1) | 3 (2) | 2 (3) | 0.6 |
| Carboplatin and paclitaxel | 7 (1) | 4 (1) | 2 (1) | 1 (1) | 1 |
| Other | 13 (2) | 8 (1) | 3 (2) | 2 (3) | 0.6 |
Statistically significant values are reported in bold.
No RT = no radiotherapy; CRT = conventional radiotherapy; SBRT = stereotactic body radiotherapy.
Survival analysis
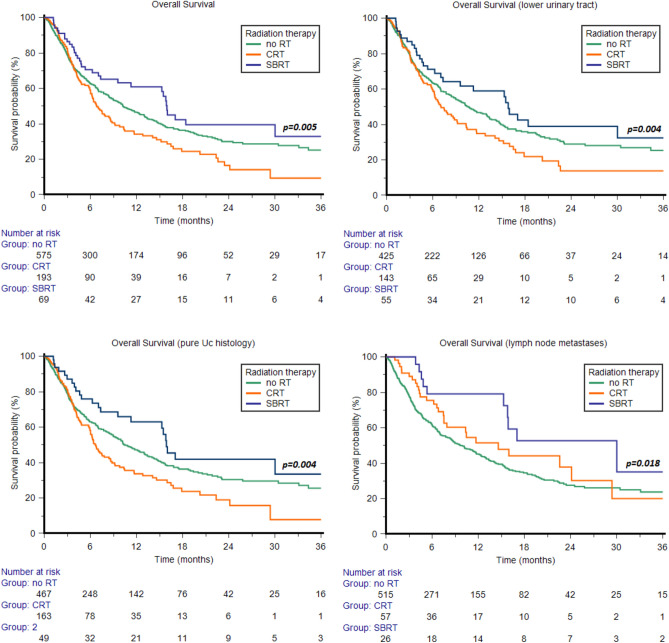
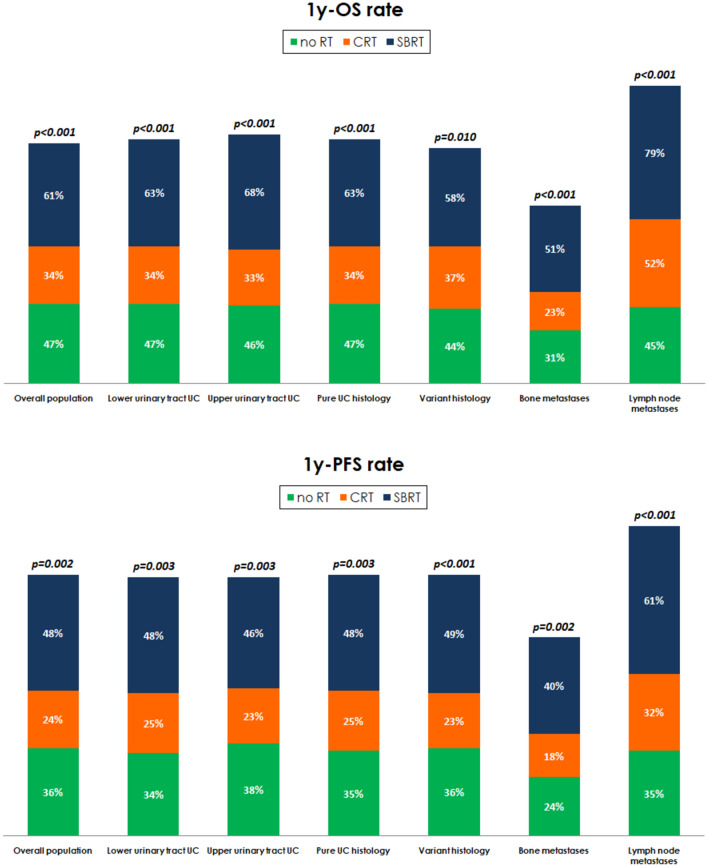
In the overall study population, the median OS was 9.8 months (95% CI 8.4–11.4). Median OS was 10.2 months (95% CI 8.7–12.4) in the no RT subgroup, 6.8 months (95% CI 6.0–8.6) in patients who underwent CRT, and 16.0 months (95% CI 9.6–30.0) in those treated by SBRT (p = 0.005, Fig. 1), with an 1y-OS rate of 47%, 34% and 61%, respectively (p < 0.001, Fig. 2).
Figure 1.
Median Overall Survival in the overall study population and in patients with tumors of the lower urinary tract, pure urothelial carcinoma (UC) histology or lymph node metastases.
Figure 2.
Overall Survival (1y-OS rate) and Progression-Free Survival rate at 1y (1y-PFS rate) stratified by clinico-pathological characteristics.
In the 623 patients with UC of the lower urinary tract, SBRT was associated with a longer median OS (15.8 months, 95% CI 7.3–46.4) compared to those treated by CRT (7.1 months, 95% CI 6.0–9.2) or those who did not receive radiotherapy (10.7 months, 95% CI 8.7–13.2, p = 0.004, Fig. 1), with an 1y-OS rate of 63%, 34% and 47% (Fig. 2). Furthermore, no statistically significant differences were found in patients with upper tract UC (SBRT: 16.0 months, 95% CI 3.9–46.4; CRT: 8.6 months, 95% CI 4.3–22.6; no RT: 8.4 months, 95% CI 6.4–13.3, p = 0.844). The 1y-OS rate was significantly higher in the SBRT subgroup (68% vs 33% vs 46%, Fig. 2).
In patients with pure UC histology, the median OS was 6.7 months (95% CI 5.9–8.6), 15.9 months (95% CI 9.6–30.0) and 10.5 months (95% CI 9.0–13.0) in patients who underwent CRT/SBRT and in cohort B respectively (p = 0.004, Fig. 1). The 1y-OS rate of 34%, 63% and 47% (p < 0.001, Fig. 2). Conversely, no statistically significant differences in terms of median OS were observed in the 158 patients with variant UC histology (6.4 months, 95% CI 4.3–29.4, 16.0 months, 95% CI 4.1–16.0, and 10.0 months, 95% CI 6.6–14.0, p = 0.272). The1y-OS rate was significantly improved by SBRT (Fig. 2).
The median OS of patients with bone metastases was 5.5 months (95% CI 3.9–6.7), 5.9 months (95% CI 3.8–6.4) and 16.0 months (95% CI 4.6–18.4) in cohort B patients and in patients undergoing CRT/SBRT respectively (p = 0.145), with a significantly improved the 1y-OS rate in patients receiving SBRT (Fig. 2).
The median OS of patients with metastases to lymph nodes was higher in those receiving SBRT (30.0 months, 95% CI 15.3–30.0) compared to those treated by CRT (14.6 months, 95% CI 7.5–24.1) and those who did not receive RT (10.0 months, 95% CI 8.0–11.8, p = 0.018, Fig. 1), with an 1y-OS rate of 79%, 52% and 45% (p < 0.001, Fig. 2).
In the overall study population, the median PFS was 5.8 months (95% CI 4.8–6.4), while it was 4.8 months (95% CI 3.6–6.3), 9.6 months (95% CI 4.3–17.3) and 5.8 months (95% CI 4.5–6.8) in patients who underwent CRT, SBRT and no RT, respectively (p = 0.060). The 1y-PFS rate was significantly higher (48%) in the SBRT subgroup (Fig. 2).
In patients with lower tract UC (LTUC), the median PFS was 5.1 months (95% CI 3.5–6.7), 10.2 months (95% CI 4.3–17.3) and 6.2 months (95% CI 4.5–7.0) in patients who underwent CRT, SBRT and no RT, respectively (p = 0.109). As for upper tract UC (UTUC), no statistically significant differences were found between patients treated by CRT (4.2 months, 95% CI 3.2–6.9), SBRT (5.7 months, 95% CI 2.1–20.0) or without RT (5.0 months, 95% CI 3.8–8.5, p = 0.608). The 1y-PFS rate was significantly improved by SBRT in both patients with LTUC and UTUC (Fig. 2).
In patients with pure UC histology, the median PFS was 5.3 months (95% CI 3.5–6.4), 10.2 months (95% CI 5.3–18.8) and 6.2 months (95% CI 4.5–7.0) in patients who underwent CRT, SBRT and no RT, respectively (p = 0.077). In patients with UC histologic variants, the median PFS was 4.5 months (95% CI 3.2–7.9), 5.3 months (95% CI 2.2–44.0) and 5.0 months (95% CI 3.5–7.0) in patients who underwent CRT, SBRT and no RT (p = 0.696). The 1y-PFS rate was significantly improved by SBRT in both patients with pure UC (p = 0.003, Fig. 2) and histologic variants (p < 0.001, Fig. 2).
In patients with bone metastases, the median PFS was 3.6 months (95% CI 3.2–4.2), while no significant differences in terms of median PFS were found between those who did not receive RT (3.5 months, 95% CI 2.8–4.0) and those treated by CRT (3.6 months, 95% CI 3.0–5.8) or SBRT (3.9 months, 95% CI 2.2–17.3, p = 0.518). The 1y-PFS rate was higher in patients treated by SBRT (p = 0.002, Fig. 2).
Accordingly, no significant differences in terms of median PFS was found between patients with metastases to the lymph nodes in the no RT subgroup (5.3 months, 95% CI 4.1–6.4) and treated by CRT (7.9 months, 95% CI 4.5–12.0) or SBRT (18.8 months, 95% CI 5.3–46.0, p = 0.100). The 1y-PFS rate was higher in patients treated by SBRT (p < 0.001, Fig. 2).
Tumor response to therapy and DoR
In the overall study population, 9% of patients experienced CR, 22% PR, 25% SD and 44% PD. In Table 2 we reported the rate of CR, PR, SD and PD in patients who did not receive RT and treated by CRT or SBRT. The difference in terms of ORR was in favor of patients treated by SBRT (Table 2).
Table 2.
Response to pembrolizumab with or without conventional radiotherapy (CRT) or stereotactic body radiotherapy (SBRT).
| Patients | no RT | CRT | SBRT | p |
|---|---|---|---|---|
| 575(%) | 193(%) | 69(%) | ||
| Tumor Response (RECIST 1.1) | - | |||
| Complete remission (CR) | 10 | 4 | 8 | |
| Partial response (PR) | 23 | 14 | 32 | |
| Stable disease (SD) | 22 | 33 | 23 | |
| Progressive disease (PD) | 45 | 49 | 37 | |
| Overall Response Rate (CR + PR) | 33 | 18 | 40 | 0.003 |
| DoR rate at 6 months | 94 | 87 | 92 | 0.205 |
| DoR rate at 1 year | 81 | 77 | 86 | 0.262 |
Statistically significant values are reported in bold.
No RT = no radiotherapy; CRT = conventional radiotherapy; SBRT = stereotactic body radiotherapy; DoR = duration of response.
In the subgroup of patients who experienced CR or PR, no statistically significant differences were found in terms of median DoR between those who did not received RT (NR, 95% CI NR–NR) or were treated by CRT (19.0 months, 95% CI 12.3–24.1) or SBRT (25.4 months, 95% CI 15.5–46.0, p = 0.090). Analogously, the rate of DoR at 6 months and 1y were not statistically different among the three subgroups (Table 2).
Prognostic factors
At univariate analysis for OS, ECOG-PS, liver and bone metastases, synchronous metastatic disease and CRT were significantly associated with OS (Table 3). At multivariate analysis, ECOG-PS, liver and bone metastases, synchronous metastatic disease confirmed their prognostic role (Table 3).
Table 3.
Univariate and multivariate analyses.
| Overall survival | Univariate Cox regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex (females vs males) | 1.19 (0.98–1.46) | 0.074 | ||
| Age ≥ 65y (Y vs N) | 1.08 (0.89–1.32) | 0.408 | ||
| ECOG-PS (≥ 2 vs 0–1) | 2.93 (2.39–3.58) | < 0.001 | 2.68 (2.16–3.33) | < 0.001 |
| Smokers vs no-smokers | 0.85 (0.58–1.24) | 0.401 | ||
| Histology (mixed vs pure UC) | 1.03 (0.82–1.30) | 0.782 | ||
| Upper vs Lower urinary tract | 0.95 (0.64–1.40) | 0.778 | ||
| Synchronous metastatic disease (yes vs no) | 1.44 (1.19–1.73) | < 0.001 | 1.23 (1.01–1.50) | 0.039 |
| Distant lymph node (Y vs N) | 0.93 (0.76–1.14) | 0.498 | ||
| Lung metastases (Y vs N) | 1.17 (0.97–1.42) | 0.146 | ||
| Liver metastases (Y vs N) | 1.81 (1.19–2.73) | 0.005 | 1.80 (1.18–2.72) | 0.006 |
| Bone metastases (Y vs N) | 1.66 (1.38–201) | < 0.001 | 1.57 (1.27–1.92) | < 0.001 |
| Geographical areas (Europe vs no Europe) | 1.12 (0.79–1.59) | 0.532 | ||
| Conventional RT (Y vs N) | 1.30 (1.05–1.60) | 0.015 | 0.99 (0.79–1.24) | 0.961 |
| SBRT (Y vs N) | 0.74 (0.52–1.06) | 0.100 | ||
Statistically significant values are reported in bold.
ECOG-PS = Eastern Cooperative Oncology Group-Performance Status; UC = urothelial carcinoma; RT = radiotherapy; SBRT = stereotactic body radiotherapy.
Discussion
This manuscript reports the outcomes of mUC patients treated with pembrolizumab and radiotherapy. Differently from the pivotal trial, KEYNOTE 0454, our overall dataset population presents with higher proportion of pure transitional carcinomas, upper tract tumors, synchronous disease, and ECOG PS 2, but lower liver metastasis.
Patients treated in real world have higher ORR and PFS, but comparable complete responses rate, progressive disease as best response, and OS. The addition of CRT did not improve OS, PFS and ORR either in the whole patient population or in any of the subgroups analysed (lower tract, upper tract, pure transitional, variant histologies, bone and node metastasis). On the other hand, the use of SBRT improved OS in the overall population, as well as in some of the subgroups analysed (lower tract, pure transitional, bone and node metastasis) of our case series. The absence of OS benefit in the UTUC and variant histologies could be explained by the small sample size. The 1y-OS benefit was observed in all patients and subgroups. The SBRT did not improve PFS in the overall population or in any of the subgroups, but the 1-year PFS rate was statistically higher with the addition of SBRT. The ORR was increased with the use of SBRT. The higher ORR with no benefit in the PFS raises questions about the possibility of an abscopal effect. Our real-world data does not support any abscopal effect and is in line with a recent prospective randomized phase 2 trial that failed to demonstrate an improvement in patients receiving SBRT with abscopal intent29.
Patients with CR + PR have the same DoR, regardless of the use or not of any radiation strategy. The better control of the disease is a well-recognized outcome for patients achieving CR or PR and the higher number of patients achieving an objective response in the SBRT group may explain, at least partially, the benefit in terms of OS and PFS.
Our analyses are biased by its retrospective nature, based on data retrieved from patients’ charts, and the heterogeneity of treatment strategies empowered across different sites around the world, as well as differences in the demographics characteristics. In addition, it is not possible to perform a propensity score and the use of SBRT has been decided according local guidelines of the respective centres. Patients in the SBRT group had less synchronous disease, which is known to be characterised by a better prognosis30. Patients receiving any radiation strategy have higher proportion of bone and node metastases, and none had liver metastasis. On the contrary, liver metastases were presented in the 27% of patients not receiving radiation therapy. The presence of liver metastases is indeed a known factor associated with worse prognosis31–34. The radiation groups (CRT and SBRT) had more patients progressing during chemotherapy, again a recognized bad prognostic factor35. Finally, we are missing important data such as radiation delivery, dose-fraction in the conventional and the SBRT group and timing of RT. The pre-selection of patients is another important bias. We did not record if patients received CRT or SBRT with palliative or ablative intent, nor if the treatment involved only the primary tumor, only the metastases, or both. Our results, differently from Fukushima et al.11 and Fischer-Valuck et al. series12 showed worse overall survival for the patients receiving chemoradiation when compared to patients receiving chemotherapy alone or SBRT. Usually, an ablative intent is recommended for patients who achieve a very good partial response, aiming towards a complete response, or to treat one or more non-responsive lesions, in order to improve the overall extent of response. Indeed, CR or PR is an important signal to predict better outcomes in patients with mUC. This benefit is shown in all trials with ICI in second or latter lines3,36–38.
The role of subsequent therapies is another important issue.
Although there is no statistically difference in ECOG PS 2 between groups, CRT group has a higher percentage of poor performance patients. It is reasonable to assume that patients in the CRT group were more symptomatic, have high-burden metastatic disease, and thus received radiation with palliative intent. The phase IV SAUL study showed that ECOG PS 2 is the most relevant factor associated with bad prognosis24.
Lastly, the access to better radiation techniques, as SBRT, is influenced by the cost and geographic distribution of this technology39,40.
Conclusion
This dataset is the first and biggest real-world data analyzing the outcomes and the importance of radiation therapy in patients with metastatic urothelial carcinoma receiving pembrolizumab. Our study showed that the use of SBRT may play a role in a subset of patients to increase the control of the disease and maybe increase the overall survival. Unfortunately, there are no biomarkers to select which patient should receive or not SBRT. Given the lack of randomized trials on the role of radiation therapy in urothelial cancer patients treated with immunotherapy, the results from this real-world analysis could be useful for decision-making.
Acknowledgements
We want to sincerely thank the ARON Working Group for the support received in this analysis and for the continuous efforts in the creation of a global network aimed to share our clinical experiences in the use of new drugs for patients with genitourinary tumors.
Author contributions
M.R., A.S. and M.S. designed the study and wrote the main manuscript text; M.R. and M.S. prepared tables and figures. All authors provided critical feedback and helped shape the research and analysis.
Data availability
The datasets used and/or analysed during this study are available from the last author upon reasonable request.
Competing interests
Mimma Rizzo has received honoraria as a speaker/consultant by MSD, Astrazeneca, Bristol-Myers Squibb, Gilead, Janssen and Merck Serono, all unrelated to the present paper. Andrey Soares has received Honoraria: Janssen, Pfizer, Bayer, AstraZeneca, Astellas Pharma, Merck Serono, Sanofi, Ipsen, Adium; Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, AstraZeneca, MSD, Bristol-Myers Squibb, Adium, Ipsen; Research Funding: Bristol-Myers Squibb (Inst), Astellas (Inst), AstraZeneca (Inst); Travel, Accommodations, Expenses: Bayer, Janssen, Ipsen, Adium, MSD. Enrique Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho, and Thermo Fisher Scientific. EG has received research grants from Pfizer, Astra Zeneca, Astellas, and Lexicon Pharmaceuticals. Ondrej Fiala received honoraria from Roche, Janssen, MSD, Pierre Fabre, GSK and Pfizer for consultations and lectures unrelated to this project. Patrizia Giannatempo has received research support from Ipsen, Astra Zeneca e MSD and honoraria for speaker engagements, advisory roles from Astellas, MSD, Janssen, Pfizer. Francesco Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD and Pfizer outside the submitted work. Fernando Sabino Marques Monteiro has received research support from Janssen, Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp Dome. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, Exelixis, Ipsen, Merck and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai and MSD. Ravindran Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies; Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas and Bayer. Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas and Bayer, all unrelated to the present paper. The other authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mimma Rizzo and Andrey Soares.
These authors jointly supervised this work: Ravindran Kanesvaran and Matteo Santoni.
References
- 1.Meeks, J. J. et al. Checkpoint inhibitors in urothelial carcinoma-future directions and biomarker selection. Eur. Urol.84, 473–483. 10.1016/j.eururo.2023.05.011 (2023). 10.1016/j.eururo.2023.05.011 [DOI] [PubMed] [Google Scholar]
- 2.Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature515, 558–562. 10.1038/nature13904 (2014). 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med376, 1015–1026. 10.1056/NEJMoa1613683 (2017). 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fradet, Y. et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol30, 970–976. 10.1093/annonc/mdz127 (2019). 10.1093/annonc/mdz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuky, J. et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol38, 2658–2666. 10.1200/JCO.19.01213 (2020). 10.1200/JCO.19.01213 [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt, J. et al. Putative biomarkers of clinical benefit with pembrolizumab in advanced urothelial cancer: results from the KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res28, 2050–2060. 10.1158/1078-0432.CCR-21-3089 (2022). 10.1158/1078-0432.CCR-21-3089 [DOI] [PubMed] [Google Scholar]
- 7.Abuodeh, Y., Venkat, P. & Kim, S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer40, 25–37. 10.1016/j.currproblcancer.2015.10.001 (2016). 10.1016/j.currproblcancer.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Herrera, F. G., Bourhis, J. & Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin67, 65–85. 10.3322/caac.21358 (2017). 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro Gomes, J. et al. Analysis of the abscopal effect With Anti-PD1 therapy in patients with metastatic solid tumors. J Immunother39, 367–372. 10.1097/CJI.0000000000000141 (2016). 10.1097/CJI.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 10.Miranda, A. F. et al. Metastasis-directed radiation therapy after radical cystectomy for bladder cancer. Urol Oncol39, 790.e1-790.e7. 10.1016/j.urolonc.2021.05.005 (2021). 10.1016/j.urolonc.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 11.Fukushima, H. et al. Impact of radiotherapy to the primary tumor on the efficacy of pembrolizumab for patients with advanced urothelial cancer: A preliminary study. Cancer Med9, 8355–8363. 10.1002/cam4.3445 (2020). 10.1002/cam4.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer-Valuck, B. W. et al. Association between local radiation therapy to the primary bladder tumor and overall survival in patients with metastatic urothelial cancer receiving systemic chemotherapy. Eur Urol Oncol5, 246–250. 10.1016/j.euo.2022.02.001 (2022). 10.1016/j.euo.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sano, T. et al. Efficacy and tolerability of second-line pembrolizumab with radiation therapy in advanced urothelial carcinoma. Anticancer Res43, 2119–2126. 10.21873/anticanres.16373 (2023). 10.21873/anticanres.16373 [DOI] [PubMed] [Google Scholar]
- 14.Nakamori, K. et al. Concurrent palliative radiation with pembrolizumab for platinum-refractory urothelial carcinoma is associated with improved overall survival. Clin Transl Radiat Oncol39, 100558. 10.1016/j.ctro.2022.12.001 (2022). 10.1016/j.ctro.2022.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundahl, N. et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur Urol75, 707–711. 10.1016/j.eururo.2019.01.009 (2019). 10.1016/j.eururo.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Palma, D. A. et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol38, 2830–2838. 10.1200/JCO.20.00818 (2020). 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips, R. et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol6, 650–659. 10.1001/jamaoncol.2020.0147 (2020). 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://clinicaltrials.gov; Accessed on November 28th 2023
- 19.Massari, F. et al. Global real-world experiences with pembrolizumab in advanced urothelial carcinoma after platinum-based chemotherapy: the ARON-2 study. Cancer Immunol Immunother.73(6), 106. 10.1007/s00262-024-03682-w (2024). 10.1007/s00262-024-03682-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoni, M. et al. Bone targeting agents, but not radiation therapy, improves survival in patients with bone metastases from advanced urothelial carcinoma receiving pembrolizumab: results from the ARON-2 study. Clin Exp Med.23(8), 5413–5422. 10.1007/s10238-023-01235-6 (2023) (Epub 2023 Nov 2). 10.1007/s10238-023-01235-6 [DOI] [PubMed] [Google Scholar]
- 21.Fiala, O. et al. Use of concomitant proton pump inhibitors, statins or metformin in patients treated with pembrolizumab for metastatic urothelial carcinoma: data from the ARON-2 retrospective study. Cancer Immunol Immunother.72(11), 3665–3682. 10.1007/s00262-023-03518-z (2023) (Epub 2023 Sep 7). 10.1007/s00262-023-03518-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo, A. et al. ARON Working Group Pembrolizumab in patients with advanced upper tract urothelial carcinoma: A real-world study from ARON-2 project. Clin Exp Metastasis.10.1007/s10585-024-10296-0 (2024) (Epub ahead of print). 10.1007/s10585-024-10296-0 [DOI] [PubMed] [Google Scholar]
- 23.Santoni, M. et al. Real-world effectiveness of pembrolizumab as first-line therapy for cisplatin-ineligible patients with advanced urothelial carcinoma: the ARON-2 study. Cancer Immunol Immunother.72(9), 2961–2970. 10.1007/s00262-023-03469-5 (2023) (Epub 2023 May 29). 10.1007/s00262-023-03469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz, L. H. et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer62, 132–137. 10.1016/j.ejca.2016.03.081 (2016). 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamias, A. et al. Definition and Diagnosis of Oligometastatic Bladder Cancer: A Delphi Consensus Study Endorsed by the European Association of Urology, European Society for Radiotherapy and Oncology, and European Society of Medical Oncology Genitourinary Faculty. Eur Urol.84(4), 381–389. 10.1016/j.eururo.2023.05.005 (2023) (Epub 2023 May 20). 10.1016/j.eururo.2023.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Aboudaram, A. et al. Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis. Cancers (Basel).15(4), 1161. 10.3390/cancers15041161 (2023). 10.3390/cancers15041161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longo, N. et al. Metastasis-Directed Radiation Therapy with Consolidative Intent for Oligometastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Cancers (Basel).14(10), 2373. 10.3390/cancers14102373 (2022). 10.3390/cancers14102373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzese, C. et al. Stereotactic Body Radiation Therapy in the Management of Oligometastatic and Oligoprogressive Bladder Cancer and Other Urothelial Malignancies. Clin Oncol (R Coll Radiol).33(1), 50–56. 10.1016/j.clon.2020.07.008 (2021) (Epub 2020 Jul 25). 10.1016/j.clon.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 29.Spaas, M. et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: The CHEERS phase 2 randomized clinical trial. JAMA Oncol9, 1205–1213. 10.1001/jamaoncol.2023.2132 (2023). 10.1001/jamaoncol.2023.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschini, M. et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int117, 604–610. 10.1111/bju.13146 (2016). 10.1111/bju.13146 [DOI] [PubMed] [Google Scholar]
- 31.Bellmunt, J. et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol28, 1850–1855. 10.1200/JCO.2009.25.4599 (2010). 10.1200/JCO.2009.25.4599 [DOI] [PubMed] [Google Scholar]
- 32.Bamias, A. et al. SAUL, a single-arm study of atezolizumab for chemotherapy-pretreated locally advanced or metastatic carcinoma of the urinary tract: outcomes by key baseline factors, PD-L1 expression and prior platinum therapy. ESMO Open6, 100152. 10.1016/j.esmoop.2021.100152 (2021). 10.1016/j.esmoop.2021.100152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merseburger, A. S. et al. Safety and Efficacy of Atezolizumab in Understudied Populations with Pretreated Urinary Tract Carcinoma: Subgroup Analyses of the SAUL Study in Real-World Practice. J Urol206, 240–251. 10.1097/JU.0000000000001768 (2021). 10.1097/JU.0000000000001768 [DOI] [PubMed] [Google Scholar]
- 34.Tufano, A. et al. Prognostic Significance of Organ-Specific Metastases in Patients with Metastatic Upper Tract Urothelial Carcinoma. J Clin Med11, 5310. 10.3390/jcm11185310 (2022). 10.3390/jcm11185310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonpavde, G. et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol63, 717–723. 10.1016/j.eururo.2012.11.042 (2013). 10.1016/j.eururo.2012.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balar, A. V. et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol34, 289–299. 10.1016/j.annonc.2022.11.012 (2023). 10.1016/j.annonc.2022.11.012 [DOI] [PubMed] [Google Scholar]
- 37.Powles, T. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet391, 748–757. 10.1016/S0140-6736(17)33297-X (2018). 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 38.Siefker-Radtke, A. O. et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: Cohort 2 of the randomized phase III THOR trial. Ann Oncol10.1016/j.annonc.2023.10.003 (2023). 10.1016/j.annonc.2023.10.003 [DOI] [PubMed] [Google Scholar]
- 39.Zubizarreta, E., Van Dyk, J. & Lievens, Y. Analysis of global radiotherapy needs and costs by geographic region and income level. Clin Oncol (R Coll Radiol)29, 84–92. 10.1016/j.clon.2016.11.011 (2017). 10.1016/j.clon.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 40.Maitre, P. et al. Modern radiotherapy technology: obstacles and opportunities to access in low- and middle-income countries. JCO Glob Oncol8, e2100376. 10.1200/GO.21.00376 (2022). 10.1200/GO.21.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during this study are available from the last author upon reasonable request.