Abstract
The VDAC (voltage-dependent anion channel) plays a central role in apoptosis, participating in the release of apoptogenic factors including cytochrome c. The mechanisms by which VDAC forms a protein-conducting channel for the passage of cytochrome c are not clear. The present study approaches this problem by addressing the oligomeric status of VDAC and its role in the induction of the permeability transition pore and cytochrome c release. Chemical cross-linking of isolated mitochondria or purified VDAC with five different reagents proved that VDAC exists as dimers, trimers or tetramers. Fluorescence resonance energy transfer between fluorescently labelled VDACs supports the concept of dynamic VDAC oligomerization. Mitochondrial cross-linking prevented both permeability transition pore opening and release of cytochrome c, yet had no effect on electron transport or Ca2+ uptake. Bilayer-reconstituted purified cross-linked VDAC showed decreased conductance and voltage-independent channel activity. In the dithiobis(succinimidyl propionate)-cross-linked VDAC, these channel properties could be reverted to those of the native VDAC by cleavage of the cross-linking. Cross-linking of VDAC reconstituted into liposomes inhibited the release of the proteoliposome-encapsulated cytochrome c. Moreover, encapsulated, but not soluble cytochrome c induced oligomerization of liposome-reconstituted VDAC. Thus the results indicate that VDAC exists in a dynamic equilibrium between dimers and tetramers and suggest that oligomeric VDAC may be involved in mitochondria-mediated apoptosis.
Keywords: apoptosis, cytochrome c, mitochondria, oligomerization, permeability transition pore, voltage-dependent anion channel (VDAC)
Abbreviations: ANT, adenine nucleotide translocator; CM-cellulose, carboxymethylcellulose; DFDNB, 1,5-difluoro-2,4-dinitrobenzene; DMS, dimethyl suberimidate; DSP, dithiobis(succinimidyl propionate); DTT, DL-dithiothreitol; EGS, ethylenglycolbis(succinimidylsuccinate); EITC, eosin 5-isothiocyanate; FRET, fluorescence resonance energy transfer; HK, hexokinase; LDAO, lauryl-(dimethyl)-amineoxide; β-OG, n-octyl-β-D-glucopyranoside; OMM and IMM, outer and inner mitochondrial membranes; PLB, planar lipid bilayer; PTP, permeability transition pore; RuR, Ruthenium Red; VDAC, voltage-dependent anion channel
INTRODUCTION
Mitochondria are potent integrators and co-ordinators of programmed cell death or apoptosis. After an apoptotic stimuli, various proteins that normally reside in the intermembrane space of mitochondria, including cytochrome c, apoptosis-inducing factors and certain procaspase proteins, are released and initiate the activation of caspases, the protease mediators of cell death [1–3]. It is still not clear how these apoptosis initiators (among them cytochrome c) are released, although different models have been proposed [3,4]. While some models predict that release occurs on rupture of the OMM (outer mitochondrial membrane) as a result of swelling of the mitochondrial matrix, other models predict the formation of a pore large enough to allow the passage of cytochrome c and other proteins into the cytosol, without damage to the OMM [4]. The finding that cytochrome c can leak from intact mitochondria [5–8] supports models predicting specific permeability of the OMM. A good candidate for a pore-forming protein is the VDAC (voltage-dependent anion channel) at the OMM, previously suggested to participate in the release of cytochrome c [1,3,9–15].
One suggested mechanism for cytochrome c release involves the induction of the mitochondrial PTP (permeability transition pore), a large channel that spans both the OMM and IMM (inner mitochondrial membrane) [1,10]. Current models suggest that the PTP is formed by a direct association between VDAC in the OMM, ANT (adenine nucleotide translocator) in the IMM and cyclophilin D in the matrix, although other proteins may also contribute [1,3,10,11,13,14]. As a consequence of PTP opening, mitochondria can no longer fulfil their vital metabolic and redox maintenance functions, become depleted of ATP and lose their ability to act as Ca2+ storage organelles.
As a core component of the PTP [10,13,16], VDAC reconstituted into bilayers presents features that are compatible with those observed for PTP, including serving as the putative pore for cytochrome c release [13,15]. These include acting as a voltage-dependent, non-selective channel with a pore diameter of 3 nm [12,17,18]. Given its pore dimensions and non-selective nature, its ability to transport and bind Ca2+ [19] (all functions known to be essential for PTP opening), the involvement of VDAC in the regulation of PTP opening and subsequent release of apoptogenic components is quite probable [10,14,16]. However, the molecular mechanisms by which VDAC could form a protein-conducting channel that would allow passage of cytochrome c are still not clear [10,14,16].
Despite the fact that functional studies of VDAC provide no compelling reason for invoking oligomerization [20,21], one possible mechanism for VDAC-mediated cytochrome c release is the formation of a channel through oligomerization of VDAC. Indeed, evidence consistent with oligomerization of VDAC has been presented; the hydrodynamic properties of purified rat liver VDAC suggest that isolated VDAC exists as dimers [22] or oligomers [23], whereas cross-linking of yeast OMM revealed the existence/formation of VDAC dimers, trimers and oligomers [24]. A low-resolution (15 Å; 1 Å=0.1 nm) surface structure of VDAC, obtained by metal shadowing and cryoelectron microscopy of human VDAC crystals grown in the presence of phospholipids showed a dimeric organization of VDAC [25]. Still, the possible function of VDAC oligomerization has not been addressed.
In the present study, the oligomeric state of purified and membrane-embedded VDAC and its possible function in the release of cytochrome c were investigated. The results show that VDAC exists as dimers to tetramers and suggest that VDAC oligomerization may serve as a control mechanism for the release of cytochrome c.
EXPERIMENTAL
Materials
BSA, CM-cellulose (carboxymethylcellulose), cytochrome c (horse heart), n-decane, DFDNB (1,5-difluoro-2,4-dinitrobenzene), DTT (DL-dithiothreitol), EITC (eosin 5-isothiocyanate), FITC, Hepes, mannitol, leupeptin, PMSF, reactive red agarose, soya-bean asolectin, Tris, Triton X-100, xanthine and xanthin oxidase were purchased from Sigma (St. Louis, MO, U.S.A.). LDAO [lauryl-(dimethyl)-amineoxide] and RuR (Ruthenium Red) were obtained from Fluka (Buchs, Switzerland). Hydroxyapatite (Bio-Gel HTP) was purchased from Bio-Rad (Hercules, CA, U.S.A.) and Celite was from Merck (Darmstadt, Germany). β-OG (n-octyl-β-D-glucopyranoside) was from Bachem (Bubendorf, Switzerland). DSP [dithiobis(succinimidyl propionate)], DMS (dimethyl suberimidate), EGS [ethylenglycolbis(succinimidylsuccinate)] and Sulpho-EGS were from Pierce (Rockford, IL, U.S.A.). Monoclonal anti-VDAC antibodies raised against the N-terminal region of 31HL human porin (clone no. 173/045) [26] were from Calbiochem (San Diego, CA, U.S.A.). Cytochrome c monoclonal antibodies were obtained from Pharmingen (San Diego, CA, U.S.A.). Horseradish peroxidase-conjugated goat anti-mouse antibodies were from Protos Immunoresearch (San Francisco, CA, U.S.A.). [45Ca2+] was purchased from NEN Life Science Products (Boston, MA, U.S.A.).
Mitochondrial preparation
Mitochondria were prepared from rat liver according to the standard procedure [27]; briefly, a liver from Wistar rat was homogenized in cold buffer H [0.25 M sucrose and 10 mM Hepes (pH 7.4) containing protease inhibitor cocktail; Sigma] by ten strokes at 900 rev./min in a Teflon/glass homogenizer. The homogenate was centrifuged for 10 min at 1000 g. The supernatant was centrifuged for 15 min at 12000 g, and the pellet was resuspended in buffer H to obtain crude mitochondria. For further purification, crude mitochondria were layered on 25% (v/v) Percoll prepared in buffer H and then centrifuged for 1 h at 100000 g (SW28 Beckman rotor). Mitochondria were resuspended in buffer H and centrifuged again for 20 min at 100000 g. Mitochondria were resuspended in buffer H stored in ice and used within 4–6 h. Succinate–cytochrome c oxidoreductase activity, as a measure of the intactness of the mitochondria, was 0 and 62.5±3.5 nmol/mg (n=6) before and after the osmotic shock respectively. Thus, in most experiments, the intactness of the mitochondria was between 80 and 99%.
Protein concentration was determined by the biuret method [28].
Mitochondrial Ca2+ accumulation
Ca2+ accumulation by freshly isolated rat liver mitochondria (0.5 mg/ml) was assayed for 1–20 min at 30 °C in the presence of 0.2 mM CaCl2 (containing [45Ca2+], 3×104 c.p.m./nmol), 220 mM mannitol, 70 mM sucrose, 0.5 mM nitrilotriacetic acid, 5 mM succinate, 0.15 mM Pi and 15 mM Tris/HCl (pH 7.2). Ca2+ uptake was terminated by rapid Millipore filtration followed by a wash with 5 ml of 0.15 M KCl.
Mitochondrial swelling
Ca2+-induced mitochondrial swelling was assayed in freshly prepared mitochondria under the same conditions as employed for Ca2+ accumulation, except that the reaction was performed at 24 °C. Swelling was initiated by the addition of Ca2+ (0.2 mM) to the sample cuvette. Absorbance changes at 520 nm were monitored every 15–20 s.
Cross-linking experiments
Rat liver mitochondria (1 mg/ml) or purified VDAC (0.2 mg/ml) were incubated with different concentrations of the various cross-linking reagents for 10 min at 30 °C in a solution containing 10 mM Tricine, at pH 8.2 (EGS, Sulpho-EGS, DFDNB and DSP) or pH 8.6 (DMS). All cross-linkers, except DMS and Sulpho-EGS, were dissolved in DMSO immediately before use. The DMSO concentration in control and reagent-containing samples was up to 2% (v/v). Samples were treated with SDS/PAGE sample buffer, lacking 2-mercaptoethanol when DSP was used.
Release of cytochrome c
Cytochrome c release was assayed under conditions that either prevent or promote the induction of PTP (as reflected by mitochondrial swelling). Mitochondria were incubated in a KCl medium containing 150 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 3 mM KH2PO4, 20 mM Hepes (pH 7.4) and, where indicated, 5 mM succinate and 0.2 mM CaCl2 [29]. Treated mitochondria were centrifuged at 20000 g and the membranal and soluble fractions were subjected to SDS/PAGE [30] and immunoblot analysis [31] using anti-cytochrome c antibodies.
Purification of VDAC and VDAC-cross-linked products
VDAC was extracted from mitochondria with Triton X-100 and purified using hydroxyapatite and reactive red agarose as described in [32] or extracted with LDAO and purified using LDAO and hydroxyapatite followed by CM-cellulose, in which LDAO was replaced by β-OG [12]. VDAC-cross-linked products were isolated from mitochondria subsequent to treatment with DSP by solubilization with LDAO and chromatography on hydroxyapatite and CM-cellulose, as described previously [12]. To cleave DSP-cross-linked VDAC, samples were exposed to 20 mM DTT for 30 min at 37 °C and subjected to SDS/PAGE and immunostaining.
Reconstitution of VDAC in FITC-cytochrome c-encapsulated liposomes
Cytochrome c (2 mM) in 100 mM sodium carbonate buffer, pH 9.0, was FITC-labelled by incubation for 2 h at 30 °C with 0.3% (w/v) FITC in DMSO (10% final concentration) under stirring. To remove free FITC, labelled cytochrome c was dialysed (24 h, 4 °C) against dialysis buffer (50 mM KCl, 5 mM MgCl2, 5 mM NaHCO3 and 20 mM Hepes, pH 7.3) with four changes. Liposomes were prepared by twice sonicating soya-bean asolectin (50 mg/ml) in dialysis buffer for 30 s. After sonication, FITC-cytochrome c was added; the mixture was sonicated for an additional 30 s and exposed to two freeze–thaw cycles. The samples were centrifuged for 15 min at 150000 g and the pellet was resuspended in dialysis buffer. Incorporation of VDAC was performed by incubation of LDAO/β-OG-purified VDAC with cytochrome c-loaded liposomes for 20 min at 4 °C, followed by three freeze–thaw cycles and mild sonication. The proteoliposomes were collected by centrifugation at 150000 g for 20 min and resuspended in dialysis buffer. Cytochrome c release from VDAC-containing and -free liposomes, in the absence and presence of xanthine (100 μM) and xanthine oxidase (20 m-units/ml), was performed essentially as described in [33].
Gel electrophoresis and immunoblot analyses
SDS/PAGE was performed by the method of Laemmli [30] and gels were stained with Coomassie Brilliant Blue. Western-blot analysis was performed by standard procedures [31]. For immunostaining, the membranes were blocked with 5% (w/v) non-fat dry milk and 0.1% Tween 20 in Tris-buffered saline, incubated with monoclonal anti-VDAC antibodies (1:7000) or anti-cytochrome c antibodies (1:2000), followed by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG as a secondary antibody (1:10000). Antibody binding was detected by chemiluminescence reagents obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Single-channel recording and analysis
Reconstitution of purified VDAC or its cross-linked products into a PLB (planar lipid bilayer) and channel recording and analysis were performed as described previously [19]. Purified VDAC or its cross-linked products were added to the chamber defined as the cis side. Currents were recorded under voltage-clamp mode using a bilayer clamp BC-525B amplifier and measured with respect to the trans side of the membrane (ground).
FRET (fluorescence resonance energy transfer)
VDAC (0.2–0.3 mg/ml) was incubated with FITC (100 μM) or EITC (100 μM) in the dark (20 min) at room temperature (∼24 °C) in a solution containing 0.3% (w/v) β-OG and 10 mM Tricine, pH 8.2. Free FITC and EITC were removed by using Sephadex-G-50 chromatography–centrifugation [34] equilibrated with 10 mM Tricine (pH 8.2) and 0.3% β-OG. FITC- or EITC-labelled VDAC (0.1–0.15 mg/ml) were mixed in the presence of 2% β-OG (conditions leading to the dissociation of oligomeric VDAC to monomers), incubated for 1 h at 30 °C and subjected to centrifugation–chromatography [35]. Fluorescence measurements were carried out using a PerkinElmer LS 55 fluorimeter, with emission spectra recorded as indicated in the legend to Figure 3 to reflect FRET [35].
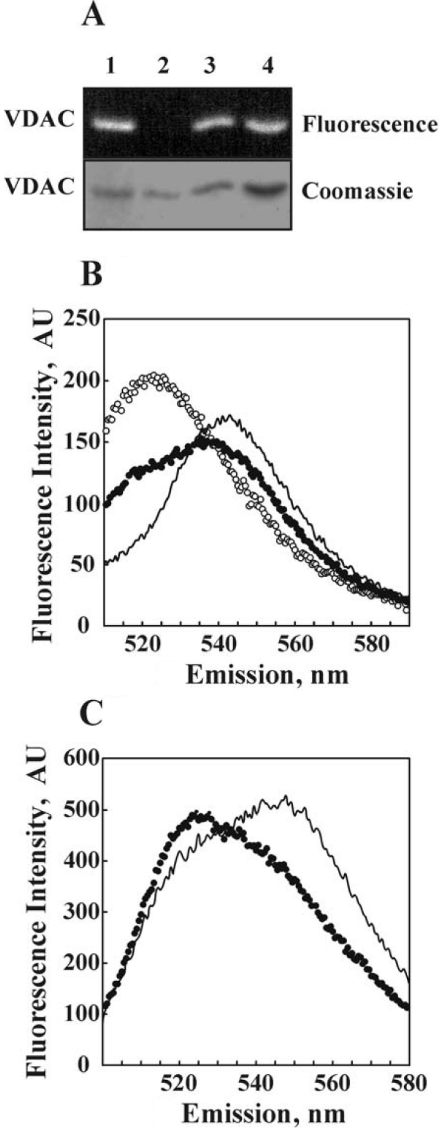
Figure 3. Dynamic association/dissociation of purified VDAC as demonstrated with FRET.
VDAC was purified using β-OG and labelled with FITC or EITC. Oligomers labelled by both fluorophores were formed as described under the Experimental section. (A) SDS/PAGE of purified VDAC labelled with FITC (lane 1), EITC (lane 2), a mixture of FITC- and EITC-VDAC (lane 3) and the FITC- and EITC-VDAC mixture containing added unlabelled VDAC (lane 4), followed by Coomassie Blue staining (Coomassie) or exposure to UV light to visualize the fluorescence. (B) The emission spectra of FITC- and EITC-VDAC were measured separately, with their computed sum presented (○). The emission spectrum of a mixture of FITC- and EITC-VDAC (●), and that of the FITC- and EITC-VDAC mixture after subjecting to dissociation–association conditions (——), are presented. FRET is evident by the quenching of the donor (FITC) emission and an increase in acceptor (EITC) emission. (C) Emission spectra of FITC- and EITC-VDAC ‘hetro-oligomers’ formed in the absence (——) or presence of an approx. 2-fold excess of unlabelled VDAC (●). This experiment is representative of four similar experiments. AU, arbitrary units.
RESULTS
Chemical cross-linking of membranal VDAC
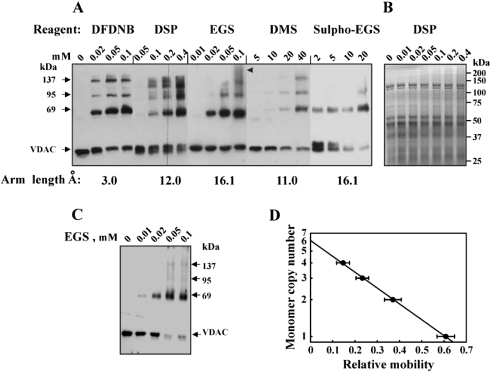
Chemical cross-linking of isolated rat liver mitochondrial proteins with various non-cleavable and cleavable cross-linking reagents of increasing space arm lengths (3–16 Å) that target primary amines was performed to obtain information on the oligomeric state of VDAC. Cross-linking of isolated mitochondria with the water-insoluble cross-linker DFDNB, EGS or DSP (a thiol-cleavable reagent) or with the water-soluble reagents Sulpho-EGS or DMS, in a hypo-osmotic medium or iso-osmotic medium, resulted in the appearance of three distinct, anti-VDAC antibody-labelled protein bands corresponding to molecular masses of 69, 95 and 137 kDa (Figures 1A and 1C). Non-cross-linked VDAC (most probably VDAC1) migrates as a 32 kDa protein band. The complete cross-linking of mitochondrial VDAC was obtained under iso-osmotic conditions (Figure 1C). This may suggest that contact between outer- and inner-membrane proteins influences VDAC oligomerization. In some mitochondrial preparations, cross-linking resulted in the appearance of small amounts of higher molecular-mass complexes, probably containing VDAC together with other proteins (see Figure 1A, arrowhead). Depending on the cross-linking reagent used, an intramolecular cross-linked VDAC monomer was also observed, reflected by a shift in the mobility of the monomeric VDAC as well as in the diffuse nature of the cross-linked product bands (e.g. as seen with DSP; Figure 1A). On the basis of the estimated molecular masses of the cross-linked products and using monomer copy number analysis [36] (Figure 1D), the 69, 95 and 137 kDa protein bands are consistent with homodimers, trimers and tetramers of VDAC respectively. The specificity of the VDAC cross-linking was suggested by a Coomassie staining profile of the cross-linked mitochondria (Figure 1B), which revealed that only a minor overall degree of protein cross-linking was obtained in the treated mitochondria. These results suggest that at least a fraction of the VDAC in the membrane is organized into tetramers.
Figure 1. Cross-linking of VDAC in mitochondria.
Mitochondria (1 mg/ml) were incubated for 10 min at 30 °C in 10 mM Tricine, pH 8.2 (or pH 8.6 for DMS) (A) or under iso-osmotic medium used in the assay of cytochrome c release (C) with the indicated concentration of the cross-linking reagent: DFDNB, DMS, EGS, Sulpho-EGS or DSP. Sample buffer for electrophoresis containing 2-mercaptoethanol, except for DSP (cleavable reagent), was added, and the samples were subjected to SDS/PAGE (10% gel), stained with Coomassie Blue (B) or Western-blot analysis using anti-VDAC antibodies. The molecular masses of VDAC-containing adducts are indicated. (D) Semi-logarithmic plot of the monomer copy number versus the corresponding relative electrophoretic mobility of the cross-linked mitochondrial VDAC species (n=6). The arrowhead in (A) indicates high molecular masses, most probably representing VDAC-containing complexes with other proteins. The arm length of each cross-linker is given.
The oligomeric state of purified VDAC
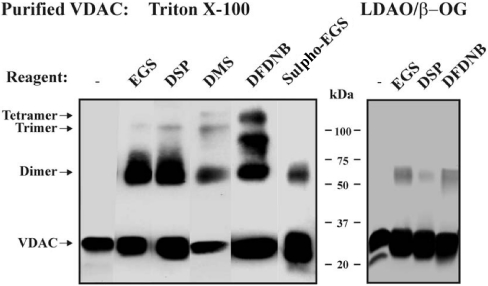
Having established by chemical cross-linking that mitochondrial VDAC monomers appear to associate intimately, the oligomeric organization of purified VDAC in solution was analysed. To cross-link detergent-solubilized purified VDAC, Triton X-100- or LDAO/β-OG-purified VDAC [12] were exposed to DFDNB, DSP, EGS, Sulpho-EGS or DMS in the presence of 0.2% Triton X-100 or 0.3% β-OG. As with membranal VDAC, two major cross-linked products of 69 kDa (dimers) and 95 kDa (trimers) were obtained. With DMS and DFDNB, an additional 137 kDa band (tetramer) was also detected (Figure 2). No VDAC-cross-linked products were obtained in the presence of 1% SDS (results not shown). For LDAO/β-OG-purified VDAC, any amino group containing LDAO that remained associated with the protein would probably affect the level of cross-linking, owing to its putative interaction with the cross-linking reagent. These findings indicate that purified VDAC can adopt a stable oligomeric structure in detergent solution.
Figure 2. Cross-linking of purified VDAC.
Triton X-100- and LDAO/β-OG-purified VDACs (0.2–0.3 mg/ml) were incubated for 10 min at 30 °C in 10 mM Tricine, pH 8.3 or pH 8.6 (DMS), with the cross-linking reagent, EGS (100 μM), DSP (50 μM), DMS (4 mM), DFDNB (50 μM), Sulpho-EGS (100 μM), and subjected to SDS/PAGE and immunostaining. The VDAC-crosslinked products are indicated.
VDAC self-association as studied by FRET
FRET [36] was next employed to obtain evidence on the dynamic behaviour of VDAC oligomerization. In these experiments, purified VDAC was labelled with either FITC or EITC as donor and acceptor fluorophores respectively. Direct interaction of the fluorescent reagents FITC and EITC with amino acid residues of the purified VDAC protein rather than with any of the associated free amino groups-containing LDAO molecules, introduced during VDAC purification, was confirmed by SDS/PAGE (Figure 3A).
Successful formation of dually labelled VDAC oligomers (i.e. labelled with both FITC and EITC) was demonstrated by FRET measurements presented in Figures 3(B) and 3(C). To confirm efficient FRET, the sum of the emission spectra of FITC-VDAC and EITC-VDAC (measured separately) was compared with the emission spectrum of the incubated FITC-VDAC and EITC-VDAC mixture. Energy transfer was evidenced by a quenching of the donor emission at 523 nm and an increase in the acceptor fluorescence at 543 nm (Figure 3B). FRET efficiency was increased on preincubation of labelled FITC- and EITC-VDAC under conditions allowing for VDAC dissociation and subsequent reassociation (1 h incubation in the presence of 2% β-OG, followed by removal of the unbound detergent; [34]). The enhanced FRET signals indicate that FITC- and EITC-VDAC joined to form dimers or higher oligomeric combinations. FRET intensity was decreased on the addition of an excess of unlabelled VDAC during the dissociation–reassociation process (Figure 3C). Interactions between the labelled VDAC monomers were less stable in the presence of detergents such as C12E9 (0.5%) or SDS (1%), as reflected by a diminished FRET intensity (results not shown).
Bilayer-reconstituted cross-linked VDAC displays subconductance states in a non-voltage-dependent manner
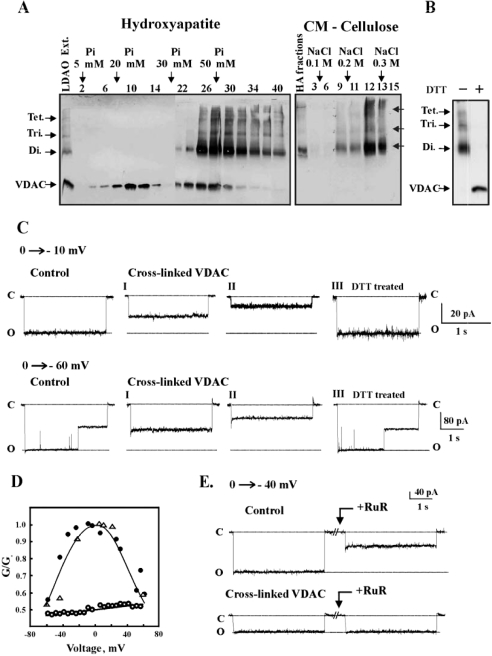
To test the effect of VDAC oligomerization on its channel activity, mitochondria were first cross-linked with the cleavable reagent DSP. DSP-cross-linked VDAC was then separated from non-cross-linked VDAC (Figure 4A). A protein fraction containing, as determined by immunostaining, VDAC dimers, trimers and tetramers (but not monomers) was isolated (Figure 4A) and reconstituted into a PLB (Figure 4C). The currents produced in response to voltages stepped up from a holding potential of 0 mV to the value indicated in each current trace were recorded (Figure 4C). As reported previously [12,19], a single-channel conductance of native, non-cross-linked VDAC of 36 pA (at 10 mV, in 1 M KCl) was obtained. As opposed to native VDAC, DSP-cross-linked VDAC showed stable, long-lived subconducting states of either 18 or 10 pA (labelled I and II), as recorded in several separate experiments, performed under the same conditions (10 mV, 1 M KCl). When a larger voltage step was applied (0 to −60 mV), transition from the main conductance state to the subconductance states occurred in the non-cross-linked VDAC, whereas the stable subconducting state was persistent in the cross-linked VDAC (Figure 4C). Moreover, in contrast with native VDAC, the channel conductance of the cross-linked VDAC was constant over the voltage range −60 to +60 mV, showing no voltage dependence (Figure 4D). Cleavage of the thiol bound in the DSP-cross-linked VDAC to monomers on incubation with DTT (see Figure 4B), however, converted the conductance and voltage-dependence properties of the protein into those of non-cross-linked VDAC (Figures 4C and 4D). This suggests that cross-linking of VDAC, rather than modification of an amino acid residue(s), is responsible for the modified VDAC channel properties.
Figure 4. Reconstitution of non-cross-linked and cross-linked VDAC into PLB.
(A) Mitochondria were incubated with the cleavable cross-linking reagent DSP (200 μM) for 30 min at 30 °C and separated from the free reagent by centrifuging at 20000 g. VDAC and its cross-linked products were solubilized with 2% LDAO and purified on hydroxyapatite (HA) column, while cross-linked and non-cross-linked VDAC were eluted with different sodium orthophosphate concentrations. Fractions 32–40 from the HA column were collected and subjected to further separation from residual non-cross-linked VDAC using CM-cellulose. VDAC and its cross-linked products were visualized by immunostaining using anti-VDAC antibodies. Tet., tetramer; Tri., trimer; Di., dimer. (B) Immunostaining of purified cross-linked VDAC-containing adducts before and after preincubation for 30 min at 37 °C with 20 mM DTT. (C) Purified native VDAC, cross-linked VDAC (fraction 14 from the CM-cellulose column, see A) and cross-linked VDAC preincubated with DTT (see B) were reconstituted into PLB. Currents through non-cross-linked VDAC (control) or cross-linked VDAC before (I, II) and after DTT treatment (III) in response to a voltage step from 0 to −10 mV or from 0 to −60 mV were recorded. The broken lines indicate current levels at open (o) or closed (c) states. (D) Multi-channel recordings of the average steady-state conductance of non-cross-linked VDAC (●) and cross-linked VDAC, before (○) and after DTT treatment (△), as a function of voltage. Relative conductance was determined as the ratio of conductance at a given voltage (G) to maximal conductance (Go). (E) Currents through bilayer-reconstituted VDAC or cross-linked VDAC in response to a voltage step from 0 to −40 mV were recorded before and 10 min after the addition of 20 μM RuR. The experiments are representative of 3–4 similar experiments.
Finally, as reported previously for purified VDAC [12,19], RuR almost completely inhibited the channel activity of native VDAC, yet had no effect on the conductance of cross-linked VDAC (Figure 4E).
Cross-linking of mitochondria prevents PTP opening and release of cytochrome c
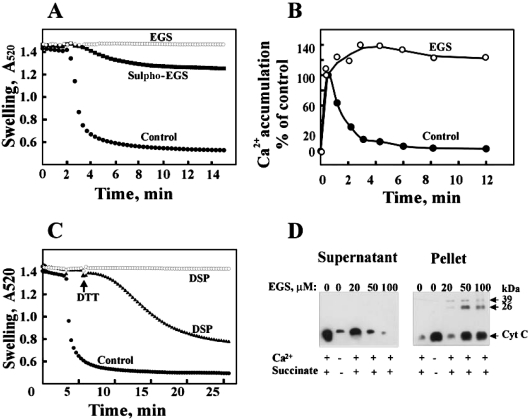
The possible involvement of VDAC oligomerization in the assembly/opening of the PTP and the release of cytochrome c were examined by testing the effect of exposure of mitochondria to cross-linking reagents on mitochondrial swelling, Ca2+ accumulation and release of cytochrome c, events that reflect PTP opening [10–13]. Addition of EGS or the membrane-impermeable reagent Sulpho-EGS, 2 min before PTP induction by Ca2+ overload of the mitochondria prevented mitochondrial swelling (Figure 5A). Inhibition of PTP opening by EGS was also revealed when the PTP was monitored for transient Ca2+ accumulation. In untreated mitochondria, Ca2+ accumulation reached a maximal level and then rapidly decreased by approx. 98% (Figure 5B), whereas, in the presence of the cross-linker, no efflux of the accumulated Ca2+ was observed. Moreover, higher amounts of Ca2+ were accumulated by mitochondria exposed to the cross-linking reagent, suggesting that prevention of PTP opening by cross-linking is probably not due to inhibition of electron transport, loss of membrane potential or failure of the Ca2+ uniporter, all required for mitochondrial Ca2+ transport and subsequent PTP opening.
Figure 5. PTP opening and cytochrome c release are prevented by chemical cross-linking of mitochondria.
(A) Ca2+-dependent mitochondrial swelling, monitored by the decrease in absorbance at 520 nm, was assayed in the absence or presence of the indicated cross-linking reagent [EGS (100 μM) or Sulpho-EGS (200 μM)]. (B) Rat liver mitochondria (0.5 mg/ml) were assayed for succinate-supported [45Ca2+] accumulation in the absence or presence of EGS (150 μM), as described in the Experimental section. (C) Mitochondrial swelling was assayed in the absence or presence of DSP (150 μM) and, at the indicated time (indicated by an arrow), DTT (10 mM) was added. (D) Release of cytochrome c was assayed after incubation of mitochondria for 10 min in the absence or presence of EGS (0–100 μM) and, as indicated, in the absence or presence of 0.2 mM Ca2+ and 5 mM succinate, conditions that induced or inhibited PTP opening respectively. After centrifugation at 20000 g, equivalent amounts of the supernatant and pellet were assayed for cytochrome c release using anti-cytochrome c antibodies. Monomeric cytochrome c (Cyt c) and 26 and 39 kDa anti-cytochrome c antibodies cross-reactive bands that may represent cross-linked dimers and trimers of cytochrome c are indicated. These experiments are representative of 3–4 similar experiments.
Ca2+-dependent PTP opening was also completely prevented by DSP. DSP-closed PTP could, however, be reopened on the addition of DTT, presumably owing to cleavage of an S–S bond containing DSP-based adduct (Figure 5C). Moreover, the reversibility of DSP inhibition of PTP opening by DTT treatment again suggests that mitochondrial cross-linking had no effect on these energy- and Ca2+-dependent reactions required for PTP opening (Figure 5C). Finally, the addition of EGS, DSP or the charged Sulpho-EGS to mitochondria in which PTP opening had been induced by atractyloside (a reagent known to interact with ANT and stabilize it in its open configuration [37]) inhibited PTP opening (results not shown). This result, together with the observed effect of the membrane-impermeable Sulpho-EGS in preventing PTP opening, suggests that the effect of cross-linking on PTP opening is not mediated by ANT.
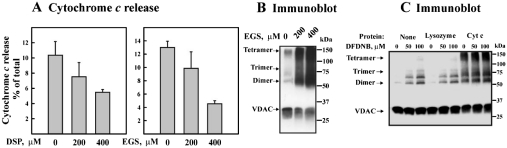
The effect of cross-linking of mitochondrial VDAC on cytochrome c release was next analysed by Western blotting using specific monoclonal anti-cytochrome c antibodies. Such an analysis revealed that cytochrome c could be released from mitochondria on the induction of PTP (in the presence of Ca2+ and succinate). This release was, however, completely prevented by treatment with 100 μM EGS, cytochrome c remaining in the mitochondrial pellet (Figure 5D). Under the conditions used in this experiment, VDAC was almost completely cross-linked to dimers, trimers and tetramers (see Figure 1C). Inhibition of cytochrome c release caused by EGS cross-linking of mitochondria was not due to the formation of large oligomers of cytochrome c that could not be released, since only a small fraction of the cytochrome c population was cross-linked to form dimers (26 kDa) and trimers (39 kDa) in such experiments (Figure 5D).
Cross-linking of VDAC reconstituted into liposomes inhibits cytochrome c release
To assess further whether cross-linking of mitochondrial VDAC, but not other PTP components, was responsible for the diminished cytochrome c release, purified VDAC, reconstituted into FITC-cytochrome c-loaded liposomes, was employed. The VDAC-proteoliposomes were exposed to DSP, and release of FITC-cytochrome c induced by O2•−, as generated by xanthine and xanthine oxidase [33], was measured (Figure 6A). Although, as shown previously [33], liposomes encapsulated with FITC-cytochrome c were slightly leaky, VDAC-proteoliposomes displayed a 2–3-fold increase in the fluorescence released into the supernatant when compared with VDAC-free liposomes. Cross-linking of VDAC-proteoliposomes with DSP or EGS, however, inhibited the release of the encapsulated cytochrome c. The efficiency of the cross-linking was confirmed by immunoblot analysis of liposome-reconstituted VDAC exposed to either EGS or DSP (Figure 6B). Surprisingly, a small fraction of the VDAC appeared as dimers, trimers and higher oligomers without chemical cross-linking (Figure 6B). A protein band with mobility higher than that of the trimers and lower than that of the dimers was also observed and most probably represents intra-molecularly cross-linked VDAC.
Figure 6. Inhibition of cytochrome c movement through VDAC-reconstituted liposomes by cross-linking and cytochrome c-induced oligomerization of VDAC.
(A) Plain and VDAC-reconstituted liposomes loaded with FITC-conjugated cytochrome c (see the Experimental section) were incubated for 20 min with or without DSP or EGS at the indicated concentration and subjected to centrifugation for 20 min at 150000 g. Pellets were resuspended in dialysis buffer and incubated with or without xanthine and xanthine oxidase for 30 min followed by centrifugation for 20 min at 150000 g. FITC-cytochrome c fluorescence in the supernatants and pellets was measured. VDAC-dependent cytochrome c release is expressed as a percentage of the total cytochrome c in each sample (liposome pellet and supernatant) (n=5). (B) Immunostaining of VDAC, incorporated into liposomes before and after exposure to EGS. (C) VDAC, reconstituted into plain liposomes or cytochrome c- or lysozyme-encapsulated liposomes, was incubated with or without DFDNB followed by SDS/PAGE and Western-blot analysis using anti-VDAC antibodies.
Cytochrome c stimulates VDAC oligomerization
To investigate whether the effect of cytochrome c in stabilizing VDAC oligomers is specific, the effects of cytochrome c and other proteins on the oligomeric state of soluble and liposome-reconstituted VDAC were analysed. Cytochrome c added to purified VDAC in solution did not affect the formation of VDAC oligomers either with or without cross-linking (results not shown). On the other hand, when VDAC was reconstituted into liposomes, cytochrome c stimulated the formation of VDAC oligomers (Figure 6C). In VDAC-liposomes containing encapsulated cytochrome c, but not in VDAC-liposomes free of cytochrome c, VDAC dimers, trimers and tetramers were observed even without chemical cross-linking and after SDS/PAGE (Figure 6C). No VDAC oligomers were obtained when lysozyme (Figure 6C) or ovalbumin (results not shown), either encapsulated within or added to VDAC-liposomes, replaced cytochrome c, unless cross-linking was performed, thereby pointing to the specific effect of cytochrome c in encouraging VDAC oligomerization. Thus it appears that luminal cytochrome c is capable of shifting the equilibrium between monomeric and oligomeric VDAC towards the direction of highly stable oligomeric forms.
DISCUSSION
The experiments reported here provide an insight into the oligomeric status of VDAC and the possible function of VDAC oligomerization in the release of cytochrome c from mitochondria.
VDAC has a dynamic oligomeric structure
Isolated mitochondria or detergent-solubilized purified VDAC, either in solution or reconstituted into liposomes, was cross-linked with five cross-linking reagents bearing different spacer lengths and membrane permeabilities; as a result, VDAC dimers, trimers and tetramers were obtained (Figures 1, 2 and 6). Furthermore, cross-linking by DFDNB (with a 3 Å distance between its two reactive groups) indicates that a relatively intimate contact exists between monomers in the VDAC oligomeric complex. The dynamic nature of the assembly of VDAC monomers into oligomers was demonstrated by the FRET obtained between purified VDAC labelled with either FITC or EITC (Figure 3), reflecting the formation of ‘hetero’-oligomers of FITC- and EITC-labelled VDAC. The results indicate that both soluble purified VDAC and membrane-embedded VDAC can assemble into tetramers and suggest that a dynamic equilibrium between the monomeric and tetrameric states of VDAC exists. Indeed, in other studies using purified rat liver or Neurospora crassa, VDAC was suggested to exist as a dimer, and also may be in a trimeric form [22,23,38]. Using brain mitochondria [39] or recombinant human VDAC [40], the existence of VDAC dimers to tetramers was also reported.
In vivo, VDAC oligomerization may play a role in the interactions between VDAC and associated proteins that serve as regulators of apoptosis. Several observations support this idea. Oligomerization of the pro-apoptotic protein Bax before [41] or during its binding to VDAC has been proposed [16]. HK-I (hexokinase-I) binding to mitochondria and subsequent prevention of PTP opening showed a high degree of co-operativity [42], consistent with the formation of HK tetramers during binding to mitochondrial VDAC [43]. In fact, Bax and HK compete for VDAC-binding sites [29]. It has also been shown that mitochondrial creatine kinase, on complexing with VDAC, is stabilized in an octameric form composed of four dimers [44]. Thus the fact that numerous VDAC-interacting proteins bind VDAC as oligomers lends support to VDAC itself existing in a high-order structure. Moreover, tetrameric VDAC could serve as the trigger for oligomerization of these proteins.
VDAC oligomerization and channel properties
It is accepted that monomeric VDAC serves as the functional channel [20,21]. Our results show that cross-linked VDAC possesses decreased channel conductance and voltage-independent channel activity (Figure 4). These effects may result from chemical modification of those amino acids that reacted with the cross-linker. However, cleavage of the S–S bond in DSP-cross-linked VDAC returned the channel properties to those of native VDAC, even though part of the DSP molecule remained attached to the cross-linked amino acid residues. Thus the fixation of VDAC into an oligomeric structure by the cross-linking reagent, and not the chemical modification of amino acid residues, is responsible for the altered channel properties. The results, however, showed that cross-linking of VDAC prevented the movement of cytochrome c, but only partially prevented the movement of small ions such as Na+ or Cl−. In this regard, it has been recently shown that a new class of PTP inhibitors that bind to VDAC and prevent PTP opening do not affect VDAC channel activity [9].
VDAC alone is sufficient for the release of cytochrome c
Previous studies [16,33] demonstrated the release of cytochrome c through purified VDAC reconstituted into liposomes in which cytochrome c had been encapsulated. In a similar system, the release of cytochrome c was inhibited on cross-linking of VDAC by DSP or EGS (Figure 6). In isolated mitochondria, the inhibition of cytochrome c release by cross-linking with the membrane-impermeable Sulpho-EGS (Figure 5) may indicate that the effect of cross-linking in preventing PTP opening is a VDAC-related event and does not involve the inner-membrane ANT component of the PTP. In fact, on ANT cross-linking with o-phenantroline and Cu2+, PTP induction rather than inhibition was obtained [45]. Furthermore, it has been recently shown that mitochondria lacking ANT can still undergo PTP opening and release of cytochrome c [46] and that bongkrekic acid, an inhibitor of ANT and PTP opening, prevented dexamethasone-induced nuclear damage and caspase-3 processing, but not the release of cytochrome c [47]. These results taken together suggest that VDAC alone is sufficient for the release of cytochrome c. It is anticipated that VDAC-mediated cytochrome c release would, however, be modulated by protein–protein interactions involving other components of the PTP, such as ANT and/or the ANT–cyclophilin D complex. Indeed, it has been shown that disruption of the ANT–VDAC interaction unmasks a latent pathway for cytochrome c [5], which may allow for VDAC oligomerization and cytochrome c accessibility. It is possible that VDAC is involved in cytochrome c arrangement at the mitochondrial periphery, since it has recently been observed that HK-VDAC–ANT complexes contain cytochrome c that could be released by Bax [48].
Possible involvement of VDAC oligomerization in the release of cytochrome c from mitochondria
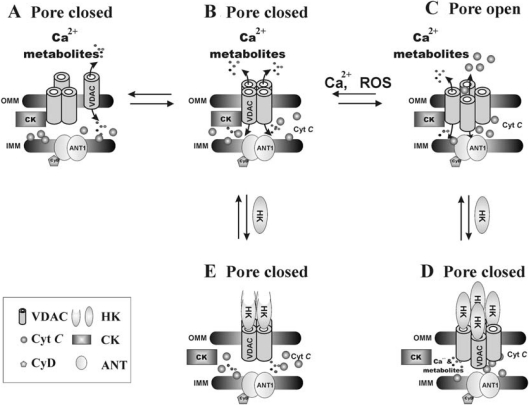
Release of cytochrome c from mitochondria is considered to be a key initial step in the apoptotic process, although the manner of release and precise mechanisms regulating cytochrome c release remain unknown. Several models suggest that the release exclusively involves an increase in OMM permeability owing to the formation of a channel large enough to account for the release of proteins such as cytochrome c [4]. When considering models of protein release through the PTP [1,3,10,14] and more specifically through the VDAC component of the PTP [10,12,13,15,16], the molecular masses of the released proteins (12–100 kDa) and the VDAC pore diameter (∼3 nm) make it difficult to imagine direct transport of these proteins through the VDAC channel. On the basis of our results, a novel concept can be proposed in which a versatile mechanism involving modulation of the oligomeric structure of VDAC would control pore opening or assembly (Figure 7). Several observations support the suggestion of a model based on tetrameric VDAC-mediated cytochrome c release. The finding that fixation of mitochondria-embedded or liposome-reconstituted VDAC into oligomers by cross-linking prevented the release of cytochrome c (Figures 5 and 6) could be explained by a disruption of this protein release pathway, as a result of cross-link-induced rigidity in the normally flexible VDAC tetramer. Interestingly, a similar model presenting oligomeric VDAC as the prime cytochrome c release channel and its pore regulation by Bax has been suggested [49]. Although the proposal that the large pore/path formed between VDAC protomers in the tetrameric complex serves as the route for cytochrome c to cross the OMM requires further study, evidence for this concept already exists. For example, in recent studies on the effect of the reagent As2O3, its apoptosis-inducing effect was attributed to an induction of homodimerization of VDAC molecules. This effect could be prevented by overexpression of the anti-apoptotic protein Bcl-XL [50]. In addition, a novel high-conductance mitochondrial channel linked to apoptosis showing electrophysiological properties similar to those of VDAC has been described previously [51].
Figure 7. A model for oligomeric VDAC as pore formation in the OMM and its regulation by HK-I.
PTP is composed of VDAC in the OMM and ANT in the IMM. VDAC, arranged as tetramers, forms large pores capable of conducting cytochrome c (C). Assembly of VDAC tetramers may be activated by conditions leading to PTP opening/assembly, such as Ca2+ overload or reactive oxygen species (ROS). HK-I tetramer formation and binding to VDAC leads to closure of both intrasubunit pores and the pore between VDAC subunits (D, E), thus inhibiting both VDAC conductance and PTP opening [42]. The interaction of HK or creatine kinase with VDAC is based on the finding that no VDAC complex containing both proteins was isolated [58].
Our results (Figure 6) also show that VDAC oligomerization is encouraged in the presence of cytochrome c. Interaction of cytochrome c with VDAC has been demonstrated previously in negatively stained crystalline arrays of fungal mitochondrial VDAC [52], and cytochrome c was found attached to the isolated HK–VDAC–ANT complex [48]. Cytochrome c is attached to the mitochondrial inner membrane and is present in loosely and tightly bound pools (the latter attached to cardiolipin) [53]. Thus dissociation of cytochrome c from the anionic cardiolipin would be required before its release. The signalling mechanism responsible for such cytochrome c translocation and induction of VDAC oligomerization are not yet known. For proteoliposomes (Figure 6), the concentration of membrane-unbound free cytochrome c is high. However, in the mitochondria, its dissociation from the cardiolipin at the inner membrane could be induced by cardiolipin peroxidation [53–56] or by Ca2+ interaction with cardiolipin [55].
Finally, the dynamic equilibrium between VDAC monomeric and oligomeric states could be regulated not only by cytochrome c, but also by additional factors such as Ca2+, oxidative stress or low ATP concentrations or by interactions with other proteins known to act as modulators of PTP opening [1–3,10–12,14]. For instance, interaction of tetrameric HK-I with VDAC decreased channel conductance and prevented PTP opening and release of cytochrome c [42]. The interaction of the Bcl-2 family members, proteins that act as pro- or anti-apoptotic proteins, with VDAC and the subsequent effect on the release of cytochrome c [12,16,33] can also be explained by a modulation of the VDAC oligomeric state. Indeed, it has been demonstrated recently that Bax increases the pore size of rat brain mitochondrial VDAC in the presence of tBid [57].
To conclude, the results presented here demonstrate the existence of a dynamic VDAC oligomerization and specify VDAC as a major regulator of mitochondria-mediated apoptosis. VDAC oligomerization induced by cytochrome c may mediate the release of the latter from mitochondria. For a fuller understanding of the mechanism underlying VDAC oligomeric assembly and its role in the release of cytochrome c further study is required, including direct monitoring of the oligomerization process in live cells under physiological and apoptosis-inducing conditions.
Acknowledgments
This research was supported by grants from the Israel Science Foundation, administered by The Israel Academy of Science and Humanities.
References
- 1.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Phys. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 2.Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Halestrap A. P., Doran E., Gillespie J. P., O'Toole A. Mitochondria and cell death. Biochem. Soc. Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 4.Martinou J. C., Desagher S., Antonsson B. Cytochrome c release from mitochondria: all or nothing. Nat. Cell Biol. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 5.Doran E., Halestrap A. P. Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem. J. 2000;348:343–350. [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher G. C., Xue L., Passingham S. K., Tolkovsky A. M. Death commitment point is advanced by axotomy in sympathetic neurons. J. Cell Biol. 2000;150:741–754. doi: 10.1083/jcb.150.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluck R. M., Esposti M. D., Perkins G., Renken C., Kuwana T., Bossy-Wetzel E., Goldberg M., Allen T., Barber M. J., Green D. R., et al. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Ahsen O., Renken C., Perkins G., Kluck R. M., Bossy-Wetzel E., Newmeyer D. D. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesura A. M., Pinard E., Schubenel R., Goetschy V., Friedlein A., Langen H., Pocic P., Forte M. A., Bernardi P., Kemp J. A. The voltage-dependent anion channel is the target for a new class of inhibitors of the mitochondrial permeability transition pore. J. Biol. Chem. 2003;278:49812–49818. doi: 10.1074/jbc.M304748200. [DOI] [PubMed] [Google Scholar]
- 10.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 11.Lemasters J. J., Qian T., Bradham C. A., Brenner D. A., Cascio W. E., Trost L. C., Nishimura Y., Nieminen A. L., Herman B. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J. Bioenerg. Biomembr. 1999;31:305–319. doi: 10.1023/a:1005419617371. [DOI] [PubMed] [Google Scholar]
- 12.Shoshan-Barmatz V., Gincel D. The voltage-dependent anion channel: characterization, modulation and role in mitochondrial function in cell life and death. Cell Biochem. Biophys. 2003;39:279–292. doi: 10.1385/CBB:39:3:279. [DOI] [PubMed] [Google Scholar]
- 13.Szabo I., De Pinto V., Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 14.Zamzami N., Kroemer G. Apoptosis: mitochondrial membrane permeabilization – the (w)hole story? Curr. Biol. 2003;13:R71–R73. doi: 10.1016/s0960-9822(02)01433-1. [DOI] [PubMed] [Google Scholar]
- 15.Zoratti M., Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto Y., Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- 17.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes porins. Biochim. Biophys. Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 18.Hodge T., Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J. Membr. Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 19.Gincel D., Zaid H., Shoshan-Barmatz V. Calcium binding and translocation by voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S., Blachly-Dyson E., Colombini M., Forte M. Determination of the number of polypeptide subunits in a functional VDAC channel from Saccharomyces cerevisiae. J. Bioenerg. Biomembr. 1992;24:27–31. doi: 10.1007/BF00769527. [DOI] [PubMed] [Google Scholar]
- 21.Rostovtseva T. K., Liu T. T., Colombini M., Parsegian V. A., Bezrukov S. M. Positive cooperativity without domains or subunits in a monomeric membrane channel. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7819–7822. doi: 10.1073/pnas.140115397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden M., Gellerfors P. Hydrodynamic properties of porin isolated from outer membranes of rat liver mitochondria. Biochim. Biophys. Acta. 1983;736:125–129. doi: 10.1016/0005-2736(83)90177-3. [DOI] [PubMed] [Google Scholar]
- 23.Krause J., Hay R., Kowollik C., Brdiczka D. Cross-linking analysis of yeast mitochondrial outer membrane. Biochim. Biophys. Acta. 1986;860:690–698. doi: 10.1016/0005-2736(86)90568-7. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller R., Freitag H., Harmey M. A., Benz R., Neupert W. A water-soluble form of porin from the mitochondrial outer membrane of Neurospora crassa. Properties and relationship to the biosynthetic precursor form. J. Biol. Chem. 1985;260:8188–8193. [PubMed] [Google Scholar]
- 25.Dolder M., Zeth K., Tittmann P., Gross H., Welte W., Wallimann T. Crystallization of the human, mitochondrial voltage-dependent anion-selective channel in the presence of phospholipids. J. Struct. Biol. 1999;127:64–71. doi: 10.1006/jsbi.1999.4141. [DOI] [PubMed] [Google Scholar]
- 26.Babel D., Walter G., Gotz H., Thinnes F. P., Jurgens L., Konig U., Hilschmann N. Studies on human porin. VI. Production and characterization of eight monoclonal mouse antibodies against the human VDAC ‘Porin 31HL’ and their application for histotopological studies in human skeletal muscle. Biol. Chem. Hoppe Seyler. 1991;372:1027–1034. doi: 10.1515/bchm3.1991.372.2.1027. [DOI] [PubMed] [Google Scholar]
- 27.Johnson D., Lardy H. Isolation of liver or kidney mitochondria. Methods Enzymol. 1967;10:94–96. [Google Scholar]
- 28.Gornall B. D., Bradwell C. S., David M. M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–752. [PubMed] [Google Scholar]
- 29.Pastorino J. G., Shulga N., Hoek J. B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gincel D., Silberberg S. D., Shoshan-Barmatz V. Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J. Bioenerg. Biomembr. 2000;32:571–583. doi: 10.1023/a:1005670527340. [DOI] [PubMed] [Google Scholar]
- 33.Madesh M., Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J. Biol. Chem. 1977;252:2891–2899. [PubMed] [Google Scholar]
- 35.Selvin P. R. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- 36.Hames B. D. One-dimensional polyacrylamide gel electrophoresis. In: Hames B. D., Rickwood D., editors. Gel Electrophoresis, A Practical Approach. 2nd edn. New York: Oxford University Press; 1990. pp. 1–139. [Google Scholar]
- 37.Klingenberg M. Dialectics in carrier research: the ADP/ATP carrier and the uncoupling protein. J. Bioenerg. Biomembr. 1993;25:447–457. doi: 10.1007/BF01108402. [DOI] [PubMed] [Google Scholar]
- 38.Guo X. W., Mannella C. A. Conformational change in the mitochondrial channel, VDAC, detected by electron cryo-microscopy. Biophys. J. 1993;64:545–549. doi: 10.1016/S0006-3495(93)81399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoshan-Barmatz V., Zalk R., Gincel D., Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y., Jiang C., Chen Q., Tang H. One-step on-column affinity refolding purification and functional analysis of recombinant human VDAC1. Biochem. Biophys. Res. Commun. 2003;303:475–482. doi: 10.1016/s0006-291x(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 41.Mikhailov V., Mikhailova M., Pulkrabek D. J., Dong Z., Venkatachalam M. A., Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 42.Azoulay-Zohar H., Israelson A., Abu-Hamad S., Shoshan-Barmatz V. In self-defense: hexokinase promotes VDAC closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie G., Wilson J. E. Tetrameric structure of mitochondrially bound rat brain hexokinase: a crosslinking study. Arch. Biochem. Biophys. 1990;276:285–293. doi: 10.1016/0003-9861(90)90040-6. [DOI] [PubMed] [Google Scholar]
- 44.Schlattner U., Dolder M., Wallimann T., Tokarska-Schlattner M. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J. Biol. Chem. 2001;276:48027–48030. doi: 10.1074/jbc.M106524200. [DOI] [PubMed] [Google Scholar]
- 45.Zazueta C., Reyes-Vivas H., Zafra G., Sanchez C. A., Vera G., Chavez E. Mitochondrial permeability transition as induced by cross-linking of the adenine nucleotide translocase. Int. J. Biochem. Cell Biol. 1998;30:517–527. doi: 10.1016/s1357-2725(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 46.Kokoszka J. E., Waymire K. G., Levy S. E., Sligh J. E., Cal J., Jones D. P., MacGregor G. R., Wallace D. C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature (London) 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sade H., Khandre N. S., Mathew M. K., Sarin A. The mitochondrial phase of the glucocorticoid-induced apoptotic response in thymocytes comprises sequential activation of adenine nucleotide transporter (ANT)-independent and ANT-dependent events. Eur. J. Immunol. 2004;34:119–125. doi: 10.1002/eji.200324650. [DOI] [PubMed] [Google Scholar]
- 48.Vyssokikh M., Zorova L., Zorov D., Heimlich G., Jurgensmeier J., Schreiner D., Brdiczka D. The intra-mitochondrial cytochrome c distribution varies correlated to the formation of a complex between VDAC and the adenine nucleotide translocase: this affects Bax-dependent cytochrome c release. Biochim. Biophys. Acta. 2004;1644:27–36. doi: 10.1016/j.bbamcr.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Debatin K.-M., Poncet D., Kroemer G. Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y., Shi Y., Tian C., Jiang C., Jin H., Chen J., Almasan A., Tang H., Chen Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlov E. V., Priault M., Pietkiewicz D., Cheng E. H., Antonsson B., Manon S., Korsmeyer S. J., Mannella C. A., Kinnally K. W. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mannella C. A., Ribeiro A. J., Frank J. Cytochrome c binds to lipid domains in arrays of mitochondrial outer membrane channels. Biophys. J. 1987;51:221–226. doi: 10.1016/S0006-3495(87)83327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ott M., Robertson J. D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrosillo G., Ruggiero F. M., Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 55.Brustovetsky N., Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- 56.Piccotti L., Buratta M., Giannini S., Gresele P., Roberti R., Corazzi L. Binding and release of cytochrome c in brain mitochondria is influenced by membrane potential and hydrophobic interactions with cardiolipin. J. Membr. Biol. 2004;198:43–53. doi: 10.1007/s00232-004-0654-2. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee J., Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 2004;323:310–314. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 58.Vyssokikh M., Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim. Pol. 2003;50:389–404. [PubMed] [Google Scholar]