Abstract
Background
IQOS was the first heated tobacco product to receive Food and Drug Administration (FDA) authorisation for ‘reduced exposure’ marketing claims, which has been exploited globally.
Methods
In November–December 2021, we conducted a survey-based 3×3 factorial experiment among US (n=1128) and Israeli adults (n=1094). We presented: (1) reduced exposure, reduced risk and control messaging and (2) 2 variations of FDA endorsement and control messaging. Each participant was randomly assigned to evaluate 2 ads (displayed on different ad imagery), then completed assessments of perceived relative harm, exposure and disease risk and likelihood of personally trying or suggesting IQOS to smokers. Ordinal logistic regression examined messaging conditions and their interactions, on the 5 outcomes, respectively, adjusting for covariates.
Results
Control (vs reduced exposure) messaging resulted in higher perceived relative harm (adjusted OR (aOR)=1.29, 95% CI=1.12 to 1.48), exposure (aOR=1.34, 95% CI=1.17 to 1.54) and disease risk (aOR=1.23; 95% CI=1.08 to 1.40), and lower likelihood of suggesting IQOS to smokers (aOR=0.85; 95% CI=0.74 to 0.97). Reduced risk (vs exposure) messaging resulted in lower perceived relative harm (aOR=0.86; 95% CI=0.75 to 0.99). One FDA endorsement message (‘IQOS (completed) the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers’) was associated with greater likelihood of suggesting IQOS to smokers, relative to control (aOR=1.19; 95% CI=1.04 to 1.37). No interactions between risk/exposure messaging and FDA endorsement messaging were found. Additionally, Israeli participants, cigarette users and men perceived lower relative harm and exposure and greater likelihood of trying or suggesting IQOS to smokers.
Conclusions
Regulators must monitor direct and indirect advertising content of modified risk tobacco product-authorised products and prevent potentially harmful misinterpretations.
INTRODUCTION
To enter or stay in the US market, tobacco manufacturers must submit a Premarket Tobacco Product Application (PMTA) to the Food and Drug Administration (FDA) for any new tobacco product (or modified product) commercially marketed after February 2007.1 Manufacturers can also apply for modified risk tobacco product (MRTP) authorisation, which authorises use of marketing claims indicating ‘reduced risk’ (ie, less health risks than cigarettes) or ‘reduced exposure’ (ie, lower exposure to harmful substances than cigarettes).2 To date, FDA has provided MRTP authorisation to 15 products, including heated tobacco products (HTPs).2
HTPs (ie, electronic devices that heat tobacco) have growing market share globally.3 IQOS, by Philip Morris (PM), is the global HTP leader,4 first released in 2014 and now sold in >60 countries.5 In April 2019, IQOS received FDA authorisation to enter the US market; FDA then authorised IQOS to use marketing claims indicating ‘reduced exposure’ (but not ‘reduced risk’) in July 2020.2 6 During this time, IQOS established its US market, expanding across four states (ie, Georgia, Virginia, North Carolina, South Carolina)7 and growing consumer interest and use.8 9 However, PM had to discontinue IQOS sales in the USA in November 2021 due to a patent-infringement lawsuit,10 11 but has stated its intent to resume IQOS sales in the USA12 and to expand its HTP portfolio globally.13
To inform regulatory decisions, FDA needs data to estimate the impact of products authorised as modified risk or exposure products and their related marketing on consumer perceptions and behaviour, and ultimately public health. While manufacturers’ applications to FDA must include data to estimate product and marketing impact, independent research suggests that these findings often underestimate such impact,14–22 thus calling for ongoing comprehensive surveillance by independent researchers.
FDA’s MRTP authorisations have been criticised domestically14 and globally23 24 for several reasons. First, some authorised language is concerning. Many consumers misinterpret the authorised IQOS messaging regarding reduced exposure claims as indicating reduced risk.25–27 Furthermore, IQOS’ FDA authorised reduced exposure marketing content uses language regarding ‘switching completely’ from traditional cigarettes to IQOS, which may not be accurately perceived by consumers.25–27 Another concern is PM’s exploitation of FDA’s MRTP authorisation, which has been used to both influence consumers and to minimise government regulation of IQOS.28 Since July 2020, media reports in several countries cite PM as mischaracterising FDA’s MRTP decision as evidence that IQOS is a reduced harm product29–39 or that IQOS is ‘FDA approved’,40 despite such claims being forbidden in the USA.41 IQOS ads that reference FDA approvals imply reduced risk, as found in some ads in Israel, for example: ‘IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers’ and ‘the US FDA decision shows that IQOS is a fundamentally different product compared with cigarettes such that it does not burn tobacco, but heats it’. Such communication has implications globally, as it is often distributed online or via social media, and thus could potentially impact consumers in the USA and other countries, including countries that have imposed strict regulations on IQOS marketing and sales42 or banned its entry into their markets altogether.43 44
In summary, there is a critical need for independent research to examine FDA MRTP authorised advertising language— specifically reduced risk and reduced exposure messaging—and its impact on consumer perceptions and behaviours. In fact, according to the Tobacco Control Act, a reduced exposure MRTP order should only be issued if the applicant demonstrates that the proposed product labelling and marketing is not mistaken by consumers to indicate reduced harm.45 Furthermore, given PM’s exploitation of the FDA MRTP authorisation globally, such research must also examine how consumers perceive these types of messages—and if such messages potentially bolster the effects of reduced risk or exposure messaging. Cross-country research is particularly important to shed light on the potential impact of different social and regulatory contexts on consumer perceptions of the product and its advertising.
This study examined the impact of IQOS advertising with reduced exposure versus reduced risk messaging, as well as content exploiting FDA’s authorisation of modified exposure messaging, among 2222 US and Israeli adults. Specifically, we conducted a survey-based 3×3 factorial experiment systematically manipulating the presence of: (1) reduced exposure versus reduced risk (and control) messaging and (2) two variations of FDA endorsement (and control) messaging. Outcomes included perceived harm relative to cigarettes, perceived exposure to harmful chemicals, perceived risk of disease and likelihood of personally trying IQOS or recommending it to cigarette smokers.
METHODS
Data sources
This factorial design experiment was embedded within a cross-sectional online survey of US and Israel participants, administered in October–December 2021. Eligibility criteria included: (1) ages 18–45 years (to capture adults most likely aware of, interested in or with prior use of new tobacco products like IQOS8 46–50) and (2) able to speak English (US), or Hebrew or Arabic (Israel); in Israel, an additional criterion was having an Israeli ID. Our target sample size was 2000 total participants (1000/country). We aimed to recruit roughly equal sample sizes of males and females in each country and to oversample individuals representing racial/ethnic minority groups and tobacco users. The final sample included 2222 participants (USA, n=1128; Israel, n=1094).
US-based sample
The US survey was recruited by Ipsos, primarily using KnowledgePanel,51 a probability-based web panel designed to be representative, and recruited via a combination of random digit dialling and address-based sampling. KnowledgePanel members are incentivised by KnowledgePanel points redeemable for cash (typically ~5000 points—equivalent to US$5—for completing a 25 min survey). As is standard with KnowledgePanel surveys, multiple prompts (ie, on days 3, 6, 14, 21, 28 and 35) were made to encourage participation. Of 4960 panellists recruited, 2397 (48.3%) completed eligibility screening and 1095 (45.7%) of those eligible completed the survey.
To meet subgroup recruitment targets, Ipsos also recruited an opt-in (ie, off-panel) convenience sample of Asian tobacco users, using banner ads, web pages and email invitations. Those who clicked on online ads completed eligibility screening (ie, gender, race/ethnicity, tobacco use). Of 353 individuals screened and eligible, 33 (9.3%) completed the survey.
Israel-based sample
The Israeli survey was entirely conducted using opt-in sample, using the same approach specified above. Of 2970 individuals who completed the eligibility screening and were eligible, 1094 (36.8%) completed the survey.
Experimental design and measures
The survey focused on tobacco use and related factors. The survey was professionally translated to Hebrew and Arabic, back-translated into English and then examined by two bilingual reviewers to verify comparability across translations. Survey content was parallel across countries/languages, except for specific sociodemographics (ie, origin, religiosity). Israel-based participants could choose to take the survey in Hebrew or Arabic. The survey took ~25 min to complete.
Experimental design
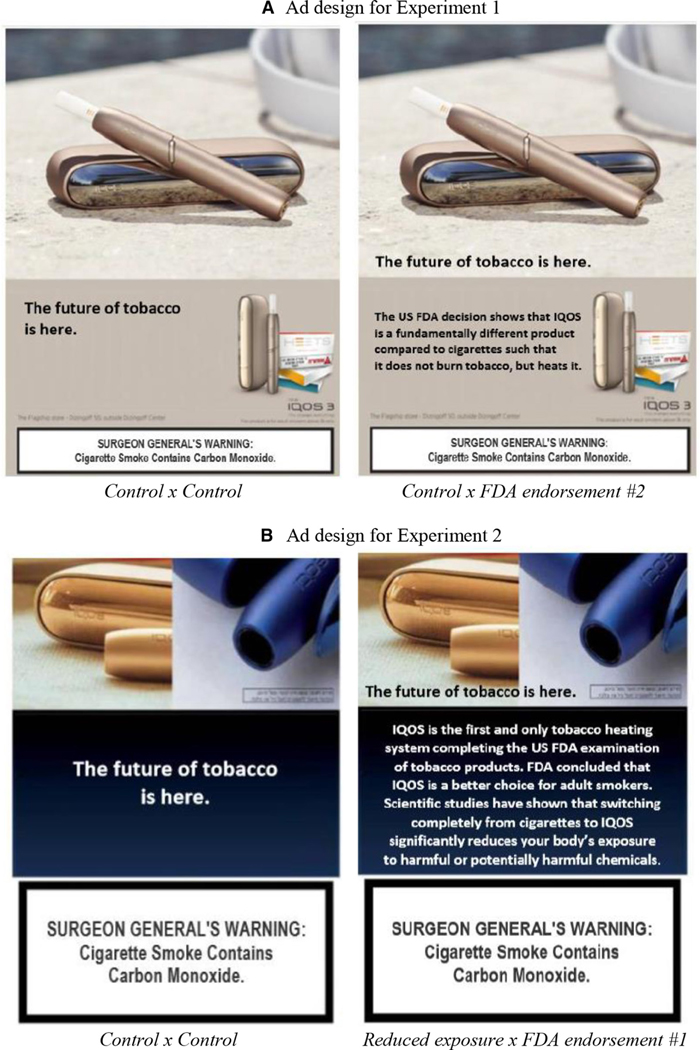
Table 1 summarises each of the nine experimental conditions, which were applied to two actual IQOS ad designs (figure 1A and B). The control conditions were absent messages regarding reduced risk/exposure messaging or FDA endorsement; all ads included the headline ‘The future of tobacco is here’, which was an ad headline used both in the USA (in English)52 and in Israel (in Hebrew).53
Table 1.
IQOS ad experimental conditions
| FDA endorsement* | Reduced exposure or risk† | ||
|---|---|---|---|
| Control | Reduced exposure | Reduced risk | |
| Control | ► The future of tobacco is here. | ► The future of tobacco is here. ► Scientific studies have shown that switching completely from cigarettes to IQOS significantly reduces your body’s exposure to harmful or potentially harmful chemicals. |
► The future of tobacco is here. ► Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system can reduce the risks of tobacco-related diseases. |
| FDA endorsement 1 | ► The future of tobacco is here. ► IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers. |
► The future of tobacco is here. ► IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers. ► Scientific studies have shown that switching completely from cigarettes to IQOS significantly reduces your body’s exposure to harmful or potentially harmful chemicals. |
► The future of tobacco is here. ► IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers. ► Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system can reduce the risks of tobacco-related diseases. |
| FDA endorsement 2 | ► The future of tobacco is here. ► The US FDA decision shows that IQOS is a fundamentally different product compared with cigarettes such that it does not burn tobacco, but heats it. |
► The future of tobacco is here. ► The US FDA decision shows that IQOS is a fundamentally different product compared with cigarettes such that it does not burn tobacco, but heats it. ► Scientific studies have shown that switching completely from cigarettes to IQOS significantly reduces your body’s exposure to harmful or potentially harmful chemicals. |
► The future of tobacco is here. ► The US FDA decision shows that IQOS is a fundamentally different product compared with cigarettes such that it does not burn tobacco, but heats it. ► Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system can reduce the risks of tobacco-related diseases. |
Figure 1.
Example IQOS ads and ad messaging in English*. (A) Ad design for experiment 1. (B) Ad design for experiment 2. *Similar ads in Hebrew and Arabic were used in the experiment for the Israeli participants; instead of ‘SURGEON GENERAL’S WARNING’, ‘MINISTRY OF HEALTH WARNING’ was used. FDA, Food and Drug Administration.
Reduced exposure and risk messages were those submitted by PM to FDA for reduced exposure and reduced risk authorisation: (1) reduced exposure: “Scientific studies have shown that switching completely from cigarettes to IQOS significantly reduces your body’s exposure to harmful or potentially harmful chemicals” and (2) reduced risk: ‘Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system can reduce the risks of tobacco-related diseases’. 54
FDA endorsement messages were based on ad content in Israel (via Ifat marketing surveillance data53): (1) ‘IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers’ and (2) ‘The US FDA decision shows that IQOS is a fundamentally different product compared with cigarettes such that it does not burn tobacco, but heats it’.
Each participant evaluated two ads—one ad displayed using imagery in figure 1A and one ad using imagery in figure 1B. Participants were randomised to determine which condition they were assigned to evaluate for each set of ads. Thus, each individual ad was assessed by ~250 participants, and each condition (represented in two ads) was evaluated by ~500 participants.
Outcomes
After presenting each ad, we assessed participants’ perceived relative harm, exposure and disease risk and likelihood of personally trying or suggesting IQOS to smokers. Specifically, they were asked to ‘consider the ad above’ and respond to the following five questions, respectively: (1) “Compared with cigarettes, how harmful to your health do you think IQOS is?” (1=much less, 2=somewhat less, 3=equally/same, 4=somewhat more, 5=much more); (2) “Do you think that using IQOS would expose you to: 1=almost no harmful chemicals, 2=a few harmful chemicals, 3=some harmful chemicals, 4=a lot of harmful chemicals”; (3) “If you used IQOS regularly for the next 10 years, how likely do you think it is that you would eventually develop serious health problems? (If you currently smoke cigarettes, imagine that you switched completely to IQOS for the next 10 years and used it as frequently as you smoke cigarettes.) (1=not at all likely to 7=extremely likely); (4) “If one of your best friends was to offer you IQOS, would you try it?” (1=not at all to 7=extremely) and (5) “How likely are you to recommend IQOS to a friend or family member who smokes cigarettes?” (1=not at all to 7=extremely).
Covariates
Sociodemographic factors included: age; gender; sexual orientation (heterosexual, other); race/ethnicity (in the USA: white, black, Asian, Hispanic; in Israel: Jewish, Arab); nativity; educational attainment (<college degree (or other), ≥college degree); household income (US$ or New Israeli Shekels (NIS)); employment status (employed, other); relationship status (married/living with partner, other) and children in the home.
To assess tobacco use, participants were then asked, “In your lifetime, have you ever used: Traditional, ordinary cigarettes? E-cigarettes, vaping products or other electronic nicotine delivery devices (excluding IQOS or similar products)? HTPs, such as IQOS? Hookah/waterpipe/nargila? Cigar products? Pipe tobacco? Smokeless tobacco?” (yes vs no). Among those reporting lifetime use, past 30-day use of the respective product was assessed (no=0; yes ≥1). To characterise participants, we report ever and current (past 30-day) use of cigarettes, e-cigarettes, HTPs and other tobacco products (which included hookah, cigars, pipe and smokeless tobacco).
Data analysis
First, descriptive and bivariate analyses (χ2 for categorical variables, t-tests or analysis of variance tests for continuous variables) were conducted to characterise participants overall, by country and by current cigarette use status. Next, we conducted bivariate analyses examining average responses to the five outcome variables by experimental conditions, using Tukey’s honestly significant difference (HSD) test for post hoc comparisons. Because each participant (n=2222) evaluated 2 ads, the total N for bivariate and regression analyses was 4444, as the responses were treated as experimental outcomes.
Then, ordinal logistic regression analyses were conducted to examine the impact of the messaging approaches, respectively, on our five experimental outcomes (ie, perceived relative harm, exposure to chemicals and disease risk; likelihood of personally trying IQOS or recommending it to others who smoke), controlling for order of presentation as well as country of residence, cigarette use status and sex and applying robust clustered SE to adjust for the fact that each participant rated two messages. For the reduced risk/exposure messaging, we chose reduced exposure messaging as the reference group, as for all outcomes (except perceived relative harm), it represented the condition with the midrange mean (between control and reduced risk messaging) and thus provided the opportunity to determine if reduced exposure messaging outperformed the control condition and if reduced risk outperformed reduced exposure messaging. For FDA endorsement messaging, the control was used as the reference group. We then added the interaction terms for reduced risk/exposure messaging and FDA endorsement messaging in a subsequent block for each outcome, and used the likelihood ratio test for each model to determine overall significance. Additional analyses were conducted to assess interactions between reduced exposure/risk messaging and FDA endorsement messaging, respectively, by: (1) country, (2) cigarette use status and (3) sex. To determine the significance of interaction terms, nested likelihood ratio tests were conducted comparing the main effect models to full models including interaction terms. Analyses were conducted using Stata V.15.1 (StataCorp, College Station, Texas, USA); the significance level was set at α=0.05.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
RESULTS
Participant characteristics
Table 2 summarises participant characteristics overall (n=2222), by country (USA n=1128; Israel n=1094) and by current cigarette use status (current use: USA n=253 (22.4%), Israel n=428 (39.1%)). The sample was on average 32.19 years old (SD=7.74), 50.3% female and 15.2% sexual minority, with racial/ethnic diversity (56.2% racial/ethnic minority in the USA, 12.8% Arabs in Israel)
Table 2.
US and Israel participant characteristics and bivariate analyses examining correlates of current cigarette use, n=2222
| Variables | USA, n=1128 | Israel, n=1094 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Total | Current cigarette use* | P value | Total | Current cigarette use* | P value | |||
| No | Yes | No | Yes | ||||||
| N=2222 | N=1128 (50.8%) | N=848 (75.2%) | N=253 (22.4%) | N=1094 (49.2%) | N=664 (60.7%) | N=428 (39.1%) | |||
| M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | |||
| Sociodemographics | |||||||||
| Age, M (SD) | 32.19 (7.74) | 34.11 (7.23) | 33.79 (7.28) | 35.04 (6.95) | 0.016 | 30.21 (7.76) | 29.81 (7.87) | 30.80 (7.56) | 0.040 |
| Female, n (%) | 1118 (50.3) | 562 (49.8) | 423 (49.9) | 122 (48.2) | 0.643 | 556 (50.8) | 367 (55.3) | 189 (44.2) | <0.001 |
| Sexual minority, n (%) | 337 (15.2) | 144 (12.8) | 103 (12.2) | 36 (14.2) | 0.385 | 193 (17.6) | 129 (19.4) | 64 (15.0) | 0.058 |
| Race/Ethnicity, n (%) | |||||||||
| White, non-Hispanic | 493 (22.2) | 493 (43.7) | 360 (42.5) | 122 (48.2) | 0.253 | -- | -- | -- | |
| Black, non-Hispanic | 284 (12.8) | 284 (25.2) | 224 (26.4) | 55 (21.7) | -- | -- | -- | ||
| Asian, non-Hispanic | 177 (8.0) | 177 (15.7) | 130 (15.3) | 42 (16.6) | -- | -- | -- | ||
| Hispanic | 174 (7.8) | 174 (15.4) | 134 (15.8) | 34 (13.4) | -- | -- | -- | ||
| Jewish | 954 (42.9) | -- | -- | -- | 954 (87.2) | 581 (87.5) | 371 (86.7) | 0.693 | |
| Arab | 140 (6.3) | -- | -- | -- | 140 (12.8) | 83 (12.5) | 57 (13.3) | ||
| Foreign born, n (%) | 234 (10.5) | 105 (9.3) | 86 (10.1) | 17 (6.7) | 0.101 | 129 (11.8) | 79 (11.9) | 50 (11.7) | 0.914 |
| Education <college degree, n (%) | 1114 (50.1) | 644 (57.1) | 450 (53.1) | 176 (69.6) | <0.001 | 470 (43.0) | 283 (42.6) | 186 (43.5) | 0.785 |
| Income, n (%) | |||||||||
| ≤US$24 999 or ≤30 000 NIS | 387 (17.4) | 183 (16.2) | 112 (13.2) | 65 (25.7) | <0.001 | 204 (22.1) | 128 (24.4) | 75 (18.9) | 0.007 |
| US$25 000–US$149 999 or 30 001–192 000 NIS | 1304 (58.7) | 735 (65.2) | 556 (65.6) | 162 (64.0) | 569 (61.6) | 301 (57.3) | 268 (67.5) | ||
| ≥US$150 000 or ≥192 001 NIS | 360 (16.2) | 210 (18.6) | 180 (21.2) | 26 (10.3) | 150 (16.3) | 96 (18.3) | 54 (13.6) | ||
| Employed, n (%) | 1589 (71.5) | 825 (73.1) | 637 (75.1) | 166 (65.6) | 0.003 | 764 (69.8) | 441 (66.4) | 322 (75.2) | 0.002 |
| Married/Living with partner, n (%) | 1186 (53.4) | 601 (53.3) | 461 (54.4) | 127 (50.2) | 0.244 | 585 (53.5) | 329 (49.5) | 255 (59.6) | 0.001 |
| Children, n (%) | 1125 (50.6) | 529 (46.9) | 393 (46.3) | 123 (48.6) | 0.525 | 596 (54.5) | 360 (54.2) | 235 (54.9) | 0.823 |
| Lifetime use, n (%) | |||||||||
| Cigarettes | 1202 (54.1) | 592 (52.5) | 312 (36.8) | 253 (100) | <0.001 | 610 (55.8) | 180 (27.1) | 428 (100) | <0.001 |
| E-cigarettes | 831 (37.4) | 396 (35.1) | 206 (24.3) | 173 (68.4) | <0.001 | 435 (39.8) | 157 (23.6) | 276 (64.5) | <0.001 |
| IQOS | 307 (13.8) | 76 (6.7) | 30 (3.5) | 41 (16.2) | <0.001 | 231 (21.1) | 73 (11.0) | 157 (36.7) | <0.001 |
| Other tobacco (hookah, cigars, pipe, smokeless) | 1209 (54.4) | 564 (50.0) | 346 (40.9) | 198 (78.3) | <0.001 | 645 (59.0) | 299 (45.0) | 344 (80.4) | <0.001 |
| Current (past 30-day) use, n (%) | |||||||||
| E-cigarettes | 445 (20.3) | 170 (15.5) | 77 (9.2) | 90 (36.3) | <0.001 | 275 (25.2) | 64 (9.7) | 210 (49.1) | <0.001 |
| IQOS | 172 (7.8) | 36 (3.2) | 3 (0.4) | 31 (12.3) | <0.001 | 136 (12.5) | 26 (3.9) | 110 (25.7) | <0.001 |
| Other tobacco (hookah, cigars, pipe, smokeless) | 487 (22.3) | 168 (15.4) | 66 (7.9) | 99 (39.4) | <0.001 | 319 (29.2) | 109 (16.5) | 210 (49.1) | <0.001 |
Missing current smoking status on 27 participants in the USA and 2 in Israel.
M, mean; NIS, New Israeli Shekels.
Bivariate analyses
Bivariate analyses examining responses to the messaging (table 3) indicated significant differences in reduced risk and exposure messaging conditions across all outcomes except likelihood of personally trying IQOS if offered by a best friend. Post hoc pairwise tests (Tukey’s HSD) found that both experimental conditions (vs control) resulted in lower perceived relative harm, lower perceived exposure and greater likelihood of suggesting IQOS to smokers. However, only the reduced exposure messaging (vs control) resulted in lower perceived disease risk. Only one significant difference was found between the two experimental conditions: the reduced risk (vs reduced exposure) messaging condition resulted in lower perceived relative harm.
Table 3.
Bivariate analyses characterising responses to ad stimuli across outcomes
| Ad messaging, n (%) | Perceived relative harm a |
Perceived exposure b |
Perceived disease risk c |
Likelihood to personally try d |
Likelihood to suggest to smokers e |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Reduced risk and exposure messaging | *** | *** | * | * | |
|
| |||||
| Reduced exposure | 2.66 (1.05) | 2.81 (0.85) | 4.19 (2.09) | 2.76 (2.08) | 2.85 (1.96) |
|
| |||||
| Reduced risk | 2.56 (1.00) | 2.83 (0.86) | 4.28 (2.09) | 2.66 (2.07) | 2.83 (2.02) |
|
| |||||
| Control | 2.75 (0.99) | 2.95 (0.86) | 4.42 (2.17) | 2.64 (2.04) | 2.66 (1.93) |
|
| |||||
| FDA endorsement messaging | |||||
|
| |||||
| FDA endorsement 1 | 2.65 (1.02) | 2.85 (0.86) | 4.31 (2.08) | 2.79 (2.10) | 2.88 (2.00) |
|
| |||||
| FDA endorsement 2 | 2.67 (1.00) | 2.86 (0.88) | 4.27 (2.12) | 2.63 (2.03) | 2.75 (1.93) |
|
| |||||
| Control | 2.64 (1.03) | 2.88 (0.85) | 4.31 (2.15) | 2.64 (2.06) | 2.72 (1.98) |
P<0.05,
P<0.01,
P<.001 for ANOVA tests. See table 1 for reduced risk/exposure and FDA endorsement messages.
1=much less (compared with cigarettes) to 5=much more;
1=almost no harmful chemicals to 4=a lot of harmful chemicals;
1=not at all likely (to develop serious health problems) to 7=extremely likely;
“f one of your best friends was to offer you IQOS, would you try it?”; 1=not at all (likely to try if offered by friend) to 7=extremely;
1=not at all (likely to recommend to friend or family member who smokes cigarettes) to 7=extremely. Post hoc pairwise tests (Tukey’s HSD): risk versus exposure messaging: perceived relative harm: risk <exposure; exposure<control; risk<control. Perceived exposure: risk<control; exposure<control. Perceived disease risk: exposure <control. Likelihood to suggest to smokers: risk>control; exposure>control.
ANOVA, analysis of variance; FDA, Food and Drug Administration; HSD, honestly significant difference; M, mean.
No significant differences were found across the FDA endorsement messages (vs control) in relation to perceived relative harm, exposure and disease risk or likelihood of personally trying or suggesting IQOS to smokers.
Multivariable analyses
Control (vs reduced exposure) messaging resulted in higher perceived relative harm (control adjusted OR (aOR)=1.29, 95% CI=1.12 to 1.48), exposure (control aOR=1.34, 95% CI=1.17 to 1.54) and disease risk (control aOR=1.23, 95% CI=1.08 to 1.40), and in lower likelihood of suggesting IQOS to smokers (control aOR=0.85, 95% CI=0.74 to 0.97; table 4). Reduced risk (vs reduced exposure) messaging resulted in lower perceived relative harm (aOR=0.86, 95% CI=0.75 to 0.99).
Table 4.
Ordinal logistic regression results of experiment exposing US and Israeli adults to IQOS ads with reduced risk or exposure messaging (vs control) and two variations of FDA endorsement messaging (vs control) in relation to perceived relative harm, exposure and disease risk and likelihood of personally trying or suggesting IQOS to smokers
| Variables | Perceived relative harm |
Perceived exposure |
Perceived disease risk |
Likelihood to personally try* |
Likelihood to suggest to smokers |
|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Order (ref: 2) | 1.00 (0.94 to 1.07) | 1.12 (1.05 to 1.19) | 1.15 (1.09 to 1.21) | 1.03 (0.98 to 1.08) | 0.95 (0.91 to 0.99) |
|
| |||||
| Sociodemographics | |||||
|
| |||||
| Country USA (ref: Israel) | 1.91 (1.65 to 2.21) | 1.78 (1.54 to 2.07) | 1.09 (0.95 to 1.26) | 0.54 (0.46 to 0.64) | 0.40 (0.34 to 0.47) |
|
| |||||
| Current (past 30-day) cigarette use (ref: non-use) | 0.85 (0.72 to 0.99) | 0.52 (0.44 to 0.60) | 1.01 (0.89 to 1.15) | 8.69 (7.28 to 10.37) | 3.29 (2.79 to 3.87) |
|
| |||||
| Male (ref: female) | 0.81 (0.71 to 0.94) | 0.78 (0.68 to 0.90) | 0.80 (0.69 to 0.92) | 1.34 (1.14 to 1.57) | 1.17 (1.01 to 1.36) |
|
| |||||
| Experimental conditions | |||||
|
| |||||
| Reduced risk/exposure messaging (ref: reduced exposure†) | |||||
|
| |||||
| Reduced risk | 0.86 (0.75 to 0.99) | 1.04 (0.91 to 1.18) | 1.07 (0.95 to 1.21) | 0.97 (0.84 to 1.12) | 1.01 (0.88 to 1.16) |
|
| |||||
| Control | 1.29 (1.12 to 1.48) | 1.34 (1.17 to 1.54) | 1.23 (1.08 to 1.40) | 0.94 (0.82 to 1.08) | 0.85 (0.74 to 0.97) |
|
| |||||
| FDA endorsement (ref: control) | |||||
|
| |||||
| FDA endorsement 1 | 1.01 (0.88 to 1.16) | 0.94 (0.82 to 1.07) | 1.00 (0.88 to 1.14) | 1.14 (0.99 to 1.31) | 1.19 (1.04 to 1.37) |
|
| |||||
| FDA endorsement 2 | 1.08 (0.94 to 1.24) | 0.98 (0.86 to 1.13) | 0.98 (0.86 to 1.11) | 0.98 (0.85 to 1.14) | 1.04 (0.91 to 1.20) |
See table 1 for reduced risk/exposure and FDA endorsement messages. Bold text indicates statistically significant aORs (P<0.05).
“If one of your best friends was to offer you IQOS, would you try it?”
Models were adjusted for clustering at the individual level using robust clustered SE, given that each participant rated two messages. aOR and 95% CI for reduced risk messaging versus. control as follows: perceived relative harm aOR=0.67, 95% CI=0.58 to 0.76; perceived exposure aOR=0.77, 95% CI=0.67 to 0.88; perceived disease risk aOR=0.87, 95% CI=0.77 to 0.99; likelihood to personally try if offered by a best friend aOR=1.03, 95% CI=0.89 to 1.19; likelihood to suggest to smokers aOR=1.19, 95% CI=1.04 to 1.36.
aOR, adjusted OR; FDA, Food and Drug Administration; ref, referent group.
Only one significant finding was found for FDA endorsement messaging. FDA endorsement 1 was associated with greater likelihood of suggesting IQOS to smokers (aOR=1.19, 95% CI=1.04 to 1.37).
One interaction effect between the two experiments (reduced risk/exposure messaging×FDA endorsement messaging) was found (online supplemental table 1): participants exposed to the reduced risk message combined with FDA endorsement 1 perceived particularly high disease risk (aOR=1.43, 95% CI=1.04 to 1.97).
Regarding sociodemographics, Israeli (vs US) participants, current cigarette users (vs non-users) and men (vs women), respectively, reported lower perceived relative harm and exposure and greater likelihood of personally trying if offered by a best friend or suggesting IQOS to smokers. Men also reported lower perceived disease risk.
Analyses exploring potential moderating roles of country, current cigarette use status and sex, respectively, on messaging effects indicated no significant interactions. We also explored changes to findings if models included lifetime IQOS use and current other tobacco use, respectively (online supplemental tables 2 and 3). Both were related to lower perceived exposure and greater likelihood of trying or suggesting IQOS to smokers; other findings did not change
DISCUSSION
This study responds to the critical need of providing independent research regarding the impact of products authorised as modified risk or exposure products and their related marketing on consumer perceptions and behaviour, and ultimately public health.14–22 Findings from our experimental design indicated that, relative to the control condition, reduced exposure messaging resulted in lower perceived relative harm, exposure and disease risk, and in greater likelihood of suggesting IQOS to smokers. Moreover, compared with reduced exposure messaging, reduced risk messaging only resulted in lower perceived relative harm but no differences in any other outcomes (eg, perceived exposure and disease risk, and in greater likelihood of trying or suggesting IQOS to smokers). These results suggest that consumers do not clearly disentangle the differences in the reduced risk versus reduced exposure messaging, as noted in prior research.25–27
Moreover, this study examined if ad content that emphasises— and potentially stretches the limits—of authorised language also had an impact on consumer perceptions. Specifically, we tested two messages from real-world ads in Israel 53 that exploited FDA’s MRTP decision29–39 to determine their impact on consumer perceptions of IQOS.53 Participants were more likely to report they would suggest IQOS to smokers when they viewed an ad that said: ‘IQOS is the first and only tobacco heating system completing the US FDA examination of tobacco products. FDA concluded that IQOS is a better choice for adult smokers’. This is intuitive, as the main suggestion is to do so, and there is a role for harm reduction in today’s tobacco market. However, analyses of on our 3×3 factorial design explored potential interactions between MRTP messaging focused on reduced risk and reduced exposure and FDA endorsement messaging used by IQOS but found no interaction. Thus, it is concerning that ad messages that directly indicate that consumers should suggest IQOS to smokers is powerful regardless of reduced risk or reduced exposure messaging. There is also the remaining concern that smokers who try to switch to IQOS may not accurately interpret the notion of ‘switching completely’ from traditional cigarettes to IQOS, which is an essential component of the intended harm reduction efforts.25–27
Message framing, or emphasising certain characteristics and/or minimising others to enhance appeal of an object or idea to a consumer, can influence consumer perceptions.55 This study provides further evidence for the success of PM in framing IQOS as a reduced risk product, regardless of the scientific evidence. Previous studies from South Korea, where IQOS use is prevalent, found a common framing of IQOS as ‘reduced harm’ in the news.56 57 Thus, MRTP decisions and regulations should consider the possibility of gaps between scientific knowledge, intention and public framing. Current findings show that participants do not adequately distinguish between reduced exposure and reduced risk language—therefore not meeting the criteria for using this language in IQOS marketing45—and that PM further exploits the potential to unduly influence consumers by misrepresenting FDA authorisation in other countries. These concerns underscore the need for FDA to reconsider such language, explore alternative language that helps consumers understand this distinction and ensure that independent research (like the current study) guides their oversight of marketing for products that receive MRTP authorisation.
Regarding sociodemographics, Israeli participants, current cigarette users and men reported lower perceived relative harm and exposure and greater likelihood of personally trying if offered by a friend or suggesting IQOS to smokers. Men also reported lower perceived disease risk. Findings regarding gender and cigarette use status align with findings in the USA and elsewhere that suggest higher use rates among men and cigarette users.8 58 59 Regarding differences by country, Israel was one of the first countries where IQOS emerged and initially was unregulated and then regulated under weak legislation. In the USA, IQOS established its market in only a few states and was discontinued after only 2 years in the USA (shortly after our survey). Thus, consumer perceptions may have been impacted by their prior exposure to IQOS and its marketing.
Current findings have implications for tobacco control worldwide. First, PM’s use of FDA ‘reduced exposure’ authorisation to frame IQOS for its purposes in other countries highlights the need for stronger global collaborations to regulate and monitor HTP messaging and for FDA to participate with other authorities to ensure that its decisions are not used as marketing tactics in other countries. This type of monitoring must address communications distributed online, via social media, and via other informal channels that may reach and impact consumers in the USA and other countries that have imposed strict regulations on IQOS marketing and sales42 or banned its entry into their markets altogether.43 44
Limitations and strengths
Study limitations include limited generalisability given recruitment of participants via an online panel in the USA and via blended online methods in Israel and for subgroups (Asians) in the USA, potential differences between those who participated versus chose not to, our restricted age range (ages 18–45 years) and limited ability to conduct more in-depth analyses within current cigarette and/or IQOS users due to small subgroup sample sizes. However, the study is strengthened by the use of a randomised factorial experiment design, the use of actual IQOS ad language and a heterogenous sample with regard to sociodemographics and tobacco use behaviours, which provide strong internal validity.
CONCLUSIONS
Results from this study provide experimental evidence that, in a sample of US and Israeli adults, reduced exposure and risk (vs control) messaging decreased perceptions of harms, exposure and disease risk, and increased the likelihood of recommending IQOS to smokers. Moreover, participants did not discern reduced exposure from reduced risk messaging in relation to these outcomes. Finally, exploiting FDA MRTP authorisation in ads has the potential to amplify the effects of MRTP language on risk perceptions. Regulators and researchers worldwide must monitor direct and indirect advertising content of MRTP-authorised products and take actions to prevent potentially harmful misinterpretations.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
IQOS emerged in the USA in 2019 and received Food and Drug Administration (FDA) authorisation to use ‘reduced exposure’ claims in its marketing in 2020.
Reduced exposure messaging may be misinterpreted to indicate reduced risk.
Philip Morris has used FDA-modified exposure authorisation to promote IQOS globally.
WHAT THIS STUDY ADDS
Findings indicated that reduced exposure (vs control) messaging resulted in lower perceived relative harm, exposure and disease risk, and in greater likelihood of suggesting IQOS to smokers.
Reduced risk and reduced exposure messaging yielded similar results in relation to control messaging, with reduced risk messaging only decreasing perceived relative harm compared with reduced exposure messaging.
Messaging stating that ‘FDA concluded that IQOS is a better choice for adult smokers’ increased the likelihood of suggesting IQOS to smokers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Findings underscore concerns regarding Philip Morris’ use of FDA authorised and unauthorised claims, and potential misinterpretations.
Regulators and researchers should monitor advertising content of products authorised to use modified risk or exposure messaging in advertisements.
Acknowledgements
The authors acknowledge and thank Orly Manor, PhD and members of the External Advisory Board for their input to the study’s design, methodology and interpretation of results (by alphabetical order): Joanna Cohen PhD, Nadav Davidovitch MD, MPH, PhD, Milka Donchin MD, MPH, Anat Gesser-Edelsburg PhD, Michael Eriksen ScD, Haim Geva Haspil, Lisa Henriksen PhD, Elad Godinger LLM and Shira Kislev.
Funding
This work was supported by the US National Cancer Institute (R01CA239178-01A1; Multiple Principal Investigators [MPIs]: CJB, HL). CJB is also supported by other US National Institutes of Health funding, including the National Cancer Institute (R01CA215155-01A1; Principal Investigator [PI]: CJB; R01CA179422-01; PI: CJB), the Fogarty International Center (R01TW010664-01; MPIs: CJB, Kegler), the National Institute of Environmental Health Sciences/Fogarty (D43ES030927-01; MPIs: CJB, Caudle, Sturua) and the National Institute on Drug Abuse (R01DA054751-01A1; MPIs: CJB, Cavazos-Rehg). KFR is supported by the National Institute on Drug Abuse (F32DA055388-01; PI: KFR; R25DA054015; MPIs: Obasi, Reitzel), the Oklahoma Tobacco Settlement Endowment Trust (TSET) contract #R22-03, and the National Cancer Institute grant awarded to the Stephenson Cancer Center (P30CA225520).
HL had received fees for lectures from Pfizer Israel Ltd (distributor of a smoking cessation pharmacotherapy in Israel) in 2017. YB-Z has received fees for lectures from Pfizer, Novartis NCH and GSK Consume Health (distributors of pharmacotherapy in Israel) in the past (2012–July 2019). LCA receives royalties for the sale of Text2Quit.
Footnotes
Competing interests No other conflicts of interest are declared.
Patient and public involvement statement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Consent obtained directly from patient(s).
Ethics approval This study was approved by Institutional Review Boards of George Washington University (IRB# NCR213416) and Hebrew University (27062021).
Participants gave informed consent to participate in the study before taking part.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
REFERENCES
- 1.Tobacco Control Legal Consortium. The FDA’s premarket review of tobacco products fails to fully protect public health. St. Paul, MN: Public Health Law Center, 2017. [Google Scholar]
- 2.US Food and Drug Administration, Marketing Order. FDA submission tracking numbers (STNs): PM0000424-PM0000426, PM0000479, IQOS system holder and Charger and Marlboro Heatsticks, 2019. Available: https://www.fda.gov/tobacco-products/premarket-tobacco-product-applications/premarket-tobacco-product-marketing-orders [Accessed 06 Aug 2020].
- 3.Ratajczak A, Jankowski P, Strus P, et al. Heat not burn tobacco product—a new global trend: impact of heat-not-burn tobacco products on public health, a systematic review. Int J Environ Res Public Health 2020;17:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg CJ, Bar-Zeev Y, Levine H. Informing iQOS regulations in the United States: a synthesis of what we know. Sage Open 2020;10. doi: 10.1177/2158244019898823. [Epub ahead of print: 09 01 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International PM. In how many countries do you sell your cigarette brands? 2020. Available: https://www.pmi.com/faq-section/faq/in-how-many-countries-do-you-sell-your-cigarette-brands#
- 6.US Food and Drug Administration. Exposure modification modified risk granted orders, FDA submission tracking numbers (STNs): MR0000059-MR0000061, MR0000133, 2020, 2020. Available: https://www.fda.gov/media/139797/download [Accessed 06 Aug 2020].
- 7.Philip Morris International. GetIQOS. com, 2021. Available: https://www.getiqos.com/pages/home/where-to-find-iqos/store-locator.html
- 8.Berg CJ, Romm KF, Patterson B, et al. Heated tobacco product awareness, use, and perceptions in a sample of young adults in the United States. Nicotine Tob Res 2021;23:1967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czoli CD, White CM, Reid JL, et al. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob Control 2020;29:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Press Associated. Altria: IQOS cigarette alternative off market through 2022, 2021. Available: https://www.usnews.com/news/best-states/virginia/articles/2022-01-27/altria-iqos-cigarette-alternative-off-market-through-2022
- 11.Gretler C, Decker S. Philip Morris IQOS imports barred from U.S.; deadline passes, 2021. Available: https://www.bloomberg.com/news/articles/2021-11-29/philip-morris-iqos-imports-barred-from-u-s-as-deadline-passes
- 12.Gretler C. Philip Morris plans U.S. IQOS-Stick production after import ban; 2022. https://www.bloomberg.com/news/articles/2022-02-10/philip-morris-plans-u-s-iqos-stick-production-after-import-ban
- 13.Philip Morris International. PMI aims to become a majority smoke-free business by 2025, 2021. Available: https://www.pmi.com/our-transformation/pmi-aims-to-become-a-majority-smoke-free-business-by-2025
- 14.Glantz SA. Heated tobacco products: the example of IQOS. Tob Control 2018;27:s1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helen G St, Jacob P III, Nardone N, et al. IQOS: examination of philip morris international’s claim of reduced exposure. Tob Control 2018;27:s30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glantz SA. PMI’s own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes. Tob Control 2018;27:s9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun L, Moazed F, Matthay M, et al. Possible hepatotoxicity of IQOS. Tob Control 2018;27:s39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Max WB, Sung H-Y, Lightwood J, et al. Modelling the impact of a new tobacco product: review of philip morris international’s population health impact model as applied to the IQOS heated tobacco product. Tob Control 2018;27:s82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabuchi T, Kiyohara K, Hoshino T, et al. Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan. Addiction 2016;111:706–13. [DOI] [PubMed] [Google Scholar]
- 20.Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control 2017;26:609–10. [DOI] [PubMed] [Google Scholar]
- 21.McKelvey K, Popova L, Kim M, et al. IQOS labelling will mislead consumers. Tob Control 2018;27:s48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Toukhy S, Baig SA, Jeong M, et al. Impact of modified risk tobacco product claims on beliefs of US adults and adolescents. Tob Control 2018;27:s62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lempert LK, Bialous S, Glantz S. FDA’s reduced exposure marketing order for IQOS: why it is not a reliable global model. Tob Control 2022;31:e83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO statement on heated tobacco products and the US FDA decision regarding IQOS, 2020. Available: https://www.who.int/news/item/27-07-2020-who-statement-on-heated-tobacco-products-and-the-us-fda-decision-regarding-iqos [Accessed 06 Aug 2020].
- 25.Yang B, Massey ZB, Popova L. Effects of modified risk tobacco product claims on consumer comprehension and risk perceptions of IQOS. Tob Control 2022;31:e41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKelvey K, Baiocchi M, Halpern-Felsher B. PMI’s heated tobacco products marketing claims of reduced risk and reduced exposure may entice youth to try and continue using these products. Tob Control 2020;29:e18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen-Sankey JC, Kechter A, Barrington-Trimis J, et al. Effect of a hypothetical modified risk tobacco product claim on heated tobacco product use intention and perceptions in young adults. Tob Control 2021. doi: 10.1136/tobaccocontrol-2021-056479. [Epub ahead of print: 31 May 2021]. [DOI] [PMC free article] [PubMed]
- 28.Berg CJ, Abroms LC, Levine H, et al. IQOS marketing in the US: the need to study the impact of FDA modified exposure authorization, marketing distribution channels, and potential targeting of consumers. Int J Environ Res Public Health 2021;18:10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MaltaTo. Philip Morris receives FDA authorisation to market IQOS in US, 2020. Available: https://timesofmalta.com/articles/view/philip-morris-receives-fda-authorisation-to-market-iqos-in-us.811917 [Accessed 10 Oct 2020].
- 30.Leyco C, Bulletin M. Philip Morris urges PH to adopt US FDA finding, 2020. Available: https://mb.com.ph/2020/09/07/philip-morris-urges-ph-to-adopt-us-fda-finding/ [Accessed 10 Oct 2020].
- 31.Times Nyasa. Malawi: agriculture Minister says smokeless cigarette technologies to boost Malawi tobacco industry, 2020. Available: https://allafrica.com/stories/202009100285.html [Accessed 10 Oct 2020].
- 32.Africa All. Zimbabwe: heating system to rescue tobacco industry, 2020. Available: https://allafrica.com/stories/202009070903.html [Accessed 10 Oct 2020].
- 33.Mushava E, Zimbabwe N. Boost for Zim’s tobacco industry NewsDay Zimbabwe; 2020. https://www.newsday.co.zw/2020/07/boost-for-zims-tobacco-industry/ [Accessed 31 Aug 2020].
- 34.Satigui S. Africa: Modified Risk Tobacco Product - Historic FDA Decision and Lessons for Africa All Africa; 2020. https://allafrica.com/stories/202007090930.html [Accessed 31 Aug 2020].
- 35.Han-soo L. FDA designation of IQOS as modified risk should change regulatory regime Korea Biomedical Review; 2020. http://www.koreabiomed.com/news/articleView.html?idxno=9180 [Accessed 10 Oct 2020].
- 36.Byung-yeul B. Philip Morris’ IQOS gets FDA approval to market as modified risk product The Korea Times; 2020. https://www.koreatimes.co.kr/www/tech/2020/07/694_292616.html [Accessed 31 Aug 2020].
- 37.Chow T. FDA statement on IQOS may prevent ban on alternative smoking products in Hong Kong Harbour Times; 2020. https://harbourtimes.com/?s=FDA+statement+on+IQOS+may+prevent+ban+on+alternative+smoking+products+in+Hong+Kong [Accessed 31 Aug 2020].
- 38.Gilmore AB, Braznell S. US regulator adds to confusion around heated tobacco products. BMJ 2020;370:m3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobacco Tactics, University of Bath. PMI promotion of IQOS using FDA MRTP order, 2020. Available: https://tobaccotactics.org/wiki/pmi-iqos-fda-mrtp-order/ [Accessed 10 Oct 2020].
- 40.Klein Y. For the first time in history: the FDA has Approved the electric cigarette, 2021. Available: https://www.bhol.co.il/news/1116473
- 41.US Food and Drug Administration. FDA Authorizes Marketing of IQOS Tobacco Heating System with ‘Reduced Exposure’ Information, 2020. Available: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information
- 42.Campaign for Tobacco-Free Kids. Heated tobacco products: global regulation, 2020. Available: https://www.tobaccofreekids.org/assets/global/pdfs/en/HTP_regulation_en.pdf [Accessed 06 Aug 2020].
- 43.Greenhalgh EM. Heated tobacco (‘heat-not-burn’) products. In: Scollo MM, WinstanleyH, eds. Tobacco in Australia: facts and issues. Melbourne: Cancer Council Victoria, 2018. http://www.tobaccoinaustralia.org.au/chapter-18-harm-reduction/indepth-18c-non-combustible-cigarettes/18c-5-legal-status-in-australia [Google Scholar]
- 44.Patanavanich R, Glantz S. Successful countering of tobacco industry efforts to overturn thailand’s ends ban. Tob Control 2021;30:e10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Food and Drug Administration. Section 911 of the Federal Food, Drug, and Cosmetic Act - Modified Risk Tobacco Products. Washington, DC: US Food and Drug Administration, 2018. [Google Scholar]
- 46.Phan L, Strasser AA, Johnson AC, et al. Young adult correlates of IQOS curiosity, interest, and likelihood of use. Tob Regul Sci 2020;6:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karim MA, Talluri R, Chido-Amajuoyi OG, et al. Awareness of heated tobacco products among US adults - health information national trends survey, 2020. Subst Abus 2022;43:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyman AL, Weaver SR, Popova L, et al. Awareness and use of heated tobacco products among US adults, 2016–2017. Tob Control 2018;27:s55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi J, Lee CM, Hwang S-S, et al. Prevalence and predictors of heated tobacco products use among male ever smokers: results from a Korean longitudinal study. BMC Public Health 2021;21:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gravely S, Fong GT, Sutanto E, et al. Perceptions of harmfulness of heated tobacco products compared to combustible cigarettes among adult smokers in Japan: findings from the 2018 ITC Japan survey. Int J Environ Res Public Health 2020;17. doi: 10.3390/ijerph17072394. [Epub ahead of print: 01 04 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The GfK Group. Knowledge panel design summary. Palo Alto, CA, 2017. https://silo.tips/download/knowledgepanel-design-summary [Google Scholar]
- 52.Berg CJ, Romm KF, Bar-Zeev Y, et al. IQOS marketing strategies in the USA before and after us FDA modified risk tobacco product authorisation. Tob Control 2021. doi: 10.1136/tobaccocontrol-2021-056819. [Epub ahead of print: 19 Oct 2021]. [DOI] [PMC free article] [PubMed]
- 53.Khayat A. IQOS adspend and marketing messages in paid ads in Israel (December 2016 – August 2020) Poster at European Public Health Conference, Virtual; 2021. [Google Scholar]
- 54.PMI Research and Development. PMI research and development, study report THS-PBA-05-REC-US: quantitative assessement of THS 2.2 label, labeling and marketing material with reduced exposure claims, 2016. Available: https://digitalmedia.hhs.gov/tobacco/static/mrtpa/732pba/PBA05REC.zip
- 55.Entman RM. Framing: toward clarification of a fractured paradigm. J Commun 1993;43:51–8. [Google Scholar]
- 56.Jun J, Kim S-H, Thrasher J, et al. Heated debates on regulations of heated tobacco products in South Korea: the news valence, source and framing of relative risk/benefit. Tob Control 2022;31:e57–63. [DOI] [PubMed] [Google Scholar]
- 57.Kim S-H, Jun J, Thrasher JF, et al. News media presentations of heated tobacco products (HTPs): a content analysis of newspaper and television news coverage in South Korea. J Health Commun 2021;26:299–311. [DOI] [PubMed] [Google Scholar]
- 58.Marynak KL, Wang TW, King BA, et al. Awareness and ever use of “heat-not-burn” tobacco products among U.S. adults, 2017. Am J Prev Med 2018;55:551–4. [DOI] [PubMed] [Google Scholar]
- 59.Nyman AL, Weaver SR, Popova L, et al. Awareness and use of heated tobacco products among US adults, 2016–2017. Tob Control 2018;27:s55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.